Abstract
The Japanese pear (Pyrus pyrifolia Nakai) is one of the most widely grown fruit trees in Japan, and it has been used throughout Japan’s history. The commercial production of pears increased rapidly with the successive discoveries of the chance seedling cultivars ‘Chojuro’ and ‘Nijisseiki’ around 1890, and the development of new cultivars has continued since 1915. The late-maturing, leading cultivars ‘Niitaka’ and ‘Shinko’ were released during the initial breeding stage. Furthermore, systematic breeding by the Horticultural Research Station (currently, NARO Institute of Fruit Tree Science, National Agriculture and Food Research Organization (NIFTS)) began in 1935, which mainly aimed to improve fruit quality by focusing on flesh texture and black spot disease resistance. To date, 22 cultivars have been released, including ‘Kosui’, ‘Hosui’, and ‘Akizuki’, which are current leading cultivars from the breeding program. Four induced mutant cultivars induced by gamma irradiation, which exhibit some resistance to black spot disease, were released from the Institute of Radiation Breeding. Among these cultivars, ‘Gold Nijisseiki’ has become a leading cultivar. Moreover, ‘Nansui’ from the Nagano prefectural institute breeding program was released, and it has also become a leading cultivar. Current breeding objectives at NIFTS mainly combine superior fruit quality with traits related to labor and cost reduction, multiple disease resistance, or self-compatibility. Regarding future breeding, marker-assisted selection for each trait, QTL analyses, genome-wide association studies, and genomic selection analyses are currently in progress.
Keywords: fruit quality, disease resistance, self-compatibility, variety, marker-assisted selection
Introduction
Pears belong to the genus Pyrus (Rosaceae, Pyrinae), and are commercially important fruit trees. There are at least 29 widely recognized primary species and 11 naturally occurring interspecific hybrid taxa, which are distributed in temperate Asia, Europe, and mountainous areas of northern Africa (USDA 2015). The genus Pyrus is believed to have arisen during the Tertiary period in the mountainous regions of western China, and dispersal is believed to have followed both eastern and western mountain chains. Vavilov (1951) listed three centers of diversity for cultivated pears, which he regarded as centers of origin: the Chinese center where P. pyrifolia Nakai (synonymous with P. serotina Rehd.) and P. ussuriensis Maxim. are grown; the central Asiatic center where P. communis L. or intermediate forms between P. communis and P. ×bretschneideri Rehd. (Yu and Zhang 1979) are grown; and the Near Eastern center where P. communis is grown. Regarding the infrageneric classification of the genus Pyrus, Koehne (1890) proposed a system that divided the genus into two sections based on the persistence of calyx on the mature fruit. However, Bailey (1916) proposed a system that divided the genus into occidental and oriental pear groups, and this system was approved by several authors (Challice and Westwood 1973, Lee 1948, Rubtsov 1944). Recent investigations of chloroplast DNA polymorphisms (Iketani et al. 1998, Zheng et al. 2014) indicated that the oriental and occidental Pyrus species may have evolved independently, and these results also support the latter classification system.
Regarding deciduous tree fruits, worldwide pear production is second to that of apples. In 2013, 25,208 kilotons of pear fruit, accounting for 3.7% of all fruit production, was produced in over 80 countries (FAOSTAT, http://faostat.fao.org/). Furthermore, Japan ranked tenth in the world following pear production totaling 294,000 tons, which accounted for 1.2% of the total global production. The Japanese pear, P. pyrifolia is the main cultivated species in Japan, and it accounts for 87.3% of the total planted area allocated to pears. Moreover, the value of Japanese pear production was estimated at more than 70 billion yen, which ranks fourth after citrus, apple, and grape production among tree fruit crops.
Global pear breeding was reviewed by Layne and Quamme (1975), Bell et al. (1996), Bell and Itai (2011), Brewer and Palmer (2011) and Dondini and Sansavini (2012). Regarding Japanese pear breeding and genetics, Machida (1979) summarized the history and achievements until the 1970s. Thereafter, Abe and Kotobuki (2004) reviewed Japanese pear breeding and summary reports on the inheritance of individual Mendelian genes in the species. This review focuses on advances in Japanese pear breeding, and it emphasizes the history of improvement, achievement, and recent genetic and genomic progress regarding important breeding traits.
Historical background
Japanese pears have been utilized throughout Japan’s history. For instance, the seeds of pears that were consumed as food were found during excavations of the Toro Ruins in Shizuoka prefecture (Tokai district), which were from the late stage of the Yayoi Period (1st century AD). With respect to the cultivation of pears, the first evidence was found in the Chronicles of Japan (720 AD), which state that the cultivation of fruits and nuts (including pears and vegetables) was promoted during the Jito Tenno era (686–696 AD) in order to fight famine. Furthermore, the concept of cultivars and cultural techniques were developed during the middle of the Edo era (1603–1867). ‘Shokokusanbutsuchou’, first mentioned in 1735, was the first recorded Japanese pear cultivar, and it was mentioned along with over 100 pear cultivars (Kajiura 2013). Commercial pear orchards were established in the late Edo era, and over 1,000 cultivar names have since been recorded (Kajiura and Sato 1990). With the successive discoveries of two chance seedlings, ‘Nijisseiki’ and ‘Chojuro’ (syn. ‘Choujuurou’) around 1890 (Kajiura 1996, Kotobuki 1996a), commercial pear production substantially increased (Kajiura 1994, Machida 1979).
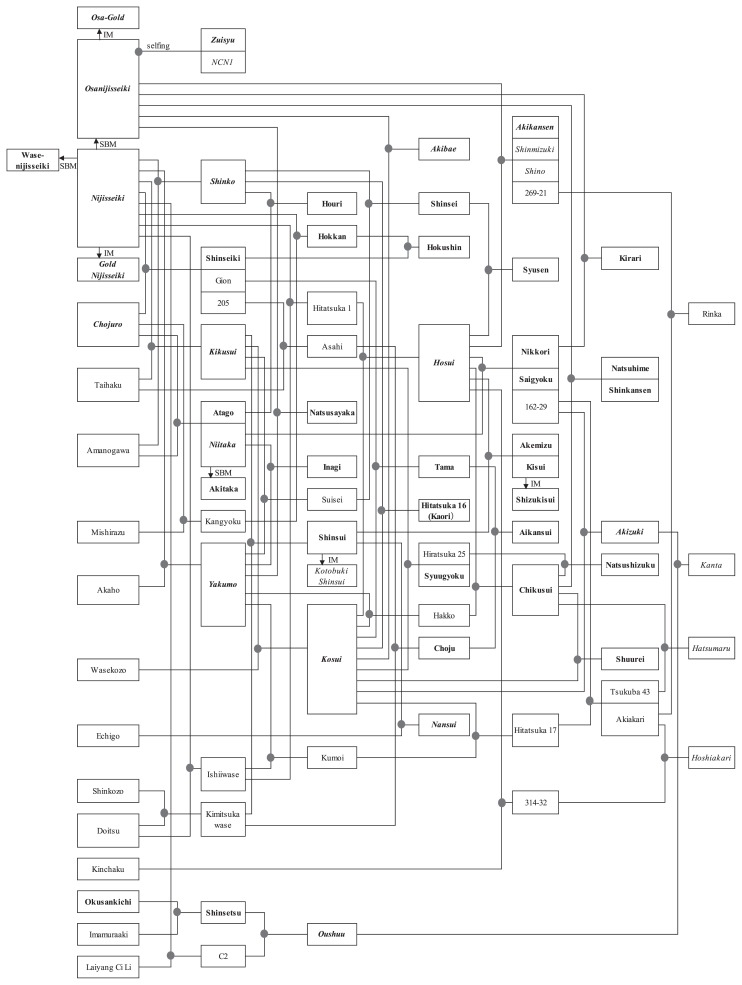
Because of its superior flesh texture, ‘Nijisseiki’ accounted for more than 30% of the total area planted with Japanese pears from the end of 1940’s to the early 1980’s (Machida 1979, Ministry of Agriculture, Forestry and Fisheries (MAFF) 1979, 1985, 1987). This cultivar flourished despite its extreme susceptibility to black spot disease; hence, extensive spraying of fungicides (>20 treatments during a growing season) and bagging was required for its cultivation. Furthermore, it is noteworthy that since ‘Nijisseiki’ and its bud mutant were frequently used as cross-parents, almost all of the recent cultivars bred in Japan descended from ‘Nijisseiki’ (Fig. 1). On the other hand, because of its high productivity and disease resistance, ‘Chojuro’ occupied more than 30% of the total planted area until the early 1970’s (Machida 1979, MAFF 1979), despite inferior flesh texture compared to ‘Nijisseiki’.
Fig. 1.
Pedigree of the cultivars cultivated in Japan in 2012 and those referred in this review. Cultivars cultivated in 2012 based on the Ministry of Agriculture, Forestry, and Fisheries statistics are represented in bold. Cultivars referred in this review are represented in italics. IM: induced mutant, SBM: spontaneous bud mutant, NCN1: Nashi chuukanbohon nou 1 gou.
Pioneers of Japanese pear breeding in Japan
The first Japanese pear breeding was conducted by Tanikawa from 1909 to 1925 at the Horticultural Research Station, presently known as the NARO Institute of Fruit Tree Science, National Agriculture and Food Research Organization (henceforth NIFTS), and it included crossing Japanese pears with European pears and Chinese pears, respectively (Machida 1979). However, no commercial cultivar was obtained. A breeding program planned by Kikuchi was started in the Kanagawa Prefectural Horticultural Experiment Station in 1915, and it used 23 Japanese, one Chinese, and nine European cultivars and hybrids. The main achievement of this program was the release of ‘Niitaka’, ‘Kikusui’, and ‘Yakumo’ cultivars. ‘Niitaka’ has become a leading cultivar, and ‘Yakumo’ and ‘Kikusui’ which is a cross-parent of ‘Kosui’ played important roles in subsequent breeding programs in Japan. Several years later, a breeding program was initiated in Okayama and Niigata prefectures, which resulted in the release of ‘Shinseiki’ in 1945 and ‘Shinko’ in 1941, respectively; ‘Shinko’ has since become a leading cultivar.
‘Niitaka’
‘Niitaka’ was released as the original cross-seedling of ‘Amanogawa’ and ‘Imamuraaki’ in 1927 by Kikuchi. However, Takasaki et al. (2004) questioned this parent-offspring relationship based on the mode of inheritance of the S-genotype, and Sawamura et al. (2008) elucidated its parentage as ‘Amanogawa’ and ‘Chojuro’ using both S-RNase and SSR markers (Fig. 1). ‘Niitaka’ ripens from the end of September to early October, and it is resistant to black spot disease. The flower has little pollen. The fruit is orbicular in shape with russet skin, and it weighs approximately 700 g. ‘Niitaka’ is productive, and the yield is around 40–50 ton/ha. Tourist farms, which have increased since the 1980s, aimed to cultivate late-maturing cultivars beside the early-maturing ‘Kosui’ and the mid-maturing ‘Hosui’ in order to extend the product period. Among the late-maturing cultivars, ‘Niitaka’ matched the demand because of its superior fruit quality, large fruit size, long shelf life, and harvesting time that is relatively close to that of ‘Hosui’. The ‘Niitaka’ cultivation area reached approximately 1,500 ha during the early 2000s, but it decreased to 1,060 ha in 2012 (MAFF 2015).
‘Shinko’
‘Shinko’ was originally released as the open-pollinated seedling of ‘Nijisseiki’. However, Sawamura et al. (2008) elucidated its parentage as ‘Nijisseiki’ and ‘Amanogawa’ using S-RNase and SSR-markers (Fig. 1). ‘Shinko’ ripens after ‘Niitaka’, and it is resistant to black spot disease. The fruit is fusiform in shape with russet skin, and it weighs about 400 g. ‘Shinko’ is productive, and the yield is almost comparable with that of ‘Niitaka’. The ‘Shinko’ cultivation area reached 400 ha in the early 2000s, but it decreased to 250 ha in 2012 (MAFF 2015).
Initiation of organized breeding at NIFTS
An organized Japanese pear breeding program supported by governmental funds was initiated in 1935 by NIFTS. The breeding objective was to develop black spot disease resistance with excellent flesh texture that was superior to that of ‘Nijisseiki’.
Fruit quality improvement has been the most important objective of Japanese pear breeding programs. Japanese pear fruit quality parameters include texture, sweetness, acidity, juiciness, flavor, and appearance. Among these traits, improvement of fruit texture has been desirable because the Japanese pear is also called the “sand pear” because of the gritty texture of the traditional cultivars. Mori (1953) noted that the frequency of excellent texture similar to ‘Nijisseiki’ is extremely low unless both parents exhibit textures comparable to ‘Nijisseiki’. Based on histological and chemical studies by Machida and Maeda (1966) and Machida and Tashiro (1968), Japanese pear texture is known to be more strongly influenced by flesh firmness than by grit stone density. Investigations of the relationship between flesh firmness and sensory evaluation for texture revealed that the texture of soft fruits was superior to that of firm ones. Therefore, firmness measurement was proposed as a simple method of quantitative texture expression. In addition, Machida and Kozaki (1975, 1976) estimated the heritability of four traits (flesh firmness, Brix, pH, and fruit weight) based on statistical genetic analyses, which used randomly selected combinations of hybrid seedlings from the breeding program. The flesh firmness showed the highest heritability (h2 = 0.60–0.70), followed by both Brix and pH (0.50–0.60) and fruit weight (0.05–0.10).
These results contributed to the development of a selection system and breeding strategy at NIFTS. Regarding flesh firmness, the difference between the selection criterion and the average of Japanese pear cultivar at the time was large and heritability was high; therefore, flesh firmness became the most important breeding objective with high selection efficiency. Because of its high heritability, Brix was also considered an important objective following flesh firmness. However, despite its high heritability, pH was not considered important, because the selection criterion was almost equivalent to those of the main cultivars. Fruit weight was not considered an important selection objective for similar reasons. As a result, recently released cultivars seem to be bred successfully based on texture improvement (Kajiura 2002, Machida and Kozaki 1975).
Regarding black spot disease resistance, the disease in Japanese pear is caused by the fungus Alternaria gaisen Nagano (Simmons 1993, Simmons and Roberts 1993) (synonymous with A. kikuchiana Tanaka, Alternaria alternata (Fries) Keissler (Japanese pear pathotype)). The fungal pathogen produces host-specific toxins AK I and II, which are responsible for necrosis on leaves, shoots, and fruits (Nakashima et al. 1982, 1985, Nishimura et al. 1978, Ohkawa and Torikata 1967, Otani et al. 1972, 1975, 1985). Tanaka (1933) reported several black spot-resistant varieties among the commonly cultivated ones in Japan based on field observations and inoculation experiments, and Kozaki (1973) analyzed the genetic behavior of black spot-resistant cultivars. Among the 160 Pyrus genotypes, 129 were identified as resistant, including ‘Kosui’ and ‘Hosui’, and 31 genotypes were susceptible, including ‘Nijisseiki’. The mode of inheritance of black spot resistance was elucidated based on Mendelian laws, and the susceptibility allele (A) was dominant. In addition, all of the investigated genotypes were heterozygous, and these results efficiently contributed to the development of black spot resistance.
After obtaining this knowledge, NIFTS released seven cultivars until the 1970s (Kajiura et al. 1967, 1969, 1974, Kotobuki 1996b), and five of the seven cultivars were black spot resistant. Among these, ‘Kosui’ and ‘Hosui’ became leading cultivars, and they were cultivated throughout the country because of their excellent texture and taste.
‘Kosui’ (syn. ‘Kousui’)
‘Kosui’ originated from a cross between ‘Kikusui’ and ‘Wasekozo’, and was released in 1959 (Fig. 1). The ripening time is near middle to late August, and it is resistant to black spot disease. The fruit is orbicular to oblate in shape with partly russet skin, and it weighs 350 g. ‘Kosui’ is moderately productive, and the yield is around 30–35 ton/ha. The fruit flesh is soft, juicy, and sweet with very fine texture. The ‘Kosui’ cultivation area reached approximately 6,800 ha in the mid-1990s, but it decreased to 4,400 ha in 2012 (MAFF 2015). ‘Kosui’ is the principal cultivar in Japan.
‘Hosui’ (syn. ‘Housui’)
‘Hosui’ was released in 1972 as the cross-seedling of ‘Ri-14’ and ‘Yakumo’ (Kajiura et al. 1974). However, Machida et al. (1982) reported that this parent-offspring relationship included discrepancies regarding fruit skin color and S-genotype. Using 61 SSR markers and chloroplast DNA, Sawamura et al. (2004) found that ‘Kosui’ was the female parent of ‘Hosui’, and ‘Hiratsuka 1’ (‘I-33’) was the male parent (Fig. 1). ‘Hosui’ ripens near early to mid-September, and it is resistant to black spot disease. The fruit is round in shape with russet skin, and weighs 450 g. ‘Hosui’ is productive, and the yield is around 35–40 ton/ha. The fruit flesh is crisp, juicy, and sweet with some acidity. ‘Hosui’ sometimes exhibits a disorder termed “watercore” (Kajiura et al. 1976), which is more likely to occur during years with cool summers (Inomata et al. 1993). Advanced maturity in the fruit flesh compared to that of the fruit skin is a key factor associated with the development of watercore (Inomata et al. 1993, 1999, Sakuma et al. 1995, 2000). The ‘Hosui’ cultivation area reached approximately 4,200 ha in the mid-1990s, but it decreased to 3,000 ha, by 2012 (MAFF 2015).
Breeding after diffusion of ‘Kosui’ and ‘Hosui’ and the registration trends of new cultivars to MAFF, Japan
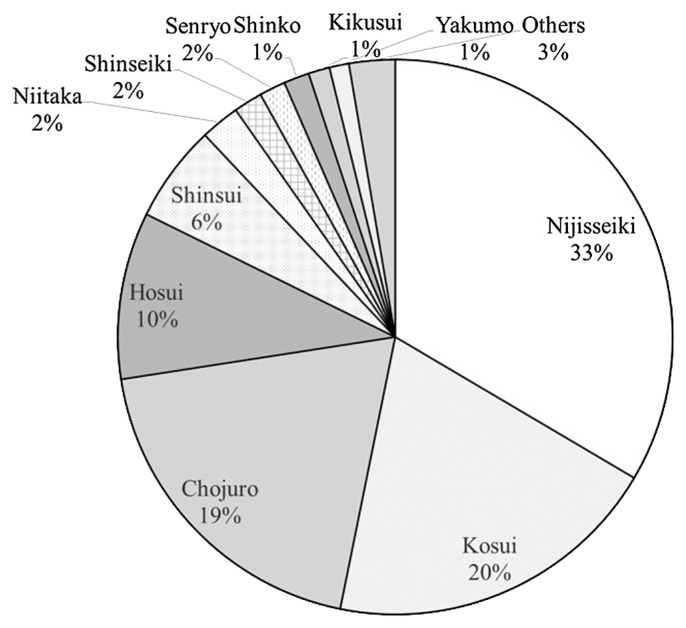
Around 1980, the total Japanese pear production mass and cultivated area in Japan reached 500 kilotons and 20,000 ha, respectively, with the spread of ‘Kosui’ and ‘Hosui’ and the plateauing of ‘Nijisseiki’ cultivation. In 1982, the share of cultivated areas among leading cultivars and others was as follows: ‘Nijisseiki’ (33.0%), ‘Kosui’ (20.0%), ‘Chojuro’ (19.0%), ‘Hosui’ (10.0%), ‘Shinsui’ (6.0%), ‘Niitaka’ (2.0%), ‘Shinseiki’ (2.0%), ‘Senryo’ (2.0%), ‘Kikusui’ (1.0%), and ‘Yakumo’ (1.0%) (MAFF 1983) (Fig. 2). Subsequently, many cultivars exhibiting superior fruit quality with maturing times differing from ‘Kosui’ and ‘Hosui’ were developed, including those at NIFTS (Kotobuki et al. 1991a, 1991b, 1991c, 1994, 2002, 2004a, 2004b, 2004c, Machida et al. 1984a, Saito et al. 2009, 2014a, 2014b, 2015a, 2015b, 2015c).
Fig. 2.
Varietal shares of Japanese pear production in Japan (1982) based on statistics of the Ministry of Agriculture, Forestry, and Fisheries (1983).
As an alternate strategy, in 1962, mutation breeding for Japanese pear using gamma irradiation was initiated by the Institute of Radiation Breeding. Since the 1980s, several induced mutants that show some degree of resistance to black spot disease have been selected from ‘Nijisseiki’, ‘Osanijisseiki’, ‘Shinsui’, and ‘Kisui’ using chronic or acute gamma irradiation (Masuda et al. 1997, Sanada 1988, Sanada et al. 1988). Among these, four cultivars were released as ‘Gold Nijisseiki’ (Kotobuki et al. 1992), ‘Osa Gold’ (Masuda et al. 1998), ‘Kotobuki Shinsui’ (Kitagawa et al. 1999), and ‘Shizukisui’ (Sawano et al. 2011) (Fig. 1).
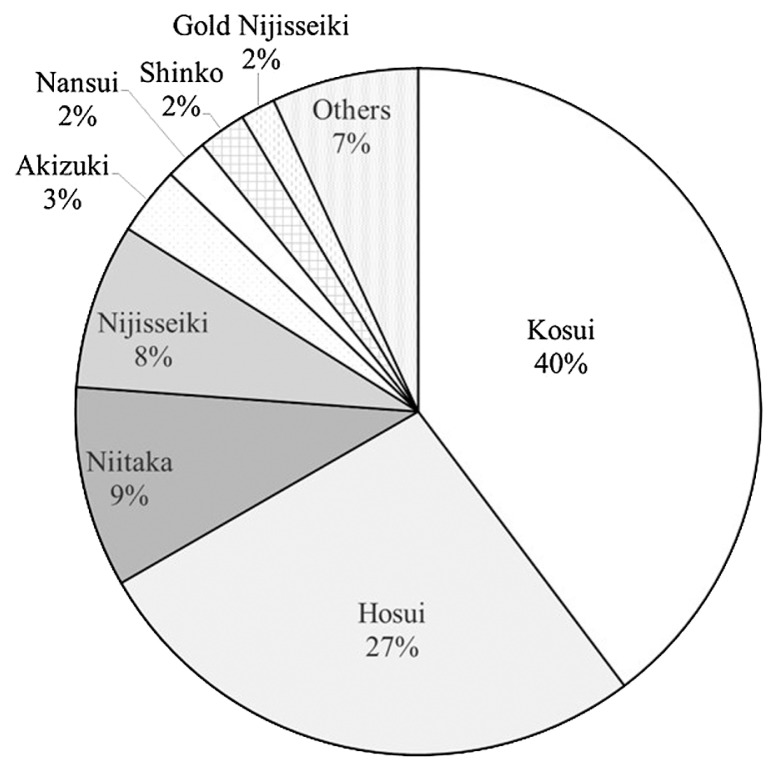
In 1978, Japan joined the International Union for the Protection of New Varieties of Plants (UPOV) and enacted the Plant Variety Protection and Seed Act. Based on data accessible through MAFF web pages (until July 2015), 97 newly developed Japanese pear cultivars have been registered. Among these, 74 resulted from crossing, five from chance seedlings, six from open-pollination, eight from spontaneous bud sports of existing cultivars, and four from mutation breeding. Sixty-one of 74 cross-bred cultivars were the offspring of Japanese pears, and 13 cultivars descended from Chinese or European pears. When registered cultivars were classified by harvesting time, including early (30), early mid (10), mid (29), mid-late (12), and late (16). The data indicated interest in the development of early ripening cultivars among breeders. Forty-four of 97 cultivars are registered by public institutions such as prefectural, university, and government institutes, excluding NIFTS. Moreover, 38 cultivars are registered by private breeders, and 15 cultivars are registered by NIFTS. From these registered cultivars, ‘Akizuki’, ‘Nansui’, and ‘Gold Nijisseiki’ have become leading cultivars. The varietal share of the cultivated areas in 2012 among leading cultivars and others was as follows: ‘Kosui’ (40.0%), ‘Hosui’ (27.0%), ‘Niitaka’ (9.0%), ‘Nijisseiki’ (8.0%), ‘Akizuki’ (3.0%), ‘Nansui’ (2.0%), ‘Shinko’ (2.0%), and ‘Gold Nijisseiki’ (2.0%) (MAFF 2015) (Fig. 3). During the 30 years after 1982, the total production area decreased by approximately 22.0%, but the selection for planting and concentration associated with ‘Kosui’, ‘Hosui’, and ‘Niitaka’ has continued. As a result, the ‘Chojuro’ cultivation area decreased to 66 ha, and it was replaced by ‘Kosui’ and ‘Hosui’. Similarly, the ‘Nijisseiki’ cultivation area and the varietal share decreased by about 85.0% and 75.0%, respectively.
Fig. 3.
Varietal shares of Japanese pear production in Japan (2012) based on statistics of the Ministry of Agriculture, Forestry, and Fisheries (2015).
‘Akizuki’
‘Akizuki’ was released in 1998 by NIFTS, and it originated from the cross between 162-29 (‘Niitaka’ × ‘Hosui’) and ‘Kosui’ (Kotobuki et al. 2002) (Fig. 1). ‘Akizuki’ ripens between ‘Hosui’ and ‘Niitaka’, and it is resistant to black spot disease. The fruit is oblate in shape with russet skin, and weighs about 500 g, which makes the fruit larger than that of ‘Hosui’. ‘Akizuki’ is productive, and the yield is almost comparable with that of ‘Hosui’. The ‘Akizuki’ cultivation area increased due to superior fruit appearance and fruit quality, which is comparable to ‘Kosui’ and ‘Hosui’, and the total area reached 360 ha (fifth position next to ‘Nijisseiki’) (MAFF 2015).
‘Nansui’
‘Nansui’ was released by Nanshin Agricultural Experiment Station in Nagano prefecture in 1990 (Ogawa et al. 2002), and it originated from the cross between ‘Echigo’ and ‘Shinsui’ made in 1973 (Fig. 1). ‘Nansui’ ripens between ‘Hosui’ and ‘Niitaka’, and the fruit is oblate in shape with russet skin, weighing approximately 400 g. The ‘Nansui’ cultivation area reached 263 ha, accounting for about 24.0% of the total in Nagano prefecture (MAFF 2015), and this area has slightly increased despite susceptibility to black spot disease, which is associated with high sugar content and long storage capability.
‘Gold Nijisseiki’
‘Gold Nijisseiki’, which is a mutant induced by chronic gamma irradiation, was released in 1991 by the Institute of Radiation Breeding (Fig. 1). ‘Gold Nijisseiki’ shows resistance to black spot that is intermediate between that of ‘Chojuro’ and ‘Nijisseiki’, and the intermediate degree of resistance inherited by the offspring as well as incomplete recessive mutations were suggested to be induced in the L-II cell layer (Sanada et al. 1994). Other agronomic characteristics of ‘Gold Nijisseiki’ are the same as those of the original ‘Nijisseiki’, except for a slightly later harvest time. The ‘Gold Nijisseiki’ cultivation area reached 400 ha in the early 2000s, but it decreased to 183 ha in 2012 (MAFF 2015).
Recent Japanese pear breeding, genetics, and the development of DNA markers for specific characteristics
The current Japanese pear breeding objectives at NIFTS mainly focus on combining superior fruit quality with multiple disease resistance (e.g., resistance to pear scab and black spot diseases) and/or self-compatibility. Development of cultivars with early ripening or attractive appearance is also important, and avoiding inbreeding depression is essential for future breeding. In order to efficiently breed cultivars with desirable characteristics, genetic study and development of DNA markers for these traits have recently been actively promoted.
Fruit quality
Abe et al. (1995) estimated the heritability of four traits (flesh firmness, Brix, pH, and fruit weight) based on statistical genetic analyses, which used randomly selected combinations of hybrid seedlings from the breeding program at NIFTS from 1989 to 1991. Fruit weight showed the highest heritability (0.57–0.82), followed by the pH of fruit juice (0.58–0.69), the soluble solid content (0.37–0.50) and flesh firmness (0.14–0.56). The estimated heritability for flesh firmness was much lower than that estimated by Machida and Kozaki (1976). This may be due to the decrease in genetic variance among cross-seedling populations that resulted from intensive selection associated with flesh firmness, which has progressed in the breeding program. Since the value of flesh firmness was in the range of 18–27 newtons (4–6 pounds) for the commercial cultivar of Japanese pear, the value of flesh firmness 23 of newtons (5 pounds) was adopted as the selection criterion for flesh firmness and seedlings with flesh firmness more than 23 newtons were discarded in the breeding program at NIFTS (Machida et al. 1984b). As a result, the heritability for fresh firmness was low, and the frequency of cross-seedlings that bore fruits with flesh firmness below the threshold of 23 newtons was high in this study. This result suggested that flesh firmness was not an effective selection criterion in the recent breeding program. Currently, both sugar content and composition are important as well as flesh firmness. In mature pear fruit, the major storage sugars include sucrose, glucose, fructose, and sorbitol (Kajiura et al. 1979, Yamaki and Moriguchi 1989). Since the sweetness of fructose and sucrose are higher than that of glucose and sorbitol, cultivars with fruit containing high levels of fructose and sucrose are desirable. Kajiura et al. (1979) investigated the sugar content and composition of 79 East Asian pear genotypes, and the more recently selected or discovered cultivars contained higher total sugar levels. These results suggested that the fruit sugar content had been improved through artificial selection. Almost all cultivars found in Chiba or Kanagawa prefectures in the Southern Kanto area before and after the Meiji Restoration (late 19th century) showed a higher sucrose percentage than the older native cultivars. Among them, ‘Nijisseiki’ and ‘Chojuro’ showed the highest sucrose content, and the sugar composition of newly bred cultivars differed from that of ‘Nijisseiki’ and ‘Chojuro’. Moriguchi et al. (1992) investigated the relationship between the accumulated sucrose content and the activity of enzymes that metabolize sucrose, including sucrose synthase (SS), sucrose-phosphate synthase (SPS), and acid invertase. Among 23 pear cultivars, SS activity was closely correlated with sucrose content, while SPS activity was weakly correlated. However, the relationship between soluble acid invertase activity and sucrose content was not significant. These results indicated that SS and SPS are important determinants of sucrose accumulation in Asian pear fruit, and that a decrease in soluble acid invertase activity is not absolutely required for sucrose accumulation. The acquisition of genetic and genomic information associated with sugar content and composition is currently underway.
Resistance to pear scab disease
The severity of pear scab disease has increased in most cultivars, particularly ‘Kosui’, during recent years. This disease is caused by two fungal species, Venturia nashicola Tanaka et Yamamoto (Tanaka and Yamamoto 1964) and V. pirina Aderh. V. pirina infects European pears, and V. nashicola infects Japanese pears; however, none of the commercial cultivars are resistant. Ishii et al. (1992) found that P. pyrifolia cultivars ‘Kinchaku’ and ‘Suishu’, P. ×bretshneideri cultivars ‘Hongli’ and ‘Mili’, P. communis cultivars ‘Flemish Beauty’ and ‘La France’, P. ussuriensis Maxim. var. aromatica (Nakai et Kikuchi) Ohwi cultivar ‘Iwateyamanashi’, and several Pyrus species are highly resistant to V. nashicola, with no visible symptoms after inoculation via conidial suspension. Abe and Kurihara (1993a) confirmed that ‘Kinchaku’, ‘Hong Li’, ‘La France’, ‘Iwateyamanashi’, and several other Pyrus cultivars are highly resistant to V. nashicola. Inheritance analyses indicated that the highly resistant phenotype found in ‘Kinchaku’, ‘Hongli’, and ‘Mili’ is controlled by a single dominant gene (Vn), and the genotype of each cultivar is heterozygous (Abe and Kotobuki 1998a). Allelism tests suggested that necrotic reactions to V. nashicola, which display symptoms without sporulation, found in several Japanese and Chinese cultivars are controlled by polygenes (Abe and Kotobuki 1998b). Additionally, European pear cultivars ‘Bartlett’ and ‘La France’ that are highly resistant to V. nashicola are controlled by a single dominant gene, and the genotype of each cultivar is homozygous (Abe et al. 2000). Iketani et al. (2001) detected six RAPD markers associated with resistance to pear scab disease by constructing genetic linkage maps of the Japanese pear cultivars ‘Kinchaku’ and ‘Kosui’. Furthermore, Terakami et al. (2006) identified the position of the scab resistance gene, which is designated Vnk in the Japanese pear cultivar ‘Kinchaku’. Vnk was located near the central region of LG1, and it was linked to eight DNA markers. Among these markers, Gonai et al. (2009) reported that STS-OPW2 and STS-OPO9 could be useful for marker-assisted selection (MAS) for pear scab resistance via the introduction of Vnk from ‘Kinchaku’. Breeding for scab resistance using ‘Kinchaku’ as a cross-parent was initiated in the late 1980s at NIFTS. A new cultivar, ‘Hoshiakari’ (selected from the progeny of ‘Kinchaku’), which is resistant to V. nashicola, was released in 2015 (Saito et al. 2015c) (Fig. 1).
Resistance to black spot disease
Banno et al. (1999) reported the identification of amplified polymorphic DNA (RAPD) markers associated with susceptibility to black spot disease in the cultivar ‘Osa Nijisseiki’. Iketani et al. (2001) detected two RAPD markers related to black spot disease susceptibility in the cultivar ‘Kinchaku’ by constructing genetic linkage maps. Terakami et al. (2007) identified the exact positions and linkage groups of the genes associated with susceptibility to black spot disease in two cultivars ‘Osanijisseiki’ (Ani) and ‘Nansui’ (Ana). Ani and Ana were located at the top regions of Lg11, and were linked to two SSR markers, CH04h02 and CH03d02.
Self-compatibility
Japanese pear exhibits gametophytic self-incompatibility, which is controlled by a single locus with multiple S-haplotypes (Kikuchi 1929). Therefore, in a pear orchard, cross-compatible pollinizer cultivars bearing an S-haplotype that differs from either of the main cultivar haplotypes are interplanted, or cross-compatible pollination is achieved via artificial pollination. To achieve a stable fruit set without the need for interplanting or artificial pollination, self-compatibility has become an important objective in Japanese pear breeding programs. Each S-haplotype contains separate genes that determine the allelic identity of both the pistil and pollen. The pistil S-gene of the Japanese pear encodes a ribonuclease called S-ribonuclease (S-RNase) (Ishimizu et al. 1996, Sassa et al. 1992, 1993). S-allele-associated stylar glycoproteins with RNase activity (S-RNases) have been identified by protein analyses (Sassa et al. 1992, 1993). Moreover, 13 S-RNase alleles, including S1, S9, S12, S30, and Se-RNase, have been cloned from Japanese pear cultivars (Castillo et al. 2002, Ishimizu et al. 1998, Kim et al. 2007, Norioka et al. 1996, Okada et al. 2009, Takasaki et al. 2004). Multiple F-box protein genes termed S locus F-box brothers (SFBB), which are located around S2-RNase and S4-RNase or linked to S1-RNase, S3-RNase, S4-RNase, S5-RNase, and S6-RNase, have been identified as good candidates for the pollen S gene(s) (Kakui et al. 2007, 2011, Okada et al. 2008, 2011, Sassa et al. 2007). It is predicted that each SFBB included in an S-haplotype recognizes non-self S-RNases and mediates their degradation through the ubiquitin-26S proteasome system, but it is not able to inactivate self S-RNases (Kubo et al. 2010, Sijacic et al. 2004).
‘Osanijisseiki’ arose as a natural mutant from ‘Nijisseiki’ (S2S4) in an orchard in Tottori prefecture locates in Sanin district, and was released as the first self-compatible (SC) Japanese pear cultivar in 1979 (Furuta and Imai 1987). Crossing experiments revealed that ‘Osanijisseiki’ self-compatibility is due to mutation in styles but not pollen, and the S-genotype of this cultivar was referred to as S2S4sm (sm: stylar-part mutant) (Sato 1993). ‘Osanijisseiki’ styles produce the transcript and protein of S2-RNase, but the S4-RNase (Ishimizu et al. 1996, Norioka et al. 1996) is not produced because of the loss of at least 4 kb, which includes the S4-RNase of the S4sm haplotype (Sassa et al. 1997). Okada et al. (2008) revealed that the S4sm haplotype lacks 34 predicted open reading frames (ORFs), including the S4-RNase and a pollen-specific F-box protein gene, which results from the deletion of 236 kb spanning from 48 kb upstream to 188 kb downstream of S4-RNase. Alternatively, S4sm pollen not only recognizes the pistil S4 but also S1, resulting in both S4S4sm and S1S4sm genotypes that show self-incompatibility (Saito et al. 2011). ‘Osanijisseiki’ has been used as a parent to breed several new SC cultivars, including ‘Akibae’, ‘Zuishyu’ (Tanabe et al. 2001), ‘Akikansen’ (Kitagawa et al. 2014), ‘Shinmizuki’, ‘Shino’ (Matsumoto et al. 2014), and ‘Nashi Chuukanbohon Nou 1 Gou’ (NCN1) (Saito et al. 2015a) (Fig. 1). ‘Osa Gold’, which is an induced black spot-resistant mutant from ‘Osanijisseiki’ via chronic gamma irradiation, was also released (Masuda et al. 1998) (Fig. 1). Several methods using DNA markers have been developed that are capable of specifically discriminating S1–S9, Sk, and S4sm (Ishimizu et al. 1999, Kim et al. 2007, Nashima et al. 2014, 2015, Okada et al. 2008, Takasaki et al. 2004).
In addition, a pollen mutated, self-compatible Japanese pear selection (designated 415-1) was obtained from the progeny of ‘Kosui’ that was pollinated with pollen collected from a chronically gamma-irradiated ‘Kosui’ tree. Reciprocal crosses with self-incompatible cultivars with the same S-alleles indicated that this mutant is a partial pollen mutation that lost its pollen self-incompatibility function but retained its stylar self-incompatibility function (Sawamura et al. 2013). Mase et al. (2014) indicated that this selection has a segmental duplication, which encompasses the S5-haplotype that is usually inherited together with the S4 chromosome. Thus, this selection is predicted to produce S-heteroallelic pollen grains that are capable of breaking down self-incompatibility via competitive interactions.
Harvest time (early ripening)
Harvest time is a very important characteristic for pears because it increases the competitiveness of a cultivar by differentiating it from other cultivars in the market. For instance, there is a strong need for early ripening cultivars, particularly when Japanese pear demand peaks during the first half of August in Japan. After examining 43 cross combinations between Japanese pears or Japanese and Chinese pears, Mori (1953) reported that the average harvest time in a family was nearly that observed in both parents. Abe et al. (1993b, 1995) elucidated that the fruit ripening date (FRD) of 20 full-sib families was polygenically inherited with low environmental variance and extremely high narrow sense heritability (0.95). In addition, Abe et al. (1993c) reported the relationship between ripening time and fruit weight using a seedling population and their parent. High correlations were observed between ripening time and fruit weight among families, mid-parent of ripening time and the family mean of fruit weight as well. Significant correlation was also observed within families and early ripening seedlings tended to bear small fruits in most families. Furthermore, Nishio et al. (2011) estimated the broad sense heritability of FRD in a family equal to 0.83 for three full-sib families. Both genome-wide association studies (GWAS) using 76 Japanese pears and QTL analyses using F1 populations from crosses of Japanese pear cultivars ‘Akiakari’ and ‘Taihaku’ detected the same two molecular markers (PPACS2 and BGA35) that were associated with FRD (Iwata et al. 2013a, Yamamoto et al. 2014). The detected PPACS2 and BGA35 positions were at the top of LG15 and at the bottom of LG3, respectively. PPACS2 is a 1-amino-cyclopropane-1- carboxylic acid (ACC) synthase gene, which is correlated with the ethylene levels in Japanese pears during ripening and storage (Itai et al. 1999). The results of Itai et al. (2003a) indicated that high ethylene producers tend to mature early, whereas low ethylene producers tend to mature late. This pattern links ethylene production during fruit ripening to harvest time and fruit storage potential, and it is closely related to the amount of ethylene, which is produced by ACC synthases. However, regarding BGA35, the gene and physiological function of FRD at this locus was not determined. The logarithm of odds (LOD) score and the proportion of the phenotypic variance explained were estimated as 3.32–3.87 and 13.7–15.1% in PPACS2 and 5.00–5.09 and 22.0–22.5% in BGA35, respectively. Verification of these markers using cross-populations is currently underway.
Recently, ‘Hatsumaru’, which exhibits a ripening time that is approximately 20 days earlier than that of ‘Kosui’, was developed from a cross between the early-ripening cultivar ‘Chikusui’ and the early-ripening selection Tsukuba 43 (Saito et al. 2015b) (Fig. 1). Among existing cultivars or selections, the maximum ripening time was about two weeks earlier than that of ‘Kosui’. ‘Hatsumaru’ ripens during the first half of August in the southern Tohoku region, while ‘Kosui’ ripens at the end of August. Although ‘Hatsumaru’ fruit is smaller than that of ‘Kosui’, the eating quality is comparable.
Shelf-life
Japanese pear include climacteric and non-climacteric cultivars (Itai et al. 1999, Kitamura et al. 1981). Climacteric type cultivar’s fruits showed a rapid increase in ethylene production during fruit ripening and have a short shelf life, while non-climacteric type cultivar’s fruits showed non-detectable levels of ethylene and maintained fruit quality for over one month. Therefore shelf-life of Japanese pear fruit is closely related to its level of ethylene production. Itai et al. (1999, 2003b) cloned three ACC synthase gene (PPACC1, 2 and 3) and studied their expression during fruit ripening. PPACS1 was expressed in cultivars producing high level of ethylene, and PPACS2 was expressed in cultivars showing moderate levels of ethylene production. Both restriction fragment length polymorphism (RFLP) and cleaved amplified polymorphism (CAPS) markers linked to PPACS1 and PPACS2 were identified. Each marker for identifying PPACS1 were designated A, which is linked to high levels of ethylene, and that for PPACS2 was designated B, which is linked to moderate level of ethylene. Using RFLP analysis, 35 Asian pear cultivars were classified into four RFLP types (AB, Ab, aB, ab), of which types AB and Ab show high levels, aB a moderate level, ab a low level of ethylene production during fruit ripening (Itai et al. 2003a). In addition, 152 cultivars were categorized into four marker types by CAPS analysis (Itai et al. 2008). According to the classification, there are a large number of aB and ab cultivars but a small number of AB and Ab cultivars, suggesting that cultivars which produce a high level of ethylene were decreased by artificial selection because of short shelf life. Among the current commercial cultivars in Japan, there are few ab type cultivar with early ripening, while almost all middle or late ripening cultivars have ab type. This information on marker type and using markers will be useful to develop the cultivars which combine early ripening with long shelf-life.
Fruit skin color
Since the Japanese pear is usually eaten fresh, an attractive fruit appearance is important. Therefore, fruit skin color is one of the main fruit appearance characteristics. The Japanese pear fruit skin color is classified into the following three types based on the russet amount: reddish brown (russet), intermediate (partially russet), and yellow-green. The russet skin consists of a cork layer (Kikuchi 1924), and brown russet and yellow-green pears are preferred in Japan. Kikuchi (1924, 1930) and Mori (1953) conducted cross-experiments and proposed genetic mechanisms, and they reported that two loci (R and I) control fruit skin color. According to their hypothesis, the R locus has the dominant effect on cork layer development, and the I locus (modifier) has the dominant effect of cork layer suppression. RAPD markers are linked to major genes that control Japanese pear fruit skin color. Using bulked segregant analyses of two F1 progenies of Japanese pear cultivars, Inoue et al. (2006) identified a 425-bp fragment (OPH-19425, generated by OPH-19 RAPD amplification) linked to a gene controlling fruit skin color. For instance, OPH-19425 was associated with the green skin trait, and it exhibited a recombination rate of 7.3%. QTL analyses using F1 populations from a cross of Japanese pear cultivars ‘Akiakari’ and ‘Taihaku’ detected one major QTL associated with fruit skin color, and it was also identified near the top of LG8 (Yamamoto et al. 2014).
Inbreeding
Generally, fruit breeding aims to accumulate useful genes for fruit characteristics and ease of grow, and this tends to result in inbreeding because cultivars with superior desirable traits are repeatedly used as parents (Yamada et al. 2011). Inbreeding in cross-pollinated plants causes an increase in homozygosity frequencies. The expression of deleterious recessive genes results in inbreeding depression. In fruit and berry crops, inbreeding reduces the vigor traits (Bell et al. 1981, Lyrene 1983, Morrow and Darrow 1952, Wilson 1970, Yamada 1993, Yamada et al. 1994). Moreover, intensive selection in Japanese pear breeding is mainly utilized to improve fruit quality, particularly traits associated with flesh texture, which were initiated during the breeding program at NIFTS. Since local cultivars with good texture were restricted to only a few cultivars, the cultivars used in crosses over the generations were also limited (Kajiura and Sato 1990, Machida and Kozaki 1975, 1976). As a result, almost all recently bred commercial cultivars are derived from ‘Nijisseiki’, and some local cultivars originated in the Southern Kanto area (Kajiura 2002) (Fig. 1). Therefore, they seem to be genetically close to each other. Sato et al. (2008) reported a strong negative correlation (–0.72) between plant height and inbreeding coefficients (F) in 29 Japanese pear families using one-year-old cross-seedlings. Compared with the heights of plants with F = 0, they found decreases in plant height of 8.0% for F = 0.10, 20.0% for F = 0.25, and 40.0% for F = 0.50. Therefore, they proposed that when conducting crosses among related individuals, the breeder should pay attention to plant height in the nursery to prevent the loss of vigor in new cultivars or selections. Onoue et al. (2015) estimated kinship and inbreeding among 73 Japanese pear and three Chinese pear cultivars using 207 SSR markers. Moreover, in order to estimate latent inbreeding early in the breeding program (to avoid inbreeding), they proposed the use of DNA markers as kinship estimators among cross parents instead of using coefficients of kinship (f) or inbreeding coefficients (F), which do not account for latent kinship among the parents. Diverse genetic resources are useful to tree and nut breeding programs, because they aid in the accumulation of traits of interest and in the avoidance of inbreeding depression. ‘Oushuu’, which is the offspring of an interspecific hybrid between the Chinese pear cultivar ‘Layang Ci Li’ (P. ussuriensis Maxim.) and ‘Nijisseiki’ (developed by NIFTS), is vigorous and highly productive with high fruit quality (Kotobuki et al. 2004c) (Fig. 1). ‘Kanta’, which is an offspring of ‘Oushuu’ that was released by NIFTS, also shows vigor, high productivity, and soft, juicy texture with high sugar content (Saito et al. 2014b) (Fig. 1). Thus, interspecific crossings using Chinese pears may be an effective method developing cultivars with higher levels of productivity and fruit quality in future breeding programs.
Marker-assisted selection, QTL analysis, genome-wide association studies, and genomic selection
MAS enables the selection of early stage offspring with small plant sizes using DNA markers that are linked to important breeding traits. Offspring with unnecessary genotypes are relinquished, and the remaining ones with promising genotypes, which will lead to increased numbers of offspring, are planted. MAS analyses associated with self-compatibility, resistance to pear scab and black spot diseases, and harvest time are being performed at NIFTS. Between 2009 and 2014, 9,614 seedlings were reduced to 3,396 seedlings, which is 35.0% of the overall rate and approximately three times more efficient than before MAS introduction (unpublished data). This method is expected to accelerate the development of cultivars with combinations of superior fruit quality and horticultural traits related to reduced labor and costs. Bi-parental QTL analyses were performed for seven fruit traits (harvest time, fruit skin color, flesh firmness, fruit weight, acid content, total soluble solids content, and pre-harvest fruit drop). Of the significant QTLs, those for flesh firmness, harvest time, and fruit skin color were stably detected for two successive years (Yamamoto et al. 2014). Genomic selection and GWAS analyses are also in progress. The potential of GWAS and genomic selection was assessed using a combination of 76 Japanese pear cultivars and 162 markers for nine agronomic traits (Iwata et al. 2013a). Markers showing significant associations with three traits (harvest time, resistance to black spot, and the number of spurs) were detected, suggesting that these markers are linked to major QTLs that control these traits. In addition, genome-wide predictions for genomic selection were accurate at the highest level (0.75) for harvest time, at medium levels (0.38–0.61) for flesh firmness, fruit size, and acid content, and at low levels (<0.20) for soluble solid content. Iwata et al. (2013b) proposed genomic predictions of trait segregation in a progeny population, which was based on genome-wide markers and phenotypic data of parental cultivars. This analysis resulted in a method that was used to provide objective and quantitative criteria for the determination of parental combinations and the breeding population size. QTL analyses, GWAS, genomic selection, and genomic prediction are potential ways to increase the efficiency of future Japanese pear breeding programs.
Literature Cited
- Abe, K. and Kurihara, A. (1993a) Species and varietal differences in scab resistance of pear. J. Japan. Soc. Hort. Sci. 61: 789–794. [Google Scholar]
- Abe, K., Sato, Y., Saito, T., Kurihara, A. and Kotobuki, K. (1993b) Inheritance of ripening time of fruit of Japanese pear (Pyrus pyrifolia Nakai). Japan. J. Breed. 43: 289–298. [Google Scholar]
- Abe, K., Sato, Y., Saito, T., Kurihara, A. and Kotobuki, K. (1993c) Genetic correlation between ripening time and weight of fruits in Japanese pear (Pyrus pyrifolia Nakai). Japan. J. Breed. 43: 439–447. [Google Scholar]
- Abe, K., Sato, Y., Saito, T., Kurihara, A. and Kotobuki, K. (1995) Narrow-sense heritability of fruit characters in Japanese pear (Pyrus pyrifolia Nakai). Breed. Sci. 45: 1–5. [Google Scholar]
- Abe, K. and Kotobuki, K. (1998a) Inheritance of high resistance to Venturia nashicola Tanaka et Yamamoto in Japanese pear (Pyrus pyrifolia Nakai) and Chinese pear (P. ussuriensis Maxim.). J. Japan. Soc. Hort. Sci. 67: 677–680. [Google Scholar]
- Abe, K. and Kotobuki, K. (1998b) Polygenic inheritance of necrotic reaction to pear scab (Venturia nashicola Tanaka et Yamamoto) in Japanese pear (Pyrus pyrifolia Nakai) and Chinese pear (P. ussuriensis Maxim.). J. Japan. Soc. Hort. Sci. 67: 839–842. [Google Scholar]
- Abe, K., Kotobuki, K., Saito, T. and Terai, O. (2000) Inheritance of resistance to pear scab from European pears to Asian pears. J. Japan. Soc. Hort. Sci. 69: 1–8. [Google Scholar]
- Abe, K. and Kotobuki, K. (2004) Genetics of the Japanese pear (Pyrus pyrifolia Nakai var. culta Nakai) and prospects for the improvement of Japanese pear cultivar. Recent Res. Devel. Gent. Breeding. 1: 397–412. [Google Scholar]
- Bailey, L.H. (1916) Pyrus. In: Standard Cyclopedia of Horticulture vol. V, Macmillan, New York, pp. 2865–2878. [Google Scholar]
- Banno, K., Ishikawa, H., Hamauzu, Y. and Tabira, H. (1999) Identification of a RAPD marker linked to the susceptible gene of black spot disease in Japanese pear. J. Japan. Soc. Hort. Sci. 68: 476–481. [Google Scholar]
- Bell, R.L., Janick, J., Zimmerman, R.H., van der Zwet, T. and Blake, R.C. (1981) Response of pear to inbreeding. J. Amer. Soc. Hort. Sci. 106: 584–589. [Google Scholar]
- Bell, R.L., Qaumme, H.A., Layne, R.E.C. and Skirvin, R.M. (1996) Pears. In: Janick, J. and Moore J.N. (eds.) Fruit Breeding Vol. I Tree and Tropical fruits, John Wiley & Sons, Inc., New York, pp. 441–514. [Google Scholar]
- Bell, R.L. and Itai, A. (2011) Pyrus. In: Kole, C. (ed.) Wild crop relatives: genomic and breeding resources. Springer, Berlin, pp. 147–177. [Google Scholar]
- Brewer, L.R. and Palmer, J.W. (2011) Global pear breeding programmes: Goals, trends and progress for new cultivars and new rootstocks. Acta Hortic. 909: 105–119. [Google Scholar]
- Castillo, C., Takasaki, T., Saito, T., Norioka, S. and Nakanishi, T. (2002) Cloning of the S8-RNase (S8-allele) of Japanese pear (Pyrus pyrifolia Nakai). Plant Biotechnol. 19: 1–6. [Google Scholar]
- Challice, J. and Westwood, M.N. (1973) Numerical taxonomic studies of the genus Pyrus using both chemical and botanical characters. Bot. J. Linn. Soc. 67: 121–148. [Google Scholar]
- Dondini, L. and Sansavini, S. (2012) European pear. In: Badenes, M.L. and Byrne D.H. (eds.) Fruit Breeding. Springer Science+Business Media, New York, pp. 369–413. [Google Scholar]
- FAOSTAT (2015) URL: http://faostat3.fao.org/browse/Q/QC/E Accessed 30 July 2015.
- Furuta, O. and Imai, T. (1987) Pomological characters of a new, self-fertilizing Japanese pear cultivar ‘Osa-Nijisseiki’ and the optimal fruit thinning method. Bull. Tottori Fruit Tree Exp. Stn. 10: 1–19. [Google Scholar]
- Gonai, T., Terakami, S., Nishitani, C., Yamamoto, T. and Kasumi, M. (2009) The validity of marker-assisted selection using DNA markers linked to a pear scab resistance gene (Vnk) in two populations. J. Japan. Soc. Hort. Sci. 78: 49–54. [Google Scholar]
- Iketani, H., Manabe, T., Matsuta, N., Akihama, T. and Hayashi, T. (1998) Incongruence between RFLPs of chloroplast DNA and morphological classification in east Asian pear (Pyrus spp.). Genet. Res. Crop Evol. 45: 533–539. [Google Scholar]
- Iketani, H., Abe, K., Yamamoto, T., Kotobuki, K., Sato, Y., Saito, T., Terai, O., Matsuta, N. and Hayashi, T. (2001) Mapping of disease-related genes in Japanese pear using a molecular linkage map with RAPD markers. Breed. Sci. 51: 179–184. [Google Scholar]
- Inomata, Y., Murase, S., Nagara, M., Shinokawa, T., Oikawa, S. and Suzuki, K. (1993) Studies on factors which educe watercore in Japanese pear (Pyrus pyrifolia Nakai cv. Hosui). J. Japan. Soc. Hort. Sci. 62: 262–275. [Google Scholar]
- Inomata, Y., Yaegaki, H. and Suzuki, K. (1999) The effects of polyethylene bagging, calcium carbonate treatment and difference in fruit-air temperatures on the occurrence of watercore in Japanese pear ‘Housui’. J. Japan. Soc. Hort. Sci. 68: 336–342. [Google Scholar]
- Inoue, E., Kasumi, M., Sakuma, F., Anzai, H., Amano, K. and Hara, H. (2006) Identification of RAPD marker linked to fruit skin color in Japanese pear (Pyrus pyrifolia Nakai). Sci. Hortic. 107: 254–258. [Google Scholar]
- Ishii, H., Udagawa, H., Nishimoto, S., Tsuda, T. and Nakashima, H. (1992) Scab resistance in pear species and cultivars. Acta Phytopathol. Entomol. Hung. 27: 293–298. [Google Scholar]
- Ishimizu, T., Sato, Y., Saito, T., Yoshimura, Y., Norioka, S., Nakanishi, T. and Sakiyama, F. (1996) Identification and partial amino acid sequences of seven S-RNases associated with self-incompatibility of Japanese pear, Pyrus pyrifolia Nakai. J. Biochem. 120: 326–334. [DOI] [PubMed] [Google Scholar]
- Ishimizu, T., Shinkawa, T., Sakiyama, F. and Norioka, S. (1998) Primary structural features of rosaseous S-RNases associated with gametophytic self-incompatibility. Plant Mol. Biol. 37: 931–941. [DOI] [PubMed] [Google Scholar]
- Ishimizu, T., Inoue, K., Shimonaka, M., Saito, T., Terai, O. and Norioka, S. (1999) PCR-based method for identifying the S-genotypes of Japanese pear cultivars. Theor. Appl. Genet. 98: 961–967. [Google Scholar]
- Itai, A., Kawata, T., Tanabe, K., Tamura, F., Uchiyama, M., Tomomitsu, M. and Shiraiwa, N. (1999) Identification of 1-aminocyclopropane-1-carboxylic acid synthase genes controlling the ethylene level of ripening fruit in Japanese pear (Pyrus pyrifolia Nakai). Mol. Gen. Genet. 261: 42–49. [DOI] [PubMed] [Google Scholar]
- Itai, A., Kotaki, T., Tanabe, K., Tamura, F., Kawaguchi, D. and Fukuda, M. (2003a) Rapid identification of 1-aminocyclopropane-1-carboxylate (ACC) synthase genotypes in cultivars of Japanese pear (Pyrus pyrifolia Nakai) using CAPS markers. Theor. Appl. Genet. 106: 1266–1272. [DOI] [PubMed] [Google Scholar]
- Itai, A., Tanabe, K., Tamura, F. and Tomomitsu, M. (2003b) Cloning and characterization of a cDNA encoding 1-aminocyclopropane-1-carboxylate (ACC) synthase (PPACS3) from ripening fruit of Japanese pear (Pyrus pyrifolia Nakai). J. Japan. Soc. Hort. Sci. 72: 99–106. [Google Scholar]
- Itai, A. and Fujita, N. (2008) Identification of climacteric and nonclimacteric phenotypes of Asian pear cultivars by CAPS analysis of 1-aminocyclopropane-1-carboxylate synthase genes. HortScience 43: 119–121. [Google Scholar]
- Iwata, H., Hayashi, T., Terakami, S., Takada, N., Sawamura, Y. and Yamamoto, T. (2013a) Potential assessment of genome-wide association study and genomic selection in Japanese pear Pyrus pyrifolia. Breed. Sci. 63: 125–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata, H., Hayashi, T., Terakami, S., Takada, N., Saito, T. and Yamamoto, T. (2013b) Genomic prediction of trait segregation in a progeny population: a case study of Japanese pear (Pyrus pyrifolia). BMC Genetics 14: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiura, I., Yamaki, S., Omura, M. and Shimura, I. (1976) Watercore in Japanese pear (Pyrus serotina Rehder var. ‘culta’ Rehder). I. Description of the disorder and its relation to fruit maturity. Sci. Hortic. 4: 261–270. [Google Scholar]
- Kajiura, I., Yamaki, S., Omura, M., Akihama, T. and Machida, Y. (1979) Improvement of sugar content and composition in fruits, and classifications of east Asian pears by the principal component analysis of sugar compositions in fruits. Japan. J. Breed. 29: 1–12. [Google Scholar]
- Kajiura, I. and Sato, Y. (1990) Recent progress in Japanese pear (Pyrus pyrifolia Nakai) breeding and descriptions of cultivars based on literature review. Bull. Fruit Tree Res. Stn. Extra 1: 1–36. [Google Scholar]
- Kajiura, I. (1994) Nashi (Japanese pear). In: Konishi, K., Iwahori S., Kitagawa H. and Yakuwa T. (eds.) Horticulture in Japan, Asakura Publishing, Tokyo, pp. 40–47. [Google Scholar]
- Kajiura, I. (1996) Nijisseiki. In: Kozaki, I., Ueno I., Tsuchiya S. and Kajiura I. (eds.) The Fruits in Japan. Yokendo, Tokyo, pp. 60–61. [Google Scholar]
- Kajiura, I. (2002) Studies on the recent advances and future trends of Asian pear in Japan. Acta Hortic. 587: 113–124. [Google Scholar]
- Kajiura, I. (2013) Japanese pear. In: Ukai, Y. and Osawa R. (eds.) History of plant breeding in Japan. Yushokan, Tokyo, pp. 413–440. [Google Scholar]
- Kajiura, M., Kanato, K., Machida, Y. and Kozaki, I. (1967) New Japanese pear variety ‘Shinsui’. Bull. Hort. Res. Stn. A 6: 69–76. [Google Scholar]
- Kajiura, M., Kanato, K., Machida, Y., Kozaki, I., Tashiro, Y. and Nakaya, E. (1969) New Japanese pear variety ‘Hayatama’. Bull. Hort. Res. Stn. A 8: 7–14. [Google Scholar]
- Kajiura, M., Kanato, K., Machida, Y., Maeda, M., Kozaki, I., Tashiro, T., Kishimoto, O. and Seike, K. (1974) New Japanese pear cultivar ‘Hakko’ and ‘Hosui’. Bull. Fruit Tree Res. Stn. A 1: 1–12. [Google Scholar]
- Kakui, H., Tsuzuki, T., Koba, T. and Sassa, H. (2007) Polymorphism of SFBB-γ and its use for S genotyping in Japanese pear (Pyrus pyrifolia). Plant Cell Rep. 26: 1619–1625. [DOI] [PubMed] [Google Scholar]
- Kakui, H., Kato, M., Ushijima, K., Kitaguchi, M., Kato, S. and Sassa, H. (2011) Sequence divergence and loss-of-function phenotypes of S locus F-box brothers genes are consistent with non-self recognition by multiple pollen determinants in self-incompatibility of Japanese pear (Pyrus pyrifolia). Plant J. 68: 1028–1038. [DOI] [PubMed] [Google Scholar]
- Kikuchi, A. (1924) On the origin of Japanese pear and the inheritance of the skin colors of their fruits. Japan. J. Genet. 3: 1–27. [Google Scholar]
- Kikuchi, A. (1929) Investigations in 1927 and 1928. 1. Paterclinical incompatibility in the Japanese pear. J. Okitsu Hortic. Soc. 24: 1–6. [Google Scholar]
- Kikuchi, A. (1930) On skin color of the Japanese pear, and its inheritance. Contr. Inst. Plant Ind. 8: 1–50. [Google Scholar]
- Kim, H., Kakui, H., Koba, T., Hirata, Y. and Sassa, H. (2007) Cloning of a new S-RNase and development of a PCR-RFLP system for the determination of the S-genotypes of Japanese pear. Breed. Sci. 57: 159–164. [Google Scholar]
- Kitagawa, K., Nagara, M., Uchida, M., Inoue, K., Murata, K., Masuda, T., Yoshioka, T. and Kotobuki, K. (1999) A new Japanese pear cultivar ‘Kotobuki Shinsui’. Bull. Tottori Hortic. Exp. Stn. 3: 1–13. [Google Scholar]
- Kitagawa, K., Inoue, K., Murata, K., Yoshida, A., Murao, K., Kadowaki, T. and Takahama, S. (2014) New Japanese pear cultivars ‘Shinkansen’ and ‘Akikansen’. Bull. Tottori Pref. Agr. Forest Res. Inst. Hort. Res. Center 1: 11–18. [Google Scholar]
- Kitamura, T., Iwata, T., Fukushima, T., Furukawa, Y. and Ishiguro, T. (1981) Studies on the maturation-physiology and storage of fruits and vegetables. II. Respiration and ethylene production in reference to species and cultivars of pear fruit. J. Japan. Soc. Hort. Sci. 49: 608–616. [Google Scholar]
- Koehne, E. (1890) Die Gattung der Pomaceen. Wissenshaftliche Beitrage zum Programm des Falk-Realgymnasium zu Berlin, Nr. 95: 1–33, Taf. 1–2. [Google Scholar]
- Kotobuki, K., Sato, Y., Kozaki, I., Omura, M., Kozono, T., Seike, K., Kajiura, I., Kanato, K., Machida, Y., Chiba, T.et al. (1991a) New Japanese pear cultivar ‘Syuugyoku’. Bull Fruit Tree Res. Stn. 21: 1–14. [Google Scholar]
- Kotobuki, K., Sato, Y., Abe, K., Omura, M., Kozono, T., Seike, K., Kajiura, I., Kanato, K., Machida, Y., Kurihara, A.et al. (1991b) New Japanese pear cultivar ‘Chikusui’. Bull. Fruit Tree Res. Stn. 21: 15–28. [Google Scholar]
- Kotobuki, K., Sato, Y., Abe, K., Omura, M., Ogata, T., Kozono, T., Seike, K., Kajiura, I., Kanato, K., Machida, Y.et al. (1991c) New Japanese pear cultivar ‘Yasato’. Bull Fruit Tree Res. Stn. 21: 29–41. [Google Scholar]
- Kotobuki, K., Sanada, T., Nishida, T., Fujita, H. and Ikeda, F. (1992) ‘Gold Nijisseiki’, a new Japanese pear cultivar resistant to black spot disease induced by chronic irradiation of gamma-rays. Bull. Nat. Inst. Aglobiol. Resour. 7: 105–120. [Google Scholar]
- Kotobuki, K., Sato, Y., Abe, K., Saito, T., Omura, M., Kajiura, I., Ogata, T., Kozono, T., Seike, K., Machida, Y.et al. (1994) ‘Hougetsu’, a new Japanese pear cultivar. Bull. Fruit Tree Res. Stn. 26: 1–14. [Google Scholar]
- Kotobuki, K. (1996a) Chojuro. In: Kozaki, I., Ueno I., Tsuchiya S. and Kajiura I. (eds.) The fruits in Japan. Yokendo, Tokyo, pp. 56–57. [Google Scholar]
- Kotobuki, K. (1996b) Kousui. In: Kozaki, I., Ueno I., Tsuchiya S. and Kajiura I. (eds.) The fruits in Japan. Yokendo, Tokyo, pp. 48–49. [Google Scholar]
- Kotobuki, K., Saito, T., Machida, Y., Sato, Y., Abe, K., Kurihara, A., Ogata, T., Terai, O., Nishibata, T., Kozono, T.et al. (2002) New Japanese pear cultivar ‘Akizuki’. Bull. Natl. Inst. Fruit Tree Sci. 1: 11– 21. [Google Scholar]
- Kotobuki, K., Saito, T., Machida, Y., Sato, Y., Masuda, R., Abe, K., Kurihara, A., Ogata, T., Terai, O., Nishibata, T.et al. (2004a) New Japanese pear cultivar ‘Akiakari’. Bull. Natl. Inst. Fruit Tree Sci. 3: 21–30. [Google Scholar]
- Kotobuki, K., Saito, T., Machida, Y., Kajiura, I., Sato, Y., Masuda, R., Abe, K., Kurihara, A., Ogata, T., Terai, O.et al. (2004b) New Japanese pear cultivar ‘Shuurei’. Bull. Natl. Inst. Fruit Tree Sci. 3: 31–40. [Google Scholar]
- Kotobuki, K., Saito, T., Machida, Y., Kajiura, I., Sato, Y., Masuda, R., Abe, K., Kurihara, A., Ogata, T., Terai, O.et al. (2004c) New Japanese pear cultivar ‘Oushuu’. Bull. Natl. Inst. Fruit Tree Sci. 3: 41–51. [Google Scholar]
- Kozaki, I. (1973) Black spot disease resistance in Japanese pear, I: Heredity of the disease resistance. Bull. Hort. Res. Stn. A 12: 17– 26. [Google Scholar]
- Kubo, K., Entani, T., Takara, A., Wang, N., Fields, A.M., Hua, Z., Toyoda, M., Kawashima, S., Ando, T., Isogai, A.et al. (2010) Collaborative non-self recognition system in S-RNase-based self-incompatibility. Science 330: 796–799. [DOI] [PubMed] [Google Scholar]
- Layne, R.E. and Quamme, H.A. (1975) Pears. In: Janick, J. and Moore J.N. (eds.) Advances in fruit breeding. Purdue University Pres; West Lafayette, Indiana, pp. 38–70. [Google Scholar]
- Lee, S.H. (1948) A taxonomic survey of the oriental pears. Proc. Amer. Soc. Hortic. Sci. 51: 152–156. [Google Scholar]
- Lyrene, P. (1983) Inbreeding depression in rabbiteye blueberries. HortScience 18: 226–227. [Google Scholar]
- Machida, Y. and Maeda, M. (1966) Studies on the texture of pear fruit I. Factors responsible for the texture in Japanese pear varieties part I. Fruit firmness and density of stone cell cluster. Bull. Hort. Res. Stn. A 5: 121–129. [Google Scholar]
- Machida, Y. and Tashiro, T. (1968) Studies on the texture of pear fruit II. Factors responsible for the texture in Japanese pear varieties part I. Cell wall substances. Bull. Hort. Res. Stn. A 7: 93–110. [Google Scholar]
- Machida, Y. and Kozaki, I. (1975) Quantitative studies on the fruit quality for Japanese pear (Pyrus serotina Rehder) breeding I. Statistical analyses of cultivar populations. J. Japan. Soc. Hort. Sci. 44: 235– 240. [Google Scholar]
- Machida, Y. and Kozaki, I. (1976) Quantitative studies on the fruit quality for Japanese pear (Pyrus serotina Rehder) breeding II. Statistical analyses of a hybrid seedling population. J. Japan. Soc. Hort. Sci. 44: 325–329. [Google Scholar]
- Machida, Y. (1979) Trend of breeding works for Japanese pear in Japan. JARQ 13: 234–237. [Google Scholar]
- Machida, Y., Sato, Y., Kozaki, I. and Seike, K. (1982) S-genotype of several cultivars of Japanese pear and question of the parents of ‘Hosui’. Abstr. J. Japan. Soc. Hort. Sci. Autumn Meet.: 58–59. [Google Scholar]
- Machida, Y., Kajiura, I., Sato, Y., Kotobuki, K., Kozaki, I., Seike, K. and Kanato, K. (1984a) New Japanese pear ‘Shinsei’. Bull. Fruit Tree Res. Stn. A 11: 9–13. [Google Scholar]
- Machida, Y., Kajiura, I., Sato, Y., Kotobuki, K., Kozaki, I. and Kozono, T. (1984b) Genetic information on flesh firmness and the characteristics of selected clones of Japanese pear: Results of the fifth Japanese pear breeding program. Bull. Fruit Tree Res. Stn. A 11: 35–42. [Google Scholar]
- Mase, N., Sawamura, Y., Yamamoto, T., Takada, N., Nishio, S., Saito, T. and Iketani, H. (2014) A segmental duplication encompassing S-haplotype triggers pollen-part self-compatibility in Japanese pear (Pyrus pyrifolia). Mol. Breed. 33: 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda, T., Yoshioka, T., Inoue, K., Murata, K., Kitagawa, K., Tabira, H., Yoshida, A., Kotobuki, K. and Sanada, T. (1997) Selection of mutants resistant to black spot disease by chronic irradiation of gamma-rays in Japanese pear ‘Osanijisseiki’. J. Japan. Soc. Hort. Sci. 66: 85– 92. [Google Scholar]
- Masuda, T., Yoshioka, T., Sanada, T., Kotobuki, K., Nagara, M., Uchida, M., Inoue, K., Murata, K., Kitagawa, K. and Yoshida, A. (1998) A new Japanese pear cultivar ‘Osa Gold’, resistant mutant to the black spot disease of Japanese pear (Pyrus pyrifolia Nakai) induced by chronic irradiation of gamma-rays. Bull. Natl. Inst. Agrobiol. Resour. 12: 1–11. [Google Scholar]
- Matsumoto, T., Nomizu, T. and Nedu, K. (2014) New self-compatible Japanese pear cultivars ‘Shinmizuki’ and ‘Shino’. Hort. Res. (Japan) 13 (Supple) 1: 45. [Google Scholar]
- Ministry of Agriculture, Forestry and Fisheries (MAFF) (1979) Current survey of district fruit production in 1978, MAFF, Tokyo, Japan. [Google Scholar]
- Ministry of Agriculture, Forestry and Fisheries (MAFF) (1983) Current Survey of District Fruit Production in 1982, MAFF, Tokyo, Japan. [Google Scholar]
- Ministry of Agriculture, Forestry and Fisheries (MAFF) (1985) Current Survey of District Fruit Production in 1984, MAFF, Tokyo, Japan. [Google Scholar]
- Ministry of Agriculture, Forestry and Fisheries (MAFF) (1987) Current Survey of District Fruit Production in 1986, MAFF, Tokyo, Japan. [Google Scholar]
- Ministry of Agriculture, Forestry and Fisheries (MAFF) (2015) Agricultural statistics. http://www.maff.go.jp/j/tokei/index.html Accessed 30 July 2015.
- Mori, H. (1953) Studies on the inheritance of the main characteristics of deciduous fruit trees (peach, Japanese pear and Japanese persimmon). Bull. Nat. Inst. Agric. Sci. E 2: 1–66. [Google Scholar]
- Moriguchi, T., Abe, K., Sanada, T. and Yamaki, S. (1992) Levels and role of sucrose synthase, sucrose-phosphate synthase, and acid invertase in sucrose accumulation in fruit of Asian pear. J. Amer. Soc. Hort. Sci. 117: 274–278. [Google Scholar]
- Morrow, E.B. and Darrow, G.M. (1952) Effects of limited inbreeding in strawberries. Proc. Am. Soc. Hortic. Sci. 59: 269–276. [Google Scholar]
- Nakashima, T., Ueno, T. and Fukami, H. (1982) Structure elucidation of AK-toxins, host-specific phytotoxic metabolites produced by Alternaria kikuchiana Tanaka. Tetrahedron Lett. 23: 4469–4472. [Google Scholar]
- Nakashima, T., Ueno, T., Fukami, H., Taga, T., Masuda, H., Osaki, K., Otani, H., Kohmoto, K. and Nishimura, S. (1985) Isolation and structures of AK-toxin I and II, host-specific phytotoxic metabolites produced by Alternaria alternata Japanese pear pathotype. Agric. Biol. Chem. 49: 807–815. [Google Scholar]
- Nashima, K., Terakami, S., Kunihisa, M., Nishitani, C., Saito, T. and Yamamoto, T. (2014) Allele-specific LAMP markers for S-RNase genes in Japanese pear. DNA Polymor. 22: 56–59. [Google Scholar]
- Nashima, K., Terakami, S., Nishio, S., Kunihisa, M., Nishitani, C., Saito, T. and Yamamoto, T. (2015) S-genotype identification based on allele-specific PCR in Japanese pear. Breed. Sci. 65: 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, S., Sugihara, M., Kohmoto, K. and Otani, H. (1978) Two different phases in pathogenicity of the Alternaria pathogen causing black spot disease of Japanese pear. J. Fac. Agr. Tottori Univ. 13: 1–10. [Google Scholar]
- Nishio, S., Yamada, M., Sawamura, Y., Takada, N. and Saito, T. (2011) Environmental variance components of fruit ripening date as used in both phenotypic and marker-assisted selection in Japanese pear breeding. HortScience 46: 1540–1544. [Google Scholar]
- Norioka, N., Norioka, S., Ohnishi, Y., Ishimizu, T., Oneyama, C., Nakanishi, T. and Sakiyama, F. (1996) Molecular cloning and nucleotide sequences of cDNAs encoding S-allele specific stylar RNases in a self-incompatible cultivar and its self-compatible mutant of Japanese pear, Pyrus pyrifolia Nakai. J. Biochem. 120: 335–345. [DOI] [PubMed] [Google Scholar]
- Ogawa, H., Usuda, A., Miyashita, T., Makita, H., Ito, T., Tsukahara, K., Shimazu, T. and Maejima, T. (2002) New Japanese pear cultivar ‘Nansui’. Acta Hort. 587: 303–306. [Google Scholar]
- Ohkawa, M. and Torikata, T. (1967) Studies on the resistance of Japanese pears to black spot disease fungus (Alternaria kikuchiana Tanaka). IV. The isolation of host specific toxin produced by Alternaria kikuchiana Tanaka. J. Japan. Soc. Hort. Sci. 36: 1–6. [Google Scholar]
- Okada, K., Tonaka, N., Moriya, Y., Norioka, N., Sawamura, Y., Matsumoto, T., Nakanishi, T. and Takasaki-Yasuda, T. (2008) Deletion of a 236 kb region around S4-RNase in a stylar-part mutant S4sm-haplotype of Japanese pear. Plant Mol. Biol. 66: 389–400. [DOI] [PubMed] [Google Scholar]
- Okada, K., Castillo, C., Sawamura, Y., Nakanishi, T. and Takasaki-Yasuda, T. (2009) S-genotype assignments of local cultivars in Japanese pear ‘Senryo’, ‘Kuroki’ and ‘Hogyoku’. J. Japan. Soc. Hort. Sci. 78: 55–60. [Google Scholar]
- Okada, K., Tonaka, N., Taguchi, T., Ichikawa, T., Sawamura, Y., Nakanishi, T. and Takasaki-Yasuda, T. (2011) Related polymorphic F-box protein genes between haplotypes clustering in the BAC contig sequences around the S-RNase of Japanese pear. J. Exp. Bot. 62: 1887–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoue, N., Yamada, M., Yamamoto, T., Terakami, S., Nishitani, C., Kunihisa, M., Takada, N., Nishio, S., Sawamura, Y. and Saito, T. (2015) Kinship and inbreeding estimates based on microsatellite markers in breeding of Japanese pear (Pyrus pyrifolia Nakai). Euphytica 205: 539–555. [Google Scholar]
- Otani, H., Nishimura, S. and Kohmoto, K. (1972) Nature of specific susceptibility to Alternaria kikuchiana in Nijisseiki cultivar among Japanese pears (I). J. Fac. Agr. Tottori Univ. 7: 5–12. [Google Scholar]
- Otani, H., Nishimura, S., Kohmoto, K., Yano, K. and Seno, T. (1975) Nature of specific susceptibility to Alternaria kikuchiana in Nijisseiki cultivar among Japanese pears (V). Role of host-specific toxin in early step of infection. Ann. Phytopath. Soc. Japan 41: 467–476. [Google Scholar]
- Otani, H., Kohmoto, K., Nishimura, S., Nakashima, T., Ueno, T. and Fukami, H. (1985) Biological activities of AK-toxins I and II, host-specific toxins from Alternaria alternata Japanese pear pathotype. Ann. Phytopath. Soc. Japan 51: 285–293. [Google Scholar]
- Rubtsov, G.A. (1944) Geographical distribution of the genus Pyrus and trends and factors in its evolution. Amer. Nat. 78: 358–366. [Google Scholar]
- Saito, T., Kotobuki, K., Abe, K., Sawamura, Y., Sato, Y., Terai, O., Shoda, M., Takada, N., Nishibata, T., Kurihara, A.et al. (2009) New Japanese pear cultivar ‘Natsushizuku’. Bull. Natl. Inst. Fruit Tree Sci. 9: 11–22. [Google Scholar]
- Saito, T., Sato, Y., Sawamura, Y., Shoda, M., Takasaki-Yasuda, T. and Kotobuki, K. (2011) Dual recognition of S1 and S4 pistils by S4sm pollen in self-incompatibility of Japanese pear (Pyrus pyrifolia Nakai). Tree Genet. Genomes. 8: 689–694. [Google Scholar]
- Saito, T., Sawamura, Y., Kotobuki, K., Takada, N., Hirabayashi, T., Sato, A., Shoda, M., Nishio, S., Terai, O., Nishibata, T.et al. (2014a) ‘Rinka’, a new Japanese pear cultivar. Hort. Res. (Japan) 13 (Suppl) l: 268. [Google Scholar]
- Saito, T., Sawamura, Y., Takada, N., Kotobuki, K., Hirabayashi, T., Sato, A., Shoda, M., Nishio, S., Kato, H., Kashimura, Y.et al. (2014b) ‘Kanta’, a new Japanese pear cultivar. Hort. Res. (Japan) 13 (Suppl) l: 269. [Google Scholar]
- Saito, T., Kotobuki, K., Sato, Y., Abe, K., Machida, Y., Kurihara, A., Kajiura, I., Terai, O., Shoda, M., Sawamura, Y.et al. (2015a) New Japanese pear cultivar ‘Nashi chuukanbohon nou 1 gou’, with the homozygote of haplotype for self-compatibility (Pyrus pyrifolia Nakai). Bull. Natl. Inst. Fruit Tree Sci. 20: 1–9. [Google Scholar]
- Saito, T., Sawamura, Y., Takada, N., Kotobuki, K., Nishio, S., Terai, O., Hirabayashi, T., Sato, A., Shoda, M., Abe, K.et al. (2015b) ‘Hatsumaru’, a new Japanese pear cultivar. Hort. Res. (Japan) 14 (Suppl) l: 269. [Google Scholar]
- Saito, T., Sawamura, Y., Takada, N., Kotobuki, K., Nishio, S., Hirabayashi, T., Sato, A., Shoda, M., Kato, H., Terai, O.et al. (2015c) ‘Hoshiakari’, a new Japanese pear cultivar. Hort. Res. (Japan) 14 (Suppl) l: 270. [Google Scholar]
- Sakuma, F., Umeya, T., Tahira, K., Katagiri, S. and Hiyama, H. (1995) Effects of high temperature and/or gibberellin treatments during early fruit development on the occurrence of watercore in Japanese pear (Pyrus pyrifolia Nakai cv. Hosui). J. Japan. Soc. Hort. Sci. 64: 243–249. [Google Scholar]
- Sakuma, F., Katagiri, S., Tahira, K., Umeya, T. and Hiyama, H. (2000) Effects of high temperature and/or controlling transpiration by bagging and/or spraying an anti-transpirant on the occurrence of watercore in Japanese pear ‘Housui’ (Pyrus pyrifolia Nakai). J. Japan. Soc. Hort. Sci. 69: 283–289. [Google Scholar]
- Sanada, T. (1988) Selection of resistant mutants to black spot disease of Japanese pear by using host-specific toxin. Japan. J. Breed. 38: 198–204. [Google Scholar]
- Sanada, T., Nishida, T. and Ikeda, F. (1988) Resistant mutant to black spot disease of Japanese pear ‘Nijisseiki’ induced by gamma rays. J. Japan. Soc. Hort. Sci. 57: 159–166. [Google Scholar]
- Sanada, T., Sagisaka, K., Soejima, J., Moriguchi, T., Teramoto, S. and Kotobuki, K. (1994) Inheritance of intermediate resistance to black spot disease in an induced Japanese pear mutant, ‘Gold Nijisseiki’. J. Japan. Soc. Hort. Sci. 62: 689–693. [Google Scholar]
- Sassa, H., Hirano, H. and Ikehashi, H. (1992) Self-incompatibility-related RNases in styles of Japanese pear (Pyrus serotina Rehd.). Plant Cell Physiol. 33: 811–814. [Google Scholar]
- Sassa, H., Hirano, H. and Ikehashi, H. (1993) Identification and characterization of stylar glycoproteins associated with self-incompatibility genes of Japanese pear, Pyrus serotina Rehd. Mol. Gen. Genet. 241: 17–25. [DOI] [PubMed] [Google Scholar]
- Sassa, H., Hirano, H., Nishio, T. and Koba, T. (1997) Style-specific self-compatible mutation caused by deletion of the S-RNase gene in Japanese pear (Pyrus serotina). Plant J. 12: 223–227. [Google Scholar]
- Sassa, H., Kakui, H., Miyamoto, M., Suzuki, Y., Hanada, T., Ushijima, K., Kusaba, M., Hirano, H. and Koba, T. (2007) S locus F-box brothers: multiple and pollen-specific F-box genes with S haplotype-specific polymorphisms in apple and Japanese pear. Genetics 175: 1869– 1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, A., Sawamura, Y., Takada, N. and Hirabayashi, T. (2008) Relationship between inbreeding coefficients and plant height of 1-year-old seedlings in crosses among Japanese pear (Pyrus pyrifolia Nakai) cultivars/selections. Sci. Hortic. 117: 85–88. [Google Scholar]
- Sato, Y. (1993) Breeding of self-compatible Japanese pear. In: Hayashi, T.et al. (eds.) Techniques on gene diagnosis and breeding in fruit trees. Fruit Tree Research Station, Tsukuba, pp. 241–247. [Google Scholar]
- Sawamura, Y., Saito, T., Takada, N., Yamamoto, T., Kimura, T., Hayashi, T. and Kotobuki, K. (2004) Identification of parentage of Japanese pear ‘Housui’. J. Japan. Soc. Hort. Sci. 73: 511–518. [Google Scholar]
- Sawamura, Y., Takada, N., Yamamoto, T., Saito, T., Kimura, T. and Kotobuki, K. (2008) Identification of parent-offspring relationship in 55 Japanese pear cultivars using S-RNase allele and SSR markers. J. Japan. Soc. Hort. Sci. 77: 364–373. [Google Scholar]
- Sawamura, Y., Mase, N., Takada, N., Sato, A., Nishitani, C., Abe, K., Masuda, T., Yamamoto, T., Saito, T. and Kotobuki, K. (2013) A self-compatible pollen-part mutant of Japanese pear produced by crossing ‘Kosui’ with pollen from gamma-irradiated ‘Kosui’. J. Japan. Soc. Hort. Sci. 82: 222–226. [Google Scholar]
- Sawano, I., Suzuki, K., Kamata, N., Nakajima, T., Kuroyanagi, E., Taneishi, M. and Hisada, H. (2011) Breeding Japanese pear ‘Shizukisui’ with resistance to black spot disease. Bull. Shizuoka Res. Inst. Agr. Forest. 4: 45–49. [Google Scholar]
- Sijacic, P., Wang, X., Skirpan, A.L., Wang, Y., Dowd, P.E., McCubbin, A.G., Huang, S. and Kao, T.H. (2004) Identification of the pollen determinant of S-RNase-mediated self-incompatibility. Nature 429: 302– 305. [DOI] [PubMed] [Google Scholar]
- Simmons, E.G. (1993) Alternaria themes and variations (63–72). Mycotaxon 48: 91–107. [Google Scholar]
- Simmons, E.G. and Roberts, R.G. (1993) Alternaria themes and variations (73). Mycotaxon 48: 109–140. [Google Scholar]
- Takasaki, T., Okada, K., Castillo, C., Moriya, Y., Saito, T., Sawamura, Y., Norioka, N., Norioka, S. and Nakanishi, T. (2004) Sequence of the S9-RNase cDNA and PCR-RFLP system for discriminating S1- to S9-allele in Japanese pear. Euphytica 135: 157–167. [Google Scholar]
- Tanabe, K., Tamura, F., Itai, A. and Hayashi, S. (2001) New Japanese pear cultivars, ‘Akibae’, ‘Zuishyu’ and ‘Shinjyu’. J. Japan Soc. Hort. Sci. 70 (Suppl 1): 220. [Google Scholar]
- Tanaka, S. (1933) Studies on black spot disease of Japanese pear (Pyrus serotina Rehd.). Mem. Coll. Agr. Kyoto Imp. Univ. 28: 1–31. [Google Scholar]
- Tanaka, S. and Yamamoto, S. (1964) Studies on pear scab II. Taxonomy of the causal fungus of Japanese pear scab. Ann. Phytopath. Soc. Japan 29: 128–136. [Google Scholar]
- Terakami, S., Shoda, M., Adachi, Y., Gonai, T., Kasumi, M., Sawamura, Y., Iketani, H., Kotobuki, K., Patocchi, A., Gessler, C.et al. (2006) Genetic mapping of the pear scab resistance gene Vnk of Japanese pear cultivar Kinchaku. Theor. Appl. Genet. 113: 743–752. [DOI] [PubMed] [Google Scholar]
- Terakami, S., Adachi, Y., Iketani, H., Sato, Y., Sawamura, Y., Takada, N., Nishitani, C. and Yamamoto, T. (2007) Genetic mapping of genes for susceptibility to black spot disease in Japanese pears. Genome 50: 735–741. [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture, Agricultural Research Service (2015) Germplasm resources information network (GRIN), GRIN taxonomy for plants. http://www.ars-grin.gov/cgi-bin/npgs/html/tax_search.pl Accessed 30 July 2015.
- Vavilov, N.I. (1951) The origin, variation, immunity and breeding of cultivated plants. Ronald, New York. [Google Scholar]
- Wilson, D. (1970) Black currant breeding: a progeny test of four cultivars and a study of inbreeding effects. J. Hortic. Sci. 45: 239–247. [Google Scholar]
- Yamada, M. (1993) Persimmon breeding in Japan. JARQ 27: 33–37. [Google Scholar]
- Yamada, M., Yamane, H. and Ukai, Y. (1994) Genetic analysis of Japanese persimmon fruit weight. J. Amer. Soc. Hort. Sci. 119: 1298– 1302. [Google Scholar]
- Yamada, M. (2011) Cross breeding in woody fruit crops. Yokendo, Tokyo. [Google Scholar]
- Yamaki, S. and Moriguchi, T. (1989) Seasonal fluctuation of sorbitol-related enzymes and invertase activities accompanying maturation of Japanese Pear (Pyrus serotina Rehder var. culta Rehder) fruit. J. Japan. Soc. Hort. Sci. 57: 602–607. [Google Scholar]
- Yamamoto, T., Terakami, S., Takada, N., Nishio, S., Onoue, N., Nishitani, C., Kunihisa, M., Inoue, E., Iwata, H., Hayashi, T.et al. (2014) Identification of QTLs controlling harvest time and fruit skin color in Japanese pear (Pyrus pyrifolia Nakai). Breed. Sci. 64: 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, D.J. and Zhang, P. (1979) Sinkiang pears, a new series of cultivars of pears in China. Acta Hort. Sinica 6: 27–32. [Google Scholar]
- Zheng, X., Cai, D., Potter, D., Postman, J., Liu, J. and Teng, Y. (2014) Phylogeny and evolutionary histories of Pyrus L. revealed by phylogenetic trees and networks based on data from multiple DNA sequences. Mol. Phylogenet. Evol. 290: 225–237. [DOI] [PubMed] [Google Scholar]