Abstract
Background
Wear particle-induced peri-implant loosening is the most common complication affecting long-term outcomes in patients who undergo total joint arthroplasty. Wear particles and by-products from joint replacements may cause chronic local inflammation and foreign body reactions, which can in turn lead to osteolysis. Thus, inhibiting the formation and activity of osteoclasts may improve the functionality and long-term success of total joint arthroplasty. The aim of this study was to interfere with CXC chemokine receptor type 2 (CXCR2) to explore its role in wear particle-induced osteolysis.
Material/Methods
Morphological and biochemical assays were used to assess osteoclastogenesis in vivo and in vitro. CXCR2 was upregulated in osteoclast formation.
Results
Local injection with adenovirus-mediated siRNA targeting CXCR2 inhibited titanium-induced osteolysis in a mouse calvarial model in vivo. Furthermore, siCXCR2 suppressed osteoclast formation both directly by acting on osteoclasts themselves and indirectly by altering RANKL and OPG expression in osteoblasts in vitro.
Conclusions
CXCR2 plays a critical role in particle-induced osteolysis, and siCXCR2 may be a novel treatment for aseptic loosening.
MeSH Keywords: Osteoclasts; Osteonecrosis; RNA, Small Interfering
Background
Total joint arthroplasty (TJA) is the criterion standard treatment for end-stage joint diseases to relieve pain and restore function. Although extensive efforts have been made to improve the quality and efficacy of TJA, wear particle-induced osteolysis and subsequent aseptic loosening (AL) remain the most common factors that limit the long-term success of arthroplasty surgery and lead to the need for revision surgery. The crucial factor contributing to AL is an adverse tissue response to wear particles that are generated at the surface of the prosthesis, which results in bone loss and promotes osteoclast formation at the site of the bone implant [1,2].
Previous studies revealed that the central event during particle-induced osteolysis is osteoclast formation, which is modulated by the monocytes/macrophages and osteoblasts. Osteoclasts are derived from monocyte/macrophage precursors when nuclear factor-kB ligand (RANKL) is overexpressed. RANKL signaling plays an important role in the activation of osteoclasts. Osteoprotegerin (OPG) can inhibit RANKL signaling because it is a decoy receptor secreted by osteoblasts. Therefore, osteoblasts play a part in osteolysis by regulating RANKL and OPG production. Indeed, macrophages produce growth factors (e.g., M-CSF, GM-CSF, and VEGF), inflammatory factors (e.g., TNF-α, IL-1β, IL-6, and PGE2) and chemotactic factors (e.g., IL-8, CCL3, and CCL2) in response to prosthesis material, all of which induce receptor activation and RANKL expression to stimulate bone destruction. Recently, IL-8 and its receptors have become popular therapeutic agents for wear particle-induced osteolysis; these chemotactic cytokines also mediate osteoclastogenesis [3,4].
IL-8, also known as CXCL8, is a member of the CXC chemokine family and is the primary cytokine secreted by macrophagocytes. It is strongly expressed in primary human breast cancers, and its expression is strongly correlated with elevated bone resorption in breast cancer patients [5]. IL-8 induces RANKL expression in stromal osteoblastic cells and promotes the differentiation of human peripheral blood mononuclear cells into bone-resorbing osteoclasts in a manner that is independent of RANKL [6]. Human osteoclasts express high levels of IL-8, which promotes osteoblast-mediated osteoclast maturation. The coupled receptors for IL-8 are CXC-chemokine receptor type 1 (CXCR1) and type 2 (CXCR2), which are located on the surface of macrophages, epithelial cells, mast cells, and endothelial cells [7]. CXCR2 is a potent neutrophil chemoattractant that regulates neutrophil homeostasis and recruitment, and is released from bone marrow as a response to injury and acute inflammation [8,9]. The IL-8/CXCR2 signaling pathway is upregulated in many cancers, including pancreatic, prostate, renal, and nasopharyngeal cancer [10,11]. The role of IL8/CXCR2 signaling in particle-induced osteolysis appears to be mostly detrimental, since CXCR2 was associated with osteoclast differentiation, bone resorption, and the promotion of aseptic-loosening. Furthermore, CXCR2-deficient mice exhibited suppressed neutrophil recruitment and decreased tissue injury [12,13]. However, it remains unclear whether IL-8 plays a role in osteoclastogenesis via CXCR2 or pathological osteolysis.
The objective of the current study was to investigate the effects of IL8/CXCR2 signaling on wear particle-induced osteolysis. We hypothesized that silencing CXCR2 might attenuate osteoclast formation.
Material and methods
Reagents
Soluble recombinant mouse RANKL was obtained from Prospec Systems (East Brunswick, NJ). Anti-TRAP and -CXCR2 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and RANKL, TRAP, CtsK, NFATc1, and OPG antibodies were purchased from Cell Signaling Technology (Beverly, MA). Alpha-minimum essential medium (α-MEM) was purchased from Hyclone (Logan, UT). Fetal bovine serum (FBS) was obtained from Gibco (Carlsbad, CA).
Cell culture
For primary cultures, bone marrow-derived macrophages (BMMs) were obtained from BALB/c mice (4–6 weeks old). Cells were cultured in α-MEM supplemented with 10% FBS and 1% penicillin/streptomycin for 24 h. Floating cells were removed, and the adherent cells were cultured in 5% CO2 at 37°C for 12 h. They were then treated with adenovirus-mediated CXCR2 or MS siRNA, macrophage colony-stimulating factor (M-CSF), and RANKL, and the culture media were replaced every 2 days until mature osteoclasts had formed. Primary mouse osteoblasts were acquired from female BALB/c mice calvaria using standard methods. They were treated with adenovirus-mediated CXCR2 or MS siRNA, M-CSF, and RANKL, as indicated. RAW264.7 cells were purchased from the American Type Culture Collection (ATCC TIB-71, Manassas, VA), and cultured in α-MEM supplemented with 10% FBS and 1% penicillin/streptomycin for 24 h. Floating cells were removed, and the adherent cells were cultured in 5% CO2 at 37°C for 12 h. They were then treated with or without RANKL for 7 days.
Flow cytometric detection of CXCR2+ cells
Treated RAW264.7 cells were incubated in mouse BD Fc Block (BD Biosciences, San Jose, CA) on ice for 20 min. The cells were divided into aliquots and stained with anti-CXCR2-fluorescein isothiocyanate (FITC) (all from BD Biosciences, San Jose, CA). The percentage of Sample 1 (CT) and Sample 2 (CXCR2+) cells was determined using flow cytometry on a FACSCalibur™ (BD Biosciences) device and analyzed with FlowJo software (Tree Star Inc., Ashland, OR).
Ti-particle-induced calvarial osteolysis model
A mouse calvarial osteolysis model was established to measure the osteolysis-suppressing effects of siCXCR2 in vivo. We purchased 4–6-week-old female BALB/c mice weighing ~30 g from the Animal Experiment Center of Chongqing Medical University. Mice received local calvaria injections of 100 μl Ti particles and were then randomly divided into 3 study groups that received injections of adenovirus expressing CXCR2 siRNA (CXCR2 group), missense siRNA (MS group), or virus-free culture medium (control group). Two mice died, but no adverse reactions were observed during the experiment. The mice were sacrificed by ether inhalation after 3 weeks and the calvaria were excised and fixed in 4% paraformaldehyde for TRAP, hematoxylin and eosin (H&E) staining, and micro-CT.
Tartrate-resistant acid phosphatase (TRAP) staining
Cells were fixed with 4% paraformaldehyde, washed with distilled water, and stained for TRAP in a humid and light-protected incubator for 1 h using the Leukocyte Acid Phosphatase Assay kit (Sigma). TRAP-positive cells containing 2 or more nuclei were regarded as osteoclasts.
A microscope (80i Eclipse, Nikon, Japan) was used to capture images of TRAP-stained pouch sections and observe TRAP-positive osteoclast-like cells (400× magnification). Due to the blurred boundaries of TRAP-positive cells stained dark red in the calvaria sections, analysis software (Image-Pro Plus 6.0) was used to quantify the number of TRAP-positive osteoclast-like cells on the bone-membrane interface by comparing the dark red colored area with the total implanted bone area (400× magnification).
2 Bone resorption pit assay
Bone slices were treated with RANKL (50 ng/ml) and M-CSF (30 ng/ml) and then incubated with adenovirus-mediated CXCR2 siRNA or MS siRNA. Bone slice images were captured using a scanning electron microscope (SEM Hitachi S-4800, CamScan, Tokyo, Japan) at 10 kV. Three fields of each bone slice were randomly selected and the pit areas were quantified using Image J software.
Micro-CT
Calvariae were analyzed using high-resolution micro-CT (Skyscan1072, Skyscan, Aartselaar, Belgium) with an isometric resolution of 9 mm and X-ray energy settings of 80 kV and 80 mA. A 2×2 mm square region of interest (ROI) with the midline suture was selected for further quantitative and qualitative testing after reconstruction. The number of pores, body volume/tissue volume (BV/TV), and bone mineral density (BMD) in the ROI were measured.
Western blotting
Protein lysates were generated from the cell cultures using standard techniques. The protein concentrations were determined using bicinchoninic acid (BCA), and lysates were separated using 8–12% SDS-PAGE gel electrophoresis and electrotransferred to PVDF membranes. The membranes were blocked with 5% non-fat milk and then probed with primary antibodies followed by peroxidase-labelled secondary antibodies. Finally, the protein bands were visualized using enhanced chemiluminescence (ECL, KeyGen, Nanjing, China).
Real-time PCR
Total RNA was extracted from cells using Trizol reagent (Invitrogen) and reverse transcribed into single-stranded cDNA using BioTeke Super RT kit (Bioteke Corp., Beijing, China) according to the manufacturer’s instructions. The target genes were PCR amplified using a LightCycler™ 96 Real-time PCR system (Roche, Nutley, NJ, USA) and normalized to GAPDH as the internal control. The sample-cycling conditions were as follows: 95°C for 60 s, 40 cycles of 95°C for 10 s, 60°C for 30 s, 72°C for 30 s, and 60°C for 30 s. The sequences of the primers were: CtsK forward, 5′-CTTCCAATACGTGCAGCAGA-3′ and reverse, 5′-TCTTCAGGGCTTTCTCGTTC-3′; TRAP forward, 5′-CTGGAGTGCACGATGCCAGCGACA-3′ and reverse, 5′-TCCGTGC TCGGCGATGGACCAGA-3′; RANKL forward, 5′-AGAAGGACG GGACATCGC-3′ and reverse, 5′-AGGTGGTAGGAGGCAGAGG-3′; and c-Fos forward, 5′-CCAGTCAAGAGCATCAGCAA-3′ and reverse, 5′-AAGTAGTGCAGCCCGGAGTA-3′ (Metabion; Martinsried, Germany).
Statistical analysis
Statistical analyses were performed using ANOVA with SPSS. Data are expressed as means ± standard deviations (SDs) from at least 3 independent experiments. * P<0.05 or ** P<0.01 were considered to indicate statistical significance.
Results
CXCR2 is upregulated during RANKL-induced osteoclastogenesis in RAW264.7 cells
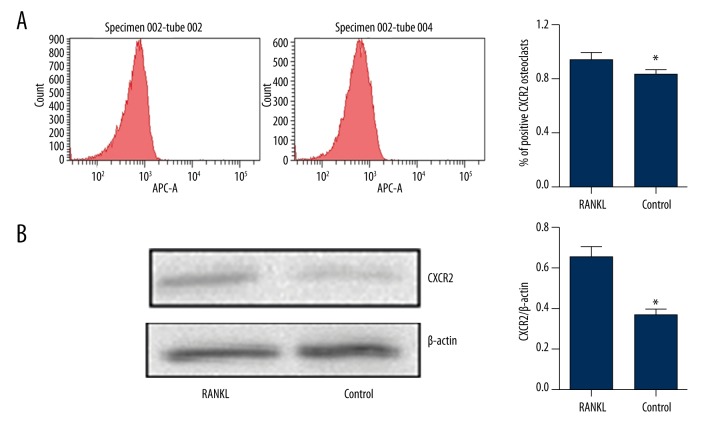
To determine CXCR2 expression during osteoclastogenesis, RAW264.7 cells were treated with or without RANKL for 7 days. Flow cytometry revealed that RANKL treatment increased the expression of cell-surface CXCR2 (Figure 1A). Western blotting demonstrated that CXCR2 levels were higher in whole-cell extracts (Figure 1B). Therefore, CXCR2 might play an important role in osteoclast formation.
Figure 1.
CXCR2 is upregulated during RANKL-induced osteoclastogenesis in RAW264.7 cells. RAW264.7 cells were treated with M-CSF (30 ng/ml) with or without RANKL (100 ng/ml) for 7 days. (A) CXCR2-positive stained cells were analyzed using FACS with anti-CXCR2 antibodies. (B) Total CXCR2 protein levels were increased in the RANKL group. Western blotting was used to analyze CXCR2 protein expression in cell lysates. Thirty micrograms of protein was loaded into each lane; β-actin was used as a loading control. * P<0.05.
CXCR2 inhibition by siRNA in vitro
CXCR2 expression in primary osteoclasts was inhibited using siRNA. Bone marrow mononuclear cells were treated with adenovirus-mediated MS or CXCR2 siRNA and then cultured in the presence of RANKL and M-CSF. Western blotting (Figure 2) showed that CXCR2 levels were significantly downregulated in the CXCR2 group compared with the control and MS groups. Treatment with siRNA suppressed CXCR2 protein levels by ~70%. This suggests that adenovirus-mediated CXCR2 siRNA is a highly efficient inhibitor of CXCR2 expression.
Figure 2.
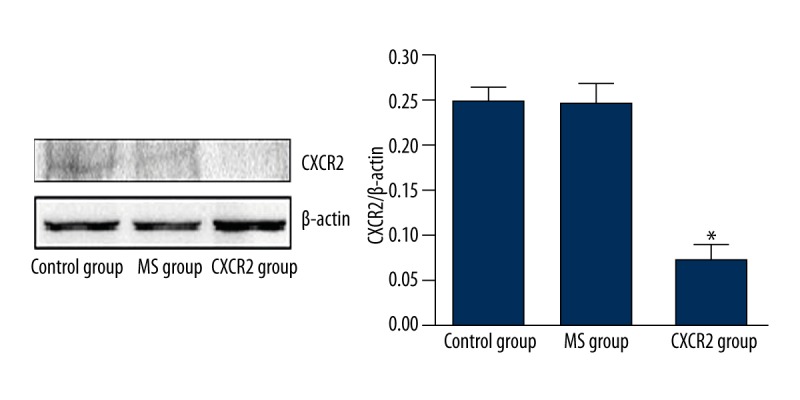
Silencing CXCR2 using adenovirus-mediated CXCR2 siRNA in osteoclasts in BMMs. Western blotting revealed that the expression of CXCR2 protein was significantly reduced in the CXCR2 group compared with the MS and control groups. The experiment was performed in triplicate. * P<0.05.
CXCR2 siRNA inhibits titanium particle-induced osteolysis in vivo
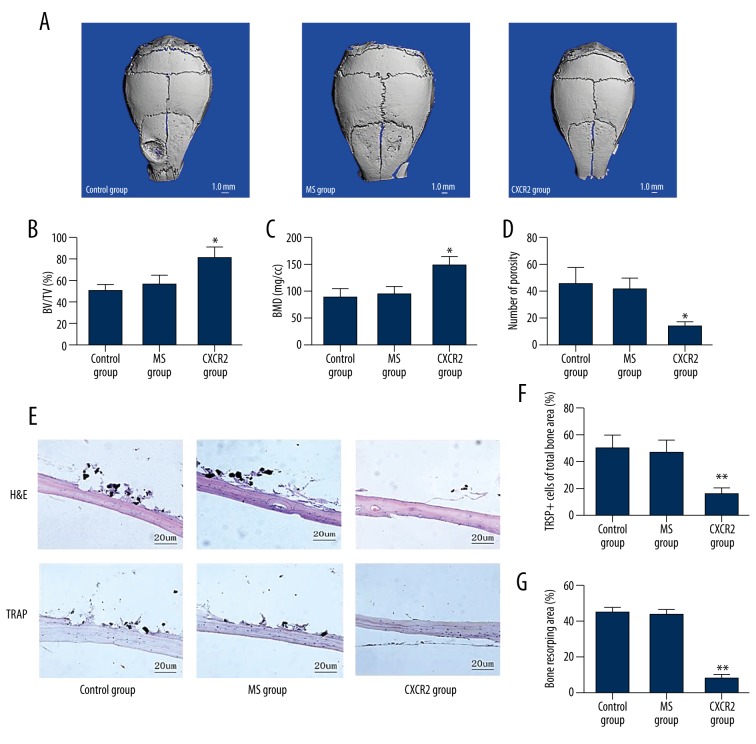
The effects of siCXCR2 on pathological osteolysis were determined using a Ti-particle-induced calvarial osteolysis model in vivo. The MS and control groups showed significant calvarial osteolysis compared with the CXCR2 group. Bone erosion was reduced when siCXCR2 was injected together with the Ti particles (Figure 3A). Quantitative analysis confirmed that siCXCR2 injection increased the BV/TV and BMD (Figure 3B, 3C) and also lowered the bone porosity compared with the MS and control groups in the calvaria (Figure 3D).
Figure 3.
Adenovirus-mediated CXCR2 siRNA attenuates titanium particle-induced mouse calvarial osteolysis in vivo. (A) Representative three-dimensional images of micro-computed tomography (CT) from each group. (B) Bone volume/tissue volume ratio (BV/TV). (C) Bone mineral density (BMD). (D) Porosity. (E) Histological staining to determine the effects of inhibiting siCXCR2 on titanium (Ti) particle-induced mouse calvarial osteolysis. Histological slices were stained with hematoxylin and eosin (H&E) and tartrate-resistant acid phosphatase (TRAP). (F) Histomorphometric analysis of the number of TRAP-positive multinucleated cells; (G) Histomorphometric analysis of the bone resorbing area. * P<0.05.
Histological analysis confirmed that siCXCR2 attenuated Ti particle-induced osteolytic bone loss (Figure 3E). TRAP-positive osteoclasts accumulated along the eroded bone surface in the MS and control groups, but there were fewer osteoclasts and the osteolysis surface area was reduced in the siCXCR2 group (Figure 3F, 3G). These data were consistent with the micro-CT results, revealing that local injection with siCXCR2 can effectively treat Ti particle-induced osteolysis.
CXCR2 signaling in osteoclastogenesis in vitro
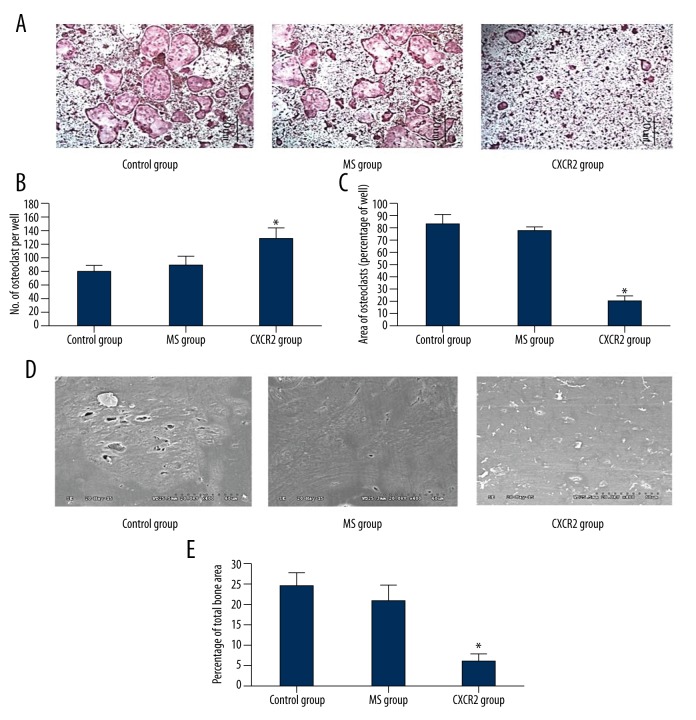
Our previous observations suggested that siCXCR2 attenuated titanium particle-induced osteoclastogenesis. However, the mechanism of action of CXCR2 on osteoclastogenesis in osteoblasts remains unclear. Therefore, we explored the role of the CXCR2 signaling pathway in osteoclastogenesis in vitro. We first examined the effects of adenovirus-mediated CXCR2 siRNA on osteoclast formation. The number of TRAP-positive osteoclasts in the presence of RANKL decreased after treatment with adenovirus-mediated CXCR2 siRNA (Figure 4A–4C). In addition, our previous study suggested that osteoclast resorption could also be inhibited by siCXCR2. Here, the area of resorption was reduced in the CXCR2 group compared with the MS and control groups (Figure 4D, 4E).
Figure 4.
Adenovirus-mediated CXCR2 siRNA suppressed RANKL-induced osteoclastogenesis in vitro. (B, C) The number of TRAP-positive cells and area occupied by TRAP-positive cells was reduced by adenovirus-mediated CXCR2 siRNA. Marrow cells were treated with 100 ng/ml RANKL and 30 ng/ml MCSF. Representative images are shown in (A). (D) Images of bone resorption pits captured using scanning electron microscopy. (E) The bone resorption area relative to control, as determined using Image J software (Bethesda, MD). All experiments were performed in triplicate. * P<0.05, CXCR2 vs. control and MS groups.
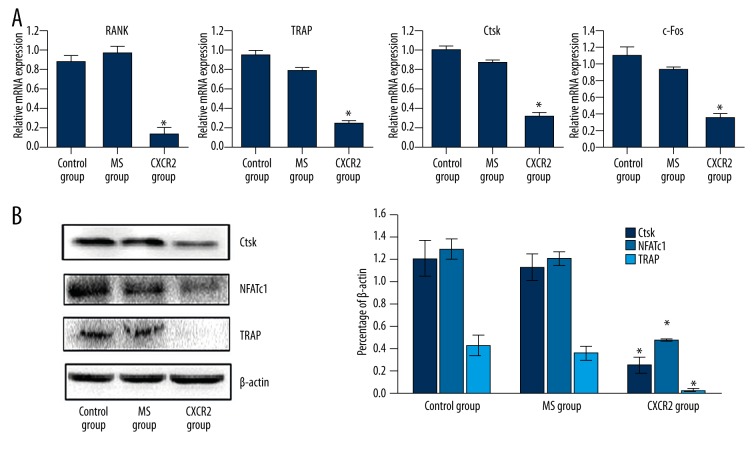
Next, real time-PCR was to measure and quantify the expression of osteoclast-related genes, including RANK, TRAP, cathepsin-K (CtsK), and c-Fos (Figure 5A), in cells transfected with adenovirus-mediated CXCR2 siRNA. We then used Western blotting to measure the protein expression of the osteoclast markers TRAP and CtsK after 7 days (Figure 5B). The expression of RANK, TRAP, CtsK, and c-Fos mRNA was markedly reduced in the CXCR2 group compared with the MS and control groups. The Western blotting results were similar (Figure 5A, 5B).
Figure 5.
Adenovirus-mediated CXCR2 siRNA inhibited RANKL-induced osteoclast-specific gene and protein expression in vitro. (A) The mRNA expression of RANK, TRAP, CtsK, and c-Fos was significantly decreased in the CXCR2 group compared with the MS and control groups. (B) Western blotting revealed that TRAP, CtsK, and NFATc1 levels were decreased in the CXCR2 group. There were no other significant differences between the MS and control groups. All experiments were performed at least 3 times. * P<0.05, CXCR2 vs. control and MS groups.
To explore further the role of CXCR2 signaling in osteoclast formation we investigated the effects of siCXCR2 on the RANKL-induced expression of Nuclear Factor of Active T cell c1 (NFATc1), a critical regulator of osteoclast formation, using Western blotting. As shown in Figure 5B, siCXCR2 decreased NFATc1 levels in the presence of RANKL and MCSF, suggesting that CXCR2 signaling affects osteoclast formation by downregulating NFATc1 expression.
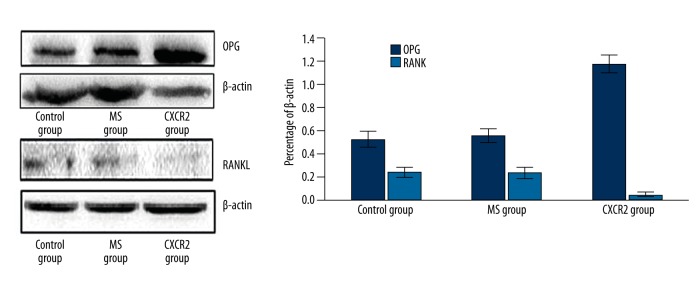
RANKL and OPG are secreted by osteoblasts and expressed on the surface of osteoblasts, and they promote and inhibit osteoclast formation and function, respectively. Therefore, the expression of OPG and RANKL was measured after treatment with siCXCR2 in primary osteoblasts. Figure 6 shows that siCXCR2 significantly increased OPG and reduced RANKL protein expression in osteoblasts.
Figure 6.
Adenovirus-mediated CXCR2 siRNA modulates RANKL and OPG expression in primary osteoblasts in vitro. Western blotting for RANKL and OPG and densitometric analysis of the resulting bands. The densitometry results were consistent with the bands. Protein expression was determined in triplicate. * P<0.05, CXCR2 vs. control and MS groups.
The current results demonstrate that silencing CXCR2 signaling directly inhibits osteoclast formation, and acts on osteoblasts indirectly to suppress osteoclast differentiation by altering OPG and RANKL expression.
Discussion
Total joint arthroplasty is a surgical intervention for the treatment of severe joint disease and trauma. However, aseptic loosening of artificial joints resulting in periprosthetic osteolysis remains the main cause of failure in artificial joint replacement, and frequently results in the need for further surgery [3,14].
The results of the current study demonstrate that CXCR2 expression is upregulated during osteoclast formation, which suggests that CXCR2 may play a vital role in osteolysis. CXCR2 is a receptor for IL-8, and is found on the surface of macrophages, epithelial cells, osteoclasts, and osteoblasts [7]. It participates in the pathological processes involved in chronic inflammation, osteolysis, and atherosclerosis [15–17]. Although the CXC chemokine family plays a vital role in recruiting osteoclasts as autocrine or paracrine moderators, few studies have linked CXCR2 signaling with osteoclast formation in titanium-induced osteoclast formation. Taken together, these data suggest that siCXCR2 reduced the bone resorption rates in a mouse calvarial model in the current study by suppressing osteoclastogenesis.
Osteoclasts play a critical role in the wear particle-induced bone resorption area, and therefore are an efficient and viable treatment target [18,19]. CXCR2 signaling is upregulated around periprosthetic tissues [20], and different wear particles (such as titanium) increase CXCR2 expression in macrophages and MSCs in localized periprosthetic tissues [21]. This can then induce osteoclast and macrophage migration to areas around implants, which accelerates osteolysis [22]. The current study revealed that there were fewer TRAP-positive cells in the siCXCR2 group compared with the MS and control groups, which suggests that siCXCR2 can inhibit osteoclastogenesis. The resulting inhibition of osteoclast differentiation and maturation resulted in dramatically reduced bone resorption pits and resorption area after treatment with siCXCR2. Furthermore, siCXCR2 also suppressed osteoclast-related gene expression. Taken together, these data suggest that the protective effects of siCXCR2 on Ti particle-induced osteolysis are modulated directly by its inhibitory effects on osteoclasts. Overall, the current findings demonstrate that Ti particle-induced osteolysis can be effectively suppressed by inhibiting CXCR2 signaling.
The dynamic balance between RANKL and OPG is crucial during the regulation of bone formation and resorption because it regulates osteoclast activation [23]. RANKL promotes osteoclast formation and differentiation, whereas OPG inhibits bone resorption. Furthermore, recent studies suggested that the balance between RANKL and OPG plays an important role in particle-induced osteolysis [24,25]. In the current study, siCXCR2-treated cells exhibited downregulated RANKL and upregulated OPG protein expression in primary osteoblasts. This suggests that siCXCR2 works on osteoblasts indirectly to suppress osteoclast differentiation by altering the balance between RANKL and OPG, which is consistent with previous findings [26]. Although the underlying pathogenesis of aseptic loosening is a complex process, the current results suggest that IL-8/CXCR2 signaling plays a role in osteoclast formation and titanium particle-induced osteolysis. Therefore, CXCR2 and its ligands are potential therapeutic targets to remedy aseptic loosening.
Conclusions
There is a growing need for more targeted approaches to the treatment and early diagnosis of harmful debris-induced inflammation [27]. Locally injected adenovirus-mediated CXCR2 siRNA could be an effective approach to treat titanium particle-induced osteolysis.
Footnotes
Source of support: This work was supported by grants from the Natural Science Foundation of China (81272005) and the Key Project of Science and Technology Commission of Chongqing (cstc2013jjB10021)
References
- 1.Gallo J, Goodman SB, Konttinen YT, et al. Osteolysis around total knee arthroplasty: A review of pathogenetic mechanisms. Acta Biomater. 2013;9:8046–58. doi: 10.1016/j.actbio.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cordova LA, Stresing V, Gobin B, et al. Heymann, Orthopaedic implant failure: Aseptic implant loosening – the contribution and future challenges of mouse models in translational research. Clin Sci. 2014;127:277–93. doi: 10.1042/CS20130338. [DOI] [PubMed] [Google Scholar]
- 3.Holt G, Murnaghan C, Reilly J, Meek RM. The biology of aseptic osteolysis. Clin Orthop Relat Res. 2007;460:240–52. doi: 10.1097/BLO.0b013e31804b4147. [DOI] [PubMed] [Google Scholar]
- 4.Goodman SB, Ma T. Cellular chemotaxis induced by wear particles from joint replacements. Biomaterials. 2010;31:5045–50. doi: 10.1016/j.biomaterials.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li C, Zhao J, Sun L, et al. RANKL downregulates cell surface CXCR6 expression through JAK2/STAT3 signaling pathway during osteoclastogenesis. Biochem Biophys Res Commun. 2012;429:156–62. doi: 10.1016/j.bbrc.2012.10.122. [DOI] [PubMed] [Google Scholar]
- 6.Bendre MS, Margulies AG, Walser B, et al. Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. Cancer Res. 2005;65:11001–9. doi: 10.1158/0008-5472.CAN-05-2630. [DOI] [PubMed] [Google Scholar]
- 7.Rose JJ, Foley JF, Murphy PM, Venkatesan S. On the mechanism and significance of ligand-induced internalization of human neutrophil chemokine receptors CXCR1 and CXCR2. J Biol Chem. 2004;279:24372–86. doi: 10.1074/jbc.M401364200. 2004. [DOI] [PubMed] [Google Scholar]
- 8.von Vietinghoff S, Asagiri M, Azar D, et al. Defective regulation of CXCR2 facilitates neutrophil release from bone marrow causing spontaneous inflammation in severely NF-kappa B-deficient mice. J Immunol. 2010;185:670–78. doi: 10.4049/jimmunol.1000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mei J, Liu Y, Dai N, et al. Cxcr2 and Cxcl5 regulate the IL-17/G-CSF axis and neutrophil homeostasis in mice. J Clin Invest. 2012;122:974–86. doi: 10.1172/JCI60588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy C, McGurk M, Pettigrew J, et al. Nonapical and cytoplasmic expression of interleukin-8, CXCR1, and CXCR2 correlates with cell proliferation and microvessel density in prostate cancer. Clin Cancer Res. 2005;11:4117–27. doi: 10.1158/1078-0432.CCR-04-1518. [DOI] [PubMed] [Google Scholar]
- 11.Horikawa T, Kaizaki Y, Kato H, et al. Expression of interleukin-8 receptor A predicts poor outcome in patients with nasopharyngeal carcinoma. Laryngoscope. 2005;115:62–67. doi: 10.1097/01.mlg.0000150675.37860.f7. [DOI] [PubMed] [Google Scholar]
- 12.Kempf T, Zarbock A, Widera C, et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 2011;17:581–88. doi: 10.1038/nm.2354. [DOI] [PubMed] [Google Scholar]
- 13.Block H, Herter JM, Rossaint J, et al. Crucial role of SLP-76 and ADAP for neutrophil recruitment in mouse kidney ischemia-reperfusion injury. J Exp Med. 2012;209:407–21. doi: 10.1084/jem.20111493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu A, Richards L, Bladen CL, et al. The biological response to nanometre-sized polymer particles. Acta Biomater. 2015;23:38–51. doi: 10.1016/j.actbio.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allegretti M, Cesta MC, Garin A, Proudfoot AE. Current status of chemokine receptor inhibitors in development. Immunol Lett. 2012;145:68–78. doi: 10.1016/j.imlet.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Therapeutic targeting of chemokine receptors by small molecules. Drug Discov Today Technol. 2012;9:e227–314. doi: 10.1016/j.ddtec.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Boppana NB, Devarajan A, Gopal K, et al. Blockade of CXCR2 signalling: A potential therapeutic target for preventing neutrophil-mediated inflammatory diseases. Exp Biol Med. 2014;239:509–18. doi: 10.1177/1535370213520110. [DOI] [PubMed] [Google Scholar]
- 18.Rao AJ, Gibon E, Ma T, et al. Revision joint replacement, wear particles, and macrophage polarization. Acta Biomater. 2012;8:2815–23. doi: 10.1016/j.actbio.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin TH, Yao Z, Sato T, et al. Goodman, Suppression of wear-particle-induced pro-inflammatory cytokine and chemokine production in macrophages via NF-kappaB decoy oligodeoxynucleotide: A preliminary report. Acta Biomater. 2014;10:3747–55. doi: 10.1016/j.actbio.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lassus J, Waris V, Xu JW, et al. Konttinen, Increased interleukin-8 (IL-8) expression is related to aseptic loosening of total hip replacement. Arch Orthop Trauma Surg. 2000;120:328–32. doi: 10.1007/s004020050475. [DOI] [PubMed] [Google Scholar]
- 21.Haleem-Smith H, Argintar E, Bush C, et al. Biological responses of human mesenchymal stem cells to titanium wear debris particles. J Orthop Res. 2012;30:853–63. doi: 10.1002/jor.22002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu Q, Shi Q, Yang H. The role of TLR and chemokine in wear particle-induced aseptic loosening. J Biomed Biotechnol. 2012;2012:596870. doi: 10.1155/2012/596870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 24.Nich C, Langlois J, Marchadier A, et al. Oestrogen deficiency modulates particle-induced osteolysis. Arthritis Res Ther. 2011;13:R100. doi: 10.1186/ar3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Zhu S, Cui J, et al. Strontium ranelate inhibits titanium-particle-induced osteolysis by restraining inflammatory osteoclastogenesis in vivo. Acta Biomater. 2014;10:4912–18. doi: 10.1016/j.actbio.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Wu NN, Mu YQ, et al. The effect of adenovirus-mediated siRNA targeting BMPR-II on UHMWPE-induced osteoclast formation. Biomaterials. 2013;34:150–59. doi: 10.1016/j.biomaterials.2012.09.059. [DOI] [PubMed] [Google Scholar]
- 27.Landgraeber S, Jager M, Jacobs JJ, Hallab NJ. The pathology of orthopedic implant failure is mediated by innate immune system cytokines. Mediators Inflamm. 2014;2014:185150. doi: 10.1155/2014/185150. [DOI] [PMC free article] [PubMed] [Google Scholar]