Abstract
Macrophage plasticity is an important feature of these innate immune cells. Macrophage phenotypes are divided into two categories, the classically activated macrophages (CAM, M1 phenotype) and the alternatively activated macrophages (AAM, M2 phenotype). M1 macrophages are commonly associated with the generation of proinflammatory cytokines, whereas M2 macrophages are anti-inflammatory and often associated with tumor progression and fibrosis development. Macrophages produce high levels of reactive oxygen species (ROS). Recent evidence suggests ROS can potentially regulate macrophage phenotype. In addition, macrophages phenotypes are closely related to their metabolic patterns, particularly fatty acid/cholesterol metabolism. In this review, we briefly summarize recent advances in macrophage polarization with special attention to their relevance to specific disease conditions and metabolic regulation of polarization. Understanding these metabolic switches can facilitate the development of targeted therapies for various diseases.
Keywords: Macrophage, Macrophage polarization, Alternative activation, Pulmonary fibrosis, Mitochondria, Reactive oxygen species, Fatty acid oxidation
Origins of Macrophages
Macrophages are innate immune cells of the mononuclear phagocyte system that play an important role in the cross-talk between the innate and adaptive immunity [1,2]. The function of macrophages varies significantly with regard to tissue specificity, such as the alveolar macrophages, the adipose tissue macrophages, Kupffer cells in the liver, and microglia cells in the central nerve system. Based on the expression of F4/80, murine tissue macrophages can trace their origin back to two sources. Macrophages that are derived within bone marrow usually express low F4/80, whereas macrophages that originate from the embryonal yolk sac typically express high level of F4/80 and are capable of proliferating in situ, a scenario seen in the radiation-induced chimeras and the bone marrow transplant mice [3–5]. To further delineate the circulating monocyte derived macrophages, studies using specific surface markers, lymphocyte antigen 6C (Ly6C), C-C chemokine receptor type 2 (CCR2), and CX3C chemokine receptor 1 (CX3CR1), two sets of monocytes are identified: the Ly6C-high and Ly6C-low monocytes [6]. The Ly6Chigh monocytes are inflammatory monocytes, which have high expression of CCR2 and a low level of CX3CR1. They are short-lived and rapidly recruited to the site of inflammation during the acute infectious process. The Ly6Clow monocytes do not express CCR2 but have high level of CX3CR1. They usually do not migrate immediately to the site of infection due to their low expression of CCR2. However, Ly6Clow monocytes are usually long-lived cells and play an important role in chronic processes, such as tumorigenesis and fibrotic remodeling.
Macrophage Polarization
Macrophage polarization is a process through which macrophages obtain different phenotypes. The phenotype of a macrophage is closely related to the microenvironment in which they reside, as macrophages are able to switch phenotypes constantly both in vivo and in vitro [7,8]. In an analogy to the T-helper-cell nomenclature, where Th1 cells are associated with the response against bacteria or viruses, and Th2 cells are associated with the response to parasitic infection and tissue remodeling, macrophages can be denoted as M1 and M2 macrophages. M1 macrophages (or classically activated macrophages, CAMs) are pro-inflammatory and have potent microbicidal and tumoricidal activity, whereas the M2 macrophages (or alternatively activated macrophages, AAMs) are involved in tumor progression and tissue remodeling, including fibrosis [9,10].
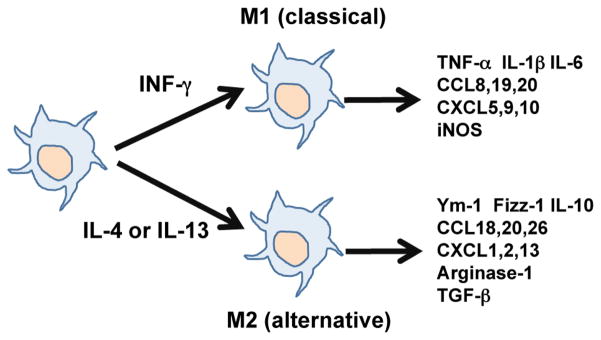
Classical macrophage activation requires priming with IFN-γ, the canonical cytokine generated by Th1 cells, and activation of the downstream transcription factors, such as signal transducer and activator of transcription 1 (STAT1), nuclear factor-kappa lightchain-enhancer of activated-B cells (NF-κB), and interferon regulatory factor 5 (IRF-5). These M1 macrophages express inflammatory genes, including TNF-α, IL-1β, and IL-6. Alternatively activated macrophages are usually activated by Th2 cytokines, IL-4 and/or IL-13. The wide range of immunosuppressive cytokines and growth factors alternatively activated macrophages produce, such IL-10, IL-1ra (IL-1 receptor antagonist), and transforming growth factor-β (TGF-β), are closely related to their ability to attenuate inflammation and promote extracellular tissue remodeling. Transcription factors involved in M2 polarization include STAT3, STAT6, IRF-4, and peroxisome proliferator-activated receptor (PPAR)-γ (Figure 1). Differential metabolism of L-arginine is characteristic of M1 and M2 macrophages. L-arginine is metabolized by iNOS to generate nitric oxide (NO) in M1 macrophages and by arginase-1 in M2 macrophages to augment the production of polyamines and L-proline, which are essential substrates for collagen synthesis [11,12].
Figure 1.
General concepts of macrophage polarization and properties of M1 and M2 macrophages. INF-γ induces M1 (classical) macrophage polarization whereas IL-4 and/or IL-13 induce M2 (alternative) macrophage polarization.
The origin of macrophages also plays a critical role in determining macrophage phenotype. L. sigmodontis infection induces M2 macrophage proliferation in situ, rather than by the recruitment and differentiation of circulating monocytes [13]. In contrast, in a LPS-induced COPD model, using MRI tracking of nanoparticles-labeled ex vivo, prepolarized bone marrow-derived macrophages, both M1 and M2 are recruited to the sites of inflammation in the lung at similar level [14].
Macrophage polarization and human diseases
The classically activated M1 macrophages boast the basic macrophage function as implied by the name given by Elie Metchnikoff in 1887. They are the dominant cells in acute infection, participating in bacteria/pathogen clearance and antigen presenting by their effective phagocytic ability. They also have compelling tumoricidal activity. M2 macrophages are actively involved in many processes associated with parasitic infection, immune tolerance, wound healing, and tumorigenesis. The function of M1 and M2 macrophages are detailed below with a particular focus of M2 macrophage and human diseases.
Inflammation, infection, and sepsis
The generation and role of alternatively activated macrophages (AAMs) has been studied extensively in helminth-related diseases [15–17]. After N. brasiliensis subcutaneous inoculation, their larvae travel to the lung and trigger a potent M2 polarization in alveolar macrophages [15]. Helminth infection not only initiates M2 polarization, but also is also capable of subverting the M1 polarization as shown in Francisella tularensis infection [18]. In an animal model of schistosomiasis, conditional macrophage/neutrophil IL-4 receptor alpha-deficient mice (LysMCre-IL-4Rα (−/flox)) show a predominant M1 polarization and more severe infection with 100% mortality [19]. Endotoxin or lipopolysaccharide (LPS) tolerance is the reduced responsiveness to LPS stimulus after repeated exposure. It is a common scenario in patients with persistent sepsis, especially in intensive care settings [20]. TNF-α production was significantly elevated in monocytes treated with one dose of LPS. However, if these cells were pre-challenged with the same dose of LPS 24 h before the second dose, the level of TNF-α production was greatly reduced [21]. Peripheral blood monocytes and macrophages from these patients often display features resembling alternative activation of monocytes, including reduced production of pro-inflammatory mediators and expression of genes involved in tissue remodeling [21,22]. Similarly, peripheral monocytes collected from septic patients have higher level of T17 and Treg cell populations with elevated CD206 and CD163 expression, suggesting LPS-tolerance and M2 polarization [23].
Wound and tissue remodeling
Wound macrophages are known to undergo alternative activation [24]. Delayed healing occurs in mice with dysfunctional M2 macrophages or deficiency of signature M2 gene expression, such as arg1 [25]. Arginase-1 is pertinent to fibrosis development as it metabolizes arginine to generate L-ornithine, which will be utilized by ornithine decarboxylase to generate L-proline and polyamines. While induction of arginase-1 by IL-4 and/or IL-13 is commonly believed to contribute to collagen deposition and fibrosis development [26,27], reports suggest that up-regulation of arginase-1 in macrophages actually inhibits fibrosis development as they compete with fibroblasts for arginine as the substrate for L-ornithine synthesis and by inhibiting Th2 cytokine production, particularly IL-13 [28]. Both IL-4 and IL-13 receptors have been shown to be essential for fibrosis development in S. mansoni granuloma formation [29]. Alternative activation of macrophages is the predominant macrophage phenotype in tissue samples from patients with chronic pancreatitis, and mice lacking IL-4Rα have less M2 macrophages and are protected from developing fibrotic changes after ceruletide injection [30]. By using an IL-4/IL-13 blocking peptide, similar anti-fibrotic effects can be achieved via inhibition of M2 polarization [30].
Cardiovascular diseases
The exact mechanism of how different macrophage phenotypes influence myocardial remodeling remains largely unknown. M2 macrophages have been shown to be crucial for post-myocardial infarction remodeling as IL-13−/− mice have significant worsening outcome in an infarction model compared to wild-type mice [31]. Another study showed that mineralocorticoid receptor knockout mice displayed a dominant M2 polarization pattern, and these mice are protected against cardiac hypertrophy, fibrosis, and vascular damage caused by angiotensin II. Additionally, aldosterone can induce M1 polarization, while eplerenone, an aldosterone antagonist, inhibits M1 activation, underscoring the cardioprotective role of M2 macrophages [32].
Pulmonary diseases
Alternatively activated macrophages are also implicated in various pulmonary disorders, including COPD, asthma, pulmonary hypertension, and pulmonary fibrosis. Plasma Chitinase-1, a signature M2 protein, has been used to quantify disease severity in COPD patients [33]. One study shows a remarkable example of a pathogenic role of IL-13 in chronic obstructive pulmonary disease (COPD) that underscores the effect of M2 macrophages. The macrophages upregulate IL-13Rα1 expression and become alternatively activated by an autocrine or paracrine mechanism [34], which leads to COPD progression. The role of different macrophage phenotypes in pulmonary hypertension remains undetermined. It is known that fibroblast-derived IL-6 polarizes alveolar macrophages into an M1 pattern and drives the development of pulmonary hypertension in a paracrine fashion, together with activation of signature M1 transcription factors, STAT3 and HIF-1α [35]. Others have found that macrophages acquire an M2 phenotype during hypoxia, and M2 macrophages lead to the proliferation of pulmonary artery smooth muscle cells. Blocking M2 polarization can potentially attenuate the progression of pulmonary hypertension by attenuating smooth muscle cell proliferation [36]. Additionally, M2 macrophages are known to be prevalent in the lungs of patients with idiopathic pulmonary fibrosis, sarcoidosis, systemic sclerosis, asbestos-induced pulmonary fibrosis, and gamma-herpes virus-induced pulmonary fibrosis [7,37,38]. Conversely, mice with predominant M1 macrophages are protected from developing asbestos-induced pulmonary fibrosis [7,39]. Similarly, in a bleomycin-induced pulmonary fibrosis model, both fibrosis and alternative activation of macrophages are prolonged in TNF-α−/− mice. Intra-tracheal delivery of recombinant TNF-α can ameliorate established pulmonary fibrosis, partially via inducing Fas-mediated fibroblast apoptosis [40,41]. Moreover, CCL-18, a signature M2 chemokine, is known to induce lung fibroblast collagen production [42], highlighting the importance of crosstalk between macrophages and fibroblasts.
Cancer
Tumor-associated macrophages (TAMs) have many properties of M2 macrophages, and they contribute to tumor local invasion through secreting proteinases, such as cathepsin [43]. GB111-NH2, an inhibitor of cathepsin, decreases expression of the classic M2 genes, fizz1 and jmjd3, resulting in tumor regression [44]. TAMs also promote angiogenesis and tumor growth through VEGF, leading to chemo-resistance [45,46]. M2 macrophages promote tumorigenesis by increasing signature M2 markers, such as CCL-18 [47]. IL-13, along with its receptors IL-13Rα2, induces TGF-β expression and contributes to tumor development by inhibiting cytotoxic T cells [48]. In contrast, blockage IL-13Rα2 via siRNA reduces metastasis and promotes survival [49]. Exposing TAMs to the canonical Th1 cytokine, INF-γ, can reprogram TAMs to acquire M1 features and regain anti-tumor activity [50]. Similarly, targeting transcription factors crucial for TAM differentiation, such as STAT3, can also achieve tumoricidal function [51]. Molecular inhibitors targeting M2 macrophages, such as the pro-apoptotic peptide [52] and anti-VEGF antibody [53], are considered to be potential candidates for cancer treatment.
Metabolic regulation of macrophage polarization
Redox status regulates macrophage polarization
The role of oxidative stress in macrophage polarization is controversial. The development of granulomas from S. mansoni exposure is not impaired in IL-4-deficient mice [54,55], as other Th2 cytokines remain elevated. In addition, wound macrophages are known to undergo alternative activation despite a deficiency of Th2 cytokines in the wound environment, and the macrophage phenotype is sustained in mice lacking IL-4R. It is not clear from these studies what induced the alternative activation.
Oxidative stress has long been known to play an important role in the development and progression of pulmonary diseases. Pro-inflammatory M1 genes, such as tnf-α, il-1β, and inos, have all been shown to be regulated by redox proteins, including Cu,Zn-SOD [56–58]. ym1 and fizz1, two signature M2 genes, are elevated in ovalbumin-challenged asthmatic mice, and their expression can be attenuated by treatment with N-acetylcysteine, a thiol-reducing agent, linking M2 polarization to oxidative stress [59]. Previous studies have shown that increases in the oxidative metabolic environment fuels alternative activation of macrophages [60], while others show that M2 macrophages generate low levels of ROS [61]. The H2O2 gradient, generated by dual oxidases (DUOX) in wound epithelium of zebrafish larvae, is known to be the chemo-attractants for macrophage recruitment [62]. IL-4-stimulated M2 macrophages have an enhanced mitochondrial oxygen-consumption rate [63], and inhibition of mitochondrial respiration by oligomycin dramatically increased the mRNA expression level of pro-inflammatory genes, such as il-6, tnf-α, and il-1β, underscoring an important role of mitochondrial respiration in M2 polarization [64].
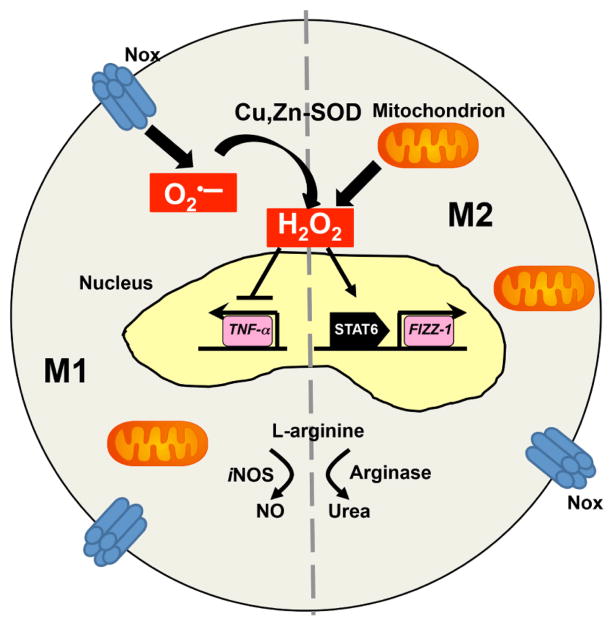
Data linking ROS to macrophage activation are emerging, but the exact role of ROS still requires further investigation. The loss of NADPH in a type I diabetes mouse model, superoxide-deficient bone marrow-derived macrophages had a marked reduction in proinflammatory M1 gene expression and showed increased M2 polarization, together with STAT6 activation [65]. Deficiency of nuclear-encoded protein NADH: ubiquinone oxidoreductase iron-sulfur protein 4 (Ndufs4), a critical component of mitochondrial complex I, is known to be related to impairment of oxidative phosphorylation [66]. Global Ndufs4 loss causes systemic inflammation with a predominant M1 polarization [67]. At the same time, a metabolic shift from fatty acid oxidation (FAO) to glycolysis was observed in Ndufs4−/− pups. Moreover, Ndufs4−/− bone marrow macrophages have significantly higher superoxide levels, which can be attenuated by MitoTEMPO to further decrease pro-inflammatory gene expression. Conversely, circulating M2 macrophages accelerate the pathological progression of amyotropic lateral sclerosis (ALS), a disease characterized with aberrant Cu,Zn-SOD function and excessive H2O2 production [68]. Over-expression of Cu,Zn-SOD, the redox protein that catalyzes the generation of H2O2, polarizes macrophages to an M2 phenotype via activation of STAT6 with a cysteine residue (Cys528) serving as the redox switch [7]. Moreover, Cu,Zn-SOD-mediated macrophage polarization can be altered by modulating H2O2 generation. As previously mentioned, differential metabolism of L-arginine is characteristic of M1 and M2 macrophages. Overexpression of Cu,Zn-SOD leads to a reduction of inos gene expression and NO synthesis, while arginase-1 expression and urea generation is enhanced [7] (Figure 2). Acute chlorine gas exposure leads to oxidation of surfactant protein and augmentation of M2 genes, such as arg1, fizz1, and ym1 [69]. Another study showed that alveolar macrophages exposed to ozone have elevated levels of both M1 and M2 genes [70]. Interestingly, one study has compared macrophage phenotype in two Nox2-deficient mouse models, gp91phox−/− and p47phox−/−. Mice deficient in p47phox−/− have a significant increase of M2 gene expression upon IL-4 stimulation and are protected from Listeria monocytogenes infection compared with gp91phox−/− mice [71]. Explanations for the differences include that macrophage polarization is driven by specific reactive oxygen species (H2O2 vs O2 •−), the different origin of ROS (membrane-bound NADPH oxidase, particularly Nox2 versus mitochondria), or the different tissue and intracellular distribution of NADPH oxidases or SODs.
Figure 2.
Redox regulation of macrophage polarization. Superoxide generated by either membrane-bound NADPH oxidase or mitochondrial electron transfer chain (ETC) will be converted to H2O2 by superoxide dismutase, which will inhibit M1 polarization and activate M2 polarization via STAT6. Revised from [7].
Redox regulation in macrophage polarization is closely related to hypoxic conditions and hypoxia-inducible factors (HIFs) activation. In murine macrophages, the expression of hypoxia-inducible factors HIF-1α and HIF-2α appears to be dependent on respective inducers. M1-promoting factors induce the expression of HIF-1α, whereas IL-4 primarily induces HIF-2α that regulates M2 polarization [72]. HIF-1α−/− macrophages exhibit diminished production of TNF-α and IL-6 in response to LPS/IFN-γ stimulation in a model of tumor spheroids [73].
Oxidative stress, particular the mitochondrial redox signal, is known to cause endoplasmic reticulum (ER) stress due to the proximal distance between mitochondria and ER [74]. Asbestos-treated macrophages, which show M2 polarization, have elevated ER stress with elevated level of binding immunoglobulin protein (BiP) and C/EBP homologous protein (CHOP) [75]. Induction of ER stress induces macrophage polarization from the M1 into the M2 phenotype leading to increased cholesterol deposition and enhanced foam cell formation [76]. MCP-1-induced protein (MCPIP), induced by either STAT6 or KLF-4, inhibits NF-κB in murine macrophages and instigates M2 polarization via induction of ER stress [77]. BiP and CHOP levels are elevated in THP-1 monocytes treated with ER-stress inducers, tunicamycin or thapsigargin, and the THP-1 cells undergo M2 polarization via the PPAR-γ pathway. Interestingly, M2 polarization could be reversed by treating with ER stress inhibitor 4-phenylbutyrate (PBA), emphasizing a potential therapeutic target [78].
Metabolism of fatty acid/cholesterol regulates macrophage polarization
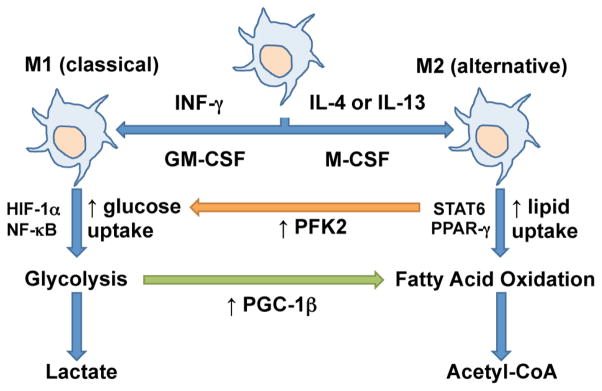
Prior data show that M2 polarization is dependent on fatty acid oxidation (FAO), whereas M1 macrophages rely on aerobic glycolysis [79]. The differences between the two metabolic pathways involve a switch in the expression of 6-phosphofructo-2-kinase/fructose-2,6-bisphostase (PFK2). M1 macrophages display a high expression of glycolytic enzymes and glycolysis-related metabolites. This shift toward aerobic glycolysis, known as the Warburg effect in cancer biology, rapidly provides immune cells with ATP and metabolic intermediates. In contrast, M2 macrophages have increased expression of genes encoding molecules in FAO and oxidative phosphorylation pathways [63]. Blocking oxidative metabolism not only selectively abrogates the ability of cells to undergo alternative activation but also potentiates the expression of M1 genes. Conversely, overexpressing PGC-1β, a key transcriptional proponent of oxidative metabolism, potentiates alternative activation and prevents classical activation by augmenting FAO [60] (Figure 3). Compared with M1 macrophages, which exert their functions over short time periods, M2 macrophages are engaged in long-term cellular activities, and the relative efficiency of FAO versus that of glycolysis is well suited to meet the metabolic requirements of their roles [80]. M2 macrophages have been shown to have longer survival compared to their M1 counterparts [63], and FAO is known to support cellular longevity [81].
Figure 3.
Metabolic regulation of macrophage polarization. M1 macrophages have increased uptake of glucose and augmented glycolysis, whereas M2 macrophages have increased uptake of lipid and augmented fatty acid oxidation. Specific cytokines and transcription factors regulate these pathways. Activation of PFK2 leads to M1 polarization while over-expressing PGC-1β leads to M2 polarization.
The isoprenoid pathway, which is essential for cholesterol metabolism, is a new target of modulating macrophage function. The use of statins has been associated with interstitial lung abnormalities in smoking individuals, a condition known to have a predominance of M2 macrophages [82]. Statins have potent anti-inflammatory properties and are known to orchestrate the immune response toward alternative activation via regulating isoprenoid biosynthesis [83]. The inhibition of farnesyltransferase, geranylgeranyltransferase I, and geranylgeranyltransferase II decreases cell survival, migration, and proliferation in many cancers [84]. Activation of Rac1 by geranylgeranylation in alveolar macrophages promotes characteristics of M2 macrophages and associates with the development of oxidative stress and pulmonary fibrosis. Digeranyl bisphosphonate (DGBP), which impairs geranylgeranylation of Rho GTPases by inhibiting geranylgeranyl diphosphate synthase, reduces mitochondrial oxidative stress and abrogates progression of pulmonary fibrosis by inhibiting Rac1 activation and its mitochondrial translocation [85].
Both the Akt pathway and the isoprenoid pathway are important in maintaining cell survival. Akt regulates apoptosis by modulating isoprenoid pathway. Akt-deficient macrophages (Akt+/−) have a significant increase of apoptosis. Akt overexpressing macrophages have a distinct M2 polarization pattern and promote fibrotic development. Conversely, Akt+/− mice are protected from developing pulmonary fibrosis [86]. Statins activate Akt and, as previously mentioned, the use of statins has been associated with interstitial lung abnormalities in smoking individuals [82,87]. Surface scavenger receptors, which are crucial for internalization of extracellular oxidized lipid particles, are capable of regulating macrophage polarization. CD36 is known to be important for triacylglycerol substrate uptake and sequential oxidative phosphorylation, which leads to M2 polarization [63]. Another surface scavenger receptor, MARCO (macrophage receptor with collagenous structure) has been shown to increase mitochondrial oxidative stress and regulates macrophage polarization. Over-expression of wild-type MACRO leads to increased M2 gene expression, while knockdown of MARCO reduces M2 gene expression. Moreover, MACRO−/− mice are protected from developing asbestos-induced pulmonary fibrosis. Inhibition of the scavenger receptor by fucoidan reduces mitochondrial H2O2 production, which inhibits macrophage M2 polarization [88]. Similarly, MARCO can limit inflammatory response as MARCO-deficient mice show an early-enhanced development of inflammation in response to influenza infection [89]. CD163, a scavenger receptor for the hemoglobin-haptoglobin complex, is expressed at high level by M2 macrophages in patients with idiopathic pulmonary fibrosis [90].
Conclusion
Macrophage polarization is a dynamic process that our immune system utilizes to maintain an immunological homeostasis. Various factors influence polarization and further investigation for metabolic regulation in shaping the macrophage differential profile is warranted. In this review, we briefly summarize recent advances in macrophage polarization with special attention to their relevance to specific disease conditions and metabolic regulation of polarization. Understanding these metabolic switches can facilitate the development of targeted therapies for various diseases related to the distinct macrophage subtype.
Acknowledgments
This publication was supported in part by National Institute of Health Grants 2R01ES015981-08 and by a Merit Review from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biological Laboratory Research and Development BX001135-04.
References
- 1.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med. 2013;210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hume DA, Ross IL, Himes SR, Sasmono RT, Wells CA, et al. The mononuclear phagocyte system revisited. J Leukoc Biol. 2002;72:621–627. [PubMed] [Google Scholar]
- 3.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt A, Sucke J, Fuchs-Moll G, Freitag P, Hirschburger M, et al. Macrophages in experimental rat lung isografts and allografts: infiltration and proliferation in situ. J Leukoc Biol. 2007;81:186–194. doi: 10.1189/jlb.0606377. [DOI] [PubMed] [Google Scholar]
- 5.Tarling JD, Lin HS, Hsu S. Self-renewal of pulmonary alveolar macrophages: evidence from radiation chimera studies. J Leukoc Biol. 1987;42:443–446. [PubMed] [Google Scholar]
- 6.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 7.He C, Ryan AJ, Murthy S, Carter AB. Accelerated development of pulmonary fibrosis via Cu,Zn-superoxide dismutase-induced alternative activation of macrophages. J Biol Chem. 2013;288:20745–20757. doi: 10.1074/jbc.M112.410720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 9.Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 10.Zaynagetdinov R, Sherrill TP, Polosukhin VV, Han W, Ausborn JA, et al. A critical role for macrophages in promotion of urethane-induced lung carcinogenesis. J Immunol. 2011;187:5703–5711. doi: 10.4049/jimmunol.1100558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Modolell M, Corraliza IM, Link F, Soler G, Eichmann K. Reciprocal regulation of the nitric oxide synthase/arginase balance in mouse bone marrowderived macrophages by TH1 and TH2 cytokines. Eur J Immunol. 1995;25:1101–1104. doi: 10.1002/eji.1830250436. [DOI] [PubMed] [Google Scholar]
- 12.Munder M, Eichmann K, Morán JM, Centeno F, Soler G, et al. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999;163:3771–3777. [PubMed] [Google Scholar]
- 13.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al Faraj A, Sultana Shaik A, Pureza MA, Alnafea M, Halwani R. Preferential macrophage recruitment and polarization in LPS-induced animal model for COPD: noninvasive tracking using MRI. PLoS One. 2014;9:e90829. doi: 10.1371/journal.pone.0090829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, et al. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18:260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuah C, Jones MK, Burke ML, McManus DP, Gobert GN. Cellular and chemokine-mediated regulation in schistosome-induced hepatic pathology. Trends Parasitol. 2014;30:141–150. doi: 10.1016/j.pt.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Salgame P, Yap GS, Gause WC. Effect of helminth-induced immunity on infections with microbial pathogens. Nat Immunol. 2013;14:1118–1126. doi: 10.1038/ni.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirey KA, Cole LE, Keegan AD, Vogel SN. Francisella tularensis live vaccine strain induces macrophage alternative activation as a survival mechanism. J Immunol. 2008;18:4159–4167. doi: 10.4049/jimmunol.181.6.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbert DR, Hölscher C, Mohrs M, Arendse B, Schwegmann A, et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 20.Cavaillon JM, Adib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care. 2006;10:233. doi: 10.1186/cc5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pena OM, Pistolic J, Raj D, Fjell CD, Hancock RE. Endotoxin tolerance represents a distinctive state of alternative polarization (M2) in human mononuclear cells. J Immunol. 2011;186:7243–7254. doi: 10.4049/jimmunol.1001952. [DOI] [PubMed] [Google Scholar]
- 22.Adib-Conquy M, Adrie C, Fitting C, Gattolliat O, Beyaert R, et al. Up-regulation of MyD88s and SIGIRR, molecules inhibiting Toll-like receptor signaling, in monocytes from septic patients. Crit Care Med. 2006;34:2377–2385. doi: 10.1097/01.CCM.0000233875.93866.88. [DOI] [PubMed] [Google Scholar]
- 23.Brunialti MK, Santos MC, Rigato O, Machado FR, Silva E, et al. Increased percentages of T helper cells producing IL-17 and monocytes expressing markers of alternative activation in patients with sepsis. PLoS One. 2012;7:e37393. doi: 10.1371/journal.pone.0037393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daley JM, Brancato SK, Thomay AA, Reichner JS, Albina JE. The phenotype of murine wound macrophages. J Leukoc Biol. 2010;87:59–67. doi: 10.1189/jlb.0409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J Immunol. 2001;167:6533–6544. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- 27.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, et al. Arginase-1-expressing macrophages suppress Th2 cytokinedriven inflammation and fibrosis. PLoS Pathog. 2009;5:e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jankovic D, Kullberg MC, Noben-Trauth N, Caspar P, Ward JM, et al. Schistosome-infected IL-4 receptor knockout (KO) mice, in contrast to IL-4 KO mice, fail to develop granulomatous pathology while maintaining the same lymphokine expression profile. J Immunol. 1999;163:337–342. [PubMed] [Google Scholar]
- 30.Xue J, Sharma V, Hsieh MH, Chawla A, Murali R, et al. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun. 2015;6:7158. doi: 10.1038/ncomms8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofmann U, Knorr S, Vogel B, Weirather J, Frey A, et al. Interleukin-13 deficiency aggravates healing and remodelling in male mice after experimental myocardial infarction. Circulation Heart failure. 2014;7:822–830. doi: 10.1161/CIRCHEARTFAILURE.113.001020. [DOI] [PubMed] [Google Scholar]
- 32.Usher MG, Duan SZ, Ivaschenko CY, Frieler RA, Berger S, et al. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest. 2010;120:3350–3364. doi: 10.1172/JCI41080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agapov E, Battaile JT, Tidwell R, Hachem R, Patterson GA, et al. Macrophage chitinase 1 stratifies chronic obstructive lung disease. Am J Respir Cell Mol Biol. 2009;41:379–384. doi: 10.1165/rcmb.2009-0122RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holtzman MJ, Byers DE, Benoit LA, Battaile JT, You Y, et al. Immune pathways for translating viral infection into chronic airway disease. Adv Immunol. 2009;102:245–276. doi: 10.1016/S0065-2776(09)01205-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El Kasmi KC, Pugliese SC, Riddle SR, Poth JM, Anderson AL, et al. Adventitial fibroblasts induce a distinct proinflammatory/ profibrotic macrophage phenotype in pulmonary hypertension. J Immunol. 2014;193:597–609. doi: 10.4049/jimmunol.1303048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vergadi E, Chang MS, Lee C, Liang OD, Liu X, et al. Early macrophage recruitment and alternative activation are critical for the later development of hypoxia-induced pulmonary hypertension. Circulation. 2011;123:1986–1995. doi: 10.1161/CIRCULATIONAHA.110.978627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pechkovsky DV, Prasse A, Kollert F, Engel KM, Dentler J, et al. Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction. Clin Immunol. 2010;137:89–101. doi: 10.1016/j.clim.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 38.Guenther JF, Cameron JE, Nguyen HT, Wang Y, Sullivan DE, et al. Modulation of lung inflammation by the Epstein-Barr virus protein Zta. Am J Physiol Lung Cell Mol Physiol. 2010;299:L771–784. doi: 10.1152/ajplung.00408.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He C, Murthy S, McCormick ML, Spitz DR, Ryan AJ, et al. Mitochondrial Cu,Zn-superoxide dismutase mediates pulmonary fibrosis by augmenting H2O2 generation. J Biol Chem. 2011;286:15597–15607. doi: 10.1074/jbc.M110.187377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Redente EF, Keith RC, Janssen W, Henson PM, Ortiz LA, et al. Tumor necrosis factor-alpha accelerates the resolution of established pulmonary fibrosis in mice by targeting profibrotic lung macrophages. Am J Respir Cell Mol Biol. 2014;50:825–837. doi: 10.1165/rcmb.2013-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frankel SK, Cosgrove GP, Cha SI, Cool CD, Wynes MW, et al. TNF-alpha sensitizes normal and fibrotic human lung fibroblasts to Fas-induced apoptosis. Am J Respir Cell Mol Biol. 2006;34:293–304. doi: 10.1165/rcmb.2005-0155OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prasse A, Pechkovsky DV, Toews GB, Jungraithmayr W, Kollert F, et al. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am J Respir Crit Care Med. 2006;173:781–792. doi: 10.1164/rccm.200509-1518OC. [DOI] [PubMed] [Google Scholar]
- 43.Bengsch F, Buck A, Günther SC, Seiz JR, Tacke M, et al. Cell type-dependent pathogenic functions of overexpressed human cathepsin B in murine breast cancer progression. Oncogene. 2014;33:4474–4484. doi: 10.1038/onc.2013.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salpeter SJ, Pozniak Y, Merquiol E, Ben-Nun Y, Geiger T, et al. A novel cysteine cathepsin inhibitor yields macrophage cell death and mammary tumor regression. Oncogene. 2015 doi: 10.1038/onc.2015.51. [DOI] [PubMed] [Google Scholar]
- 45.Shojaei F, Ferrara N. Refractoriness to antivascular endothelial growth factor treatment: role of myeloid cells. Cancer Res. 2008;68:5501–5504. doi: 10.1158/0008-5472.CAN-08-0925. [DOI] [PubMed] [Google Scholar]
- 46.Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 47.Chen J, Yao Y, Gong C, Yu F, Su S, et al. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell. 2011;19:541–555. doi: 10.1016/j.ccr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas DA, Massagué J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 49.Fichtner-Feigl S, Terabe M, Kitani A, Young CA, Fuss I, et al. Restoration of tumor immunosurveillance via targeting of interleukin-13 receptor-alpha 2. Cancer Res. 2008;68:3467–3475. doi: 10.1158/0008-5472.CAN-07-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duluc D, Corvaisier M, Blanchard S, Catala L, Descamps P, et al. Interferon-gamma reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor-associated macrophages. Int J Cancer. 2009;125:367–373. doi: 10.1002/ijc.24401. [DOI] [PubMed] [Google Scholar]
- 51.Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 52.Cieslewicz M, Tang J, Yu JL, Cao H, Zavaljevski M, et al. Targeted delivery of proapoptotic peptides to tumor-associated macrophages improves survival. Proc Natl Acad Sci U S A. 2013;110:15919–15924. doi: 10.1073/pnas.1312197110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huanga Y, Yuanb J, Righib E, Kamouna WS, Ancukiewicza M, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Nat Acad Sci U S A. 2012;109:17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chensue SW, Warmington K, Ruth JH, Lukacs N, Kunkel SL. Mycobacterial and schistosomal antigen-elicited granuloma formation in IFNgamma and IL-4 knockout mice: analysis of local and regional cytokine and chemokine networks. J Immunol. 1997;159:3565–3573. [PubMed] [Google Scholar]
- 55.Pearce EJ, Cheever A, Leonard S, Covalesky M, Fernandez-Botran R, et al. Schistosoma mansoni in IL-4-deficient mice. Int Immunol. 1996;8:435–444. doi: 10.1093/intimm/8.4.435. [DOI] [PubMed] [Google Scholar]
- 56.Chang SC, Kao MC, Fu MT, Lin CT. Modulation of NO and cytokines in microglial cells by Cu/Zn-superoxide dismutase. Free Radic Biol Med. 2001;31:1084–1089. doi: 10.1016/s0891-5849(01)00691-8. [DOI] [PubMed] [Google Scholar]
- 57.Marikovsky M, Ziv V, Nevo N, Harris-Cerruti C, Mahler O. Cu/Zn superoxide dismutase plays important role in immune response. J Immunol. 2003;170:2993–3001. doi: 10.4049/jimmunol.170.6.2993. [DOI] [PubMed] [Google Scholar]
- 58.Meissner F, Molawi K, Zychlinsky A. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat Immunol. 2008;9:866–872. doi: 10.1038/ni.1633. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L, Wang M, Kang X, Boontheung P, Li N, et al. Oxidative stress and asthma: proteome analysis of chitinase-like proteins and FIZZ1 in lung tissue and bronchoalveolar lavage fluid. J Proteome Res. 2009;8:1631–1638. doi: 10.1021/pr800685h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pelegrin P, Surprenant A. Dynamics of macrophage polarization reveal new mechanism to inhibit IL-1beta release through pyrophosphates. EMBO J. 2009;28:2114–2127. doi: 10.1038/emboj.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang SC, Everts B, Ivanova Y, O’Sullivan D, Nascimento M, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15:846–855. doi: 10.1038/ni.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Izquierdo E, Cuevas VD, Fernández-Arroyo S, Riera-Borrull M, Orta-Zavalza E, et al. Reshaping of Human Macrophage Polarization through Modulation of Glucose Catabolic Pathways. J Immunol. 2015;195:2442–2451. doi: 10.4049/jimmunol.1403045. [DOI] [PubMed] [Google Scholar]
- 65.Padgett LE, Burg AR, Lei W, Tse HM. Loss of NADPH oxidase-derived superoxide skews macrophage phenotypes to delay type 1 diabetes. Diabetes. 2015;64:937–946. doi: 10.2337/db14-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kirby DM, Crawford M, Cleary MA, Dahl HH, Dennett X, et al. Respiratory chain complex I deficiency: an underdiagnosed energy generation disorder. Neurology. 1999;52:1255–1264. doi: 10.1212/wnl.52.6.1255. [DOI] [PubMed] [Google Scholar]
- 67.Jin Z, Wei W, Yang M, Du Y, Wan Y. Mitochondrial complex I activity suppresses inflammation and enhances bone resorption by shifting macrophage-osteoclast polarization. Cell Metab. 2014;20:483–498. doi: 10.1016/j.cmet.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vaknin I, Kunis G, Miller O, Butovsky O, Bukshpan S, et al. Excess circulating alternatively activated myeloid (M2) cells accelerate ALS progression while inhibiting experimental autoimmune encephalomyelitis. PloS one. 2011;6:e26921. doi: 10.1371/journal.pone.0026921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Massa CB, Scott P, Abramova E, Gardner C, Laskin DL, et al. Acute chlorine gas exposure produces transient inflammation and a progressive alteration in surfactant composition with accompanying mechanical dysfunction. Toxicol Appl Pharmacol. 2014;278:53–64. doi: 10.1016/j.taap.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sunil VR, Patel-Vayas K, Shen J, Laskin JD, Laskin DL. Classical and alternative macrophage activation in the lung following ozone-induced oxidative stress. Toxicol Appl Pharmacol. 2012;263:195–202. doi: 10.1016/j.taap.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yi L, Liu Q, Orandle MS, Sadiq-Ali S, Koontz SM, et al. p47(phox) directs murine macrophage cell fate decisions. Am J Pathol. 2012;180:1049–1058. doi: 10.1016/j.ajpath.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takeda N, O’Dea EL, Doedens A, Kim JW, Weidemann A, et al. Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010;24:491–501. doi: 10.1101/gad.1881410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Werno C, Menrad H, Weigert A, Dehne N, Goerdt S, et al. Knockout of HIF-1alpha in tumor-associated macrophages enhances M2 polarization and attenuates their pro-angiogenic responses. Carcinogenesis. 2010;3:1863–1872. doi: 10.1093/carcin/bgq088. [DOI] [PubMed] [Google Scholar]
- 74.Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, et al. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325:477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ryan AJ, Larson-Casey JL, He C, Murthy S, Carter AB. Asbestos-induced disruption of calcium homeostasis induces endoplasmic reticulum stress in macrophages. J Biol Chem. 2014;289:33391–33403. doi: 10.1074/jbc.M114.579870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oh J, Riek AE, Weng S, Petty M, Kim D, et al. Endoplasmic reticulum stress controls M2 macrophage differentiation and foam cell formation. J Biol Chem. 2012;287:11629–11641. doi: 10.1074/jbc.M111.338673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kapoor N, Niu J, Saad Y, Kumar S, Sirakova T, et al. Transcription factors STAT6 and KLF4 implement macrophage polarization via the dual catalytic powers of MCPIP. J Immunol. 2015;194:6011–6023. doi: 10.4049/jimmunol.1402797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiu F, Catapano M, Diao L, Stanojcic M, Jeschke MG. Prolonged Endoplasmic Reticulum-Stressed Hepatocytes Drive an Alternative Macrophage Polarization. Shock. 2015;44:44–51. doi: 10.1097/SHK.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodríguez-Prados JC, Través PG, Cuenca J, Rico D, Aragonés J, et al. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185:605–614. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- 80.Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annu Rev Pathol. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu JF, Washko GR, Nakahira K, Hatabu H, Patel AS, et al. Statins and pulmonary fibrosis: the potential role of NLRP3 inflammasome activation. Am J Respir Crit Care Med. 2012;185:547–556. doi: 10.1164/rccm.201108-1574OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Youssef S, Stüve O, Patarroyo JC, Ruiz PJ, Radosevich JL, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 84.Jiang K, Coppola D, Crespo NC, Nicosia SV, Hamilton AD, et al. The phosphoinositide 3-OH kinase/AKT2 pathway as a critical target for farnesyltransferase inhibitor-induced apoptosis. Mol Cell Biol. 2000;20:139–148. doi: 10.1128/mcb.20.1.139-148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Osborn-Heaford HL, Murthy S, Gu L, Larson-Casey JL, Ryan AJ, et al. Targeting the isoprenoid pathway to abrogate progression of pulmonary fibrosis. Free Radic Biol Med. 2015;86:47–56. doi: 10.1016/j.freeradbiomed.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Larson-Casey JL, Murthy S, Ryan AJ, Carter AB. Modulation of the mevalonate pathway by akt regulates macrophage survival and development of pulmonary fibrosis. J Biol Chem. 2014;289:36204–36219. doi: 10.1074/jbc.M114.593285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, et al. The HMGCoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murthy S, Larson-Casey JL, Ryan AJ, He C, Kobzik L, et al. Alternative activation of macrophages and pulmonary fibrosis are modulated by scavenger receptor, macrophage receptor with collagenous structure. FASEB J. 2015;29:3527–3536. doi: 10.1096/fj.15-271304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ghosh S, Gregory D, Smith A, Kobzik L. MARCO regulates early inflammatory responses against influenza: a useful macrophage function with adverse outcome. Am J Respir Cell Mol Biol. 2011;45:1036–1044. doi: 10.1165/rcmb.2010-0349OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gibbons MA, MacKinnon AC, Ramachandran P, Dhaliwal K, Duffin R, et al. Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am J Respir Crit Care Med. 2011;184:569–581. doi: 10.1164/rccm.201010-1719OC. [DOI] [PubMed] [Google Scholar]