Abstract
The mouse laser-induced choroidal neovascularization (CNV) model has been a crucial mainstay model for neovascular age-related macular degeneration (AMD) research. By administering targeted laser injury to the RPE and Bruch’s membrane, the procedure induces angiogenesis, modeling the hallmark pathology observed in neovascular AMD.
First developed in non-human primates, the laser-induced CNV model has come to be implemented into many other species, the most recent of which being the mouse. Mouse experiments are advantageously more cost-effective, experiments can be executed on a much faster timeline, and they allow the use of various transgenic models. The miniature size of the mouse eye, however, poses a particular challenge when performing the procedure. Manipulation of the eye to visualize the retina requires practice of fine dexterity skills as well as simultaneous hand-eye-foot coordination to operate the laser. However, once mastered, the model can be applied to study many aspects of neovascular AMD such as molecular mechanisms, the effect of genetic manipulations, and drug treatment effects.
The laser-induced CNV model, though useful, is not a perfect model of the disease. The wild-type mouse eye is otherwise healthy, and the chorio-retinal environment does not mimic the pathologic changes in human AMD. Furthermore, injury-induced angiogenesis does not reflect the same pathways as angiogenesis occurring in an age-related and chronic disease state as in AMD.
Despite its shortcomings, the laser-induced CNV model is one of the best methods currently available to study the debilitating pathology of neovascular AMD. Its implementation has led to a deeper understanding of the pathogenesis of AMD, as well as contributing to the development of many of the AMD therapies currently available.
Keywords: Medicine, Issue 106, Laser-induced choroidal neovascularization, experimental CNV, age-related macular degeneration, neovascular age-related macular degeneration, wet AMD
Introduction
Age-related macular degeneration (AMD) is one of the leading causes of blindness in individuals over the age of 501-3. AMD can be classified into two forms: atrophic (“dry”) AMD and neovascular (“wet”) AMD. The former is characterized by geographic atrophy of the retinal pigment epithelium (RPE), choriocapillaris, and photoreceptors, while the latter is characterized by the invasion of abnormal vessels from the choroid into the outer retinal layers causing leakage, hemorrhage, and fibrosis, and ultimately leading to blindness1,2. Of the two forms, neovascular AMD accounts for the majority of vision loss1. Fortunately, this form has numerous effective pharmacological management options, whereas its atrophic counterpart currently has no proven medical treatments3. Moreover, because the neovascular form has been easily re-capitulated in an animal model, it has been more widely accessible to basic AMD research exploring the underlying pathological mechanisms in order to develop novel therapies4.
The first animal model of experimental choroidal neovascularization (CNV) was developed by Ryan et al. in non-human primates5. This model induced rupture of Bruch’s membrane via laser photocoagulation, which caused a local inflammatory response resulting in angiogenesis similar to that seen in neovascular AMD. The histopathological progression of angiogenesis post-laser induction was found to mimic neovascular AMD, which confirmed the model’s validity6. Non-human primates offer the most similar anatomy to humans, but unfortunately, are expensive to maintain, cannot be easily genetically manipulated, and have a slow time course of disease progression7. Contrastingly, rodent models are much more cost-effective to maintain, can be genetically manipulated with relative ease, and have a much faster course of disease progression (experiments can be conducted on a time scale of weeks versus months). These experiments should only be conducted in pigmented rodents as it is very difficult to visualize in albino animals.
The mouse laser-induced CNV model, first developed by the Campochiaro group in the late 90’s10, has grown to be the dominant animal model in the majority of recent studies11-16. Due to the complex and still unclear pathogenesis of CNV, the laser model has been applied in all aspects of wet AMD research ranging from studying the molecular mechanisms driving angiogenesis to evaluating new treatment modalities for future human use. For example, Sakurai et al. and Espinosa-Heidmann et al. used the laser model to investigate the effect of macrophages on the development of CNV using transgenic mice and pharmacological depletion treatments15, 16. Giani et al. and Hoerster et al. used optical-coherence tomography (OCT) to image the laser-induced CNV in an effort to characterize the progression of CNV and compare the histopathologic findings to the findings seen on OCT imaging12,17. Finally, studies involving intravitreal injection of anti-angiogenic agents have been used as pre-requisites for human trials and were vital in developing the first generation of anti-VEGF agents used in management of neovascular AMD today10,18,19.
Alternative models for experimental CNV utilize surgical methods to induce CNV. This procedure involves injecting pro-angiogenic substances (e.g. recombinant viral vectors overexpressing VEGF, subretinal injection of RPE cells and/or polystyrene beads) to mimic the increased VEGF expression seen in neovascular AMD, with the goal of causing angiogenesis8,20. However, this method yields a drastically lower incidence of neovascularization; these studies showed that CNV in C57/BL6 mice occurs in 31% of injections versus the ~70% success rate seen in the laser photocoagulation method in the same strain of mice8,14. For these reasons, and given the advantages of using rodents versus non-human primates, the mouse model of laser-induced CNV has become the standard animal model of CNV for most neovascular AMD study experiments8.
The mouse eye is a miniscule, delicate tissue to work with. Maneuvering of the eye to visualize the retina is difficult and requires much practice until mastery is achieved. This task is complicated by the fact that it must be learned with the dominant and non-dominant hand. Furthermore, after the fine movements required to visualize the retina have been learned, the coordination between both hands and the foot pedal operating the laser are important. In this paper, we sought to distill the challenges of learning all of the physical manipulations involved in the laser-induced CNV procedure into a guide that would help operators achieve rapid success with this model.
Protocol
All animals are treated in accordance with the Guide of the Care and Use of Laboratory Animals 2013 Edition, the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research, and as approved by the Institutional Animal Care and Use Committee for Northwestern University.
Note: The following procedure can be done entirely with one operator; however, it is much more efficiently conducted with two operators with the tasks split accordingly.
1. Prepare Laser and Pre-laser Station
- Position the laser and slit lamp where it can be easily accessible. Turn on laser and set to pre-determined parameters (e.g. 75 µm spot size, 100 mW power, 100 msec duration). CAUTION: Ensure operator wears all animal and laser safety personal protective equipment and pertinent laser safety signs are displayed outside of the procedure room.
- Before using any experimental animals, find ideal parameters using a calibration, non-experimental mouse. Laser parameters will depend on the laser used. Ideal parameters are defined by the lowest laser power setting that consistently causes “bubble” formation when properly focused. See video for example of lesion with proper “bubble” formation.
- Prepare the pre-laser station so that anesthesia, animal warmer, tissue wipes, and all eye drops (Tropicamide, Tetracaine, and artificial tears) are easily within reach to operator.
- Ensure that animal warmer is pre-heated to correct temperature (37 °C) before injecting anesthetic into first mouse in order to avoid anesthesia-induced hypothermia.
Place mouse stage on warmer so it can retain heat and remain warm once laser procedure begins.
2. Mouse Anesthesia and Pre-laser Preparation
Before injecting anesthesia, inspect the eye macroscopically to ensure it has no deformities or abnormalities that diminish corneal clarity (e.g. cataract).
Weigh mouse.
Record weight, sex, and animal ID number.
Using weight, calculate appropriate amount of anesthetic to be used based on guidelines given by institution (e.g. 100 mg/kg ketamine hydrochloride, 10 mg/kg xylazine OR tribromoethanol 250 mg/kg; for a 20 g mouse inject 0.20 ml xylazine/ketamine cocktail OR 0.25 ml of tribromoethanol). Have a table of pre-calculated dosages per weight in increments of 1 g in order to reduce mathematical errors.
Scruff mouse and inject anesthetic intraperitoneally based on calculations in step 2.4.
Place mouse on animal warmer and wait until mouse is completely anesthetized by checking the pedal reflex via toe pinch (approximately 3-5 min).
Roll up a tissue wipe and protect the mouse’s nasal area to prevent aspiration of liquid roll-off. Roll mouse on its side and place a drop (approximately 30 µl) of tetracaine hydrochloride into each eye for topical anesthesia. Wait 2 min for solution to take effect.
Repeat step 2.7 with one drop of topical Tropicamide for pupillary dilation. Alternatively, use phenylephrine hydrochloride (2.5%) for dilation.
Wait 2 min for solutions to take effect; keep animal on warmer during this time.
After appropriate time has elapsed, quickly place the mouse on the mouse stage and place the stage on chin rest of slit lamp.
Turn on slit lamp to the lowest light brightness and check the degree of pupillary dilation. If pupil is not adequately dilated (approximately 2.5-3 mm), return mouse to animal warmer and wait. Alternatively, administer another drop of Tropicamide. Once eye is sufficiently dilated, proceed to laser procedure.
3. Laser Procedure
Note: Ensure other persons in the room wear protective goggles when away from laser-protected slit lamp eye-piece
- Adjust the placement of mouse on the mouse-stage, so that it is ideally positioned for visualization of optic nerve (see 3.1.2).
- Orient the mouse on its holder so it lies horizontally, perpendicular to slit lamp beam, with the head at one side and tail at the other. Ideal mouse placement will make optic nerve visualization much easier once cover slip is applied.
- Slightly turn the mouse so it is at a ~170° angle with the head closer to laser operator.
- Ensure that mouse is as close as possible to slit lamp, yet still in a position where it is stable and where the operator’s hand will have enough space for fine manipulation.
- After the mouse is ideally positioned, place one drop of artificial tear solution on a 25 mm x 25 mm glass coverslip.
- Place one drop of artificial tear solution on mouse’s opposite eye – this will ensure eye is hydrated and help delay cataract formation.
Hold corner of coverslip between thumb and pointer fingers; position so that the glass is squeezed between tips of both fingers.
- Gently wrap the remaining three fingers around the animal’s body for support and hand stabilization. Position hand so that the glass coverslip can be easily placed on the mouse’s eye.
- Be sure that the wrist is stabilized on a firm surface in order to reduce hand tremor.
- Once stable position is obtained, carefully press glass coverslip (with drop of artificial tear still adhered) onto the mouse’s eye.
- Make sure the coverslip is positioned as perpendicular as possible to the laser beam in order to prevent laser beam scatter or reflection. The coverslip acts as a contact lens to flatten the cornea.
Look through slit lamp and with free hand toggle focus until retina can be visualized. The retina will have a light-yellow/red color depending on the location visualized, distinct, red vessels will be visible.
Slowly and carefully manipulate mouse head and/or coverslip until visualizing the optic nerve. The optic nerve will be yellow in color with multiple vessels radiating from it.
Once operator has confirmed visualization of optic nerve, turn on laser focusing beam.
Once laser beam has been turned on, maneuver laser focusing beam to desired position (approximately 1 disc diameter from the optic nerve).
Focus laser beam on the RPE of the eye fundus. Proper focus is achieved by having the sharpest and clearest laser beam. If aiming beam looks oval or out of focus, toggle slit lamp focus or re-position glass coverslip.
- Once the aiming beam is focused on RPE, initiate laser administration using the laser’s foot trigger.
- Be sure to avoid retinal vessels to prevent intraocular hemorrhage.
- Watch for the appearance of a bubble immediately after laser administration. The outline of the laser shot should be clear and not hazy in any way.
- If the laser shot does not result in bubble formation, or area of impact looks hazy (cloudy appearance with ill-defined circular border vs. clear, sharply defined border of a successful impact), or if hemorrhage is seen after laser administration, do NOT include these lesions for future analysis.
Repeat steps 3.10-3.12 for all desired CNV positions (usually at 3, 6, 9, and 12 o’clock positions around optic nerve). If laser inductions are applied at approximately same distance from optic nerve, refocus should not be necessary. However, due to the strong curvature of the mouse eye and small variations that may exist in the retina, refocusing the beam may be necessary between consecutive laser administrations.
Record in a notebook the location and result of each laser shot administration and result (successful, hazy, hemorrhage, etc.) of each administered shot for the eye. Be sure to place the laser in stand-by mode when not in use.
Repeat 3.1-3.14 for the mouse’s other eye, if needed, using the opposite hand for stabilization and a new coverslip.
After all desired laser shots are administered, turn off laser and slit-lamp.
Discard coverslip and place mouse on warmer for recovery from anesthesia. Macroscopically inspect eye for any injury and place a drop of artificial tear solution to keep the eye hydrated and potentially prevent future cataract development. Once mouse recovers from anesthesia, return to cage.
| AVERTIN | AVERTIN | AVERTIN | XYL/KET | XYL/KET | |
| Mouse Weight (g) | Dose (mg/kg) | Solution Concentration (mg/ml) | Anesthetic Dose (ml) | Dose (mg/kg) | Anesthetic Dose (ml) |
| 15 | 250 | 20 | 0.1875 | 100 mg/kg ketamine; 10 mg/kg xylazine | 0.15 |
| 16 | 250 | 20 | 0.2 | 100 mg/kg ketamine; 10 mg/kg xylazine | 0.16 |
| 17 | 250 | 20 | 0.2125 | 100 mg/kg ketamine; 10 mg/kg xylazine | 0.17 |
| 18 | 250 | 20 | 0.225 | 100 mg/kg ketamine; 10 mg/kg xylazine | 0.18 |
| 19 | 250 | 20 | 0.2375 | 100 mg/kg ketamine; 10 mg/kg xylazine | 0.19 |
| 20 | 250 | 20 | 0.25 | 100 mg/kg ketamine; 10 mg/kg xylazine | 0.2 |
| 21 | 250 | 20 | 0.2625 | 100 mg/kg ketamine; 10 mg/kg xylazine | 0.21 |
| 22 | 250 | 20 | 0.275 | 100 mg/kg ketamine; 10 mg/kg xylazine | 0.22 |
| 23 | 250 | 20 | 0.2875 | 100 mg/kg ketamine; 10 mg/kg xylazine | 0.23 |
| 24 | 250 | 20 | 0.3 | 100 mg/kg ketamine; 10 mg/kg xylazine | 0.24 |
| 25 | 250 | 20 | 0.3125 | 100 mg/kg ketamine; 10 mg/kg xylazine | 0.25 |
| 26 | 250 | 20 | 0.325 | 100 mg/kg ketamine; 10 mg/kg xylazine | 0.26 |
| 27 | 250 | 20 | 0.3375 | 100 mg/kg ketamine; 10 mg/kg xylazine | 0.27 |
| 28 | 250 | 20 | 0.35 | 100 mg/kg ketamine; 10 mg/kg xylazine | 0.28 |
| 29 | 250 | 20 | 0.3625 | 100 mg/kg ketamine; 10 mg/kg xylazine | 0.29 |
| 30 | 250 | 20 | 0.375 | 100 mg/kg ketamine; 10 mg/kg xylazine | 0.3 |
Table 1: XyIKet Dosage Chart.
Representative Results
Quantification of CNV lesions can be performed through analysis of flat-mounted choroids using immunofluorescence staining to label the CNV vessels. The two most frequently employed methods of tissue preparation are FITC-dextran labeling, done via perfusion immediately before animal sacrifice, or post-mortem immuno-staining with an endothelial cell marker. Both of these methods have been described previously in detail13,14,21; Figures 1 and 2 show examples of each, respectively. After confocal microscopy image acquisition, either area (2-Dimensions) or volume (3-Dimensions) can be calculated and visualized with ImageJ software or Volocity. In addition to quantification, OCT imaging can be used to visualize the CNV lesion in vivo. An example of a cross sectional image of the retina with resultant CNV is shown in Figure 3.
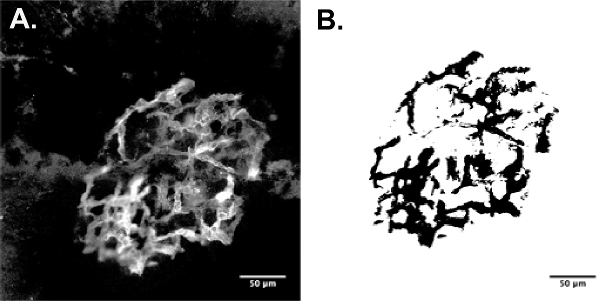 Figure 1: CNV Perfusion Staining & Area (2D) Calculation Example (25X). (A) FITC dextran perfused CNV lesion. (B) ImageJ area quantification method via thresholding. Mouse Strain: C57BL/6J. Please click here to view a larger version of this figure.
Figure 1: CNV Perfusion Staining & Area (2D) Calculation Example (25X). (A) FITC dextran perfused CNV lesion. (B) ImageJ area quantification method via thresholding. Mouse Strain: C57BL/6J. Please click here to view a larger version of this figure.
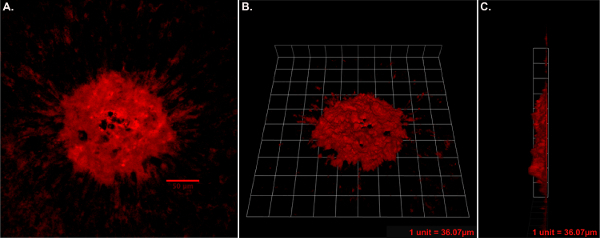 Figure 2: CNV Immunostaining & Volume (3D) Calculation Example (25X). (A) Isolectin GS-IB4 stained CNV lesion. (B) 3D reconstruction of CNV lesion en face view. (C) 3D reconstruction side view (B and C one tile width = 35 µm). Mouse Strain: C57BL/6J. Please click here to view a larger version of this figure.
Figure 2: CNV Immunostaining & Volume (3D) Calculation Example (25X). (A) Isolectin GS-IB4 stained CNV lesion. (B) 3D reconstruction of CNV lesion en face view. (C) 3D reconstruction side view (B and C one tile width = 35 µm). Mouse Strain: C57BL/6J. Please click here to view a larger version of this figure.
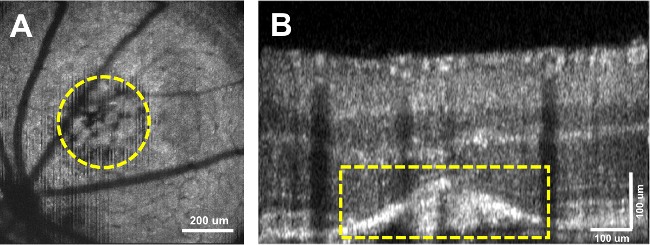 Figure 3: OCT Cross-Sectional CNV Visualization. (A) OCT en face view of CNV lesion. (B) OCT cross-sectional B-scan of retina with CNV circled in yellow. Mouse Strain: C57BL/6J. Please click here to view a larger version of this figure.
Figure 3: OCT Cross-Sectional CNV Visualization. (A) OCT en face view of CNV lesion. (B) OCT cross-sectional B-scan of retina with CNV circled in yellow. Mouse Strain: C57BL/6J. Please click here to view a larger version of this figure.
Discussion
There are multiple factors that can affect laser delivery and resultant CNV lesion development after successful laser-induction. These factors should be controlled for and standardized in order to have the most reliable results. The most pertinent of these factors are mouse selection (genotype, age, and sex), anesthetic selection, and laser settings.
The specific mouse model used can have a significant effect on the course of CNV development. The most widely used genotype is the C57BL/6 mouse. The vendor from which the animals are obtained can affect the resultant CNV size. Poor et al. demonstrated significant differences in final CNV lesion size in C57BL/6 mice from Jackson, Charles Rivers, and Taconic laboratories, with mice from Taconic developing significantly larger CNV than the other two vendors4. Therefore, purchasing from one company and using all mice from that same vendor will help to minimize external differences in CNV lesion size. Age and sex of the mouse has also been shown to be an important factor to consider when designing the experiment. Female mice develop more CNV than male mice of the same age, and older mice of both sexes develop more CNV than younger mice 22,23. Depending on the experimental parameters, operators should keep these factors in mind. For example, if operators are using the laser-CNV procedure to elucidate mechanistic and drug-development purposes, both sexes at different ages should be used to define mechanisms that are specific to the sexes and those that are not.
Proper anesthesia is a critical step for this procedure, especially during the learning process. Most IACUC approved regimens can induce anesthesia for at least 15-30 min, which is more than enough time to deliver 4-5 laser shots to each eye once the procedure has been mastered. However, if too much time elapses, anesthesia can lead to reversible lens opacification, rendering the eye optically impervious for experimental CNV24. The two main protocols used to induce anesthesia are a xylazine/ketamine cocktail or tribromoethanol (TBE) delivered intraperitoneally. Although some reports posit the advantages of TBE to xylazine/ketamine in terms of cataract formation, both eventually result in the development of a cloudy lens if the operator does not work quickly14. One way to extend the time before cataract development is to maintain hydration of the eye, as mentioned in the detailed protocol, through application of artificial tears25. This method, regardless of anesthetic substance used, should help delay cataract formation and give the operator more time to complete the procedure.
Laser settings can be a major factor in the reliable induction of CNV. Calibrating the laser power and duration are an important preliminary step before conducting the procedure on experimental animals. Laser shots that cause hemorrhage or are out of focus without bubble formation should be excluded from calculation, as this disrupts the CNV development process. If multiple vessels are ruptured causing diffuse hemorrhage, the entire eye should be excluded. Ideally, the lowest power and shortest duration of laser that consistently ruptures Bruch’s membrane causing bubble formation and a lesion with clear demarcation should be used4. Protocols experimenting with different power settings have demonstrated that increased power and duration lead to excessive tissue damage and subsequently lower rates of CNV formation4, 14,17. Furthermore, location of the laser injury can also affect CNV development. Impacts delivered approximately 1 disc diameter (DD) from the optic disk yielded significantly larger area CNV volumes than those delivered <1 DD or 2 or more DDs away23. Depending on the post-laser analysis, lesion location may or may not be crucial. For example, if an OCT image is to be obtained of all lesions, central location around the optic nerve is vital. Contrastingly, if lesions are to be analyzed via flat mount, lesion location is not as crucial. Finally, ensure that last shots are not placed too close to one another or else two lesions may “grow” together.
The CNV assay is a robust method to experimentally study neovascular AMD. The angiogenesis resulting from laser induction is similar in location and overall appearance to the angiogenesis observed in human AMD patients. However, it is far from a perfect model. Unlike the human eye, mice do not have a defined macula, and the acute injury-related angiogenesis seen in the laser-induced CNV model fundamentally differs from the genetically influenced, chronic pathology of AMD7,8,14. The mouse retinal environment is healthy and the resultant angiogenesis occurs as a reaction to the trauma caused by the laser impact, rather than genetic/environmental factors and age, as in human AMD. Contrastingly, the human retinal environment in AMD is in a state of chronic inflammation, during which abnormalities in VEGF expression and other cytokines cause CNV3. Clearly, the neovascular development in these two environments is markedly different.
In conclusion, the laser-induced CNV protocol is one that is initially difficult to perform but ultimately rewarding to master. The fine dexterity needed to hold the mouse and coverslip, as well as manipulate the coverslip and mouse head to visualize the optic nerve are exercises that require patience and practice, especially since they need to be performed well using either hands of the operator. However, once the technique is learned it can be an efficient way to implement experimental CNV and can be applied to most aspects of neovascular AMD research.
Disclosures
The authors declare they have no competing financial interests.
Acknowledgments
The authors would like to acknowledge Jonathan Chou, MD for his assistance on preparation and editing of the final manuscript and Wenzhong Liu for the OCT data. We would also like to acknowledge support from the Macula Society Research Grant (AAF), support from an unrestricted grant to Northwestern University from Research to Prevent Blindness, Inc., New York, NY, USA, and support from NIH-EY019951.
References
- Bressler NM, Bressler SB, Fine SL. Age related macular degeneration. Surv Ophthalmol. 1988;32:375–413. doi: 10.1016/0039-6257(88)90052-5. [DOI] [PubMed] [Google Scholar]
- Congdon N, et al. Causes and Prevalence of Visual Impairment Among Adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- Jager RD, Mieler WF, Miller JW. Age-Related Macular Degeneration. NEJM. 2008;358:2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- Poor SH, et al. Reliability of the Mouse Model of Choroidal Neovascularization Induced by Laser Photocoagulation. IOVS. 2014;55:6525–6534. doi: 10.1167/iovs.14-15067. [DOI] [PubMed] [Google Scholar]
- Ryan SJ. The development of an experimental model of subretinal neovascularization in disciform macular degeneration. Trans Am Ophthalmol Soc. 1979;77:707–745. [PMC free article] [PubMed] [Google Scholar]
- Miller H, Miller B, Ishibashi T, Ryan SJ. Pathogenesis of laser induced choroidal subretinal neovascularization. IOVS. 1990;31:899–908. [PubMed] [Google Scholar]
- Pennesi ME, Neuringer M, Courtney RJ. Animal models of age related macular degeneration. Molr Aspects Mede. 2012;33:487–509. doi: 10.1016/j.mam.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossniklaus HE, Kang SJ, Berglin L. Animal Models of Choroidal and Retinal Neovascularization. Prog Retin Eye Res. 2010;29:500–519. doi: 10.1016/j.preteyeres.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiss CJ. REVIEW PAPER: Animals as Models of Age Related Macular Degeneration An Imperfect Measure of the Truth. Vet Pathol Online. 2010;47:396–413. doi: 10.1177/0300985809359598. [DOI] [PubMed] [Google Scholar]
- Tobe T, et al. Targeted Disruption of the FGF2 Gene Does Not Prevent Choroidal Neovascularization in a Murine Model. Am J Pathol. 1998;153:1641–1646. doi: 10.1016/S0002-9440(10)65753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Marneros AG. Macrophages Are Essential for the Early Wound Healing Response and the Formation of a Fibrovascular Scar. Am J Pathol. 2013;182:2407–2417. doi: 10.1016/j.ajpath.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerster R, et al. In vivo and ex vivo characterization of laser induced choroidal neovascularization variability in mice. Graefes Arch Clin Exp Ophthalmol. 2012;250:1579–1586. doi: 10.1007/s00417-012-1990-z. [DOI] [PubMed] [Google Scholar]
- Jawad S, et al. The Role of Macrophage Class A Scavenger Receptors in a Laser Induced Murine Choroidal Neovascularization Model. IOVS. 2013;54:5959–5970. doi: 10.1167/iovs.12-11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert V, et al. Laser induced choroidal neovascularization model to study age related macular degeneration in mice. Nat Protocols. 2013;8:2197–2211. doi: 10.1038/nprot.2013.135. [DOI] [PubMed] [Google Scholar]
- Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J. Macrophage Depletion Inhibits Experimental Choroidal Neovascularization. IOVS. 2003;44:3578–3585. doi: 10.1167/iovs.03-0097. [DOI] [PubMed] [Google Scholar]
- Espinosa Heidmann DG, et al. Macrophage Depletion Diminishes Lesion Size and Severity in Experimental Choroidal Neovascularization. IOVS. 2003;44:3586–3592. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- Giani A, et al. In Vivo Evaluation of Laser Induced Choroidal Neovascularization Using Spectral Domain Optical Coherence Tomography. IOVS. 2011;52:3880–3887. doi: 10.1167/iovs.10-6266. [DOI] [PubMed] [Google Scholar]
- Kwak N, Okamoto N, Wood JM, Campochiaro PA. VEGF Is Major Stimulator in Model of Choroidal Neovascularization. IOVS. 2000;41:3158–3164. [PubMed] [Google Scholar]
- Reich SJ, et al. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Molr Vis. 2003;9:210–216. [PubMed] [Google Scholar]
- Baffi J, Byrnes G, Chan CC, Csaky KG. Choroidal Neovascularization in the Rat Induced by Adenovirus Mediated Expression of Vascular Endothelial Growth Factor. IOVS. 2000;41:3582–3589. [PubMed] [Google Scholar]
- Claybon A, Bishop AJR. Dissection of a Mouse Eye for a Whole Mount of the Retinal Pigment Epithelium. JoVE. 2011. p. 2563. [DOI] [PMC free article] [PubMed]
- Espinosa-Heidmann DG, et al. Age as an Independent Risk Factor for Severity of Experimental Choroidal Neovascularization. IOVS. 2002;43:1567–1573. [PubMed] [Google Scholar]
- Zhu Y, et al. Improvement and Optimization of Standards for a Preclinical Animal Test Model of Laser Induced Choroidal Neovascularization. PLoS ONE. 2014;9:e94743. doi: 10.1371/journal.pone.0094743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M, Stewart HC. Occurrence in rodents of reversible drug induced opacities of the lens. British J Ophthalmol. 1961;45:408–414. doi: 10.1136/bjo.45.6.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridder III WH, Nusinowitz S, Heckenlively JR. Causes of Cataract Development in Anesthetized Mice. Exp Eye Res. 2002;75:365–370. doi: 10.1006/exer.2002.2007. [DOI] [PubMed] [Google Scholar]


