Abstract
Aggressive behavior in Drosophila melanogaster is composed of the sequential expression of stereotypical behavioral patterns (for analysis see 1). This complex behavior is influenced by genetic, hormonal and environmental factors. As in many organisms, previous fighting experience influences the fighting strategy of flies and the outcome of later contests: losing a fight increases the probability of losing later contests, revealing "loser" effects that likely involve learning and memory 2-4. The learning and memory that accompanies expression of complex social behaviors like aggression, is sensitive to pre-test handling of animals 5,6. Many experimental procedures are used in different laboratories to study aggression 7-9, however, no routinely used protocol that excludes handling of flies is currently available. Here, we report a new behavioral apparatus that eliminates handling of flies, using instead their innate negative geotactic responses to move animals into or out of fighting chambers. In this protocol, small circular fight arenas containing a food cup are divided into two equal halves by a removable plastic slider prior to introduction of flies. Flies enter chambers from their home isolation vials via sliding chamber doors and geotaxis. Upon removal of plastic sliders, flies are free to interact. After specified time periods, flies are separated again by sliders for subsequent experimentation. All of this is done easily without handling of individual flies. This apparatus offers a novel approach to study aggression and the associated learning and memory, including the formation of "loser" effects in fly fights. In addition, this new general-purpose behavioral apparatus can be employed to study other social behaviors of flies and should, in general, be of interest for investigating experience-related changes in fundamental behavioral processes.
Keywords: Neuroscience, Issue 106, Behavior, Drosophila melanogaster, handling, aggression, "loser" effect, social memory
Introduction
Aggression in animal systems is strongly associated with the acquisition and holding of resources such as food, territory and mates. Given the important role this plays in the fitness of individuals, it is not surprising that aggression has evolved across the animal kingdom. As an adaptive trait that directly benefits individuals, a strong learning and memory component is associated with aggression. In competition for social rank, previous fighting experience influences the outcome of later contests. In general, prior losing experience increases, and winning experience decreases, the probability of losing later contests (called "loser" and "winner" effects). "Loser" effects have been observed in a wide range of species, and some reports suggest that these can last for several days while "winner" effects usually are of shorter duration 2,10,11.
The first report of aggressive behavior in fruit flies (D. ampelophila) was by Sturtevant in 1915 in a paper concerned with sex recognition and sex selection 12. Half a century later, more complete examinations of male fruit fly aggression were made, that described most of the behavioral patterns observed during fruit fly fights. These experiments were mostly carried out in small groups of male and female flies observed over hours 13-15. Recently, with the addition of powerful genetic tools, dyadic single sex fight setups and shorter times of observation, D. melanogaster has emerged as an important model system for the study of the biology of aggression 1,16. Analysis of fights between same sex pairs of male and female flies demonstrated that aggression involves stereotypical behavioral patterns that transition from one to the other in a statistically reliable manner 1,17. Some of the behavioral patterns observed are sex-specific while others are observed in fights in both sexes. Male fights go to higher intensity levels than female fights and result in the formation of dominance relationships with clear "winners" and "losers". At present, many laboratories have started investigating the biochemical 18, neural 7,19-21, and genetic 22 underpinnings of aggression. Unfortunately, a meta-analysis of studies to gain insight into fight dynamics and the formation and maintenance of dominance relationships is problematic due to the use of a multiplicity of experimental procedures in different laboratories. Essentially all of the techniques described in the literature involve handling and manipulation of flies to introduce them into arenas 19,23,24 and during the behavioral experiment 3,4 to transfer flies out of arenas. Gentle aspiration is the most common way to manipulate flies 3,4,23-25, but cold or CO2 anesthesia are also used 9,26, even though previous studies already have reported that these procedures have deleterious effects on fly behavior 27,28. A period of at least 24 hr is recommended after the use of any anesthetics to minimize their effects on behavior 29,30.
Flies learn from previous fighting experience and modify their behavioral pattern usage in new situations, suggesting that learning and memory accompany and are consequences of agonistic encounters. In that sense, fly fights resemble to operant conditioning learning situations in which flies learn that a strategy has worked and then use it more and more often during subsequent encounters. Flies change their fighting strategies after dominance relationships have been established during fights, with winners lunging more and more and losers less and less. After a period of separation, previous losers show much more submissive behavior and are highly likely to lose 2nd fights when paired with naïve flies or previous winners 3,4. However, the absence of an experimental procedure that excludes handling of flies has made detailed studies of "loser" effects difficult. In a recent study, we compared two experimental procedures routinely used in laboratories (aspiration and cold-anesthesia) to introduce flies into behavioral chambers to the new procedure that eliminates handling. The results showed that cold-anesthesia had far greater negative effects on aggression than aspiration, but even aspiration reduced the level of aggressiveness by flies. Aspiration did, however, cause highly significant effects on the learning and memory that accompany aggression. Following identical protocol with two experimental procedures (aspiration and no handling), a robust "loser" effect was only observed when the handling of flies was eliminated from the experimental procedure 5,6.
Ideally, studies of Drosophila aggression in laboratories should include environmental situations that flies normally encounter in the wild (competition for resources, territory to defend and space to escape). In addition, experimental conditions should be optimized to reliably induce, observe and interpret the behavior under examination (try to minimize animal handling, limit the use of CO2 as anesthetic, and standardize the experimental procedures). In attempting to address most or all of these issues, we designed a new behavioral apparatus that eliminates handling of flies before, during and after introducing them to behavioral arenas. With these apparatus, flies use their innate negative geotaxis to be moved and transferred into and out of the fight chambers. By eliminating handling of flies, the protocol aims to: (a) reduce the behavioral variability among individuals; (b) decrease the time necessary for flies to interact and generate clear dominance relationships (males); and (c) reliably induce strong enough behavioral changes to allow formation of "loser" effects.
This protocol describes a new experimental apparatus and a step by step procedure to analyze aggression and to allow the formation of a strong "loser" effect in D. melanogaster. We expect that this behavioral apparatus can be easily adapted for the study of other social behaviors exhibited by fruit flies.
Protocol
Note: The following is a step-by-step description of the experimental protocol we use to allow reliable triggering of aggression between pairs of male fruit flies (D. melanogaster). This procedure induces the formation of a "loser" effect. Using this protocol, a short-term "winner" effect also has been recently reported 5,6. Figure 1 shows the timeline of the behavioral experiments.

Figure 1. Timeline of aggression experiment. On day 1, late-stage male pupae (when wings become black) are isolated in freshly prepared isolation vials. On day 5 after emerging as adults, 4 day-old male flies are anesthetized with CO2 and painted with different colors for identification purposes. On day 7, food cups and fighting chambers are prepared to set up the behavioral experiments. Two 6-7 day-old adult males are loaded into the chambers and are able to interact. Please click here to view a larger version of this figure.
1. Day 1: Social Isolation of Pupae
Raise and maintain fly stocks in an environmentally controlled 12 hr light/12 hr dark cycle incubator at 25 °C and 50% relative humidity.
Prepare isolation vials: Heat fresh fly food until melted. Using a Pasteur pipette, transfer approximately 1.5 ml of melted fly food into single empty glass test tubes (1.6 x 10 cm). Allow the food to solidify.
Isolate pupae: With a fine paintbrush, gently remove a late-stage male pupa (when wings become black) from the stock vial and place it on the side of a freshly prepared food containing test tube. Close isolation vials with cotton (Figure 2).
Place isolation vials containing pupae into the 12 hr light/12 hr dark incubator at 25 °C and 50% relative humidity.
Figure 2. Social Isolation. One late-stage male pupa is placed on the side on a freshly prepared isolation vial. Isolation vials containing pupa are placed in the 12 hr light/12 hr dark incubator at 25 °C and 50% relative humidity. Please click here to view a larger version of this figure.
2. Day 5: Painting the Flies for Individual Identification Purposes
Perform this step at day 5 with 4 day-old male flies and two days prior the behavioral experiments to minimize the negative effect of CO2 anesthesia.
Anesthetize fly with CO2.
With a fine toothpick, place a small dot of acrylic paint on the thorax. Avoid placing paint on the head, wings, abdomen or legs (Figure 3).
Let paint dry for approximately 10 sec, and gently transfer flies back to their original isolation vials.
Return isolation vials to the 12 hr light/12 hr dark incubator at 25 °C and 50% relative humidity.

Figure 3. Individual identification. After anesthetizing a fly with CO2, a small drop of acrylic paint is applied on the thorax. For individual identification purpose, different colors are used (white and blue here). See discussion below about use of CO2 at this step. Please click here to view a larger version of this figure.
3. Day 7: Preparing the Apparatus
- Prepare Food Cups:
- Heat fresh fly food until it melts.
- Using a Pasteur pipette, transfer approximately 0.8 ml food to the lid of a screw cap vial (1.5 cm diameter, 1 cm height) and allow to cool. Avoid air bubbles.
- Make fresh yeast paste by mixing dry yeast with a few drops of water. When thick, apply a small drop of yeast paste to the center of the food cup surface with a toothpick (Figure 4).

Figure 4. Preparing the food cup. A food cup is prepared with fresh fly food. After the food solidifies, a dot of yeast paste is placed in the center of the food cup surface. Please click here to view a larger version of this figure.
- Prepare Individual Fighting Chambers:
- Thoroughly wash the apparatus and individual fighting chambers with water before use each time. Dry with paper towel before use.
- Place a food cup in the center of each fighting chamber and fix in place with a drop of putty.
- Cover fighting chambers with the apparatus lid (Figure 5).
- Place the apparatus into a high-humidity environment (60%) at 25 °C.
- Place a light source above the apparatus to illuminate the fighting chambers and set a video camera for recording from above.

Figure 5. Preparing the individual fighting chamber. The freshly prepared food cup with a dot of yeast paste on the surface is fixed into washed individual fighting chambers. Chambers are closed with the two-piece cover allowing insertion of an opaque plastic divider into individual fighting chambers. Please click here to view a larger version of this figure.
4. Day 7: Behavioral Experiment
Perform the behavioral experiments with 6-7 day-old male flies.
Insert plastic dividers into each fighting chamber.
Remove the cotton plug from the isolation tube and position the tube below an open hole on the side of the apparatus.
Let the fly enter into the fighting chamber by negative geotaxis, usually within a few seconds.
Close the sliding wall after the fly has left the tube and entered the chamber.
Repeat the procedure to introduce the second fly into the fight chamber on the other side of the apparatus.
Place the apparatus back in the illuminated videotaping position.
- Begin video recording and remove the plastic divider to allow flies to interact.
- For Aggression Experiments:
- In these experiments, record fights for at least 20 min to ensure the formation of strong dominance relationships.
- After the desired time, stop video recording.
- Remove flies from the fighting chambers by reversing the procedure described above.
- Thoroughly wash the behavioral apparatus with mild detergent and water after each use and rinse.
- For "Loser" Effect Experiments: Note: In these experiments, first fights are considered as the conditioning phase and second fights as the test phase. The period of rest between fights can differ but 10 min allows formation of a strong "loser" effect.
- Follow the procedure described above to perform a first fight.
- CRITICAL STEP: To induce the formation of a "loser" mentality, ensure that first fights are at least 20 min long in order to establish strong dominance relationships. After 20 min, re-insert the plastic divider into fighting chambers to separate the flies.
- After a 10 min period of rest, gently remove the plastic divider to allow flies to interact for second fights.
- At the end of the second 20 min fight, stop the video recording.
- Remove flies from behavioral chambers as above.
- Once again, thoroughly clean the behavioral apparatus.
5. Behavioral Analysis
- Transferring Movies
- Connect cameras to the computer.
- Convert flies .MTS to .mov flies and join movies clips together, using video converting software and the option "join selected clips".
- Observe and analyze each movie with video reader software.
- Scoring Behavior Note: this can be done manually or using behavioral analysis program software if available. The illustration below describes a possible manual analysis for a "loser" effect.
- Score the time of the first encounter between the flies on the food cup (encounters are interactions between pairs of flies that last at least 2 sec) and the time of appearance of the first lunge (a behavioral pattern in which one fly rises high on its hind legs and snaps down on and attempts to grab the opponent). Then, score the latency to lunge (the interval between the first encounter and the first lunge).
- Count the total number of encounters and lunges during a fight. Note: If desired, one also can score other behavioral patterns, like wing threat, boxing, fencing, retreat or the numbers of encounters before the first lunge, for example.
- Score the time of establishment of dominance (time between the first encounter and the time when one fly retreats for three consecutive times off the food cup after receiving lunges). Note which fly is the winner and which the loser of the fight.
- Calculating a "Loser" Effect
- Divide the number of fights in which previous losers lose 2nd fights (Losers that lose: LL) by the total number of fights, and multiply by 100. The percentage obtained represents the "loser" effect: [(# flies LL) / (# total fight)] x 100
- Use a Chi-square test to compare whether the "loser" effect is statistically different from the expected value of 50%.
Representative Results
This section presents the design of the behavioral chamber and the analysis of a typical set of behavioral experiments following the protocol described above measuring aggression and the formation of a "loser" effect. Also illustrated are samples of other behaviors that can be measured using this apparatus.
Figure 6 shows a schematic representation of the new apparatus. The apparatus is composed of three cylindrical wells (dimension: 22.86 mm diameter, 17 mm height) that are the fighting chambers. The two-piece cover closes the fighting chambers on the top and allows insertion of a thin opaque plastic divider that splits the chambers into two equal sections. On each side of the apparatus, sliding walls with three holes (15.88 mm diameter) allow loading of flies into the fighting chambers by negative geotaxis directly from their isolation vials. After flies enter the fighting chambers, the sliding walls are used to close the sides of the all chambers. At this point, the two flies are within the fight arenas but cannot interact due to the dividers. By removing these, the flies are free to interact and the subsequent behavioral patterns can be observed and recorded.
Aggression experiments: 38 fights between pairs of wild type CS males were performed. Different behavioral parameters were scored while flies were on food cups and the dynamics of fights were analyzed (Table 1). An average of 24 encounters (brief meetings between flies), 54 lunges (an indicator of higher intensity fighting) were observed during a 20 min time period, with the 1st lunge usually delivered during the 2nd encounter. On average, the 1st encounter, the 1st lunge and the time to establish dominance were seen 2, 4 and 8 min after the start of recording. In most cases, the last 12 min of fights consist of repetitive sequences of high intensity encounters with subordinate animals approaching, being attacked and retreating, reinforcing the dominance or submissive status of each animal.
"Loser" effect experiments: In order to search for the formation of a "loser" effect, 21 1st fights of 20 min duration were carried out between pairs of wild type CS males. The chamber sliding divider then was inserted to separate the flies. After 10 min, 2nd 20 min fights were performed between (a) previous losers and familiar winners or (b) previous losers and unfamiliar winners and these were analyzed as above (Figure 7). In both conditions, 20 previous losers lost their 2nd fights (95.5%), demonstrating a robust "loser" effect. The fight outcomes were compared to the expected value of 50-50 with a two-tailed Chi-square analysis and a highly significant "loser" effect was found. Other behavioral changes were observed in losing individuals between the 1st and 2nd fights accompanying the formation of the "loser" effect 5.
Other behaviors than aggression can be studied in the apparatus: to illustrate, courtship and locomotion experiments were performed (Table 2). For courtship, male and female flies were loaded by negative geotaxis into the behavioral chambers, dividers were removed and behavioral courtship patterns were scored. 34 experiments were performed and the latencies to court (time between the 1st encounter and the 1st courtship behavior = 7 sec) and to copulate (time between the 1st encounter and copulation = 4 min) were analyzed. Only 2 males did not succeed in copulating during the 15 min time of observation. The Courtship Vigor Index (the fraction of time that males spent courting females during a 15 min period after the 1st courtship behavioral pattern) was scored at 75% (Table 2). For locomotion either single flies or group of 10 flies were introduced into chambers and the total number of midline crossings during 5 min were counted. Single flies cross the midline of the chamber on average 73 times during 5 min, while individual flies in groups cross the midline on average 45 times/5 min (Table 2). This decrease observed when flies are in groups can be explained by the reduced space available per fly and by the time they spend interacting with each other instead of exploring the territory.
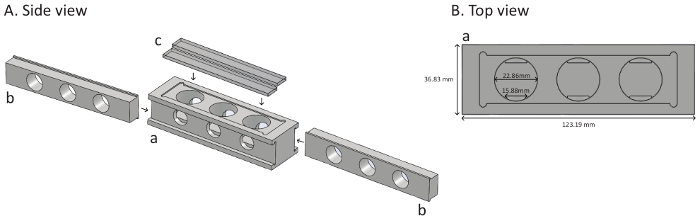 Figure 6. Schematic representation of the behavioral chamber. (A) Side view of the apparatus. The new apparatus is composed of three separate parts: (a) a plastic block containing three individuals fighting chambers; (b) two sliding walls with three holes (15.88 mm diameter) in each; and (c) the two-piece cover on the top. (B) Top view of the apparatus. The dimensions of the plastic block (a) are 36.83 mm by 123.19 mm. Each individual fighting chamber has a diameter of 22.86 mm and a height of 17 mm. Holes on the side that allow the loading of the flies have a diameter of 15.88 mm. Please click here to view a larger version of this figure.
Figure 6. Schematic representation of the behavioral chamber. (A) Side view of the apparatus. The new apparatus is composed of three separate parts: (a) a plastic block containing three individuals fighting chambers; (b) two sliding walls with three holes (15.88 mm diameter) in each; and (c) the two-piece cover on the top. (B) Top view of the apparatus. The dimensions of the plastic block (a) are 36.83 mm by 123.19 mm. Each individual fighting chamber has a diameter of 22.86 mm and a height of 17 mm. Holes on the side that allow the loading of the flies have a diameter of 15.88 mm. Please click here to view a larger version of this figure.
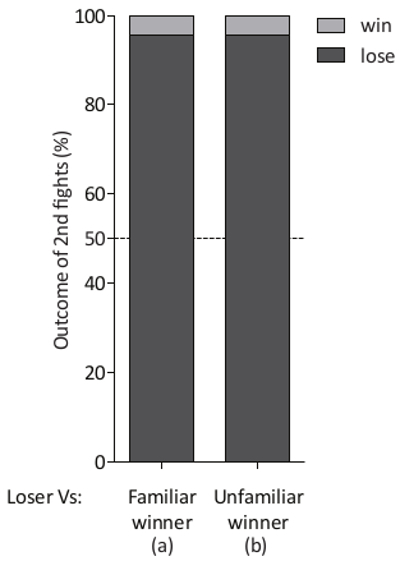 Figure 7. The formation of a robust "loser" effect. A significant "loser" effect (percentage of losers that lose 2nd fights is observed when previous losers were paired (a) with familiar winners (n = 21, *** p <0.0001) or (b) with unfamiliar winners for their 2nd fights (n = 21, *** p <0.0001) (Lose: 95.5 %, Win: 4.5%, No draws). Please click here to view a larger version of this figure.
Figure 7. The formation of a robust "loser" effect. A significant "loser" effect (percentage of losers that lose 2nd fights is observed when previous losers were paired (a) with familiar winners (n = 21, *** p <0.0001) or (b) with unfamiliar winners for their 2nd fights (n = 21, *** p <0.0001) (Lose: 95.5 %, Win: 4.5%, No draws). Please click here to view a larger version of this figure.
| Behavioral parameters | Unit | Time (sec) | n |
| Encounters | 24 ± 2 | 38 | |
| Lunges | 54 ± 6 | 38 | |
| Number of encounters before 1st lunge | 2 ± 0.3 | 38 | |
| Time of the 1st encounter | 145 ± 20 | 38 | |
| Time of the 1st lunge | 229 ± 26 | 38 | |
| Time of dominance | 475 ± 56 | 34 | |
| Latency to lunge | 87 ± 20 | 38 | |
| Latency to dominance | 342 ± 51 | 34 |
Table 1: Dynamics of agonistic interactions between pairs of male flies. Mean values ± SEM are calculated for each parameter scored as indicators of aggressiveness. The total number of encounters and lunges, and the number of encounters before the 1st lunge were scored. Times of the 1st encounter, 1st lunge and dominance, and the latencies to lunge and to dominance are presented in sec ± SEM (n >34).
| Behavioral parameters | Time (sec) | Unit | (%) | n |
| Courtship behavior | ||||
| Latency to court | 7 ± 3 | 34 | ||
| Latency to copulate | 273 ± 62 | 32 | ||
| Courtship Vigor Index | 75 ± 0.05 | 34 | ||
| Locomotion | ||||
| Total midline crosses (single fly) | 73 ± 4 | 48 | ||
| Total midline crosses (group of flies) | 45 ± 3 | 20 |
Table 2: Analysis of courtship behavior and locomotion. Mean values ± SEM are calculated for the latencies to court and copulate (in sec). A courtship vigor index (in % of time spent courting after 1st courtship display) was scored after males and females were loaded into behavioral chambers by negative geotaxis (n >32). Locomotion, when single flies or groups of 10 flies were loaded into chambers, was scored by counting the total number of midline crossings during 5 min (n = 48 and 20).
Discussion
The modern era of using Drosophila with its powerful genetic methods as a model organism for the study of aggression began about a dozen years ago with the introduction of novel experimental arenas in which reliable fighting behavior could be obtained with single pairs of flies 1,7,8,17,19. These arenas included desired resources (a food cup, potential mates) and a space for loser flies to retreat into. When results obtained with these arenas were added to previous knowledge about fruit fly fight behavior, Drosophila melanogaster was firmly established as a highly desirable model system for examining a complex behavior like aggression and is used in many laboratories. The summed results obtained revealed that: (a) flies exhibit aggression in competition for food resources, access to mates or for defense of territory 14,15; (b) fights are composed of a sequential series of stereotypical behavioral patterns 1; (c) male and female flies fight in same sex pairings (some patterns are male specific, some are female specific and some are shared by both sexes) 17; (d) only male fights go to high intensity levels and result in the formation of hierarchical relationships with clear « winner » and « loser » flies 17; (e) a single gene fruitless, of the sex determination hierarchy, determines whether flies fight like males or females 16 and (f) multiple hormonal substances including amines and peptides influence aggression in fruit flies 7,8,19,20.
Here, a novel approach is described for the study of fruit fly aggression in which handling of flies during experiments is eliminated. This offers a significant improvement in the experimental protocol as all behavioral measurements are improved 5,6, new information relating to the behavior has been gained and the learning and memory that accompanies male fly fights is greatly improved. To illustrate, only two previous studies have reported the formation of "loser" effects in this model system 3,4. In both of these, larger chambers than the ones described in this protocol were used, flies were transferred into and out of fight arenas by aspiration, and longer fight times and interval times between fights were necessary to generate clear "loser" effects. Our recent study demonstrated that eliminating handling from the experimental protocol, clear dominance relationships between pairs of D. melanogaster male flies are observed after only 20 min of interaction. This amount of time is sufficient to induce significant changes in fighting strategies in both flies during 1st fights and to reliably observe the formation of strong "loser" effects in 2nd fights. In addition, for the first time, clear short-term "winner" effects also were observed during 2nd fights 5,6.
For success using this protocol, some critical points need to be noted. To induce and observe robust aggressive behavioral patterns we recommend using 6-7 day-old male flies that have been held in social isolation since the late pupal stage. In general, the availability of resources that flies are willing to compete over (food, mates) is highly desirable in order to obtain strong fighting behavior and establishment of dominance relationships. During this protocol, flies are painted after anesthesia using CO2, and that may be of concern as our recently published study showing that anesthetizing flies seriously interferes with subsequent behavior 5. Possible ways to avoid anesthesia might be to not paint animals if it is not important to keep track of individuals. If painting is necessary, however, the anesthesia should be used 48 hr before the animals are used for experiments, or alternative approaches to identify individuals also might be used 31. To induce strong "loser" and "winner" effects, it is crucial that flies establish and maintain a dominance relationship during first fights. In the protocol presented here, using the wild-type CS strain at 25 °C, 20 min of interaction is sufficient to generate clear "winners" and "losers" and to observe changes in the fight strategies of both individuals. In experiments performed at different temperatures than 25 °C or with other genotypes than CS, however, the average time for establishing a robust dominance relationship might be different. Accordingly, the fighting period should be adjusted by performing sets of preliminary experiments. Finally, how pairs of flies are separated after their first fights might be important in preserving the memory of fight outcomes. It is probably important to reduce movement of the apparatus and to be gentle during the introduction of the plastic divider in order to minimize disturbance of the animals.
The design of the apparatus and the two-piece cover allowing separation of the behavioral chambers into two equal compartments, make it easy to separate the two flies after first fights and to introduce new opponents in 2nd fights. Also, as shown in Table 2, with small adjustments of the experimental protocol, the same apparatus can be used to study courtship, locomotion or indeed most fruit fly behaviors in what should be less stressful conditions for the experimental animals. Possible further improvements on the procedure might be to introduce some form of thermostatic control into the apparatus (piezoelectric element or a coil to circulate temperature-controlled fluid) to allow easy use of temperature sensitive reagents (shibirets1, dTRPA1 channel).
In summary, we have devised a novel apparatus that offers a new strategy for the study of aggression in fruit flies in which handling of flies prior to, during and after experiments was eliminated. All behavioral measures are improved with the new apparatus when compared to several other common ways of handling flies. Perhaps a more general comment to make is that essentially all behavioral experiments in laboratory environments involve experimenters picking up or in other ways, handling animals. Despite efforts to be gentle in this handling, this is likely to be stressful for the animals. It might be useful to devise methods to eliminate this handling whenever possible, so that animals might be observed under slightly more normal conditions.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This research was supported by grants from the National Institute of General Medical Sciences (GM099883 and GM074675) to E.A.K. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank the people from our machine shop at Harvard Medical School for the design of the behavioral apparatus (contact: http://mikesmachine.com).
Author contributions: S.T., B.C. designed the behavioral apparatus. S.T. designed and optimized the experimental protocol. S.T. and B.C. performed and analyzed the experiments. S.T., E.A.K. and B.C. wrote the paper.
References
- Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA. Fighting fruit flies: a model system for the study of aggression. Proc Natl Acad Sci U S A. 2002;99:5664–5668. doi: 10.1073/pnas.082102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y, Earley RL, Wolf LL. Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol Rev Camb Philos Soc. 2006;81:33–74. doi: 10.1017/S146479310500686X. [DOI] [PubMed] [Google Scholar]
- Yurkovic A, Wang O, Basu AC, Kravitz EA. Learning and memory associated with aggression in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2006;103:17519–17524. doi: 10.1073/pnas.0608211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn JK, Zito MF, Kravitz EA. A single social defeat reduces aggression in a highly aggressive strain of Drosophila. Proc Natl Acad Sci U S A. 2010;107:12682–12686. doi: 10.1073/pnas.1007016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trannoy S, Chowdhury B, Kravitz EA. Handling alters aggression and 'loser' effect formation in Drosophila melanogaster. Learn Mem. 2015;22:64–68. doi: 10.1101/lm.036418.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trannoy S, Kravitz EA. Learning and memory during aggression in Drosophila: handling affects aggression and the formation of a ''loser'' effect. Journal of Nature and Science. 2015;1(3):e56. [PMC free article] [PubMed] [Google Scholar]
- Alekseyenko OV, Chan YB, Li R, Kravitz EA. Single dopaminergic neurons that modulate aggression in Drosophila. Proc Natl Acad Sci U S A. 2013;110:6151–6156. doi: 10.1073/pnas.1303446110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierick HA, Greenspan RJ. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat Genet. 2007;39:678–682. doi: 10.1038/ng2029. [DOI] [PubMed] [Google Scholar]
- Williams MJ, et al. Regulation of aggression by obesity-linked genes TfAP-2 and Twz through octopamine signaling in Drosophila. Genetics. 2014;196:349–362. doi: 10.1534/genetics.113.158402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase ID, Bartolomeo C, Dugatkin LA. Aggressive interactions and inter-contest interval: how long do winners keep winning? Anim. Behav. 1994;48:393–400. [Google Scholar]
- Goessmann C, Hemelrijk C, Huber R. The formation and maintenance of crayfish hierarchies: behavioral and self-structuring properties. Behav Ecol Sociobiol. 2000;48:418–428. [Google Scholar]
- Sturtevant AH. Experiments on sex recognition and the problem of sexual selection in Drosophila. J Animal Behav. 1915;5:351–366. [Google Scholar]
- Jacobs ME. Influence of light on mating of Drosophila melanogaster. Ecology. 1960;41:182–188. [Google Scholar]
- Dow MA, von Schilcher F. Aggression and mating success in Drosophila melanogaster. Nature. 1975;254:511–512. doi: 10.1038/254511a0. [DOI] [PubMed] [Google Scholar]
- Hoffmann A. A laboratory study of male territoriality in the sibling species Drosophila melanogaster and D. simulans. Anim. Behav. 1987;35:807–818. [Google Scholar]
- Vrontou E, Nilsen SP, Demir E, Kravitz EA, Dickson BJ. fruitless regulates aggression and dominance in Drosophila. Nat Neurosci. 2006;9:1469–1471. doi: 10.1038/nn1809. [DOI] [PubMed] [Google Scholar]
- Nilsen SP, Chan YB, Huber R, Kravitz EA. Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2004;101:12342–12347. doi: 10.1073/pnas.0404693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez MP, Kravitz EA. Aggression and courtship in Drosophila: pheromonal communication and sex recognition. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013;199:1065–1076. doi: 10.1007/s00359-013-0851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseyenko OV, et al. Single serotonergic neurons that modulate aggression in Drosophila. Current biology : CB. 2014;24:2700–2707. doi: 10.1016/j.cub.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina K, et al. Tachykinin-expressing neurons control male-specific aggressive arousal in Drosophila. Cell. 2014;156:221–235. doi: 10.1016/j.cell.2013.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews JC, et al. Octopamine neuromodulation regulates Gr32a-linked aggression and courtship pathways in Drosophila males. PLoS genetics. 2014;10:e1004356. doi: 10.1371/journal.pgen.1004356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwarts L, et al. Complex genetic architecture of Drosophila aggressive behavior. Proc Natl Acad Sci U S A. 2011;108:17070–17075. doi: 10.1073/pnas.1113877108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierick HA. A method for quantifying aggression in male Drosophila melanogaster. Nat Protoc. 2007;2:2712–2718. doi: 10.1038/nprot.2007.404. [DOI] [PubMed] [Google Scholar]
- Wang L, et al. Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nat Neurosci. 2011;14:757–762. doi: 10.1038/nn.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SM, Thomas AL, Nomie KJ, Huang L, Dierick HA. Tailless and Atrophin control Drosophila aggression by regulating neuropeptide signalling in the pars intercerebralis. Nat Commun. 2014;5:3177. doi: 10.1038/ncomms4177. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Song Y, Yang CH, Jan LY, Jan YN. Female contact modulates male aggression via a sexually dimorphic GABAergic circuit in Drosophila. Nat Neurosci. 2014;17:81–88. doi: 10.1038/nn.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron JM, Huot L, Corrivault GW, Chawla SS. Effects of carbon dioxide anaesthesia on Drosophila melanogaster. J Insect Physiol. 1972;18:1869–1874. doi: 10.1016/0022-1910(72)90157-6. [DOI] [PubMed] [Google Scholar]
- Joachim D, Curtsinger JW. Genotype and anesthetic determine mate choice in Drosophila melanogaster. Behav Genet. 1990;20:73–79. doi: 10.1007/BF01070742. [DOI] [PubMed] [Google Scholar]
- Barron AB. Anaesthetising Drosophila for behavioural studies. J Insect Physiol. 2000;46:439–442. doi: 10.1016/s0022-1910(99)00129-8. [DOI] [PubMed] [Google Scholar]
- Greenspan RJ. Fly Pushing: The theory and Practice of Drosophila Genetics. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1997. [Google Scholar]
- Cabral LG, Foley BR, Nuzhdin SV. Does sex trade with violence among genotypes in Drosophila melanogaster? PLoS One. 2008;3(4):e1986. doi: 10.1371/journal.pone.0001986. [DOI] [PMC free article] [PubMed] [Google Scholar]


