Abstract
Primary cells are derived directly from tissue and are thought to be more representative of the physiological state of cells in vivo than established cell lines. However, primary cell cultures usually have a finite life span and need to be frequently re-established. Fibroblasts are an easily accessible source of primary cells. Here, we discuss a simple and quick experimental procedure to establish primary fibroblast cultures from ears and tails of mice. The protocol can be used to establish primary fibroblast cultures from ears stored at RT for up to 10 days. When the protocol is carefully followed, contaminations are unlikely to occur despite the use of non-sterile tissue stored for extended time in some cases. Fibroblasts proliferate rapidly in culture and can be expanded to substantial numbers before undergoing replicative senescence.
Keywords: Developmental Biology, Issue 107, Extraction, fibroblasts, primary cells, mouse, tails, ears
Introduction
Primary cells are derived from living tissue and cultured under in vitro conditions. It is generally assumed that primary cells more closely resemble the physiological state and genetic background of the tissue from which they originated than immortalized or tumor cell lines1. For that reason, primary cells represent a useful model for studying biological questions2,3. However, unlike established cell lines that grow indefinitely, primary cells eventually undergo senescence in culture and need to be frequently re-established.
Commonly used primary cells include fibroblasts, epithelial cells, endothelial cells, T cells, B cells, bone marrow-derived macrophages (BMDM) and bone marrow-derived dendritic cells (BMDC). Fibroblasts are often utilized as primary cell culture model. They offer key advantages over other primary cells. Cell cultures are easily established, readily maintained and require no purification of cells prior to culture. They have rapid initial proliferation and no requirement for specialized medium or activation protocols. Fibroblasts can be efficiently transfected using biological, chemical, and physical protocols4,5. There is a possibility to store ears for up to 10 days at RT prior to establishing cell cultures. Fibroblast cultures are conducive to visualization of cytoplasmic processes and suitable for reprogramming into induced pluripotent stem (iPS) cells6.
Fibroblasts are important cells of the connective tissue that secrete collagen proteins and extracellular matrix7. They provide the structural framework in many tissues8 and play an essential role in wound healing and tissue repair9,10.
Here, we describe a simple and quick (<4 hr) protocol to establish fibroblast cultures from ears and tails of mice11. The protocol requires minimal mouse experience to harvest the tissues (in contrast to other protocols12,13) and can be used to establish cultures from ears stored in medium at RT for up to 10 days.
Protocol
Mice were housed in pathogen-free conditions in compliance with the institutional guidelines until euthanization (The Institutional Animal Care and Use Committee (IACUC) guidelines at the National University of Singapore and the National Advisory Committee for Laboratory Animal Research (NACLAR) guidelines).
1. Mice
Order one mouse of the appropriate genetic background. This protocol is based on tissue derived from one C57BL/6 mouse.
2. Preparation of Complete Medium
Prepare complete medium by adding the following components to Roswell Park Memorial Institute (RPMI) 1640 medium: 10% fetal calf serum (FCS), 50 µM 2-mercaptoethanol, 100 µM asparagine, 2 mM glutamine, 1% penicillin-streptomycin solution.
3. Preparation of Enzyme Solutions
- Prepare collagenase D solution in a 15 ml conical bottom tube.
- Weigh 10 mg of collagenase D. Dissolve collagenase D in 4 ml complete medium.
- Prepare pronase solution in a 1.7 ml microcentrifuge tube.
- Weigh 10 mg of pronase.
- Add 5 µl of 1 M Tris buffer (pH 8.0). Add 1 µl of 0.5 M EDTA (pH 8.0).
- Top up with 494 µl of sterile water.
- Incubate the pronase solution at 37 °C in a water bath for 30 min.
4. Preparation of Collagenase D-pronase Mix (≤2 Tails)
Note: Perform the subsequent steps in a sterile cell culture hood.
Add 250 µl of pronase solution to 4 ml of collagenase D solution.
Pass the collagenase D-pronase mixture through a 0.2 µm syringe filter into a sterile 15 ml conical bottom tube.
5. Extraction of Fibroblasts from Ear and Tail Tissues
Euthanize mice according to the appropriate institutional guidelines.
Place autoclaved surgical instruments (scissors and forceps) in the cell culture hood.
Add 10 ml complete medium into two 10 cm cell culture dishes each.
Cut ears (~1 cm radius) and 5 cm of tail (from the tip of the tail) of a mouse with scissors and incubate for 5 min in 40 ml 70% ethanol in a sterile 50 ml conical bottom tube.
Air-dry ears and tail by placing them in an open 10 cm cell culture dish in the hood for 5 min. Once dried, transfer ear and tail pieces to two culture dishes labelled "ears" and "tail" containing 10 ml complete medium as described in step 5.3.
Remove hair from the ears and tail using scissors.
Cut ears and tail into pieces smaller than 3 mm in size using scissors.
Transfer the cut tissues into 1.8 ml cryotube vials labelled "ears" and "tail" and add sufficient collagenase D-pronase solution for the volume to reach the 1.8 ml mark on the vial.
Place the cryotube vials horizontally on a shaker and shake the samples at 200 rpm for 90 min at 37 °C.
After 90 min incubation, remove the cryotube vials from the shaker and place the vials in the hood.
Add 10 ml complete medium into two 10 cm cell culture dishes, labelled "ears" and "tail" each, and put a 70 µm cell strainer in each dish.
Place the digested ear and tail tissues in the 70 µm cell strainer in the accordingly labelled dishes prepared in step 5.11 and forcefully grind the tissues using a 10 ml syringe plunger for >5 min. Shake the cell strainer occasionally in the medium to wash cells out of the cell strainer.
Pipette the cell suspension from each dish into two 15 ml conical bottom tubes labelled "ears" and "tail". Wash the dish and strainer with additional 10 ml complete medium and add the medium to the appropriate 15 ml conical bottom tubes.
Spin down the cell suspension for 7 min at ~580 x g and 4 °C using a refrigerated cell centrifuge.
Remove supernatant, add 10 ml complete medium to the cell pellet in the 15 ml conical bottom tube and resuspend the cells.
Repeat step 5.14 and 5.15.
6. Culturing of Cell Mixture
Remove supernatant. Ensure that the cell pellet remains undisturbed.
Re-suspend cells in 10 ml complete medium and add the respective mixture to two 10 cm cell culture dishes labelled "ears" and "tail".
Add 10 µl amphotericin B solution (stock solution: 250 µg/ml) to the culture.
Incubate cells at 37 °C in a humidified 5% CO2 incubator.
On the third day, replace the medium with 10 ml fresh complete medium containing 10 µl of amphotericin B to remove debris (Figures 1 and 2).
7. Sub-culture of Fibroblasts
When culture reaches around 70% confluency (day 3-4 of culture) remove the medium and wash the cells with 5 ml sterile 1x phosphate buffered saline (PBS).
Remove PBS and add 2 ml sterile 1x trypsin-EDTA solution to the cells.
Incubate the cells for 5 min at 37 °C in a humidified 5% CO2 incubator.
After 5 min, gently tap the culture dish and add 6 ml complete medium to the cells.
Transfer the cell suspension to a 15 ml conical bottom tube and spin the tube for 5 min at ~450 x g and 4 °C using a refrigerated cell centrifuge.
Remove supernatant, and gently re-suspend the cell pellet in 5 ml complete medium.
Seed 2 x 105 cells in a 10 cm cell culture dish and incubate the cells at 37 °C in a humidified 5% CO2 incubator for 3-4 days before repeating steps 7.1 to 7.6.
8. Preparation of Ears for Shipment
Note: Perform the subsequent steps in a sterile cell culture hood.
Euthanize mice according to the appropriate institutional guidelines.
Place autoclaved scissors and forceps into the cell culture hood.
Add 50 ml complete medium into a 50 ml conical bottom tube.
Cut ears (~1 cm radius) of a mouse with scissors.
Transfer the ears into the 50 ml conical bottom tube (as prepared in step 3) using forceps.
Seal the 50 ml conical bottom tube with parafilm before shipping at RT in an appropriate box.
Representative Results
Extraction of fibroblasts from tissue results in a significant amount of tissue debris (Figure 1). In contrast to tissue debris, fibroblasts adhere to tissue culture plastic surfaces between day 1 and 3 of culture. The medium of fibroblast cultures can be safely changed on day 3 of culture, which should significantly decrease the levels of debris present in the culture (Figure 2). Fibroblasts display an elongated morphology and a clearly visible cytoplasm (Figures 1 and 2). Mitotic cells should be present from day 3 of culture onwards and cells should reach 70-80% of cell confluency within 3-4 days of culture. The yield from ear and tail tissues in a 10 cm culture dish ranges from 4 to 5 x 105 (ears) and 5 to 6 x 105 cells (tail) on day 3 of culture. Following the third day of fibroblasts isolation, the cells can be passaged and seeded at 2 x 105 cells per 10 cm culture dish.
Extraction of fibroblasts from ears stored at RT for 10 days should result in 70-80% cell confluency within 5 to 6 days of culture (Figure 3). Seeding the cells at 2 x 105 cells per 10 cm culture dish after day 5 should also give rise to approximately 1 x 106 cells within 3-4 days of culture. Long-term storage does not affect the time it takes for fibroblasts to enter senescence in our experience (data not shown).
To verify the identity of cells after 3 days of culture, cells can be labelled for the fibroblasts marker vimentin14. Using the above protocol, we routinely obtain pure fibroblast cultures as indicated by vimentin staining (Figure 4).
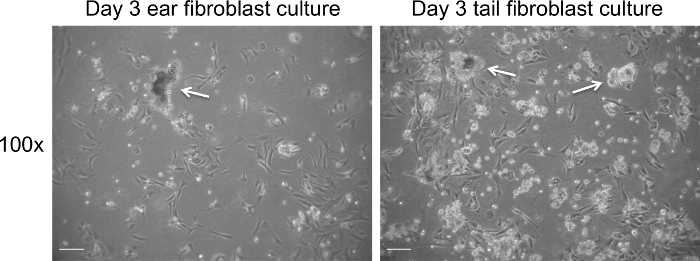 Figure 1. Fibroblast culture on day 3 post extraction prior to change of medium. Representative bright field images of cell debris (white arrows) present on day 3 of culture. Images were captured at 100× magnification using a light microscope. The scale bar represents 100 µm. Please click here to view a larger version of this figure.
Figure 1. Fibroblast culture on day 3 post extraction prior to change of medium. Representative bright field images of cell debris (white arrows) present on day 3 of culture. Images were captured at 100× magnification using a light microscope. The scale bar represents 100 µm. Please click here to view a larger version of this figure.
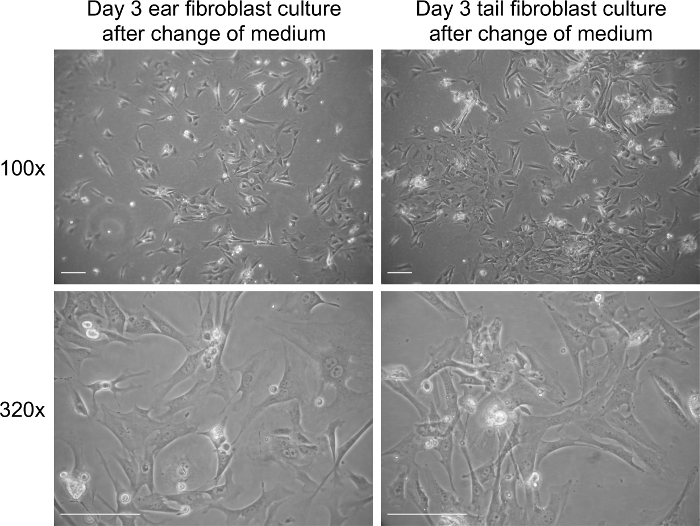 Figure 2. Fibroblast culture on day 3 post extraction after addition of new medium. Representative bright field images of fibroblasts on day 3 in culture. Images were captured at 100× and 320× magnification using a light microscope. The scale bar represents 100 µm. Please click here to view a larger version of this figure.
Figure 2. Fibroblast culture on day 3 post extraction after addition of new medium. Representative bright field images of fibroblasts on day 3 in culture. Images were captured at 100× and 320× magnification using a light microscope. The scale bar represents 100 µm. Please click here to view a larger version of this figure.
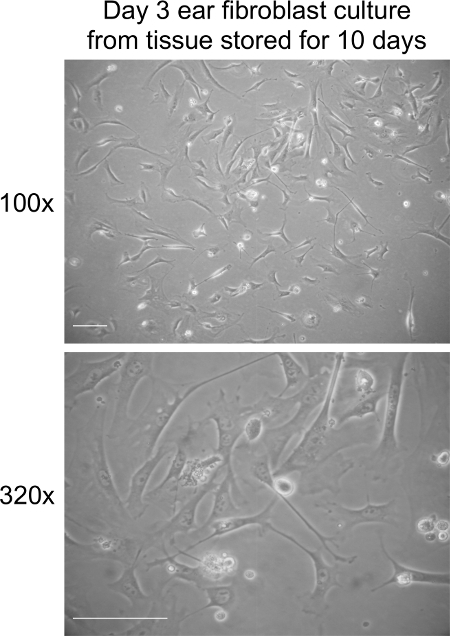 Figure 3. Fibroblast culture on day 3 post extraction from ear tissues stored at RT for 10 days. Representative bright field images of fibroblasts at day 3 in culture. Images were captured at 100× and 320× magnification using a light microscope. The scale bar represents 100 µm. Please click here to view a larger version of this figure.
Figure 3. Fibroblast culture on day 3 post extraction from ear tissues stored at RT for 10 days. Representative bright field images of fibroblasts at day 3 in culture. Images were captured at 100× and 320× magnification using a light microscope. The scale bar represents 100 µm. Please click here to view a larger version of this figure.
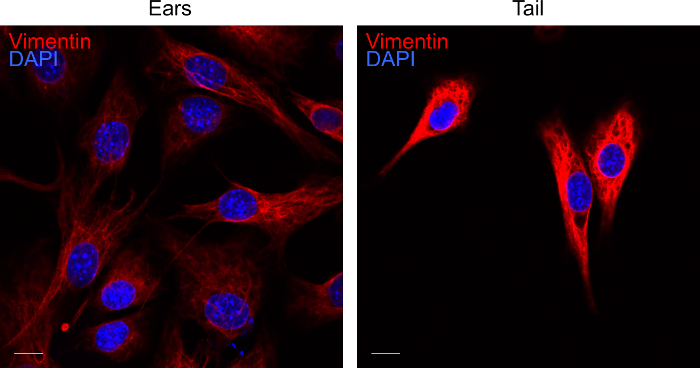 Figure 4. Labelling of fibroblast cultures for vimentin. Representative confocal image of ear and tail fibroblasts on day 3 of culture. Fibroblasts extracted from the tissues were labelled for vimentin (red) and DAPI (blue). The fluorescent images were acquired by confocal microscopy. The scale bar represents 10 µm. Please click here to view a larger version of this figure.
Figure 4. Labelling of fibroblast cultures for vimentin. Representative confocal image of ear and tail fibroblasts on day 3 of culture. Fibroblasts extracted from the tissues were labelled for vimentin (red) and DAPI (blue). The fluorescent images were acquired by confocal microscopy. The scale bar represents 10 µm. Please click here to view a larger version of this figure.
Discussion
Here we provide a simple, inexpensive and fast experimental procedure to establish primary fibroblast cultures from ears and tails of mice. The extraction should result in adherent and rapidly dividing fibroblasts within 3 days post-isolation of the tissue. An important limitation of primary cells is senescence, a permanent growth arrest15. Using the protocol, fibroblast cultures can be passaged for 5 to 6 times before fibroblasts become senescent, indicated by the flattening of cells, increase in size (2-3 times increase) and failure to expand.
When performing isolation of the fibroblasts, attention must be paid during the disruption of tissues, as insufficient cutting or digestion will result in low recovery of fibroblasts. It is possible to pool the ear and tail fibroblasts to increase the number of fibroblasts. Additional tissue including peritoneal and lung tissue processed in the same manner can be added to increase the number of fibroblasts. Though debris is present in the culture following fibroblasts extraction, it is recommended to change the medium only after the third day as fibroblasts take time to adhere to cell culture dishes.
A potential limitation of the protocol is the use of non-sterile tissues and associated possibility of microbial contamination. To reduce the risk of contamination, ears and tail are incubated in 70% ethanol before harvesting the fibroblasts. Furthermore, the antifungal amphotericin B is added to the primary culture to prevent the outgrowth of yeast and fungi, a common problem when establishing fibroblast cultures. The medium also contains penicillin and streptomycin to prevent bacterial contamination. Using these precautions, contaminations are rarely observed even when establishing fibroblasts from ears and tails that were kept at RT for several days.
One of the key advantages of this protocol is the ability to generate fibroblasts from ears that were stored in medium at RT for up to 10 days before fibroblasts isolation. We observed a modestly decreased efficiency in establishing ear fibroblast culture after 10 days of storage (70-80% confluency is reached within 5-6 days compared to 3-4 days for freshly isolated tissue). Hence, ears of mice can be exchanged by researchers using standard shipping, although express shipment is recommended. In our experience, the ease of use to obtain ears and the fact that the tissue is rarely used for experimental procedures often allows access to tissue of genetically modified mice that might be time-consuming to obtain otherwise. For tails the efficiency of recovering fibroblasts after storage can vary widely and tails should only be used if ears are not available for shipment. Finally, most people should be able to perform the protocol, as the harvest of tissue requires minimal expertise and training in handling of mice.
The protocol has only been tested using mouse tissue, but should in theory allow the generation of fibroblast cultures using tissue of other species although the protocol might need further optimization.
Disclosures
The authors declare no conflict of financial interests.
Acknowledgments
This work was supported by the NRF grant HUJ-CREATE - Cellular and Molecular Mechanisms of Inflammation.
References
- Fitzpatrick LE, McDevitt TC. Cell-derived matrices for tissue engineering and regenerative medicine applications. Biomater Sci. 2015;3(1):12–24. doi: 10.1039/C4BM00246F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenbaas B, et al. Human breast cancer cells generated by oncogenic transformation of primary epithelial cells. Genes Dev. 2001;15(1):50–65. doi: 10.1101/gad.828901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansley B, Post J, Hensley K. A comparative review of cell culture systems for the study of microglial biology in Alzheimer's disease. J Neuroinflammation. 2012;9(1):115. doi: 10.1186/1742-2094-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Dobson J. Improved transfection of HUVEC and MEF cells using DNA complexes with magnetic nanoparticles in an oscillating field. J Genet. 2012;91(2):223–227. doi: 10.1007/s12041-012-0164-4. [DOI] [PubMed] [Google Scholar]
- Li M, et al. High-efficiency transduction of fibroblasts and mesenchymal stem cells by tyrosine-mutant AAV2 vectors for their potential use in cellular therapy. Hum Gene Ther. 2010;21(11):1527–1543. doi: 10.1089/hum.2010.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Yang S. Advances in reprogramming somatic cells to induced pluripotent stem cells. Stem Cell Rev. 2010;6(3):367–380. doi: 10.1007/s12015-010-9123-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AC, Nakatsu MN, Chou W, Gershon PD, Hughes CC. The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell luman formation. Mol Biol Cell. 2011;22(20):3791–3800. doi: 10.1091/mbc.E11-05-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med. 2014;211(8):1503–1523. doi: 10.1084/jem.20140692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127(5):998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- Shen YJ, et al. Genome-derived cytosolic DNA mediates type I interferon-dependent rejection of B cell lymphoma cells. Cell Rep. 2015;11(3):460–473. doi: 10.1016/j.celrep.2015.03.041. [DOI] [PubMed] [Google Scholar]
- Seluanov A, Vaidya A, Gorbunova A. Establishing primary adult fibroblast cultures from rodents. J Vis Exp. 2010. [DOI] [PMC free article] [PubMed]
- Baglole CJ, et al. Isolation and phenotypic characterization of lung fibroblasts. Methods Mol Med. 2005;117:115–127. doi: 10.1385/1-59259-940-0:115. [DOI] [PubMed] [Google Scholar]
- Alt E, et al. Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol Cell. 2011;103(4):197–208. doi: 10.1042/BC20100117. [DOI] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24(22):2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander MR, Moll B, Rowe WP. A procedure for culture of cells from mouse tail biopsies: brief communication. J Natl Cancer Inst. 1978;60(2):477–478. [PubMed] [Google Scholar]
- Moore CB, Allen IC. Primary ear fibroblast derivation from mice. Methods Mol Biol. 2013. pp. 65–70. [DOI] [PubMed]
- Liu J, et al. Generation of stable pluripotent stem cells from NOD mouse tail-tip fibroblasts. Diabetes. 2011;60(5):1393–1398. doi: 10.2337/db10-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]


