Abstract
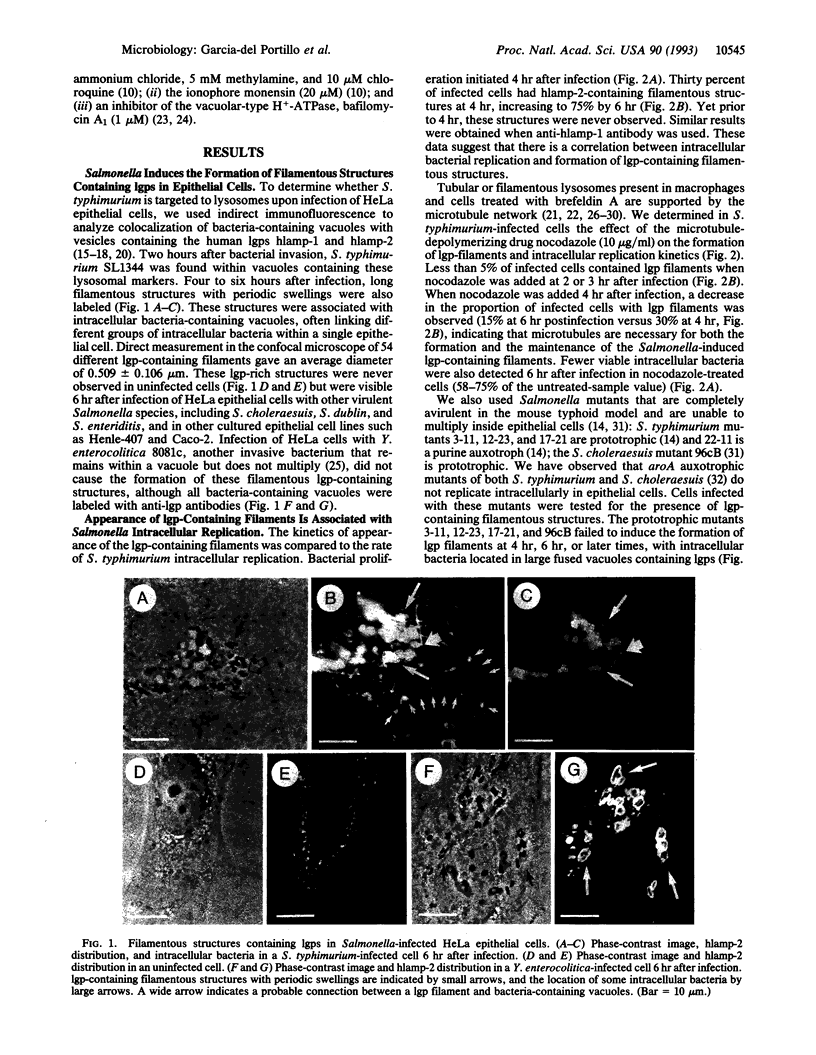
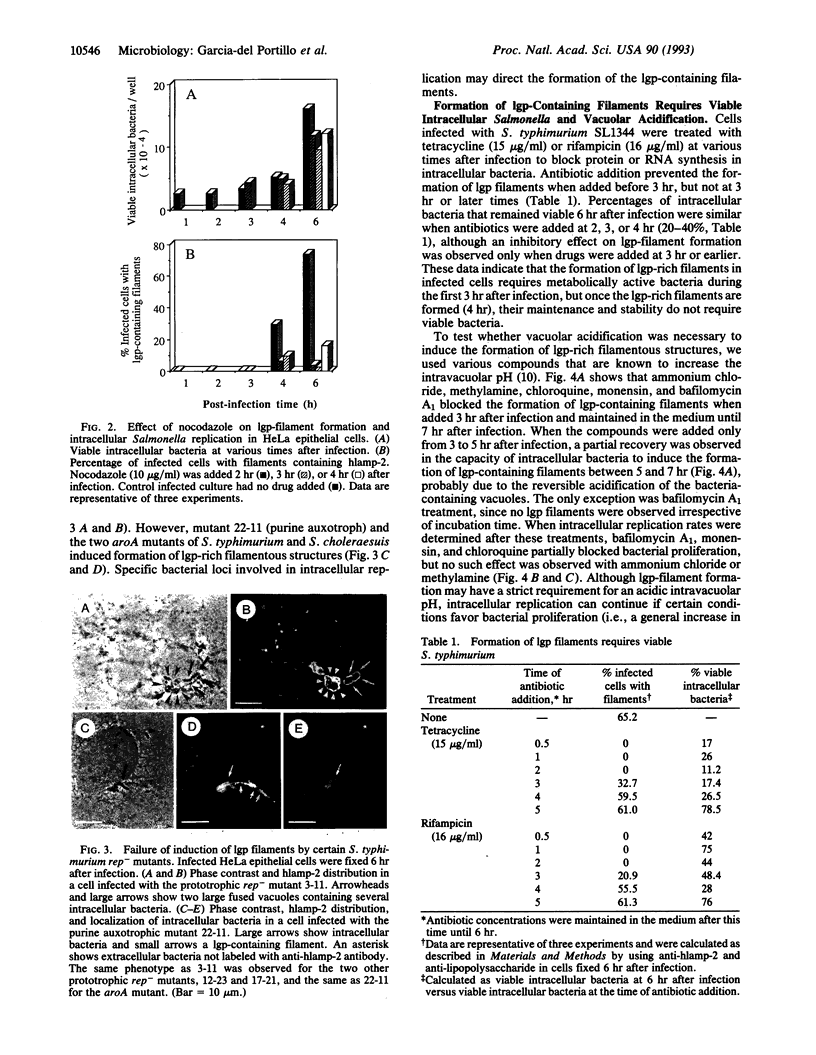
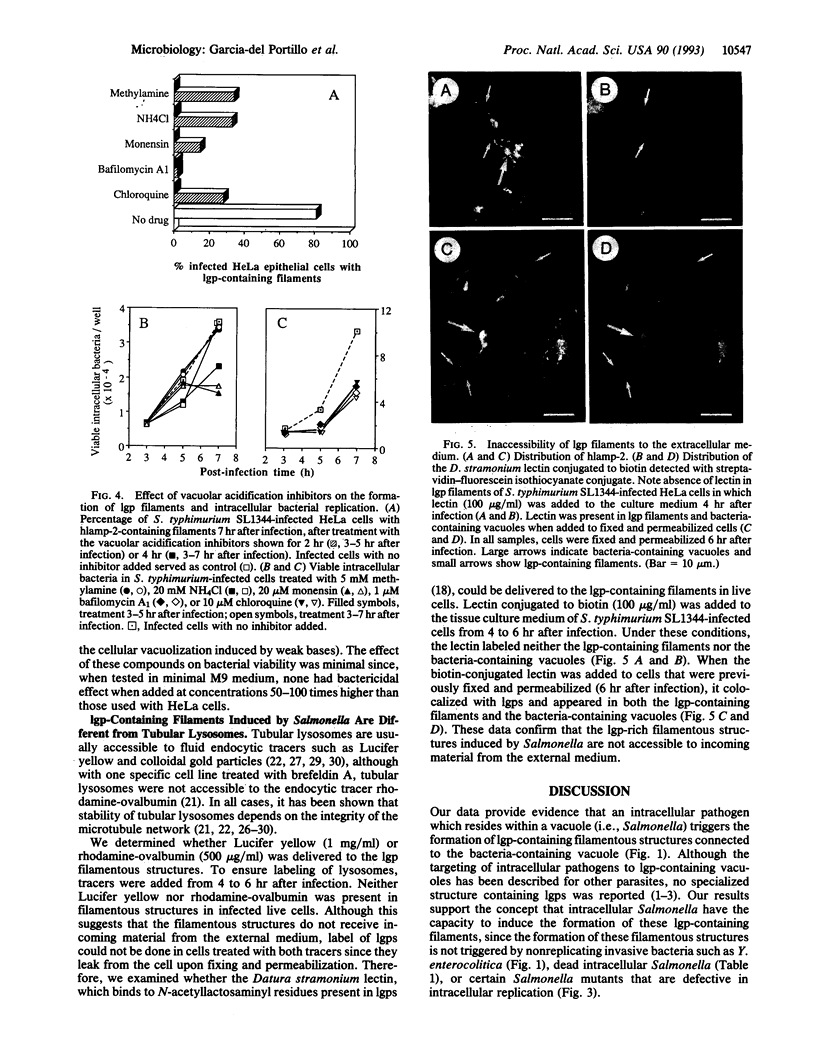
Salmonella species invade and replicate within epithelial cells in membrane-bound vacuoles. In this report we show that upon infection of HeLa epithelial cells, Salmonella typhimurium residues in vacuoles that contain lysosomal membrane glycoproteins (lgps). Four to six hours after invasion, intracellular bacteria induce the formation of stable filamentous structures containing lgps that are connected to the bacteria-containing vacuoles. Formation of these lgp-rich structures requires viable intracellular bacteria and is blocked by inhibitors of vacuolar acidification. These structures are not present in uninfected cells or in cells infected with another invasive bacteria, Yersinia enterocolitica. Tracers added to the extracellular medium are not delivered to the Salmonella-induced filaments, suggesting that these structures are different from previously described tubular lysosomes. Initiation of intracellular bacterial replication correlates with formation of these lgp-containing filaments. Certain avirulent Salmonella mutants that are defective for intracellular replication fail to induce formation of these structures. These observations suggest that Salmonella-induced filaments containing lgps are linked to intracellular bacterial replication.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akporiaye E. T., Rowatt J. D., Aragon A. A., Baca O. G. Lysosomal response of a murine macrophage-like cell line persistently infected with Coxiella burnetii. Infect Immun. 1983 Jun;40(3):1155–1162. doi: 10.1128/iai.40.3.1155-1162.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpuche Aranda C. M., Swanson J. A., Loomis W. P., Miller S. I. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10079–10083. doi: 10.1073/pnas.89.21.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman E. J., Siebers A., Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Inhibition of macrophage phagosome-lysosome fusion by Salmonella typhimurium. Infect Immun. 1991 Jul;59(7):2232–2238. doi: 10.1128/iai.59.7.2232-2238.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson S. R., Roth J., Piller F., Fukuda M. Isolation and characterization of human lysosomal membrane glycoproteins, h-lamp-1 and h-lamp-2. Major sialoglycoproteins carrying polylactosaminoglycan. J Biol Chem. 1988 Dec 15;263(35):18911–18919. [PubMed] [Google Scholar]
- Carrol M. E., Jackett P. S., Aber V. R., Lowrie D. B. Phagolysosome formation, cyclic adenosine 3':5'-monophosphate and the fate of Salmonella typhimurium within mouse peritoneal macrophages. J Gen Microbiol. 1979 Feb;110(2):421–429. doi: 10.1099/00221287-110-2-421. [DOI] [PubMed] [Google Scholar]
- Cover T. L., Reddy L. Y., Blaser M. J. Effects of ATPase inhibitors on the response of HeLa cells to Helicobacter pylori vacuolating toxin. Infect Immun. 1993 Apr;61(4):1427–1431. doi: 10.1128/iai.61.4.1427-1431.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Chatfield S., Leung K. Y., Dougan G., Falkow S. Characterization of a Salmonella choleraesuis mutant that cannot multiply within epithelial cells. Can J Microbiol. 1991 Jul;37(7):568–572. doi: 10.1139/m91-095. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Comparison of the invasion strategies used by Salmonella cholerae-suis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie. 1988 Aug;70(8):1089–1099. doi: 10.1016/0300-9084(88)90271-4. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Salmonella as an intracellular parasite. Mol Microbiol. 1989 Dec;3(12):1833–1841. doi: 10.1111/j.1365-2958.1989.tb00170.x. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Gumbiner B., Falkow S. Penetration of Salmonella through a polarized Madin-Darby canine kidney epithelial cell monolayer. J Cell Biol. 1988 Jul;107(1):221–230. doi: 10.1083/jcb.107.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M. Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J Biol Chem. 1991 Nov 15;266(32):21327–21330. [PubMed] [Google Scholar]
- Fukuda M., Viitala J., Matteson J., Carlsson S. R. Cloning of cDNAs encoding human lysosomal membrane glycoproteins, h-lamp-1 and h-lamp-2. Comparison of their deduced amino acid sequences. J Biol Chem. 1988 Dec 15;263(35):18920–18928. [PubMed] [Google Scholar]
- Hall B. F., Furtado G. C., Joiner K. A. Characterization of host cell-derived membrane proteins of the vacuole surrounding different intracellular forms of Trypanosoma cruzi in J774 cells. Evidence for phagocyte receptor sorting during the early stages of parasite entry. J Immunol. 1991 Dec 15;147(12):4313–4321. [PubMed] [Google Scholar]
- Heuser J. Changes in lysosome shape and distribution correlated with changes in cytoplasmic pH. J Cell Biol. 1989 Mar;108(3):855–864. doi: 10.1083/jcb.108.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Hollenbeck P. J., Swanson J. A. Radial extension of macrophage tubular lysosomes supported by kinesin. Nature. 1990 Aug 30;346(6287):864–866. doi: 10.1038/346864a0. [DOI] [PubMed] [Google Scholar]
- Isberg R. R. Discrimination between intracellular uptake and surface adhesion of bacterial pathogens. Science. 1991 May 17;252(5008):934–938. doi: 10.1126/science.1674624. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Fuhrman S. A., Miettinen H. M., Kasper L. H., Mellman I. Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc receptor-transfected fibroblasts. Science. 1990 Aug 10;249(4969):641–646. doi: 10.1126/science.2200126. [DOI] [PubMed] [Google Scholar]
- Kornfeld S., Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Leung K. Y., Finlay B. B. Intracellular replication is essential for the virulence of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11470–11474. doi: 10.1073/pnas.88.24.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L., Tipper C., Amherdt M., Orci L., Klausner R. D. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991 Nov 1;67(3):601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- Lowrie D. B., Aber V. R., Jackett P. S. Phagosome-lysosome fusion and cyclic adenosine 3':5'-monophosphate in macrophages infected with Mycobacterium microti, Mycobacterium bovis BCG or Mycobacterium lepraemurium. J Gen Microbiol. 1979 Feb;110(2):431–441. doi: 10.1099/00221287-110-2-431. [DOI] [PubMed] [Google Scholar]
- Mane S. M., Marzella L., Bainton D. F., Holt V. K., Cha Y., Hildreth J. E., August J. T. Purification and characterization of human lysosomal membrane glycoproteins. Arch Biochem Biophys. 1989 Jan;268(1):360–378. doi: 10.1016/0003-9861(89)90597-3. [DOI] [PubMed] [Google Scholar]
- Matteoni R., Kreis T. E. Translocation and clustering of endosomes and lysosomes depends on microtubules. J Cell Biol. 1987 Sep;105(3):1253–1265. doi: 10.1083/jcb.105.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Cytoplasmic vacuolation of mouse peritoneal macrophages and the uptake into lysosomes of weakly basic substances. J Cell Biol. 1981 Sep;90(3):656–664. doi: 10.1083/jcb.90.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phaire-Washington L., Silverstein S. C., Wang E. Phorbol myristate acetate stimulates microtubule and 10-nm filament extension and lysosome redistribution in mouse macrophages. J Cell Biol. 1980 Aug;86(2):641–655. doi: 10.1083/jcb.86.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. G., Xu S., Chakraborty P. Intracellular trafficking and the parasitophorous vacuole of Leishmania mexicana-infected macrophages. J Cell Sci. 1992 Dec;103(Pt 4):1193–1210. doi: 10.1242/jcs.103.4.1193. [DOI] [PubMed] [Google Scholar]
- Scheel J., Matteoni R., Ludwig T., Hoflack B., Kreis T. E. Microtubule depolymerization inhibits transport of cathepsin D from the Golgi apparatus to lysosomes. J Cell Sci. 1990 Aug;96(Pt 4):711–720. doi: 10.1242/jcs.96.4.711. [DOI] [PubMed] [Google Scholar]
- Stocker B. A. Auxotrophic Salmonella typhi as live vaccine. Vaccine. 1988 Apr;6(2):141–145. doi: 10.1016/s0264-410x(88)80017-3. [DOI] [PubMed] [Google Scholar]
- Straley S. C., Harmon P. A. Yersinia pestis grows within phagolysosomes in mouse peritoneal macrophages. Infect Immun. 1984 Sep;45(3):655–659. doi: 10.1128/iai.45.3.655-659.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Burke E., Silverstein S. C. Tubular lysosomes accompany stimulated pinocytosis in macrophages. J Cell Biol. 1987 May;104(5):1217–1222. doi: 10.1083/jcb.104.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Bushnell A., Silverstein S. C. Tubular lysosome morphology and distribution within macrophages depend on the integrity of cytoplasmic microtubules. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1921–1925. doi: 10.1073/pnas.84.7.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieux I., Webster P., Ravesloot J., Boron W., Lunn J. A., Heuser J. E., Andrews N. W. Lysosome recruitment and fusion are early events required for trypanosome invasion of mammalian cells. Cell. 1992 Dec 24;71(7):1117–1130. doi: 10.1016/s0092-8674(05)80061-3. [DOI] [PubMed] [Google Scholar]
- Wood S. A., Brown W. J. The morphology but not the function of endosomes and lysosomes is altered by brefeldin A. J Cell Biol. 1992 Oct;119(2):273–285. doi: 10.1083/jcb.119.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimori T., Yamamoto A., Moriyama Y., Futai M., Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem. 1991 Sep 15;266(26):17707–17712. [PubMed] [Google Scholar]






