Abstract
The insect olfactory system plays an important role in detecting semiochemicals in the environment. In particular, the antennal sensilla which house single or multiple neurons inside, are considered to make the major contribution in responding to the chemical stimuli. By directly recording action potential in the olfactory sensillum after exposure to stimuli, single sensillum recording (SSR) technique provides a powerful approach for investigating the neural responses of insects to chemical stimuli. For the bed bug, which is a notorious human parasite, multiple types of olfactory sensillum have been characterized. In this study, we demonstrated neural responses of bed bug olfactory sensilla to two chemical stimuli and the dose-dependent responses to one of them using the SSR method. This approach enables researchers to conduct early screening for individual chemical stimuli on the bed bug olfactory sensilla, which would provide valuable information for the development of new bed bug attractants or repellents and benefits the bed bug control efforts.
Keywords: Neuroscience, Issue 107, bed bug, olfaction, olfactory receptor neuron, action potential, single sensillum recording
Introduction
The common bed bug Cimex lectularius L (Hemiptera: Cimicidae), as a temporary ectoparasite, is an obligated blood-sucking insect, which means their survival, development, and reproduction require blood sources from hosts, including both humans and animals1,2. Although virus transmission has rarely been reported due to C. lectularius, the biting nuisance generated by an infestation seriously affects hosts both physically and psychologically3. The introduction and widespread use of chemical insecticides, especially DDT, lowered the risk of infestations and by the end of the 1950s infestations were at such a low level that they were no longer a serious public concern. However, a number of possible factors have led to resurgence in bed bug populations worldwide, such as the reduced use of insecticides, a decline of public awareness, increased traveling activity, and the development of resistance to insecticides4-9.
Chemical cues in the environment are detected and recognized by insects through olfactory organs such as antennae and maxillary palps. The olfactory sensilla on the insect antennae play a crucial role in detecting these chemical cues. The chemical molecules enter the antennal cuticle through pores on the cuticle surface. Odorant binding proteins in the antennal lymph bind to these chemical molecules and transport them onto the odorant receptors10. The odorant receptors and their co-receptor from the non-selective cation ion channel on the neural membrane, which will be depolarized once these chemical molecules are recognized by the odorant receptors11.
Single sensillum recording (SSR) was developed to detect the extracellular change in the action potential caused by the application of either chemical or non-chemical stimuli. By inserting a recording electrode into the sensillum lymph and a reference electrode into some other part of the insect body (usually either the compound eyes or the abdomen), the firing rate of the neurons in response to stimuli can be recorded12. Changes in the number of spikes represent the sensitivity of the insect to specific stimuli. Chemical stimuli of different identities and concentration will elicit different neural responses, with different firing rates and temporal structures, and can thus be used to investigate the coding process of the insect to specific chemicals.
For the common bed bug, both sexual forms share the same pattern of olfactory sensilla on the antennae: nine grooved peg C sensilla, 29 hair-like E (E1 and E2) sensilla, and one pair each of Dα, Dβ, Dγ smooth peg sensilla 13,14. As multiple neurons have been identified in each type of sensillum, it is not easy to distinguish the action potentials from different neurons housed in the same sensillum, so for this experiment the total numbers of action potentials were counted off-line for a 500 msec period before and after stimulation. The number of action potentials after stimulation was then subtracted from the number of action potentials before stimulation and multiplied by two in order to quantify the changes in the firing rate in each individual sensillum in spikes per second15.
Protocol
1. Preparation of Instruments, Stimuli Solutions, and Bed Bugs
Prepare a 50% KNO2 solution (w/v) in a 20 ml bottle.
- Sharpen two tungsten microelectrodes in KNO2 solution at 5 V by repeatedly dipping the tungsten electrodes in and out of the solution.
- Roughly sharpen the tungsten wire by dipping about 10 mm of the tungsten wire in and out of the KNO2 solution at the speed of 2 dips/sec for about 5 min, which can greatly consume the front end of the tungsten wire.
- Delicately sharpen the electrode by dipping about 1 mm of the wire tip in and out of the solution at the speed of 2 dips/sec for at least 1 min so as to make a fine and sharp point of the electrode. Check the electrode tip diameter under the microscope frequently until it reaches 0.2-0.5 µm, which should be fine enough to puncture the cuticle of bed bug olfactory sensillum. Note: While manually sharpening the electrode, the dipping speed of the tungsten wire into the KNO2 solution is not constant all the time. With more practice, it is much easier to keep a relatively constant speed in sharpening the electrode. The sharpening time is also uncertain depending on how fine the electrode should be. Here, an electrode tip with the diameter of ~0.2 µm is sufficient enough to puncture through the olfactory sensillum.
Dilute each of the chemical stimuli in dimethyl sulfoxide (DMSO) from the neat compound to an initial concentration of 1:10 v/v as a stock solution. Create a series of decadic dilutions depending on how many doses required in the experiment, again with DMSO, from each of the stock solutions for each chemical. Here, use 10% (+)-β-pinene and eucalyptol.
Place the unfed or seven days post-feeding adult bed bugs (either male or female) from the Ft. Dix colony (a gift from Dr. Haynes in University of Kentucky) to be used in the experiment in a petri dish. Note: There is no exact number for bed bugs placed in the Petri dish. It can be a few or a lot.
2. Bed Bug Antennae Preparation
Anaesthetize the bed bugs on ice (2-3 min).
Fix both the antennae and insect body on a microscope coverslip with double-sided tape and remove the legs with fine scissors.
Use a small pin to gently touch the antennae so as to stick them on the tape steadily.
Rest the coverslip against a small ball (~1 cm diameter) of dental wax to facilitate manipulation and adjust it to an appropriate angle (~ 90°) for the recording electrode (Figure 1).
Once secured, place the bed bug under a stereo microscope, turn on the cold light source and adjust the intensity of illumination until the antenna is clearly presented, and focus the microscope on the second flagellum of the bed bug antenna at high magnification (720X). Note: The intensity of illumination used in the experiment is not quantitated, which really depends on how the experimenter's eyes feel the intensity of illumination.
3. Single Sensillum Recording
Connect the preamplifier (10X) with the signal acquisition controller, which is connected with the computer for signal recording and visualization. Turn on the computer and launch the software, e.g., AutoSpike32 and click the "Record" mode from the menu bar. Then choose the "wave" so as to start recording the wave signals. Note: A flat line running from the left to the right of the monitor repeatedly should now be visible. Here, the recording window lasts 40 sec. Max wave recording is 10 sec. Selected sampling rate is 96000 and digital sampling rate is 240. There is 0% offset and no filtering, no rectification for the recording signals. All these parameter settings in the software can be modified as needed.
Turn on the speaker connected to the preamplifier, which is used to present the toning mode for the neuronal responses from antennal sensillum.
Insert the reference electrode into the abdomen of the stabilized bed bug. Note: The reference electrode was held by a metal stand magnetically attached to the air-table.
After the reference electrode has been connected to the bed bug's abdomen, move the recording electrode, which is connected to the preamplifier and manipulated by a micromanipulator, towards the posterior end of the bed bug's antenna.
When the recording electrode is in contact with the right tip of the antenna, switch on the microscope and locate the electrode at low magnification.
Adjust the recording electrode while gradually increasing the magnification until both the electrode and the antennal sensillum are in the same plane and clearly visible under the microscope. Note: By this time, the microscope is usually at the highest magnification.
Insert the recording electrode into the shaft of the sensillum using the micromanipulator and go a little deeper if the background noise is high compared to the action potential.
- Once clear action potentials are observed from the recorded sensillum, fill a micropipette with 10% (+)-β-pinene. Use the micropipette to deposit 10 µl aliquot of 10% (+)-β-pinene onto a filter paper strip (~3 x 15 mm) placed inside a glass Pasteur pipette.
- Connect the loaded pipette to the outlet of the pulse flow tube of the stimulus controller and place the tip of the pipette into the small hole in the tube oriented towards the antenna.
When all these connections have stabilized, depress the footswitch of the stimulus controller to deliver a 0.5 sec puff of stimulus (0.5 L/min) into the continuous humidified air stream. The recording of action potentials will be initiated simultaneously when the footswitch is depressed. The recording process will be last for 10 sec starting 1 sec before the stimulation.
Count the action potentials off-line for two 500 msec periods, one before and one after the stimulation. Subtract any change in the spike rate during the 500 msec post-stimulation from the spontaneous activity recorded during the preceding 500 msec and convert the counts into the conventional scale of spikes/s by multiplying them by 2.
4. Stimulus Replacement in the SSR
Once the footswitch has been triggered, deliver the 10% (+)-β-pinene in the pipette onto the bed bug antennae and record the response to this specific odorant for 10 sec, after which the pipette is removed.
Label another new pipette with the 0.001% eucalyptol to be tested. Place a small piece of filter paper onto which 10 µl of the stimulus has been applied, into the new pipette.
Wait for 2-5 min until the stimulus is completely vaporized in the glass pipette. Attach the pipette onto the outlet of the pulse flow tube.
Insert the pipette tip into the small hole of the tube oriented towards the antenna. Depress the footswitch and start the 10 sec recording.
Disconnect the pipette and prepare another pipette with 0.01% eucalyptol.
Test all the rest of the doses of eucalyptol (from 0.001% to 10%) on the antennal sensilla to observe the dose-dependent responses. Test from the most dilute to the least dilute doses.
Representative Results
Single sensillum recording is a powerful investigative technique used in studies of insect chemical ecology and neural physiology. Investigating the neural responses of insects to different volatile compounds, especially those thought to be ecologically related to the survival and development of the insects, not only gives us invaluable insights into the insect olfaction process, but also opens up promising new avenues that could potentially lead to the development of useful new reagents for pest control.
The common bed bug, as a notorious urban pest, has certainly attracted the attention of many researchers. Among the various study areas related to bed bugs, their olfaction mechanism is of the utmost importance for the chemical ecology of bed bugs. Previous studies have explicitly described the quantity and distribution of different types of olfactory sensillum on bed bug antennae. As shown in Figure 2A, bed bug antennae possess four segments (SC, PE, F1 and F2). The majority of the olfactory sensilla are presented on the posterior end of the 2nd flagellum (F2), but their distribution is distinctly different for each type: the D sensilla, namely Dα, Dβ and Dγ, are only located along the inner side of the antennae (Figure 2C), while the C and E (E1 and E2) sensilla are found on both sides of the antennae (Figure 2B). Therefore, in order to ensure that we record the neural response of the D sensilla, careful positioning of the antenna is essential.
Since both sexual forms of bed bug share the same pattern of sensilla types and the inner side of their antennae contains all types of sensilla, targeting this area makes it much easier to record the chemical responses of all the different types of sensilla separately on the antennae (Figure 3A). In single sensillum recording, different olfactory sensilla exhibit neural signals with distinctively different action potential types and amplitudes (Figure 3B). For example, E sensilla are known to have one or two neurons inside, while D type sensilla house more neurons than either E or C sensilla, producing more complicated action potentials than the others as a result. The amplitudes of the neural responses from C sensilla are much smaller than those of the other sensillum types.
Once the electrode connections have been set up, the neural responses from each type of sensillum to each stimulus can be recorded based on their identity and intensity. For some stimuli to which bed bugs are extremely sensitive, the neural response can be very strong and last several seconds beyond the termination of stimulation. For instance, in response to 10% (+)-β-pinene, bed bugs showed a strong reaction with a huge firing rate (≥200 spikes/sec) and super-sustained temporal dynamics compared to the control with solvent alone as the stimulus (Figure 4A and B). Different stimuli may elicit totally different neural responses from the same sensillum and different concentrations of the same stimulus are likely to generate quite different firing frequencies. As shown in Figure 5, increasing the concentration of eucalyptol raised the firing frequencies from 30 spikes/sec at 0.001% to 240 spikes/sec at 10% in a dose-dependent manner.
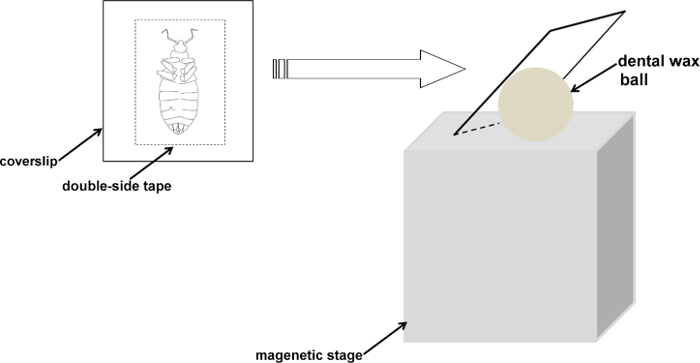 Figure 1. A schematic diagram showing the fixation procedure for bed bugs. The bed bug is stabilized on the coverslip with the antennae fixed on the tape. The assembled sample is then placed on a magnetic stage. The orientation and height of the sample can be adjusted to an appropriate angle between the bed bug antennae and the recording electrode. Please click here to view a larger version of this figure.
Figure 1. A schematic diagram showing the fixation procedure for bed bugs. The bed bug is stabilized on the coverslip with the antennae fixed on the tape. The assembled sample is then placed on a magnetic stage. The orientation and height of the sample can be adjusted to an appropriate angle between the bed bug antennae and the recording electrode. Please click here to view a larger version of this figure.
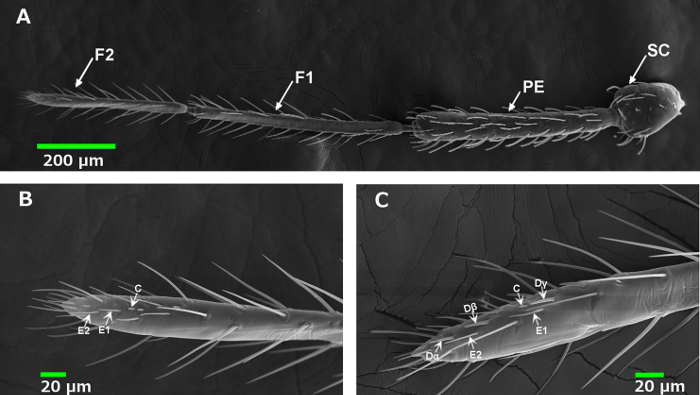 Figure 2. Distribution of olfactory sensilla on the bed bug antennae. (A) A Scanning Electrode Microscope (SEM) image of a bed bug antenna. The antenna has four segments, the Sculpus (SC), Pedecel (PE), the first flagellum (F1) and the second flagellum (F2). Most of the olfactory sensilla are located on F2, although a few olfactory sensilla have also been found on F1, which is thought to be related to their function of aggregation pheromone detection for the bed bug16. (B) A SEM image of the outer side of F2, which houses the C and E olfactory sensilla. (C) A SEM image of the inner side of F2, which was found to house all the different types of olfactory sensilla: D (Dα, Dβ, Dγ), C and E. Please click here to view a larger version of this figure.
Figure 2. Distribution of olfactory sensilla on the bed bug antennae. (A) A Scanning Electrode Microscope (SEM) image of a bed bug antenna. The antenna has four segments, the Sculpus (SC), Pedecel (PE), the first flagellum (F1) and the second flagellum (F2). Most of the olfactory sensilla are located on F2, although a few olfactory sensilla have also been found on F1, which is thought to be related to their function of aggregation pheromone detection for the bed bug16. (B) A SEM image of the outer side of F2, which houses the C and E olfactory sensilla. (C) A SEM image of the inner side of F2, which was found to house all the different types of olfactory sensilla: D (Dα, Dβ, Dγ), C and E. Please click here to view a larger version of this figure.
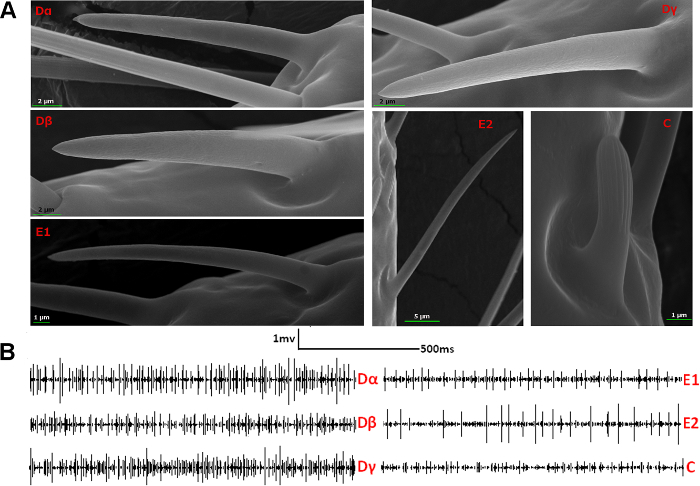 Figure 3. Typical neural signals of different types of olfactory sensilla on bed bug antennae. (A) High resolution SEM pictures of each type of olfactory sensillum on the bed bug antennae. (B) Typical neural signals of the different olfactory sensilla before exposure to a stimulus. Dα, Dβ, Dγ and C sensilla, which house multiple olfactory sensory neurons (OSNs), exhibit more complicated action potentials than E1 and E2 sensilla, which contain only one or two OSNs. The amplitudes of the action potentials from C sensilla are much smaller than those in other sensillum types. Please click here to view a larger version of this figure.
Figure 3. Typical neural signals of different types of olfactory sensilla on bed bug antennae. (A) High resolution SEM pictures of each type of olfactory sensillum on the bed bug antennae. (B) Typical neural signals of the different olfactory sensilla before exposure to a stimulus. Dα, Dβ, Dγ and C sensilla, which house multiple olfactory sensory neurons (OSNs), exhibit more complicated action potentials than E1 and E2 sensilla, which contain only one or two OSNs. The amplitudes of the action potentials from C sensilla are much smaller than those in other sensillum types. Please click here to view a larger version of this figure.
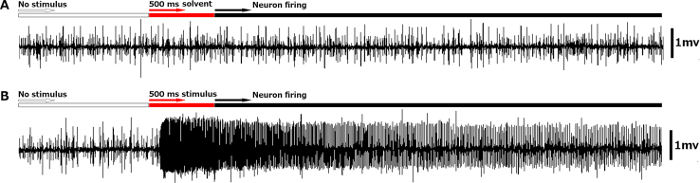 Figure 4. Representative neural response to stimuli that bed bugs are sensitive to. (A) Signal trace showing the typical neural response of an olfactory sensillum (Dγ) to the solvent, which is used as the control in the single sensillum recording. The signal traces are set to start 1 sec before the 0.5 sec puff of stimulus. The signal traces continue recording for 10 sec after initiating the stimulus puff. (B) Signal trace showing the extremely strong neural response of an olfactory sensillum (Dγ) to a botanical stimulus, 10% (+)-β-pinene. After the puff of (+)-β-pinene is delivered to the Dγ sensillum, OSNs housed inside this sensillum are fired with high frequency and a long-lasting temporal dynamic. The white bar above the signal trace indicates the 1 sec interval before stimulus exposure, the red bar above the trace corresponds to the delivery of the stimulus puff onto the olfactory sensillum, and the black bar above the trace indicates the signal recorded after the termination of stimulus puff. Please click here to view a larger version of this figure.
Figure 4. Representative neural response to stimuli that bed bugs are sensitive to. (A) Signal trace showing the typical neural response of an olfactory sensillum (Dγ) to the solvent, which is used as the control in the single sensillum recording. The signal traces are set to start 1 sec before the 0.5 sec puff of stimulus. The signal traces continue recording for 10 sec after initiating the stimulus puff. (B) Signal trace showing the extremely strong neural response of an olfactory sensillum (Dγ) to a botanical stimulus, 10% (+)-β-pinene. After the puff of (+)-β-pinene is delivered to the Dγ sensillum, OSNs housed inside this sensillum are fired with high frequency and a long-lasting temporal dynamic. The white bar above the signal trace indicates the 1 sec interval before stimulus exposure, the red bar above the trace corresponds to the delivery of the stimulus puff onto the olfactory sensillum, and the black bar above the trace indicates the signal recorded after the termination of stimulus puff. Please click here to view a larger version of this figure.
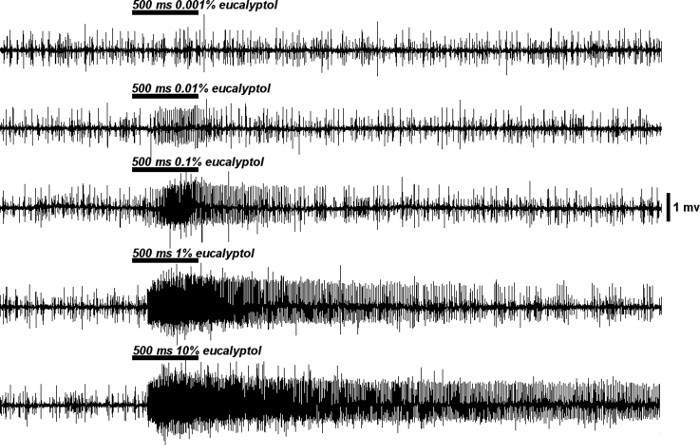 Figure 5. Representative dose-dependent responses of OSNs to the stimuli. Using another botanical stimulus, eucalyptol as an example, the Dγ sensilla displayed a dose-dependent response to different concentrations of eucalyptol. As the concentrations increased from 0.001% to 10%, the firing frequencies rose from 30 spikes/sec to 240 spikes/sec. Please click here to view a larger version of this figure.
Figure 5. Representative dose-dependent responses of OSNs to the stimuli. Using another botanical stimulus, eucalyptol as an example, the Dγ sensilla displayed a dose-dependent response to different concentrations of eucalyptol. As the concentrations increased from 0.001% to 10%, the firing frequencies rose from 30 spikes/sec to 240 spikes/sec. Please click here to view a larger version of this figure.
Discussion
The Single Sensillum Recording technique has been extensively used in testing the neural responses of insects such as fruit flies, mosquitoes and bed bugs to different chemical stimuli in the environment. These chemical stimuli are often dissolved and diluted in a common solvent in order to prepare different doses of treatments. However, different solvents can produce quite different release rates for the stimuli. Previous studies on some extensively studied insects such as Drosophila melanogaster, Anopheles gambiae, Culex quinquefasciatus and Aedes aegypti haveusually used paraffin oil as the solvent to dissolve the stimuli, as these insects are relatively insensitive to paraffin oil17-20. Paraffin oil has also been used in earlier single sensillum recording studies of bed bugs, for the same reason14. However, the most commonly used solvent may not be the best for each insect species. In the case of bed bugs, both paraffin oil and DMSO, to which bed bugs also exhibit insensitivity, have been used to dissolve the stimuli in different studies14,15, but the same doses of stimuli diluted in DMSO appear to elicit much stronger neural responses on the sensilla of bed bugs. For instance, DMSO-dissolved R-(+)-limonene and S-(-)-limonene generated neural responses of ≥70 spikes/sec from Dγ sensilla on bed bug antennae, while the paraffin oil-dissolved limonene elicited neural responses of only ≤25 spikes/sec from Dγ sensilla. This decrease in the neural responses is quite common in stimuli that have been diluted with paraffin oil, probably due to the slower release rate of paraffin oil compared with DMSO. This slower release rate reduces the amount of stimulus delivered onto the surface of the sensillum and may result in a misleading conclusion regarding the sensitivity of insects to certain semiochemicals.
Two critical steps for conducting the single sensillum recording are 1) sample preparation and 2) signal recording. For the sample preparation, since the bed bugs have very strong legs and actively moving antennae, it is very important to remove all legs and stick the antennae tightly onto the double-sided tape. In the signal recording process, sometimes, it is positionally impossible for the electrode to point into the sensillum shaft. If this is the case, the electrode may puncture the posterior end of the sensillum, which always gives a very clean and clear signal with very little background noise.
As there are multiple neurons housed in the D and C type sensilla, it is often hard to distinguish between individual neurons based on the amplitudes and shapes of the action potentials produced during SSR. However, it is still possible to see differences in the bed bug's responses to different stimuli based on the combined firing frequency of all the neurons in the same sensillum. Theoretically, bed bugs are sensitive to certain stimuli with strong stimulation while insensitive to other stimuli with weak stimulation at the same dose. Further studies integrating behavior tests and information of their neural response to these stimuli would therefore provide meaningful information on ecologically related semiochemicals for bed bugs.
In this study, we also used the SSR technique to test the neural responses of olfactory sensilla to different doses of stimuli. We observed a dose-dependent pattern in the bed bug's neural responses to different chemicals. However, considering the complex environment bed bugs live in, the actual dose of volatiles encountered by bed bugs in their normal surroundings will be very low. As a result, the semiochemicals that elicit strong neural response at low doses down to 1:105 v/v and 1:104 v/v are more likely to be biologically meaningful for bed bugs than other chemicals that only function at high doses. Therefore, those semiochemicals that act at low doses probably play an important role in the chemoreception of bed bugs, helping them to locate a host or avoid adverse factors, and will thus provide useful guidance in screening for promising bed bug attractants or repellents for use in both laboratory and field assays.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The project was supported by Award AAES 461Hatch/Multistate Grants ALA08-045 and ALA015-1-10026 to N.L.
References
- Bartonicka T, Gaisler J. Seasonal dynamics in the numbers of parasitic bugs (Heteroptera, Cimicidae): a possible cause of roost switching in bats (Chiroptera, Vespertilionidae) Parasitol Res. 2007;100(6):1323–1330. doi: 10.1007/s00436-006-0414-6. [DOI] [PubMed] [Google Scholar]
- Thomas I, Kihiczak GG, Schwartz RA. Bed bug bites: a review. Int J Dermatol. 2004;43(6):430–433. doi: 10.1111/j.1365-4632.2004.02115.x. [DOI] [PubMed] [Google Scholar]
- Anderson AL, Leffler K. Bed bug infestations in the news: a picture of an emerging public health problem in the United States. J Environ Health. 2008;70(9):24–27. [PubMed] [Google Scholar]
- Boase C. Bed bugs (Hemiptera: Cimicidae): an evidence-based analysis of the current situation. In: Robinnson W, Bajomi D, editors. Sixth international conference on urban pests. OOK-Press Kft; Budapest, Hungary. 2008. [Google Scholar]
- Doggett SL, Geary MJ, Russell RC. The Resurgence of bed bugs in Australia: with notes on their ecology and control. Environ Health. 2004;4(2):30–38. [Google Scholar]
- Ter Poorten MC, Prose NS. The return of the common bedbug. Pediatr Dermatol. 2005;22(3):183–187. doi: 10.1111/j.1525-1470.2005.22301.x. [DOI] [PubMed] [Google Scholar]
- Yoon KS, Kwon DH, Strycharz JP, Craig S, Lee SH, Clark JM. Biochemical and molecular analysis of deltamethrin resistance in the common bed bug (Hemiptera: Cimicidae) J Med Entomol. 2008;45(6):1092–1101. doi: 10.1603/0022-2585(2008)45[1092:bamaod]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Wang L, Xu Y, Zeng L. Resurgence of bed bugs (Hemiptera: Cimicidae) in mainland China. Fla Entomol. 2013;96(1):131–136. [Google Scholar]
- Haynes KF, Potter MF. Recent progress in bed bug management. In: Ishaaya I, Palli SR, Horowitz AR, editors. Advanced technologies for managing insect pests. New York: Springer; 2013. pp. 269–278. [Google Scholar]
- Carey AF, Carlson JR. Insect olfaction from model systems to disease control. Proc Natl Acad Sci. 2011;108(32):12987–12995. doi: 10.1073/pnas.1103472108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal WS. Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu Rev Entomol. 2013;58:373–391. doi: 10.1146/annurev-ento-120811-153635. [DOI] [PubMed] [Google Scholar]
- Den Otter CJ, Behan M, Maes FW. Single cell response in female Pieris brassicae. (Lepidoptera: Pieridae) to plant volatiles and conspecific egg odours. J Insect Physiol. 1980;26(7):465–472. [Google Scholar]
- Levinson HZ, Levinson AR, Muller B, Steinbrecht RA. Structural of sensilla, olfactory perception, and behavior of the bed bug, Cimex lectularius., in response to its alarm pheromone. J Insect Physiol. 1974;20(7):1231–1248. doi: 10.1016/0022-1910(74)90229-7. [DOI] [PubMed] [Google Scholar]
- Harraca V, Ignell R, Löfstedt C, Ryne C. Characterization of the antennal olfactory system of the bed bug (Cimex lectularius) Chem Senses. 2010;35(3):195–204. doi: 10.1093/chemse/bjp096. [DOI] [PubMed] [Google Scholar]
- Liu F, Haynes KF, Appel AG, Liu N. Antennal olfactory sensilla responses to insect chemical repellents in the common bed bug, Cimex lectularius. J Chem Ecol. 2014;40(6):522–533. doi: 10.1007/s10886-014-0435-z. [DOI] [PubMed] [Google Scholar]
- Olson JF, Moon RD, Kells SA, Mesce KA. Morphology, ultrastructure and functional role of antennal sensilla in off-host aggregation by the bed bug, Cimex lectularius. Arthropod Struct Dev. 2014;43(2):117–122. doi: 10.1016/j.asd.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30(2):537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- Qiu YT, Loon JJA, Takken W, Meijerink J, Smid HM. Olfactory coding in antennal neurons of the malaria mosquito, Anopheles gambiae. Chem Senses. 2006;31(9):845–863. doi: 10.1093/chemse/bjl027. [DOI] [PubMed] [Google Scholar]
- Ghaninia M, Ignell R, Hansson BS. Functional classification and central nervous projections of olfactory receptor neurons housed in antennal trichoid sensilla of female yellow fever mosquito, Aedes aegypti. Eur J Neurosci. 2007;26(6):1611–1623. doi: 10.1111/j.1460-9568.2007.05786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SR, Hanson BS, Ignell R. Characterization of antennal trichoid sensilla from female southern house mosquito, Culex quinquefasciatus Say. Chem Senses. 2009;34(3):231–252. doi: 10.1093/chemse/bjn080. [DOI] [PubMed] [Google Scholar]


