Abstract
The penta-ethyl ester prodrug of the chelating agent diethylene triamine pentaacetic acid (DTPA) referred to as C2E5, is being developed as an orally bioavailable radionuclide decorporation agent. The predicted human efficacy obtained in these experimental animals is confounded by interspecies variations of metabolism. Therefore, in the present study, carboxylesterase-mediated metabolism of [14C]-C2E5 was compared in the S9 intestinal and hepatic fractions of human, dog and rat and their respective plasma. Intestinal hydrolysis of C2E5, resulting in the formation of the tetraethyl ester of DTPA (C2E4), was only detected in human and rat. The primary metabolite in human and dog hepatic fractions was C2E4 whereas the predominant species identified in rat hepatic fractions was the triethyl ester (C2E3). Hepatic hydrolysis of C2E5 causes the formation of C2E4 in human, dog and rat and C2E3 in rat only. Minimal C2E5 hydrolysis was observed in human and dog plasma whereas in rat plasma C2E5 converted to C2E3 rapidly, followed by slower further metabolism. Both recombinant CES1 and CES2 play roles in C2E5 metabolism. Together, these data suggest that dogs may be the most appropriate species for predicting human C2E5 metabolism whereas rats might be useful for clarifying the potential toxicity of C2E5 metabolites.
Introduction
The development of medical countermeasures is a national security priority due to the diversion of radioactive materials and the threat of nuclear terrorism. A number of potential terrorist threat scenarios have been envisioned, including the deployment of a Radiological Dispersal Device (“dirty bomb”) that spreads radioactive material over a large populated area. Of particular concern are isotopes of the transuranic elements americium (Am), plutonium (Pu), and curium (Cm) due to their abundance and availability. The National Institutes of Health has been leading an effort to identify innovative therapies that can be included in the US Strategic National Stockpile for distribution to populations affected by an act of nuclear terrorism. The current strategy to reduce the absorbed radiation dose involves decorporation therapy to accelerate excretion of the contaminating radionuclide 1,2. Intravenous (i.v.) administration of either the calcium or zinc salts of diethylene triamine pentaacetic acid (Ca-DTPA/Zn-DTPA) has been approved by the US Food and Drug Administration (FDA) for contamination with Am, Pu, or Cm 3. To improve access to treatment, there is considerable interest in developing orally bioavailable chelators for the actinides. As a Class III compound in the Biopharmaceutics Classification System (high solubility, low permeability), DTPA is not a good candidate for oral delivery and can only be effectively given by i.v. administration.
Introduction of an ester linkage has been shown to enhance the systemic delivery of pharmacologically active compounds with low oral absorption due to high hydrophilicity 4-6. Therefore, in order to achieve higher oral bioavailability, a penta-ethyl ester DTPA prodrug, designated as C2E5, was synthesized. The five added ester moieties greatly increase the lipophilicity of DTPA, which is measured by increased CLogP. (CLogP of -2.7 for DTPA in contrast to ClogP of 4.7 to C2E5) Studies in rats confirmed that C2E5 shows enhanced oral bioavailability compared to DTPA, with extensive but incomplete metabolism 7 and can elicit effective radionuclide decorporation following oral 8 and transdermal delivery 9. Together, these findings suggest that this prodrug may be a useful and effective alternative treatment to DTPA. The enzymes responsible for C2E5 metabolism to DTPA have not previously been reported, but promising candidates for metabolism are the Carboxylesterases (CESs), which could sequentially cleave the five ester moieties to form the metabolites C2E4, C2E3, C2E2, C2E1, and DTPA as shown in Figure 1.
Figure 1. Proposed carboxylesterase-mediated metabolism of C2E5 to DPTA through four intermediates.
Many of the ester-based prodrugs are biotransformed by carboxylesterases (CESs) (EC 3.1.1.1), which are members of the α/β-fold hydrolase superfamily. CESs are grouped in five subfamilies (CES1–CES5) according to the homology of the amino acid sequence 10,11. The most prolific CESs belong to the CES1 and CES2 families, and are categorized as phase I enzymes important for metabolism of xenobiotics, including drugs and prodrugs. All CESs cleave esters via a two-step process that involves the formation and degradation of an acyl-enzyme intermediate. CESs exhibit ubiquitous tissue expression, but are predominantly expressed within the small intestine and liver consistent with their roles in biotransformation and detoxification. Carboxylesterase expression differs among species and organs within a single species, resulting in differences in substrate specificity 12,13. For example, rat plasma has high expression of CES whereas the human and dog are lacking of CES activities 14. In addition to the differences in expression, species differences exist regarding to CES activity. In general, it has been shown that CES2 has much greater affinity for lipophilic substrates compared to CES1 13; meanwhile, CES1 prefers small alcohol group compound and CES2 prefers bigger alcohol group compound. Those characteristics of CESs could have important implications for lipophilic ester prodrugs, such as C2E5.
The increasing threat of nuclear and biological terrorism has led the FDA to expedite the development and approval of new drug and biological products. The Animal Efficacy Rule (21 C.F.R. § 314.610, drugs) 16,17 states, “when efficacy studies in humans are unethical or infeasible, studies in animal models will typically be needed to provide the efficacy data required to support approval, licensure, clearance, or emergency authorization.” As new therapies are being developed that utilize these hydrolytic pathways, it is vital to understand the similarities and differences in CES activity between humans and preclinical models of drug disposition 18. Thus, establishment of a reliable animal model for assessing the efficacy of radionuclide decorporation therapy is critical. The goal of the current study was to identify potential species and tissue differences in the CES-mediated hydrolysis of the multi-ester prodrug C2E5.
Material and Methods
Chemicals and Materials
[14C]-diethylene triamine pentaacetic acid pentaethyl ester ([14C]-C2E5; 50 mCi/mmol) was purchased from American Radiolabeled Chemicals, Inc. (St. Louis, MO). Sprague-Dawley rat intestinal S9 fractions, isolated from a single male were purchased from Celsis In Vitro Technologies (Baltimore, MD). Pooled, mixed gender human (intestinal, hepatic), single male Beagle dog (intestinal, hepatic), and single male Sprague-Dawley (hepatic) S9 fractions were purchased from Xenotech, LLC (Lenexa, KS). All S9 fractions were prepared in the absence of phenylmethanesulfonylfluoride. Human CES1 and CES2 supersomes were obtained from BD Biosciences (Woburn, MA). Human, Beagle dog, and Sprague-Dawley rat plasma was purchased from Lampire Biological Laboratories, Inc. (Pipersville, PA). Ultima-Flo AP scintillation fluid was obtained from PerkinElmer Life and Analytical Sciences (Waltham, MA). Acetonitrile (ACN) and bis-(p-nitrophenyl) phosphate (BNPP) were purchased from Sigma Aldrich (St. Louis, MO).
C2E5 In Vitro Metabolism in Human, Dog and Rat Intestinal and Hepatic S9 Fractions and Plasma
[14C]-C2E5 (50μM) was pre-incubated in 0.1 M phosphate buffer (pH 7.4) for 5 min at 37°C. Reactions were initiated by the addition of intestinal or hepatic S9 fractions (1 mg/ml) and were maintained at 37°C. Plasma (250 μl reaction volumes) was pre-incubated for 5 min at 37°C. Reactions were initiated by the addition of 50 μM [14C]-C2E5 and were maintained at 37°C. Equivalent boiled S9 fractions were used as a blank to correct for non-enzymatic C2E5 degradation. The reactions were terminated by addition of an equal volume of ice-cold ACN followed by centrifugation at 14,000 × g for 10 min at 4°C. The supernatant was transferred to HPLC vials for subsequent analysis.
C2E5 Metabolism by Human CES1-b, CES1-c, and CES2
One μM of [14C]-C2E5 was pre-incubated in 0.1 M phosphate buffer (pH 7.4) for 5 min at 37°C. Reactions were initiated by the addition of CES1-b, CES-c and CES2 recombinant supersomes. The final enzyme concentration was 1 mg/ml and maintained at 37°C. In all instances, equivalent boiled S9 fractions were used as blanks to correct for non-enzymatic C2E5 degradation. The reactions were terminated by addition of an equal volume of ice-cold ACN followed by centrifugation at 14,000 × g for 10 min at 4°C. The supernatant was transferred to HPLC vials for subsequent analysis.
Inhibition Studies on C2E5 metabolite formation
Recombinant human supersomes of CES1-b, CES1-c, and CES2 (final concentration of 1 mg/ml) were incubated in the absence and presence of the esterase inhibitor bis (4-nitrophenyl)-phosphate (BNPP) at 100 μM. The final concentration of the organic solvent (methanol) was less than 1%.
HPLC analysis of C2E5 and Metabolites
C2E5 and its metabolites were analyzed using a Shimadzu Prominence HPLC system equipped with a Perkin Elmer Radiomatic 625TR Flow Scintillation Analyzer. Two Chromolith® Fast Gradient columns (RP-18e 50 × 2 mm Sorbent Lot No. UM8507) were used in tandem with an Alltima Alltech HP All-Guard Cartridge (C18, 5 μm particle size, 2.1 × 7.5 mm). Mobile phase A consisted of water with 0.1% TFA and mobile phase B was ACN with 0.1% TFA. Six regions of interest in the radiochromatogram were identified with a linear 14-min gradient with a flow rate of 0.3 ml/min, 5-95% B over 8 min before returning to 5% B for the remaining six min. Each sample (10 μl) was injected in quadruplicate. The activity in each region that met the threshold criteria (peak height greater than 60 cpm) was determined and expressed as a percentage of the total activity in all regions. The regions of interest were as follows: DTPA, 1.20-2.30 min; C2E1, 3.60-4.90 min; C2E2, 4.90-5.60 min; C2E3, 5.60-6.50 min; C2E4, 6.50-7.30 min; and C2E5, 7.30-9.00 min.
Data Analysis
All assays were performed in triplicate and expressed as the mean ± S.D. Data were analyzed with one-way ANOVA test by using GraphPad Prism 5 software. (***) is designated for P<0.001.
Results
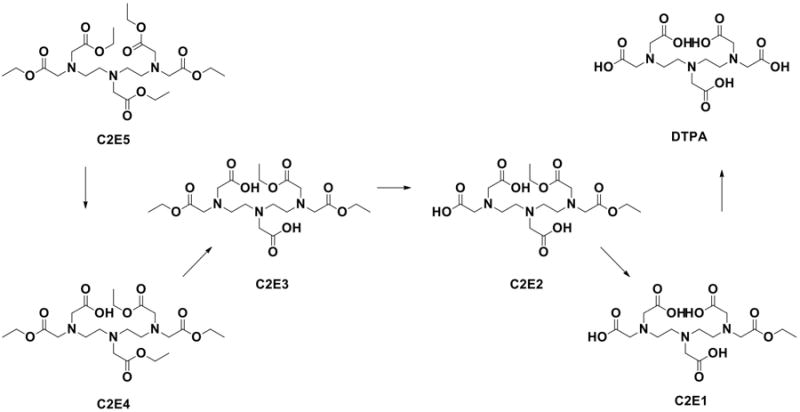
C2E5 Hydrolysis Catalyzed by Human, Dog and Rat Intestinal S9 Fractions
As shown in Fig. 2A, C2E5 hydrolysis was minimal in human intestinal S9 fractions, with approximately 84% C2E5 remaining over the 2 h incubation, whereas no hydrolysis occurred in the dog intestinal fractions. Rat intestinal fractions metabolized 85% of the C2E5. The C2E5 metabolic profile of human, dog and rat fractions was determined by a radiomatic HPLC assay and is shown in Figs. 2B-2D. In human intestinal fractions, no metabolites were detected at the early time points; however, at 2 h, a modest amount (16%) of the activity had been converted to the tetraethyl ester (C2E4) metabolite (Fig. 2B). The rapid disappearance of C2E5 in rat intestinal fractions coincided with time-dependent C2E4 formation as early as 10 min (Fig 2D). A minor amount of the triethyl ester (C2E3) was observed at later time points.
Figure 2. C2E5 Hydrolysis by Human, Dog, and Sprague-Dawley Rat Intestinal S9 Fractions.
Fifty μM [14C]-C2E5 was pre-incubated in 0.1 M phosphate buffer (pH 7.4) for 5 min at 37°C. Reactions were initiated by the addition of intestinal S9 fractions (1 mg/ml final concentration) and were incubated at 37°C. The reactions were terminated at the indicated time points by addition of an equal volume of cold acetonitrile followed by centrifugation at 14,000 × g for 10 min at 4°C. The percent (A) C2E5 remaining and (B) human, (C) dog and (D) rat metabolic profiles [C2E4 (◆); C2E3 (○); C2E2 (▲); C2E1 (□); DTPA (●)] were determined by radio-HPLC. All assays were performed in triplicate and are expressed as the mean ± S.D.
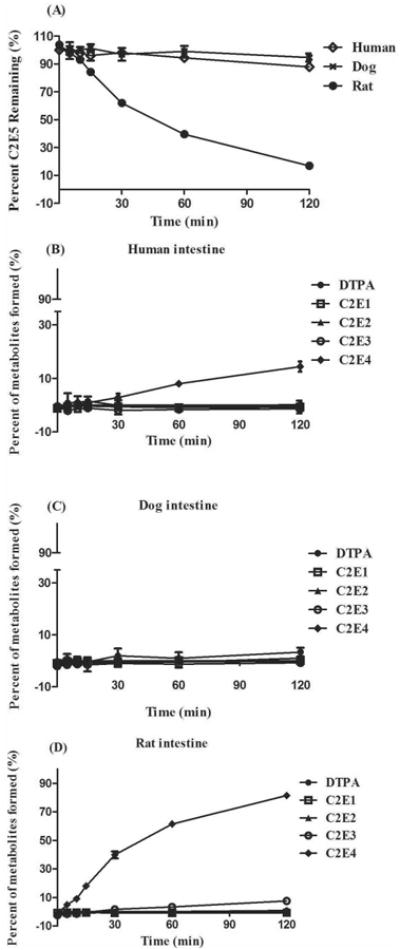
C2E5 Hydrolysis Catalyzed by Human, Dog and Rat Liver S9 Fractions
As shown in Fig. 3A, C2E5 is rapidly metabolized in liver S9 fractions from all tested species. Human and dog S9 fractions hydrolyze approximately 85% of C2E5 within the first 30 min. No further metabolism was observed over the remainder of the 2 h incubation period. Similarly, rat S9 fractions efficiently metabolized greater than 95% of C2E5 over the same incubation period. Interestingly, in all three species C2E5 hydrolysis correlated with primarily an increase in C2E4. In the case of human and dog fractions (Fig. 3B and 3C), C2E4 formation peaked at nearly 80% of the detected activity by 60 min, before a slight decrease at the 120 min time point, due to minor increases in C2E3 and the diethyl ester (C2E2). In rat fractions, C2E4 formation was complete at 10 min and C2E3 became the predominant metabolite, accounting for nearly 80% of the detected activity, from 15 min through the duration of the assay (Fig. 3D).
Figure 3. C2E5 Hydrolysis by Human, Dog, and Sprague-Dawley Rat Hepatic S9 Fractions.
Fifty μM [14C]-C2E5 was pre-incubated in 0.1 M phosphate buffer (pH 7.4) for 5 min at 37°C. Reactions were initiated by the addition of hepatic S9 fractions (1 mg/ml final concentration) and were incubated at 37°C. The reactions were terminated at the indicated time points by addition of an equal volume of cold acetonitrile followed by centrifugation at 14,000 × g for 10 min at 4°C. The percent (A) C2E5 remaining and (B) human, (C) dog and (D) rat metabolic profiles [C2E4 (◆); C2E3 (○); C2E2 (▲); C2E1 (□); DTPA (●)] were determined by radio-HPLC. All assays were performed in triplicate and are expressed as the mean ± S.D.
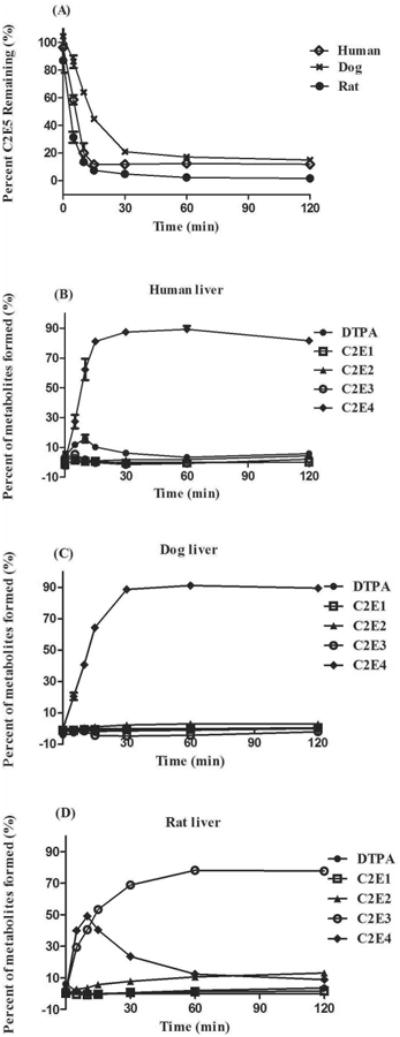
C2E5 Hydrolysis Catalyzed by Human, Dog and Rat Plasma
Species differences are clearly evident when C2E5 was incubated in plasma of human, dog and rat (Fig. 4A). Moderate (∼30%) and no C2E5 hydrolysis were observed in human and dog plasma respectively. (Fig. 4A). Human plasma generated only C2E4 as a metabolite after 2 h of incubation, comprising approximately 30% of the total activity, while dog plasma did not show any detectable metabolites within this time period (Fig. 4B and 4C). In contrast, C2E5 was rapidly hydrolyzed in rat plasma and yielded a strikingly different metabolic profile, in which C2E4 was the primary metabolite in the first min of incubation (Fig. 4D and 4E). The principle metabolite identified within the first 60 min was C2E3 which accounted for ∼60% of the activity. At the 2 h time point, there are equivalent amounts of C2E3, C2E2 and the mono-ethyl ester (C2E1) each accounting for ∼30% of the activity. The remaining activity was attributed to C2E4 and DTPA (Fig. 4E).
Figure 4. C2E5 Hydrolysis by Human, Dog, Sprague-Dawley Rat Plasma Fractions.
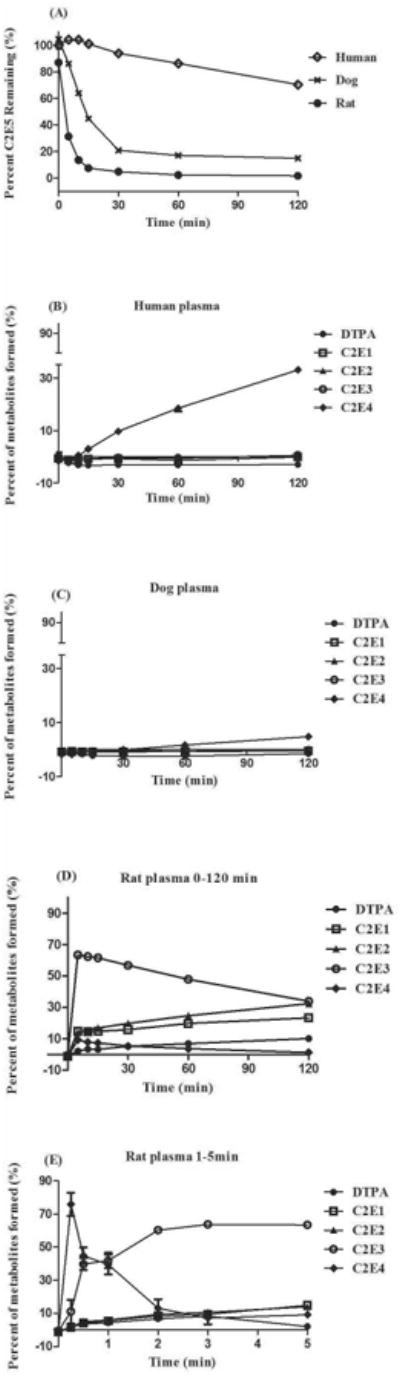
Fifty μM [14C]-C2E5 was added directly to plasma (pre-warmed for 5 min at 37°C). The reactions were terminated at the indicated time points by addition of an equal volume of cold acetonitrile followed by centrifugation (2X) at 14,000 × g for 10 min at 4°C. The percent (A) C2E5 remaining and (B) human, (C) dog and (D, E) rat metabolic profiles [C2E4 (◆); C2E3 (○); C2E2 (▲); C2E1 (□); DTPA (●)] were determined by radio-HPLC. All assays were performed in triplicate and were expressed as the mean ± S.D.
C2E5 Hydrolysis Catalyzed by Recombinant Human CES1-b, CES1-c and CES2
Incubation of 1μM [14C]-C2E5 with human CES1 or CES2 supersomes are presented in Figure 5. These data show that C2E5 was hydrolyzed (∼15%) over 60 min. CES1-b, CES1-c and CES2 all contribute to the hydrolysis of C2E5.
Figure 5. C2E5 Hydrolysis by CES1-b, CES1-c and CES2.
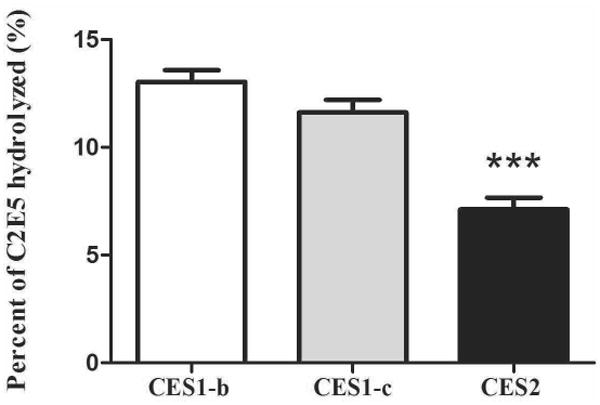
One μM [14C]-C2E5 was pre-incubated in 0.1 M phosphate buffer (pH 7.4) for 5 min at 37°C. Reactions were initiated by the addition of recombinant supersomes (1 mg/ml final concentration) and were incubated at 37°C. The reactions were terminated at the indicated time points by addition of an equal volume of cold acetonitrile followed by centrifugation at 14,000 × g for 10 min at 4°C. The disappearance of C2E5 was determined by radio-HPLC.
Inhibition Studies of C2E5 Metabolism
The inhibition effects of BNPP on C2E5 metabolite formation was investigated (Fig. 6). In the presence of the esterase inhibitor BNPP, C2E5 metabolism was significantly reduced in recombinant human CES1-b and CES1-c and human liver S9 fractions.
Figure 6. Inhibition Studies on C2E5 Hydrolysis.
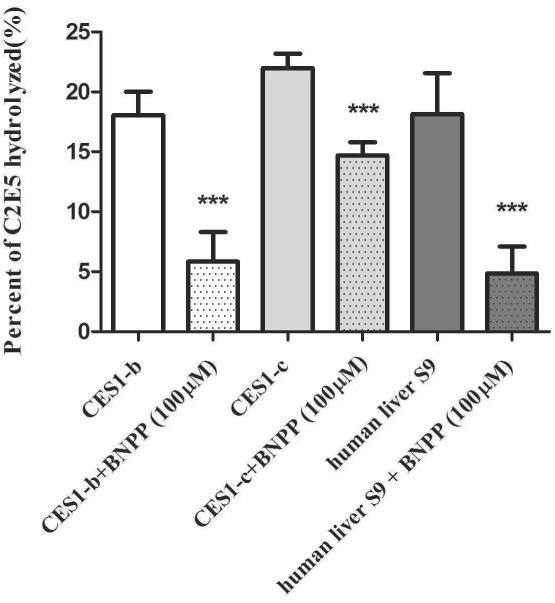
Fifty μM [14C]-C2E5 was pre-incubated in 0.1 M phosphate buffer (pH 7.4) for 15 min at 37°C with addition of 100 uM BNPP. Reactions were initiated by the addition of recombinant supersomes (1 mg/ml final concentration) and were incubated at 37°C. The reactions were terminated at the indicated time points by addition of an equal volume of cold acetonitrile followed by centrifugation at 14,000 × g for 10 min at 4°C. The disappearance of C2E5 was determined by radio-HPLC.
Discussion
The path to FDA approval for treatments against bio- and nuclear-terrorism agents is unique. Instead of relying on human studies, the FDA Animal Rule allows approval of drugs that have been shown to be effective in animal models in the absence of clinical trials. However, the predictive human efficacy obtained in experimental animals is confounded by interspecies variations in metabolism. The inhibition effects of the specific esterase inhibitor, BNPP, confirmed that C2E5 was mainly cleaved by esterase. Our results provide direct evidence that C2E5 is metabolized in a species and tissue dependent manner. Additionally, C2E5 metabolism observed in the presence of recombinant human CES isoforms (CES1-b, CES1-c and CES2) further supports the role of the CES enzyme system in C2E5 hydrolysis. The current study suggests that the dog maybe the best for modeling human C2E5 exposure whereas the rat is uniquely useful for clarifying relative capacity of C2E5 to form more metabolites.
Intestinal metabolism plays an important role in the bioavailability of oral therapeutic ester prodrugs. The expression of intestinal CES is species-dependent. For example, the small intestine contains only enzymes from the CES2 family in humans and rats whereas CES2 expression is lacking in dog13. Consistent with the CES expression profile, C2E5 hydrolysis was absent following incubation in dog intestinal S9 fractions. However, C2E5 hydrolysis was minimal in human intestinal fractions whereas rat intestinal fractions almost completely hydrolyze C2E5 (Fig. 2B and 2D). The differing rates of metabolism observed in human and rat intestinal fractions may be explained, in part, by differential CES isoform expression. Humans express only one CES2 isoform, however, rats express one major CES2 (RL4) and four minor CESs in the intestine 19. In addition to differing CES expression, it has been shown that the intestinal CES2 of rat is markedly more efficient at metabolizing ester-containing compounds than the human CES213 implying the rapid disappearance of C2E5 in rat intestinal fractions is due to the greater ability to hydrolyze C2E513. However, additional unidentified enzymes may be responsible for the distinct C2E5 metabolic profile identified in rat. Interestingly, in both human and rat intestinal S9 studies, C2E4 was the major metabolite. This may be due to the structure-activity relationship between C2E5 and CESs. Together, these results suggest that the intestine plays an important role in the CES-mediated first-pass metabolism of C2E5 in rats, but not in the dog and only to a small degree in human.
CES1 and CES2 are all expressed abundantly in human, dog, and rat liver 20. The degree of C2E5 hydrolysis and the resultant metabolic profiles are vastly different between human and dog liver S9 fractions compared to rats (Table 1). This could be due to different CES expression patterns in rats. The rat liver CES1 family includes 4 different isozymes, hydrolase A, hydrolase B, hydrolase C and rat egasyn and three minor CES2 isozymes (RL4, AY034877 and D50580). Among them, hydrolase A is the closest to the major human CES1 with about 78% homology 21. Observed species-dependent CES expression profile is probably responsible for the differences in the metabolism of C2E5 identified in human and dog when compared to rat. However, a direct comparison of the full complement of human, dog and rat CES isozymes would be needed to confirm these hypotheses.
Table 1. Metabolites of C2E5 Identified in Human, Dog and Rat S9 and Plasma Fraction.
| Species | Intestine S9 | Hepatic S9 | Recombinant S9 | Plasma |
|---|---|---|---|---|
| Human | C2E4 | C2E4 | C2E4, C2E3, DTPA | C2E4 |
| Dog | ND | C2E4 | N/A | ND |
| Rat | C2E4 | C2E4, C2E3, C2E2 | N/A | C2E4, C2E3, C2E2, C2E1, DTPA |
The formation of DTPA from C2E5 mediated by CES, and possibly other enzymes, appears to occur through four intermediates (C2E4, C2E3, C2E2, C2E1) as shown in Figure 1. C2E5 hydrolysis in S9 fractions suggests CES activities in the intestine and livers are insufficient for complete conversion to DTPA (Figs. 2 and 3). Thus, once C2E5, or a resulting metabolite, enters the systemic circulation, it is still susceptible to further metabolism by esterases present in plasma. Human and dog plasma have little or no detectable CES enzyme 15. However, in human plasma there was a moderate level of C2E5 hydrolysis even in the absence of CES activity (Fig 4A and 4B). In contrast to human liver and small intestine where greater than 90% of esterase activity is attributed to CESs 22, butyrylcholinesterase (BuChE), paraoxonase (PON), and albumin esterase, but not CESs, possess hydrolytic activity in human plasma 15. Of the three PON isoforms (PON1, PON2, PON3), only PON 1 has hydrolytic activity 23. Moreover, PON1 substrate specificity is restricted to organophosphate pesticides and nerve gasses 24,25, thus limiting the role that PON1 may play in the hydrolysis of C2E5. BuChE and albumin esterase, both of which contribute to the activation of prodrugs 26-29 are both likely to contribute to the hydrolysis of C2E5 in human plasma. The fact that C2E5 was rapidly hydrolyzed in rat plasma is consistent with the presence of relatively high levels of CES activity in rat plasma. This represented the most efficient cleavage of the esters resulting in the time-dependent formation of multiple metabolites.
C2E5, a lipophilic compound with a CLogP value of 4.7, was hydrolyzed by recombinant protein CES1 and CES2 7. Hydrolysis of C2E5 by CES1 was higher than CES2 at an enzyme concentration of 1mg/ml (Fig. 5). The difference in activity is most likely due to the structural and conformational differences between CES1 and CES2. Human CES2 has been shown to have a greater affinity for lipophilic substrates compared to human CES1, possibly due to greater conformational flexibility and a larger entrance to the active site 28, but human CES1 prefers substrates with smaller alcohol groups. C2E5, a lipophilic molecule with small alcohol groups, possesses attributes that make it suitable for metabolism by both enzymes. However, the metabolites of C2E5 might be expected to have an increasing preference for CES1 because of the decrease in lipophilicity associated with the cleaving each ester. Any difference in metabolism between CES1-b and CES1-c is small because the enzymes are structurally very similar. In contrast, CES1 and CES2 share only 48% of sequence homology 31. Bis-(4-nitrophenyl) phosphate (BNPP), an inhibitor of CES-mediated hydrolysis 32, inhibited C2E5 hydrolysis when added to samples of CES1-b, CES1-c, and human liver S9 (Fig 6). This result confirms that human recombinant proteins CESs are responsible for the hydrolysis of C2E5. Even though the inhibition is not one hundred percent, in all cases these results are consistent with the findings in literature on ester-based prodrug studies 32.
During the development of pharmaceutical products, drug disposition and toxicity are usually evaluated in several different animal species. For a drug such as C2E5, the FDA Animal Rule requires an in depth understanding of differences in metabolic capacities among species. In conclusion, we have shown that C2E5 metabolism is species specific due to differing CESs expression profiles. Together, the results suggest that the dog is a better predictive model for C2E5 metabolism in humans whereas the rat is important for understanding the potential toxicity of C2E5 metabolites.
Acknowledgments
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract Nos. HHSN266200500045C and HHSN272201000030C.
References
- 1.Menetrier F, Grappin L, Raynaud P, Courtay C, Wood R, Joussineau S, List V, Stradling GN, Taylor DM, Berard P, Morcillo MA, Rencova J. Treatment of accidental intakes of plutonium and americium: guidance notes. Appl Radiat Isot. 2005;62:829–846. doi: 10.1016/j.apradiso.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Cassatt DR, Kaminski JM, Hatchett RJ, DiCarlo AL, Benjamin JM, Maidment BW. Medical countermeasures against nuclear threats: radionuclide decorporation agents. Radiat Res. 2008;170:540–548. doi: 10.1667/rr1485.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FDA. Guidance for Industry Internal Radioactive Contamination - Development of Decorporation Agents. 2006 Retrieved August 6, 2015 www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071944.pdf.
- 4.Beaumont K, Webster R, Gardner I, Dack K. Design of ester prodrugs to enhance oral absorption of poorly permeable compounds: challenges to the discovery scientist. Curr Drug Metab. 2003;4:461–485. doi: 10.2174/1389200033489253. [DOI] [PubMed] [Google Scholar]
- 5.Imai T. Human carboxylesterase isozymes: catalytic properties and rational drug design. Drug Metab Pharmacokinet. 2006;21:173–185. doi: 10.2133/dmpk.21.173. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen NM, Bundgaard H. Glycolamide esters as biolabile prodrugs of carboxylic acid agents: synthesis, stability, bioconversion, and physicochemical properties. J Pharm Sci. 1988;77:285–298. doi: 10.1002/jps.2600770402. [DOI] [PubMed] [Google Scholar]
- 7.Sueda K, Sadgrove MP, Huckle JE, Leed MG, Weber WM, Doyle-Eisele M, Guilmette RA, Jay M. Orally administered DTPA penta-ethyl ester for the decorporation of inhaled (241) Am. J Pharm Sci. 2014;103:1563–1571. doi: 10.1002/jps.23932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadgrove MP, Leed M, Shapariya S, Madhura D, Jay M. Evaluation of a DTPA Prodrug. C2E5 as an Orally Bioavailable Radionuclide Decorporation Agent. Drug Dev Res. 2012;73:243–251. [Google Scholar]
- 9.Zhang Y, Sadgrove M, Mumper R, Jay M. Transdermal Prodrug Delivery for Radionuclide Decorporation: Nonaqueous Gel Formulation Development and In Vitro and In Vivo Assessment. Drug Dev Res. 2013;74:322–331. [Google Scholar]
- 10.Hosokawa M. Structure and catalytic properties of carboxylesterase isozymes involved in metabolic activation of prodrugs. Molecules. 2008;13:412–431. doi: 10.3390/molecules13020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satoh T, Hosokawa M. Structure, function and regulation of carboxylesterases. Chem Biol Interact. 2006;162:195–211. doi: 10.1016/j.cbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Inoue M, Morikawa M, Tsuboi M, Sugiura M. Species difference and characterization of intestinal esterase on the hydrolizing activity of ester-type drugs. Jpn J Pharmacol. 1979;29:9–16. doi: 10.1254/jjp.29.9. [DOI] [PubMed] [Google Scholar]
- 13.Taketani M, Shii M, Ohura K, Ninomiya S, Imai T. Carboxylesterase in the liver and small intestine of experimental animals and human. Life Sci. 2007;81:924–932. doi: 10.1016/j.lfs.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Williams ET, Bacon JA, Bender DM, Lowinger JJ, Guo WK, Ehsani ME, Wang X, Wang H, Qian YW, Ruterbories KJ, Wrighton SA, Perkins EJ. Characterization of the expression and activity of carboxylesterases 1 and 2 from the beagle dog, cynomolgus monkey, and human. Drug Metab Dispos. 2011;39:2305–2313. doi: 10.1124/dmd.111.041335. [DOI] [PubMed] [Google Scholar]
- 15.Li B, Sedlacek M, Manoharan I, Boopathy R, Duysen EG, Masson P, Lockridge O. Butyrylcholinesterase, paraoxonase, and albumin esterase, but not carboxylesterase, are present in human plasma. Biochem Pharmacol. 2005;70:1673–1684. doi: 10.1016/j.bcp.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Roberts R, McCune SK. Animal studies in the development of medical countermeasures. Clin Pharmacol Ther. 2008;83:918–920. doi: 10.1038/clpt.2008.23. [DOI] [PubMed] [Google Scholar]
- 17.Snoy PJ. Establishing efficacy of human products using animals: the US food and drug administration's “animal rule”. Vet Pathol. 2010;47:774–778. doi: 10.1177/0300985810372506. [DOI] [PubMed] [Google Scholar]
- 18.Mizen L, Burton G. The use of esters as prodrugs for oral delivery of beta-lactam antibiotics. Pharm Biotechnol. 1998;11:345–365. doi: 10.1007/0-306-47384-4_15. [DOI] [PubMed] [Google Scholar]
- 19.Ohura K, Tasaka K, Hashimoto M, Imai T. Distinct patterns of aging effects on the expression and activity of carboxylesterases in rat liver and intestine. Drug Metab Dispos. 2014;42:264–273. doi: 10.1124/dmd.113.054551. [DOI] [PubMed] [Google Scholar]
- 20.Satoh T, Taylor P, Bosron WF, Sanghani SP, Hosokawa M, La Du BN. Current progress on esterases: from molecular structure to function. Drug Metab Dispos. 2002;30:488–493. doi: 10.1124/dmd.30.5.488. [DOI] [PubMed] [Google Scholar]
- 21.Involvement of carboxylesterase in hydrolysis of propranolol prodrug during permeation across rat skin by Teruko Imai, Yuko Takase, HarunobuIwase and Mitsuru Hashimoto.
- 22.Imai T, Taketani M, Shii M, Hosokawa M, Chiba K. Substrate specificity of carboxylesterase isozymes and their contribution to hydrolase activity in human liver and small intestine. Drug Metab Dispos. 2006;34:1734–1741. doi: 10.1124/dmd.106.009381. [DOI] [PubMed] [Google Scholar]
- 23.Liederer BM, Borchardt RT. Enzymes involved in the bioconversion of ester-based prodrugs. J Pharm Sci. 2006;95:1177–1195. doi: 10.1002/jps.20542. [DOI] [PubMed] [Google Scholar]
- 24.Costa LG, Cole TB, Jarvik GP, Furlong CE. Functional genomic of the paraoxonase (PON1) polymorphisms: effects on pesticide sensitivity, cardiovascular disease, and drug metabolism. Annu Rev Med. 2003;54:371–392. doi: 10.1146/annurev.med.54.101601.152421. [DOI] [PubMed] [Google Scholar]
- 25.Getz GS, Reardon CA. Paraoxonase, a cardioprotective enzyme: continuing issues. Curr Opin Lipidol. 2004;15:261–267. doi: 10.1097/00041433-200406000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Jarvinen T, Poikolainen M, Suhonen P, Vepsalainen J, Alaranta S, Urtti A. Comparison of enzymatic hydrolysis of pilocarpine prodrugs in human plasma, rabbit cornea, and butyrylcholinesterase solutions. J Pharm Sci. 1995;84:656–660. doi: 10.1002/jps.2600840525. [DOI] [PubMed] [Google Scholar]
- 27.Salvi A, Carrupt PA, Mayer JM, Testa B. Esterase-like activity of human serum albumin toward prodrug esters of nicotinic acid. Drug Metab Dispos. 1997;25:395–398. [PubMed] [Google Scholar]
- 28.Udata C, Tirucherai G, Mitra AK. Synthesis, stereoselective enzymatic hydrolysis, and skin permeation of diastereomeric propranolol ester prodrugs. J Pharm Sci. 1999;88:544–550. doi: 10.1021/js980358h. [DOI] [PubMed] [Google Scholar]
- 29.Sakurai Y, Ma SF, Watanabe H, Yamaotsu N, Hirono S, Kurono Y, Kragh-Hansen U, Otagiri M. Esterase-like activity of serum albumin: characterization of its structural chemistry using p-nitrophenyl esters as substrates. Pharm Res. 2004;21:285–292. doi: 10.1023/b:pham.0000016241.84630.06. [DOI] [PubMed] [Google Scholar]
- 30.Redinbo MR, Potter PM. Mammalian carboxylesterases: from drug targets to protein therapeutics. Drug Discov Today. 2005;10:313–325. doi: 10.1016/S1359-6446(05)03383-0. [DOI] [PubMed] [Google Scholar]
- 31.Sanghani SP, Quinney SK, Fredenburg TB, Davis WI, Murry DJ, Bosron WF. Hydrolysis of irinotecan and its oxidative metabolites, 7-ethyl-10-[4-N-(5-aminopentanoic acid)-1-piperidino] carbonyloxycamptothecin and 7-ethyl-10-[4-(1-piperidino)-1-amino]-carbonyloxycamptothecin, by human carboxylesterases CES1A1, CES2, and a newly expressed carboxylesterase isoenzyme, CES3. Drug Metab Dispos. 2004;32:505–511. doi: 10.1124/dmd.32.5.505. [DOI] [PubMed] [Google Scholar]
- 32.Tabata T, Katoh M, Tokudome S, Nakajima M, Yokoi T. Identification of the cytosolic carboxylesterase catalyzing the 5′-deoxy-5-fluorocytidine formation from capecitabine in human liver. Drug Metab Dispos. 2004;32:1103–1110. doi: 10.1124/dmd.104.000554. [DOI] [PubMed] [Google Scholar]


