Abstract
The basal chordate amphioxus resembles vertebrates in having a dorsal, hollow nerve cord, a notochord and somites. However, it lacks extensive gene duplications, and its embryos are small and gastrulate by simple invagination. Here we demonstrate that Nodal/Vg1 signaling acts from early cleavage through the gastrula stage to specify and maintain dorsal/anterior development while, starting at the early gastrula stage, BMP signaling promotes ventral/posterior identity. Knockdown and gain-of-function experiments show that these pathways act in opposition to one another. Signalling by these pathways is modulated by dorsally and/or anteriorly expressed genes including Chordin, Cerberus, and Blimp1. Overexpression and/or reporter assays in Xenopus demonstrate that the functions of these proteins are conserved between amphioxus and vertebrates. Thus, a fundamental genetic mechanism for axial patterning involving opposing Nodal and BMP signaling is present in amphioxus and probably also in the common ancestor of amphioxus and vertebrates or even earlier in deuterostome evolution.
Introduction
The anterior/posterior, dorsal/ventral and left/right axes of the basal chordate amphioxus (Branchiostoma floridae) are established by the end of the gastrula stage. The anterior/posterior axis lies close to the animal/vegetal axis, which is established during oogenesis. Amphioxus eggs are small and relatively yolk-poor. The oocyte nucleus is offset toward the animal pole, while sheets of endoplasmic reticulum, which will form the pole plasm, are located near the vegetal pole (Holland and Holland, 1991; Holland and Holland, 1992). Additional asymmetries develop soon after fertilization. As the pole plasm coalesces, the sperm nucleus migrates to the vegetal pole and then, together with a cloud of mitochondria, back to the animal hemisphere to join the female chromosomes (Holland and Holland, 1992). Development is regulative with at least the first four blastomeres capable of giving rise to a gastrula (Wilson, 1893). The hollow blastula is just one cell thick with the vegetal blastomeres slightly larger than the animal ones. Gastrulation is by invagination with virtually no involution (Zhang et al., 1997). The first sign of the posterior pole of the embryo is expression of Wnt8 and Brachyury in a ring around the equator of the blastula, which marks the future blastoporal lips (Holland and Holland, 2007). Although the first morphological indication of dorsal/ventral polarity is the flattening of the neural plate at the late gastrula stage, gene expression patterns show that dorsal identity is specified by the onset of gastrulation. At this stage, although several genes are broadly expressed throughout the nascent mesendoderm (BMPs, Dkk1/2/4) or ectoderm (Distalless), others have restricted expression dorsally in either the mesoderm (Gsc) or in both mesoderm and ectoderm (Nodal, Lefty, Tsg, ADMP, Chordin) (Holland et al., 1996; Yu et al., 2007). How or exactly when dorsal/ventral polarity is established in amphioxus embryos is unknown.
For other chordates, dorsal identity in at least the Xenopus and zebrafish blastula is mediated by nuclear β-catenin in dorsal nuclei (Yost et al., 1996; Schneider et al., 1996; Wylie et al., 1996; Kelly et al., 2000; Schier and Talbot, 2005). However, Wnt/β-catenin signaling is not required for patterning pre-implantation mouse embryos (Marikawa, 2006), while in the chick, Wnt expression in the marginal zone is necessary for Vg1 to induce a primitive streak, but nuclear localization of β-catenin has not clearly been demonstrated (Skromne and Stern, 2001; Schmidt et al., 2004). Moreover, in amphioxus, while Wnt/β-catenin signaling is important for specification of posterior identity during the gastrula stage, nuclear β-catenin, which is translocated to all nuclei at the 8-cell stage, is not involved in establishing dorsal identity (Holland et al., 2005).
The expression of Nodal and its antagonist Lefty dorsally in the amphioxus gastrula and its earlier asymmetric expression suggest that Nodal signaling may play a key role both in specification of dorsal identity during cleavage stages and in its maintenance later during the gastrula stage. At the onset of gastrulation, Nodal and Lefty are expressed throughout the presumptive mesoderm, which is entirely dorsal in character during gastrulation, and in the overlying presumptive neuroectoderm (Yu et al., 2002; 2007). If Nodal were comparably expressed earlier in development, it might function in the initial steps in specification of dorsal identity. Nodal genes in other chordates are zygotically, and in some instances maternally, expressed. However, while roles in anterior/posterior (A/P) patterning are known for at least the mouse (Varlet et al., 1997; Tian and Meng, 2006), roles in specification of dorsal identity are controversial. In the zebrafish, the Nodal genes Squint and Cyclops were suggested to participate early in induction of the dorsal organizer. Maternal Squint is localized to two dorsal blastomeres in the four-cell zebrafish embryo, and its knockdown can eliminate dorsal structures (Gore et al., 2005). However, mutation of maternal Cyclops and Squint showed that they are not essential for specification of the dorsal axis before the mid-blastula transition (Bennett et al., 2007; Hagos et al., 2007). In the chick, the nodal-related gene Vg1 functions in primitive streak induction. However, Nodal has several other roles in vertebrates. In both zebrafish and Xenopus, Nodal and, at least in Xenopus, endodermal Vg1 function in mesoderm induction (Agius et al., 2000; Birsoy et al., 2006; Tian and Meng, 2006). In Xenopus, Nodal signals are important in axial patterning after the mid-blastula transition, the onset of zygotic transcription when Nodal-related factors are expressed in dorsal-vegetal cells destined to form anterior endoderm (Takahashi et al., 2000; De Robertis and Kuroda, 2004). Adding to the rather confusing picture are reports that absence of Nodal signaling before gastrulation in the mouse results in premature neural differentiation (Camus et al., 2006), while maintenance of the neuroectoderm requires signals from the axial mesendoderm previously induced by Nodal. Moreover, in the sea urchin, which is basal to amphioxus in the deuterostomes, Nodal expression in the oral (ventral) ectoderm of the larva regulates larval aboral/oral (dorsal/ventral) polarity (Duboc et al., 2004; 2008; 2010).
To study possible roles of Nodal signaling in the establishment and maintenance of dorsal identity in amphioxus embryos, we took advantage of the structural simplicity of early amphioxus embryos, which allows gene expression patterns to be clearly followed from the egg to the blastula into the gastrula, and of the relative lack of genetic redundancy, which means that knockdown of a single gene usually gives a clear phenotype. Therefore, we first determined maternal expression of amphioxus Nodal and the related Vg1 and Activin genes as well as that of Cerberus, which in Xenopus can antagonize ligands in the Nodal, BMP and Wnt families (Piccolo et al., 1999), and that of Blimp1, a transcription factor expressed in the anterior endoderm in Xenopus that positively regulates Cerberus (de Souza et al., 1999). We then upregulated signaling by Nodal/Vg1/Activin receptors by application of exogenous Activin, and downregulated it with SB505124, which blocks Type I receptor signaling downstream of the ligands (DaCosta Byfield et al., 2004). In addition, because Nodal and BMPs have been reported to antagonize one another (Tian and Meng, 2006), we overexpressed BMP2/4 and Cerberus mRNA and knocked down the endogenous functions of Blimp1 and Chordin. Finally, to determine how functionally conserved these proteins are we performed cross-species assays in Xenopus embryos and animal caps. Taken together, our results indicate that from very early cleavage stages, Nodal/Vg1 signaling probably specifies dorsal/anterior identity and that, beginning at the early gastrula stage, dorsal Nodal/Vg1 signaling is opposed by BMP signaling, which ventralizes and posteriorizes embryos. We propose that axial patterning by opposing Nodal/Vg1 and BMP signals, augmented by blastoporal Wnt/β-catenin signaling, is fundamental to chordate embryos and may have arisen even earlier in the deuterostome lineage.
Materials and methods
Amphioxus methods
Sexually mature adults of the Florida amphioxus (Branchiostoma floridae) were collected in Old Tampa Bay, Florida. Embryos were raised in a laboratory on site. Fixation and in situ hybridizations were as described (L. Z. Holland et al., 1996; Holland and Yu, 2004). Gene markers included AmphiChordin, AmphiSox1/2/3, AmphiBMP2/4, AmphiGsc, AmphiEvx, AmphiWnt3 (Schubert et al., 2001), Amphibra2, AmphiHex, AmphiNodal (Yu et al., 2007), AmphiOtx (Schubert et al., 2006), AmphiFoxQ2 (Yu et al., 2003) and AmphiCer (EU670254). Synteny analysis confirmed the identity of AmphiCer (Figs. S1, S2). AmphiVg1 (EU670255), AmphiBlimp1 (EU708968) were identified from EST sequences (Yu et al., 2008). Human activin protein was purchased from R&D Systems, Minneapolis, MN. Controls included equal concentrations of bovine serum albumin. SB505124 (Sigma/Aldrich, St. Louis, MO) which inhibits the Nodal/Vg1/Activin receptor inhibitor (DaCosta Byfield et al., 20004) was dissolved in DMSO at a concentration of 20 mM and added to embryos at a final concentration of 50 μM.
Microinjection was performed as previously described (Holland and Yu, 2004), either with the control antisense morpholino oligonucleotide (MO) (5′-CCTCCTACCTCAGTTACAATTTATA-3′), Chordin MO-A (5′-GCACAACGTGCGAGGAACAACATCC-3′) or MO-B (5′-CGGCCAGGACTTCAGAGAATGTT-3′), Blimp1 MO-A (5′-TCTGTCATCGTTGTCCCTCGCATTG–3′(Gene Tools, LLC, Philomath, OR, USA), or with mRNA of amphiBMP2/4 or amphiCerberus. Approximately 2 pl was injected into amphioxus eggs (140 μm in diameter) prior to their fertilization. MOs were dissolved at 1mM and mRNAs at 1μg/μl in 15% glycerol, 5 mg/ml Texas Red dextran (Molecular Probes, Inc., Eugene, OR, USA). In vitro translation confirmed that MOs blocked translation (Fig. S6). cDNAs were generated by PCR and cloned into the pCS2+ vector. Injection of mRNA coding for tandem dimer Tomato, derived from pCS2+tdTomato demonstrated effective translation in vivo (Fig. S3).
Xenopus assays
Embryos were staged according to the table of Nieuwkoop and Faber (1956) and transferred into 0.1X modified Barth’s saline (MBS). For animal cap assays, tissues were excised at stage 9 and cultured in 1X low calcium magnesium Ringer’s with 0.2% BSA. Injections were in 1x MBS. The xVent2 reporter vector -385xVent2-luc (Candia et al., 1997) was used to assay BMP signaling (50 pg/cell), and the xMix2 reporter vector (Huang et al., 1995) for Nodal signaling (50 pg/cell). Unless otherwise indicated, injections were into the animal 4 blastomeres at the 8 cell stage. Animal caps were excised at stage 9, cultured until siblings reached stage 10 and assayed for luciferase (Watabe et al., 1995).
Results
The present study focuses on the combinatorial roles of the TGFβ superfamily genes Nodal/Vg1/Activin in axial patterning in amphioxus. These three proteins signal via the same Smad2/3 proteins and have similar, although not entirely identical, functions (Reissmann et al., 2001; Ramis et al., 2007; Schmierer and Hill, 2007). In particular, all three can antagonize BMP signaling and vice versa (Dale et al., 1992; Jones et al., 1992; Candia et al., 1997; Lagna and Hemmati-Brivanlou, 1999; Tian and Meng, 2006), and the effects of mis-expressing any one are typically interpreted as reflecting the roles of the other two in vivo (Hoodless et al., 1999). Because expression of some key genes in these signaling pathways has either not been studied at all in amphioxus embryos or not studied before the early gastrula, we first investigated expression of Nodal, Vg1, Activin and the Nodal/Vg1 antagonist Lefty as well as Cerberus and Blimp1. Each of these genes is single copy in amphioxus.
Although Activin is present in the amphioxus genome sequence (http://genome.jgi-psf.org/Brafl1/Brafl1.info.html), there were no Activin clones in our EST libraries from unfertilized egg, gastrula, neurula, early larva and adults, and an attempt to amplify Activin by RT-PCR was unsuccessful (data not shown), indicating that if Activin is expressed at all during amphioxus early development, it is at a very low level. In contrast, the EST libraries contained cDNAs for the other four genes. Both Nodal and Vg1, but not Lefty, Cerberus or Blimp1, are maternally expressed (Fig. 1). However, maternal expression differs in that Vg1 mRNA is ubiquitous (Fig. 1A-C), while that of Nodal is lower toward the vegetal pole (Fig. 1I-N). At the mid-blastula stage, Vg1 expression is reduced but still ubiquitous, while that of Nodal remains largely restricted to the animal two-thirds of the embryo (Fig. 1C, L, M). However, by the late blastula/early gastrula, mRNA of both genes becomes restricted to the dorsal ectoderm and mesoderm of the gastrula, including the dorsal blastopore lip, the presumed gastrula organizer (Fig. 1D, O, P) (Yu et al., 2002; 2007). The Nodal domain in both ectoderm and mesoderm extends somewhat more anteriorly than that of Vg1. However as dorsal views show, by the mid-late gastrula, neither gene is expressed in the rim of the blastopore (Fig. 1E, Q). By the mid-neurula, expression of Vg1, like that of Nodal, is detected only in the anterior most left somites (Fig. 1F-H) (Yu et al., 2002).
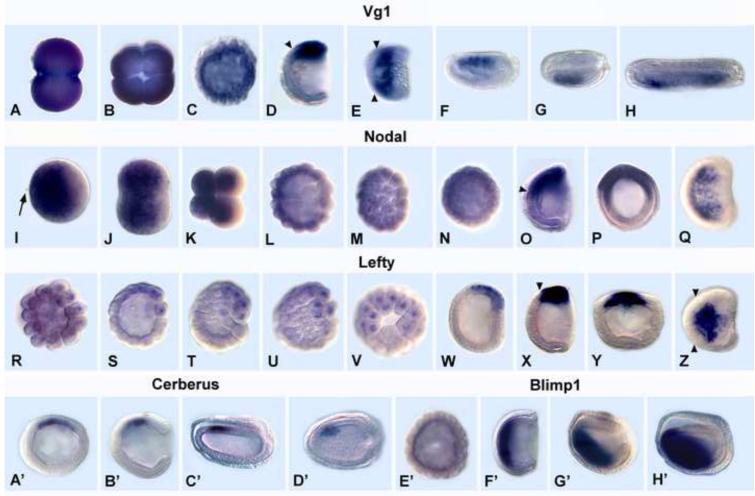
Fig. 1.
Expression of Vg1, Nodal, Lefty, Cerberus and Blimp1 in normal amphioxus embryos. Animal pole or anterior to left except as noted. Arrowheads = anterior limits of expression. (A-H) Vg1. (A) 2-cell stage. (B) 4-cell stage; dorsal view. (C) mid-blastula. (D) mid-gastrula; side view, blastopore at right. (E) Dorsal view of mid-gastrula in D. (F) Mid-neurula (15 hrs); side view. (G) Dorsal view of embryo in F. Expression in 4 left anterior somites; blastopore at right. (H) Late neurula (20 hrs); dorsal view; expression in left somites. (I-Q). Nodal. (I) Fertilized egg. Arrow shows second polar body. (J) 2-cell stage. (K) 8-cell stage. (L) Mid-blastula. (M) Surface view of blastula in K. (N) Late blastula; optical cross section. (O) Mid-gastrula. Side view; blastopore at right. (P). Blastopore view of the gastrula in O. (Q) Dorsal view of the gastrula in O. (R-Z) Lefty. (R) Very early blastula. (S) early-mid blastula. (T) Mid-blastula. (U) surface view of the embryo in T. (V) Vegetal pole view of the embryo in V. (W) Very early gastrula. (X) Mid-gastrula. (Y) Blastoporal view of late gastrula. (Z) Dorsal view of late gastrula. (A’-D’). Cerberus. (A’) Mid-gastrula; optical cross-section; animal pole at top. (B’) Mid-gastrula; blastopore at right. (C’) Early neurula; side view and (D’) dorsal view. Cerberus expression in anterior right paraxial mesoderm. (E’-H’) Blimp1. (E’) Mid-blastula. (F’). Mid-gastrula. Blastopore at right. (G’) Late gastrula. (H’) Early neurula.
The Nodal/Vg1 antagonist, Lefty, is not maternally expressed. Zygotic transcription as shown by nuclear localization of transcripts, begins at the early blastula (Fig. 1R, S). Expression is restricted to a subset of blastomeres at one side of the vegetal pole, which in some embryos is marked by a space between the blastomeres, the vegetal pore (Fig. 1V). Although there is no morphological difference between the dorsal, ventral, left or right blastomeres during the blastula stage, since Lefty is clearly co-expressed with Vg1 and Nodal by the mid-gastrula stage (Fig. 1W-Y), Lefty expression during cleavage appears to mark presumptive dorsal cells. Like Nodal and Vg1, by the mid-late gastrula, Lefty is not expressed in the posterior one-third of the embryo (Fig. 1Z). Since we found no evidence for Activin expression in the early amphioxus embryo, we conclude that Smad2/3-mediated Activin/Nodal/Vg1 signaling occurs via by Nodal and Vg1 ligands and will be hereafter be referred as Nodal/Vg1 signaling.
Cerberus, a member of the cystine-knot superfamily of secreted proteins, can inhibit signaling by Nodal, BMPs and Wnts (Piccolo et al., 1999; reviewed in Tian and Meng, 2006). Since our amphioxus EST libraries lack Cerberus clones, we used PCR to clone the entire intronless coding sequence from genomic DNA. Cerberus has very limited expression. It begins to be transcribed at the mid-gastrula in a small region of dorsal/anterior mesoderm, fated to become notochord and somites (Fig. 1A’-B’). By early neurula, expression becomes restricted to the anteriormost right somites (Fig. 1C’-D’). This pattern is consistent with a role for Cerberus in antagonism of Nodal/Vg1 and/or Wnt and/or BMP and suggests that Cerberus does not function in the earliest steps of embryonic patterning, but in the maintenance of dorsal/anterior identity.
In contrast to Vg1, Nodal and Cerberus, Blimp1 is a transcription factor that is essential for head formation in Xenopus (de Souza et al., 1999; John and Garrett-Sinha, 2009). AmphioxusBlimp1 is first expressed in the anterior endoderm of the mid-gastrula (Fig. 1F’), shifting ventrally at later stages (Fig. 1G’, H’). The evident lack of substantial overlap between the Blimp1 domain and those of Cerberus and Chordin suggests that Blimp1 probably does not directly regulate either gene in amphioxus, but might function in limiting their expression to the dorsal mesoderm.
Dorsal/anterior development in amphioxus is promoted by Nodal/Vg1/Activin signaling
To investigate the role of Nodal/Vg1/Activin in axial patterning in amphioxus, we both up- and down-regulated the signaling pathway. In addition, to determine whether Nodal/Vg1/Activin and BMP signaling oppose one another, we tested whether Activin protein could overcome the effect of overexpressing BMP2/4. For upregulation of Nodal/Vg1/Activin signaling, embryos were exposed to 10 ng/ml human Activin protein from the early blastula stage (Fig. 2). Activin, Nodal, and Vg1 signal via the same receptors (ActRIIB/ActRII, ALK4, ALK7) and downstream transcription factors (Smad2 and Smad3) (Feng and Derynck, 2005; Tian and Meng, 2006). Consequently, signaling by Activin protein is generally assumed to closely approximate that of Nodal and/or Vg1.
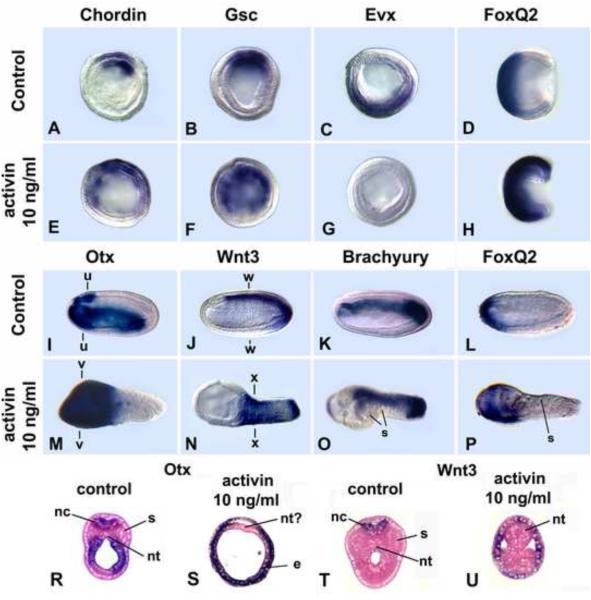
Fig. 2.
Human Activin protein dorsal/anteriorizes amphioxus embryos. 10 ng/ml human activin applied at the early blastula eliminates expression of the ventral marker Evx and expands those of the anterior marker FoxQ2 and dorsal markers Chordin and Gsc. The entire ectoderm is specified as neural; the forebrain expressing Otx is expanded. (A-C, E-G) Mid-gastrulae; optical cross-sections. (D, H) Mid-gastrulae; side views, blastopore at right. (I-P) Mid-neurulae (15 hr); side view. Anterior at left. (R-U) Cross-sections through levels indicated in I, J, M, N. nc = nerve cord, nt = notochord, s = somites, e = endoderm. Gene markers as indicated.
Activin-treated embryos were strongly dorsalized and anteriorized. In gastrulae, which were somewhat foreshortened but otherwise grossly normal (Fig. 2H), the domains of two dorsal mesoderm markers (Chordin and Gsc) were expanded ventrally (Fig. 2A, B, E, F), while Evx expression in ventral ectoderm and endoderm was eliminated (Fig. 2C, G), and that of the anterior ectodermal marker FoxQ2 was expanded (Fig. 2D, H). By the mid-neurula stage, the effects of Activin protein were obvious. The neurulae comprised an expanded anterior end lacking somites and notochord plus a narrow somite and notochord-containing trunk (Fig. 2M-P). The somites and notochord are expanded ventrally (Fig. 2O, U). Cross-sections show that the neural tube is absent (Fig. 2R-U). Gene expression shows that all of the ectoderm is neural, with the anterior neural marker Otx and anterior ectodermal marker FoxQ2 expressed throughout the ectoderm of the expanded portion (Fig. 2I,M,L,P), and the hindbrain and spinal cord marker Wnt3 expressed in the entire ectoderm of the narrow posterior part (Fig. 2J, N). Brachyury is expressed throughout the shortened notochord (Fig. 2K, O). These results indicate that Nodal/Vg1/activin signaling promotes dorsal/anterior (i.e. head) development. At higher concentrations of Activin protein, the embryos were more severely anteriorized with complete absence of the notochord and somites (data not shown).
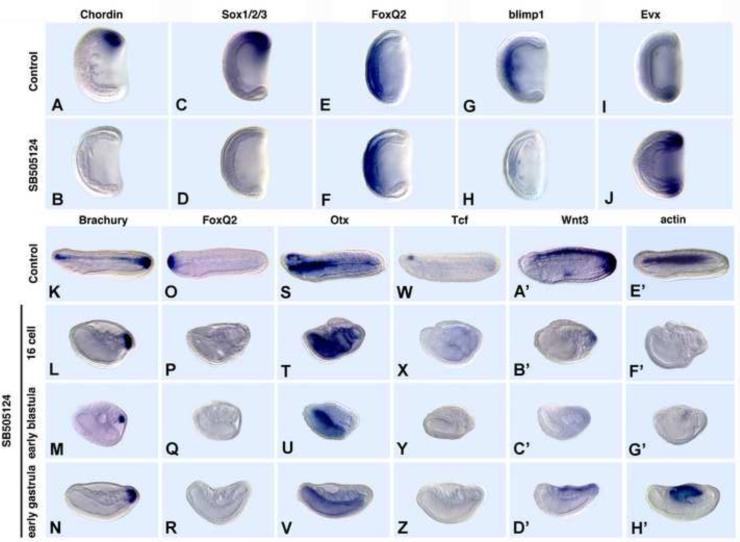
To reduce Nodal/Vg1/Activin signaling, we first attempted to knock-down Nodal function with an antisense morpholino oligonucleotide (MO). However, although it effectively blocked Nodal translation in an in vitro assay, the MO-injected embryos were normal (data not shown) presumably because of a large store of maternal Nodal mRNA and/or because Vg1, which signals through the same Smads as Nodal (Birsoy et al., 2006; White and Heasman, 2008) can compensate for lack of Nodal (Fig. 1). Therefore, we blocked the function of the single amphioxus homolog of vertebrate Alk4, Alk5 and Alk7, which are receptors for Nodal, Vg1 and Activin, with 50 μM SB505124 added at the 16-cell stage, early blastula or early gastrula (Fig. 3). Although Alk5 is also a receptor for TGFβ, no TGFβ clones were present in our EST libraries from unfertilized egg through the neurula stage, suggesting that it does not function together with Nodal and Vg1 during those stages. All the inhibitor-treated embryos gastrulated. Gastrulae treated from the early blastula stage looked grossly normal (Fig. 3 A-J). However, they lost dorsal identity and partially lost anterior identity. Thus, expression of dorsal markers, including the BMP2/4 antagonist, Chordin, which is normally expressed in both the axial mesoderm and overlying neural plate, and the neural plate specifier, Sox1/2/3, was eliminated (Fig. 3A-D), and that of the anterior endodermal marker Blimp1 is severely reduced (Fig. 3G,H). Although FoxQ2 expression was relatively normal at the gastrula stage (Fig. 3E, F), by the late neurula stage, it was completely eliminated (Fig. 3O, P). Conversely, the posterior ventral marker Evx, was upregulated and radialized (Fig. 3I, J). By the late neurula, it is clear that if SB505124 is added at either the 16-cell stage or early blastula, the embryos are severely foreshortened and completely lack dorsal/anterior identity, but have retained posterior identity. Brachyury was still expressed in the tailbud, but expression was absent from the notochord (Fig. 3K-M). Otx remained expressed in the endoderm, but not in the forebrain (Fig. 3S-U). Similarly, expression of the forebrain marker Tcf was eliminated (Fig. 3W-Y), as was Wnt3 expression in the hindbrain, spinal cord and tailbud (Fig. 3A’-C’). Moreover, expression of the somite marker, muscle actin (BFMA1) was eliminated if SB505124 is added at the 16-cell stage (Fig. 3F’), but expressed at a low level when addition was delayed until the early blastula (Fig. 3G’). The embryos were much less severely ventralized by addition of SB505124 at the early gastrula in that expression of muscle actin together with Wnt3 was not blocked if addition was postponed until the early gastrula (Fig. 3N, R, V, Z, D’, H’). These results show that blocking signaling of both maternal and zygotic Nodal and Vg1 inhibits dorsal/anterior development, whereas delaying blocking Nodal/Vg1 signaling until the gastrula stage, when transcription is all zygotic allows some development of the dorsal neural tube and somites, but not of the notochord or anterior structures. Taken together, these gain- and loss-of-function experiments with Activin protein and SB505124 are consistent with an early role for maternal Nodal/Vg1 signaling in specification of dorsal/anterior identity and a continuing requirement for zygotic Nodal/Vg1 signaling in maintenance of dorsal/anterior identity in the early gastrula.
Fig. 3.
Inhibition of Nodal/Vg1 signaling ventral/posteriorizes amphioxus embryos. 50 μM SB505124, which inhibits signaling by Nodal, Vg1 and Activin, was added at the early blastula unless otherwise indicated. Side views. Anterior to left. (A-J) Mid-gastrula stage. Expression of dorsal and anterior markers except for FoxQ2 is eliminated. (K-H’) Mid-late neurulae. The earlier SB505124 is added, the more severe the ventralization and the greater the foreshortening.
BMP signaling opposes that of Nodal/Vg1/Activin
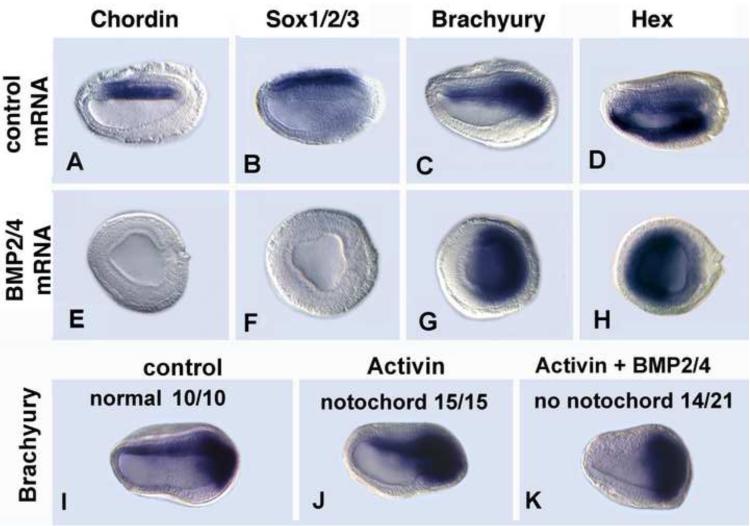
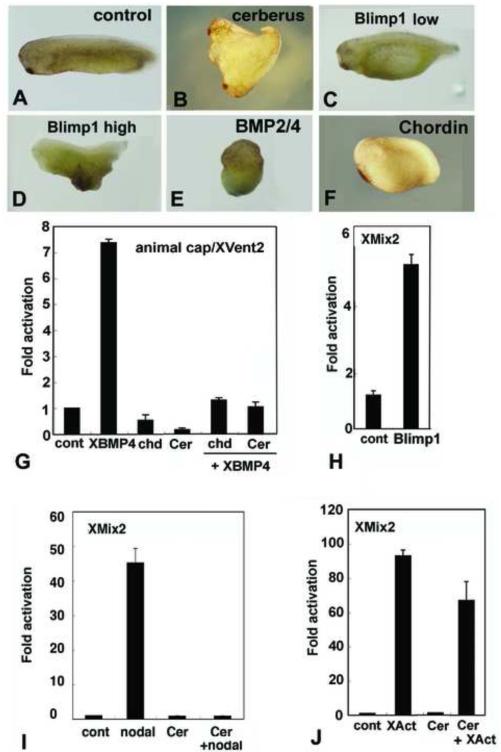
Because neither BMP2/4 nor BMP5-8 is highly expressed before the onset of gastrulation (Yu et al., 2007), they are probably not required for axial patterning at the blastula stage. However, since BMPs are known to oppose signaling by Nodal/Vg1/Activin (Dale et al., 1992; Jones et al., 1992; Candia et al., 1997) and have overlapping expression with Nodal and Vg1 in the early amphioxus gastrula, we overexpressed amphioxus BMP2/4 mRNA in embryos and tested whether it reduced the effect of added Activin (Fig. 4). Injection of amphioxus BMP2/4 mRNA alone severely ventralized and posteriorized embryos, eliminating expression of the dorsal neural plate marker Sox1/2/3 and the notochord and medial neural plate marker Chordin (Fig. 4A, B, E, F), as was expression of Otx in the forebrain and pharynx (Fig. S3). Expression of Brachyury in the notochord was also eliminated, but the domain around the blastopore was expanded (Fig. 4C, G). Moreover, the domain of Hex in the endoderm was expanded dorsally (Fig. 4D, H). These effects are comparable to those of zebrafish BMP4 protein added from the early blastula stage (Yu et al., 2007). Embryos injected with BMP2/4 mRNA and treated with human Activin protein at the blastula stage had phenotypes intermediate between those of either single treatment. The embryos were less severely foreshortened than those injected with BMP2/4 alone, and one-third of them retained the notochordal domain of Brachyury (Fig. 4I-K). These results show first, that the phenotype of embryos overexpressing BMP2/4 is the opposite (posteriorized/ventralized) of that of embryos treated with Activin protein (anteriorized/dorsalized) and suggest that at the early gastrula stage BMP signaling antagonizes that of Nodal and Vg1 to maintain dorsal/anterior and posterior/ventral identities.
Fig. 4.
Activin protein rescues the ventral/posteriorizing defects in embryos overexpressing BMP2/4. Early mid-neurulae (14 hrs). Gene markers as indicated (A-H) Injection of amphioxus BMP2/4 mRNA eliminates dorsal and anterior structures. Embryos are severely foreshortened. (I-K). Human activin protein (10 ng/ml) added to blastulae dorsal/anteriorizes embryos, causing an expanded anterior end (J). (K) Embryos injected with amphioxus BMP2/4 mRNA and treated with human Activin protein at the blastula stage are either partially rescued with restoration of a narrower anterior end and a decreased domain of posterior Brachyury expression, but no notochord (14/21 embryos) or nearly completely rescued with restoration of the notochord as well (7/21).
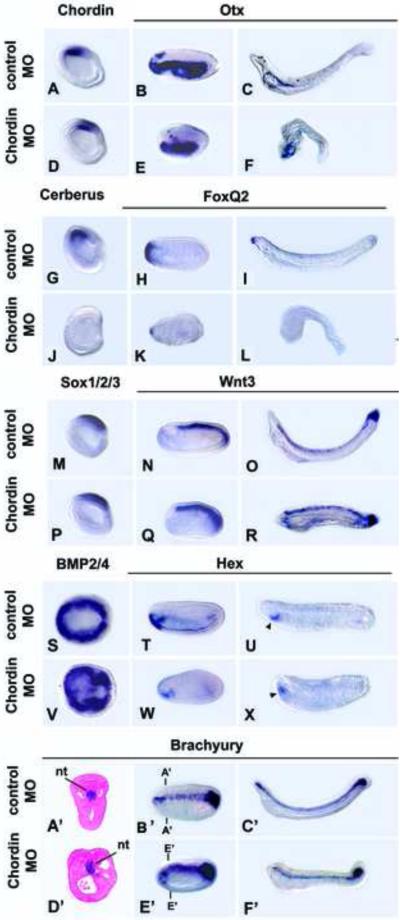
In a second strategy to test the role of BMP signaling in early amphioxus development, we knocked down function of the BMP antagonist Chordin. Amphioxus Chordin is first expressed in the dorsal mesendoderm and adjacent dorsal ectoderm at the onset of gastrulation, before Sox1/2/3 expression is detectable in the future neural plate (Yu et al., 2007). In the gastrula and neurula, the Chordin and Cerberus domains overlap with the former extending more posteriorly, and the latter more anteriorly (Figs. 1A’-B’, 5A) (Yu et al., 2007). Chordin knockdown impaired anterior development, but the phenotype was milder than from overexpressing BMP2/4 (Figs. 5A-F’, S5). The MO-injected embryos were foreshortened with truncated heads, as shown by the down-regulation of Otx (Fig. 5B, C, E, F), FoxQ2 (Fig. 5H, I, K, L), the anterior mesodermal marker Cerberus and anterior endodermal marker Hex (Fig. 5G, J, T, U, W, X). However, the embryos still had dorsal structures such as a CNS and notochord as shown by normal expression of Chordin itself (Fig. 5A, D), Sox1/2/3 (Fig. 5M, P) and Wnt3 (Fig. 5N, O, Q, R), while the Brachyury domain curved ventrally around the anterior tip of the embryo (Fig. 5A’-F’). Down-regulation of BMP2/4 in neuroectoderm, which normally occurs in the early neurula, was only partially inhibited (Fig. 5S, V), suggesting either incomplete knockdown of Chordin or the presence of other BMP antagonists. Even so, in line with loss of the forebrain/midbrain, Chordin knockdown strongly suppressed Cerberus expression in the dorsal/anterior mesoderm (Fig. 5G, J). In contrast, expression of the ventral marker Evx was unaffected (Fig. S5). In early larvae, Hex expression in the anterior endoderm is normally downregulated, but a new domain appears in the endostyle (Fig. 5T, U). In embryos in which Chordin is knocked down, the latter domain shifted anteriorly (Fig. 5W, X), in agreement with loss of the anterior part of the head. Identical results were obtained with a second Chordin morpholino (Fig. S7). In sum, the results suggest that specification of anterior identity in all tissue layers --forebrain, non-neural ectoderm, endoderm and mesoderm-- requires Chordin function.
Fig. 5.
Knockdown of Chordin truncates the head. Side views, anterior to left, except dorsal views in S,V and cross sections in A’, D’. (A, D, G, J, M, P) Mid gastrulae. (B, E, H, K, N, Q, T, W, A’, B’,D’, E’) Mid-neurulae (15 hrs). (U, X) Late neurulae (22 hrs). (C, F, I, L, O, R, U, X, C’ F’) Early larvae (36 hrs). (A,D) Chordin expression is unaffected. (B,C,E,F) Otx expression in the CNS is reduced (arrow). (G,J) Cerberus expression is eliminated. (H,K,I,L) FoxQ2 expression eliminated. (M, P) Sox1/2/3 expression in CNS unaffected. (N, O, Q, R) Wnt3 expression unaffected, but the forebrain/midbrain, which normally does not express Wnt3, is reduced. (S,V) Late gastrula; dorsal views. BMP2/4 partially down regulated in neural plate. (T,W,U,X) Hex expression in anterior endoderm reduced. Arrowhead in U,X indicates presumptive endostyle (A’, F’) Brachyury expression in notochord largely unaffected (A’-F’). In some neurulae (D’, E’), the domain extends anteriorly. A’-A’ and D’, D’ indicate levels of cross sections in A’ and D’.
The anterior mesendodermal markers Cerberus and Blimp1 have opposite roles in axial patterning in amphioxus
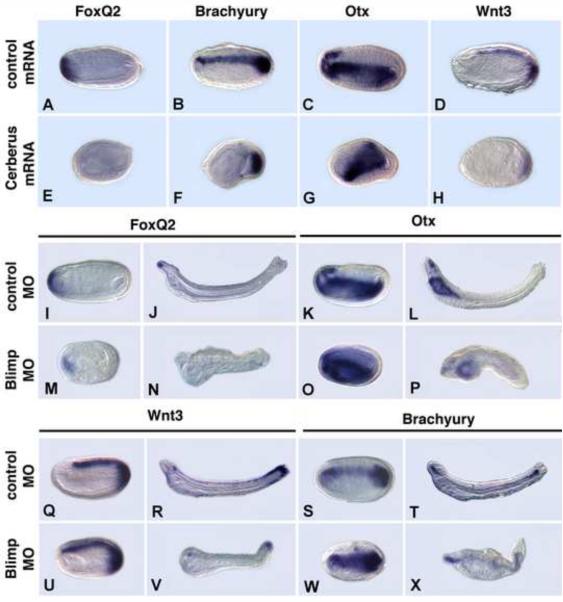
To further investigate the roles of Nodal/Vg1 and BMP signaling in axial patterning in amphioxus, we first manipulated levels of two anterior mesendodermal markers, Cerberus and Blimp1, which have both been implicated as regulating Nodal and/or BMP signaling. Although Cerberus was found to inhibit Nodal, BMP and Wnt signaling in Xenopus (Piccolo et al., 1999), it can also promote BMP signaling in the chick (Yu et al., 2008). Blimp1 can induce Cerberus expression in Xenopus embryos and ventralize zebrafish embryos, decreasing Chordin expression and potentiating BMP signaling (reviewed in John and Garrett-Sinha, 2009). Overexpression of amphioxus Cerberus strongly ventral/posteriorized embryos with loss of the CNS, notochord and anterior identity (Fig. 6A-H). Expression of anterior and dorsal markers was suppressed, and the embryos were shortened. FoxQ2 expression, Otx expression in the forebrain plus midbrain (termed the cerebral vesicle) and that of Wnt3 in the hindbrain and spinal cord was lost, together with Brachyury expression in the developing notochord (Fig. 6). The Brachyury and Wnt3 domains in the tailbud remained. This phenotype resembles, but is less severe than, that from overexpressing amphioxus BMP2/4 mRNA (Fig. 4A-H), and is the expected phenotype if Cerberus were either a Nodal/Vg1/Activin antagonist or BMP2/4 agonist.
Fig. 6.
Overexpression of Cerberus mRNA or knockdown of Blimp1 ventralizes and posteriorizes amphioxus embryos. Side views. Anterior at left. Expression of gene markers as noted. (A-H) Overexpression of Cerberus eliminates dorsal, anterior structures. Early mid-neurulae (15 hrs). (A-D) Injection of control mRNA. (E-H) Injected with Cerberus mRNA. (I-X) MO knockdown of Blimp eliminates the anterior part of the head. (I,M,K,O,Q,U,S,W) Early mid-neurulae (15 hrs). (J,N,L,P,R,V,T,X) Early larva (36 hrs). (I-L,Q-T) Embryos injected with control MO. (M-P,U-X) Injected with Blimp1 MO.
To further test the function of amphioxus Cerberus, we performed comparative experiments in Xenopus. Injection of mRNA for amphioxus Cerberus, into the ventral vegetal D4 blastomere of Xenopus embryos at the 32-cell stage induced a secondary axis with a head (sometimes partial) and a trunk (Fig. 7B), similar to injection of Xenopus Cerberus, although the latter induces a head but no trunk (Bouwmeester et al., 1996). In Xenopus animal cap assays, amphioxus Cerberus effectively repressed the ability of XBMP4 to activate an XVent2- luciferase reporter construct and blocked activation from the XMix2 reporter activated by amphioxus Nodal (Fig. 7G, I), but was only weakly effective in blocking activation driven by Xenopus Activin (Fig. 7J). Although amphioxus Cerberus inhibits both Nodal and BMP signaling in these assays, suppression of anterior development in amphioxus by overexpressing Cerberus indicates that in amphioxus, Cerberus probably preferentially antagonizes Nodal/Vg1 signaling.
Fig. 7.
Experiments in Xenopus show conserved protein functions. (A-F) Injections into marginal zones of 4-cell Xenopus embryos except for B. (A) Uninjected control. (B) 10pg/cell amphioxus Cerberus mRNA in D4 blastomere at 32 cell stage induces secondary axis. (C) 100pg/cell amphioxus Blimp1 mRNA expands ventral structures. (D) 500 pg/cell amphioxus Blimp1 mRNA reduces head structures and expands ventral ones. (E) 100pg/cell amphioxus BMP2/4 mRNA ventralizes. (F) 10pg/cell amphioxus Chordin mRNA enlarges the cement gland. (G-J) Assays of amphioxus proteins in Xenopus animal caps. (G) BMP responsive reporter assay. Amphioxus Chordin and Cerberus suppress signaling by Xenopus BMP4, as monitored by transcription from an Xvent2 reporter. (H) Amphioxus Blimp1 induces transcription from a Nodal-responsive (Xmix2) reporter construct. (I) Nodal-responsive assay. Amphioxus Cerberus suppresses amphioxus Nodal. (J) Activin response assay. Amphioxus Cerberus suppresses Xenopus Activin signaling. Experiments done twice with comparable results; error bars ± 1 s.d.
Amphioxus embryos injected with a Blimp1 MO had a similar, but milder phenotype than those overexpressing Cerberus, suggesting that the two genes have opposite functions in axial patterning. Embryos were foreshortened with either no head or a very small one (Fig. 6M-P, U-X) and with a bent notochord (Fig. 6N, P, V, X). Although the MO eliminated FoxQ2 expression, by larval stages, that of the forebrain/midbrain and pharyngeal marker Otx, although greatly reduced, was still present (Fig. 6I-P). Expression of Wnt3 was expanded to the anterior tip of the CNS (Fig. 6U), and Brachyury expression in the notochord was expanded ventrally (Fig. 6W). To further test the function of amphioxus Blimp1, we injected Blimp1 mRNA into the marginal zones of four-cell Xenopus embryos. The A/P axis was shortened and the ventral region enlarged (Fig. 7C, D) similar to Xenopus embryos injected with Xenopus blimp1 (de Souza et al., 1999). Moreover, in Xenopus animal cap assays, amphioxus Blimp1 promoted transcription from the XMix2 reporter (Fig. 7H). Taken together, these results indicate that Blimp1 is necessary for proper head development in amphioxus, and are consistent with a role for Blimp1 in both amphioxus and Xenopus in either promotion of Smad2/3-mediated (Activin/Nodal) signaling cascades or in inhibition of BMP signaling.
Discussion
Establishment of dorsal/anterior identity by asymmetric localization of Nodal signaling
Our previous work has indicated that maternal Wnt/β-catenin signaling probably does not establish the D/V axis in amphioxus (Holland et al., 2005), as it does in frogs and fish (Kofron et al., 2001; Schier and Talbot, 2005). In fact, amphioxus lacks genes for bozozok, siamois and twin, which mediate the pre-gastrular role of Wnt/β-catenin in establishing the D/V axis in both vertebrates (Schier and Talbot, 2005; Heasman, 2006). Instead, in amphioxus, nuclear β-catenin is localized to all nuclei during the blastula stage (Holland, 2002). Starting at the early gastrula, nuclear β-catenin becomes largely restricted to ectoderm around the blastopore, where it has an evolutionarily conserved role in specification of posterior identity (Onai et al., 2009).
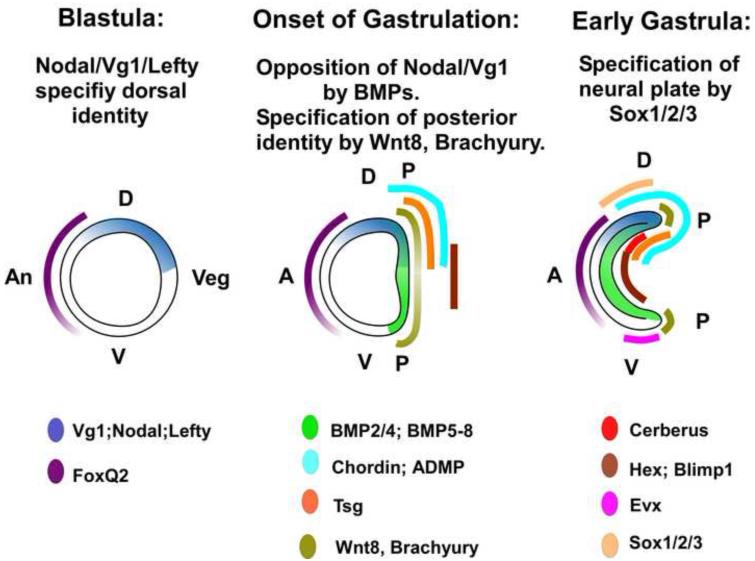
The present results show that dorsal identity in amphioxus is probably initially specified during early cleavage stages by asymmetric localization and co-expression of maternal Nodal and Vg1 mRNAs, modulated from the early-mid blastula stage by zygotic Lefty (Figs. 1A-C, I-M, R-V; 8) (Yu et al., 2002; 2007). Uregulation of Nodal/Vg1 signaling from the blastula stage by addition of Activin protein, presumably mediated by Smad2/3, severely anteriorizes and dorsalizes the embryos to the extent that all of the ectoderm is neural (i.e. Otx and Wnt3 expression is radialized) and the domains of anterior markers such as FoxQ2 are expanded. Conversely, inhibition of the pathway has the opposite effect (e.g. expression of dorsal markers such as Chordin and Sox1/2/3 and of anterior markers such as FoxQ2 is eliminated). The requirement for Nodal/Vg1 signaling for dorsal development of amphioxus embryos gradually decreases until, by the early gastrula, inhibition of Nodal signaling fails to eliminate dorsal structures, although the embryos do not elongate normally and anterior identity is still lost (Fig. 3). Maternal Nodal mRNA may be localized by cytoplasmic movements occurring shortly after fertilization in which large numbers of mitochondria accompany the sperm nucleus as it migrates from the vegetal pole to one side of the animal pole (Holland and Holland, 1992). It has been proposed for other deuterostomes that asymmetry of mitochondria sets up a respiration gradient specifying dorsal/ventral polarity (Coffman, 2009; Coffman and Denegre, 2007; Coffman et al., 2004). In sea urchins, dispersal of a concentration of mitochondria localized on the oral side of the embryo, where Nodal is normally expressed, disrupts oral/aboral polarity. Moreover, quenching mitochondrial H2O2 also inhibits Nodal activation, which is required for specification of oral identity, although increasing H2O2 does not activate Nodal (Coffman et al. 2009). To clarify a possible relationship between mitochondria, Nodal and Vg1 mRNAs in amphioxus it would be useful in the future to determine if Nodal and Vg1 proteins co-localize with their respective mRNAs and with their receptors and how localization of these proteins correlates with localization of mitochondria.
Fig. 8.
Diagram of axial patterning genes in early amphioxus development of amphioxus. In the late blastula, Nodal/Vg1 signaling, presumably opposed by Lefty marks the future dorsal side (D) of the embryo, while FoxQ2 marks the anterior ectoderm. At the onset of gastrulation, dorsal Nodal/Vg1 signaling becomes opposed by BMP signaling in the mesendoderm. BMP signaling is modulated by several dorsally and anteriorly expressed genes. Wnt8 is expressed throughout the future mesendoderm, most strongly in the rim of the forming blastopore, which will be the posterior pole of the embryo. By the early gastrula, additional modulators of Nodal/Vg1 and BMP signaling, Evx, a marker of ventral ectoderm, and the neuroectoderm marker Sox1/2/3 are first expressed. A= anterior pole, An = animal pole, P= posterior pole, V= ventral.
Maternal Nodal/Vg1 may also function in establishing dorsal/anterior identity in vertebrates, although this role has been lost in tunicates, the sister group of vertebrates (Hudson and Yasuo, 2005). In the zebrafish, a major role of the nodal-related genes Squint and Cyclops is in animal/vegetal patterning of the mesoderm (Dougan et al., 2003), although a role in D/V patterning has also been proposed (Harvey and Smith, 2009). Maternal Squint mRNA is localized to blastomeres giving rise to dorsal cells, and its knockdown in oocytes ventralized embryos (Gore et al., 2005). In addition, mutants in both maternal and zygotic Squint have both dorsal and anterior defects (Hagos et al., 2007), although mutants of zygotic Squint and Cyclops do not (Bennett et al., 2007; Pei et al., 2007). If localized maternal Nodal does help specify dorsal identity in zebrafish, it probably acts in parallel with maternal β-catenin, which establishes dorsal identity both in zebrafish and Xenopus (Moon and Kimelman, 1998; Kelly et al., 2000; Schier and Talbot, 2005). While currently there is no evidence for maternal expression of any of the several Nodal genes in Xenopus, mRNAs for both Vg1 and VegT, a T-box transcriptional regulator of Nodal genes, are maternal, and Vg1 is essential for head development (Birsoy et al., 2006; Heasman, 2006). These data, together with those from amphioxus and sea urchins, indicate that a central role for Nodal/Vg1 in dorsal specification was present at the base of the deuterostomes and has been conserved in amphioxus and possibly also in teleosts among the vertebrates.
During the gastrula stage, BMP signaling opposes Nodal/Vg1 signaling to ventral/posteriorize amphioxus embryos
In amphioxus, the role of Nodal/Vg1 signaling during the gastrula stage appears to be in maintenance of dorsal/anterior identity—a direct continuation of its role in specification of dorsal identity at earlier stages. By the onset of gastrulation, mRNAs of BMPs and their modulators such as Chordin are detectable by in situ hybridization (Fig. 8), and our experiments indicate that BMP signaling is a ventralizing and posteriorizing influence opposing Nodal/Vg1 signaling from the gastrula organizer (Fig. 4) (Yu et al., 2007). In vertebrates, Nodal/Vg1 signaling also acts to maintain dorsal/anterior identity in opposition to BMP signaling (Birsoy et al., 2006; Tian and Meng, 2006). Moreover, similar to up- and down-regulation of amphioxus Nodal/Vg1 signaling, overexpression of Xenopus nodal-related (Xnr)-2 in animal caps induces expression of anterior markers such as Cerberus as does injection of Vg1, Xnr1 and Xnr2 into two ventral blastomeres, while injection of a dominant-negative Xnr2 causes anterior deletions (Osada and Wright, 1999; Zorn et al., 1999). In addition, amphioxus embryos treated with human Activin strongly resemble Xenopus embryos in which function of BMP2, BMP4, BMP7 and ADMP, which has BMP activity, has been knocked down; the entire ectoderm is neural, expressing Otx2 in the swollen anterior part and the neural marker Sox2 throughout the ectoderm (Fig. 2) (Reversade and De Robertis, 2005), suggesting that in both, antagonism between Nodal/Vg1 and BMP signaling plays a major role in axial patterning. However, in vertebrates, Nodal genes also have roles in establishment of mesoderm and endoderm (De Robertis and Kuroda, 2004; Heasman, 2006), and suppression of both Nodal and BMP signals is essential for neural induction (Chang and Harland, 2007). As in Xenopus, the lack of Nodal signaling also results in premature neural differentiation in the mouse (Camus et al., 2006). In fact, in the mouse, BMP is expressed before gastrulation, maintaining Nodal signaling and preventing premature neural differentiation (Di-Gregorio et al., 2007). Roles for Nodal in mesoderm induction are unlikely in amphioxus as it is expressed in both presumptive mesoderm and neuroectoderm at the blastula stage. Thus, although roles for opposing Nodal/Vg1 and BMP signaling in axial patterning are evidently a conserved feature of chordates, Nodal/Vg1 signaling may have acquired additional ones in vertebrates.
Nodal/Vg1 and BMP signaling is modulated by dorsal/anteriorly expressed antagonists in amphioxus and vertebrates
Several potential modulators and regulators of Nodal/Vg1 and BMP signaling have localized expression in the amphioxus gastrula. In addition to Cerberus, Chordin and Blimp1, they include the BMP antagonists Bambi, Tsg and Tob, the nodal antagonist Lefty, as well as Tolloid-like, which encodes a metalloprotease that may degrade Chordin (Fig. 8) (Holland et al., 1997; Yu et al., 2007). Most of these genes are expressed in comparable patterns as their vertebrate homologs suggesting that regulation of Nodal/Vg1 and BMP signaling is conserved in amphioxus and vertebrates. Our experiments with Blimp1, Cerberus and Chordin are consistent with this idea.
In Xenopus, as in amphioxus, Blimp1 is required for development of the head and anterior endomesoderm (de Souza et al., 1999). However, although Xenopus Blimp1 positively regulates Cerberus, the same is not likely to be true in amphioxus as the domains of Blimp1 and Cerberus do not appear to overlap (Fig. 1A’-H’) and Blimp1 knockdown in amphioxus, like Cerberus overexpression, eliminates FoxQ2 expression (Fig. 6A, E, I, M). Consistent with these data, amphioxus Blimp1 activates transcription from a Nodal-responsive promoter (XMix2) in Xenopus animal cap assays (Fig. 7H) indicating that it can potentiate Nodal signaling. Since neither Nodal nor Vg1 is co-expressed with Blimp1, this effect is likely to be indirect. In the zebrafish, unlike Xenopus and amphioxus, overexpression of Blimp1 inhibits dorsal/anterior structures and reduces Chordin expression (Wilm and Solnica-Krezel, 2005). Thus, Blimp1 function in head formation may be conserved to some extent between amphioxus and Xenopus, but appears to have been modified in teleosts. Interestingly, Blimp1 is expressed in the invaginating mesendoderm not only in amphioxus, but also in sea urchins, where it activates Wnt8 expression and represses itself, ultimately causing downregulation of Wnt8 (reviewed in Smith et al., 2007). The same could be true of amphioxus since Wnt8 is initially co-expressed with Blimp1 in the invaginating mesendoderm but is soon down-regulated except immediately around the blastopore (Schubert et al., 2000; Yasui et al., 2001).
Expression of Cerberus in the anterior mesoderm of amphioxus, the anterior endomesoderm in Xenopus and the anterior visceral endoderm in the mouse is comparable (Fig. 1A’-C’) (Bouwmeester et al., 1996; Perea-Gomez et al., 2002). Moreover, both amphioxus Cerberus and Xenopus Cerberus induce respectively a complete secondary axis or secondary head when ventro-vegetally expressed in frog embryos (Bouwmeester et al., 1996; Piccolo et al., 1999) (Fig. 7B). Although experiments in Xenopus animal caps indicate that amphioxus Cerberus, like its vertebrate counterparts is multifunctional, overexpression in vivo suggests that during gastrulation in amphioxus, the function of Cerberus as a suppressor of Nodal/Vg1 signaling dominates its function as a BMP suppressor (Fig. 6). Later in development, like Cerl-2 expression in the mouse node (Marques et al., 2004), and Kupffer’s vesicle in the zebrafish (Hashimoto et al., 2004), expression of amphioxus Cerberus shifts to the right (Fig. 1C’,D’), where it presumably suppresses Nodal/Vg1 signals from the left (Yu et al., 2002; 2007).
Unlike Cerberus, Chordin is not multifunctional. It is only known to antagonize signaling by vertebrate BMP2, 4 and 7 (Gazzerro and Canalis, 2006). Compared to overexpressing BMP2/4, knockdown of Chordin function in amphioxus gives a relatively mild phenotype which could be due to residual Chordin translation or redundancy with another BMP antagonist, or because Chordin only antagonizes BMP2/4 and not BMP5-8, which is co-expressed with BMP2/4 (Yu et al., 2007). The emerging picture is that opposing BMP and Nodal/Vg1 signals are major players in head induction. Signaling by these pathways is modulated by secreted antagonists, most of which are expressed anteriorly and dorsally. Although these interactions are doubtlessly complex, the amphioxus embryo offers an excellent system for unraveling them because of its structural simplicity and comparative lack of genetic redundancy.
The evolution of axial patterning in chordates
The model we propose for head specification in amphioxus (and, by extension, in the common chordate ancestor of amphioxus and vertebrates) involves evolutionarily ancient roles of BMP2/4 and Wnt/β-catenin signaling plus the more recent ones for Nodal/Vg1 signaling. In cnidarians and the two major groups of bilaterians, BMPs and their antagonists are expressed asymmetrically (Matus et al., 2006a; Rentzsch et al., 2006), while Wnt genes are expressed around the blastopore (reviewed in Holland, 2002; Tanaka and Weidinger, 2008). Asymmetrical expression of these genes led to the suggestion that the cnidarian ancestor had both A/P and D/V polarity, although the latter is not mediated by BMPs (Matus et al., 2006a,b). In contrast, it is well documented that opposing BMP and Chordin signals mediate D/V patterning in both protostomes and deuterostomes (De Robertis, 2008; Mizutani and Bier, 2008) while at least in most deuterostomes, Wnt/β-catenin signaling specifies posterior identity.
Roles for Nodal, Vg1 and Lefty in axial patterning evolved at the base of the deuterostomes or possibly at the base of the Bilateria. Cnidarians lack these genes, although Nematostella has genes related to inhibin/activin (Miyazawa et al., 2002; Herpin et al., 2004). In protostomes, Nodal signaling also functions in left-right patterning, but apparently not in dorsal/ventral patterning (Grande and Patel. 2009). However, among the invertebrate deuterostomes, Nodal and Vg1 mediate D/V patterning in sea urchins, as they do in amphioxus and vertebrates. In both sea urchins and amphioxus, the Vg1, Nodal and Lefty domains overlap with one another (Fig. 8) (Duboc et al., 2008; 2010), and with that of BMP2/4. There are two minor differences. The first is that in the sea urchin, the expression domain of BMP2/4 is restricted to the oral (ventral) side, although the protein is localized to the aboral side (Duboc et al., 2010), but initially includes the entire embryo in amphioxus, being downregulated dorsally at the onset of neurulation. The second is that Nodal is expressed on the oral (ventral) side in sea urchins but dorsally in amphioxus, probably indicative of a D/V inversion at the base of the chordates. In sea urchin embryos, univin/Vg1, like amphioxus Vg1, is maternally expressed and is required for Nodal expression (Flowers et al., 2004; Duboc and Lepage, 2008). Moreover, like amphioxus embryos, those of sea urchins are radialized and ventralized by human Activin B (Flowers et al., 2004). In addition, antagonism between Nodal and BMP2/4 patterns the sea urchin embryonic D/V axis, while Nodal overexpression transforms the entire ectoderm into oral (ventral) ectoderm (Duboc et al., 2004). This is comparable to conversion of the entire amphioxus ectoderm to neuroectoderm by human Activin. Therefore, we propose that the fundamental mechanism mediating D/V polarity in deuterostomes involves two steps—first maternal Nodal/Vg1 establishes the ventral (oral) side of the sea urchin embryo or the dorsal side of the amphioxus embryo and then, opposition of Nodal/Vg1 signaling by BMPs specifies the opposite side of the embryo, thus establishing the gastrula D/V axis.
Although the focus on D/V patterning of bilaterian embryos has been on the suppression of BMP signaling by Chordin, our results indicate that antagonism between Nodal/Vg1 and BMP signaling is perhaps of more fundamental importance. In sea urchins, BMP/Nodal antagonism is required for maintenance of the aboral-oral (D/V) axis (Christiaen et al., 2007; Duboc et al., 2010), but there are conflicting reports as to whether manipulating Chordin levels affects D/V patterning. Although both Bradham et al. (2009) and Lapraz et al. (2009), report that chordin opposes BMP2/4 signaling in sea urchins, the former found no effect of altered chordin levels on D/V patterning, while the latter found that overexpression of Chordin mRNA strongly inhibited dorsal development in 30% of the embryos while the remaining 70% lacked expression of Tbx2/3 on the dorsal side. Additional experiments are needed to clarify the discrepancy.
In hemichordate embryos, which have little or no CNS and are the sister group of echinoderms, BMP2/4 and Chordin are expressed on opposite sides of the embryo (Lowe et al., 2006), but it is not known if antagonism between Nodal and BMPs is involved in D/V patterning. If Nodal expression is co-localized with Chordin, it could indicate that coupling of opposing Nodal and BMP signals to partition the ectoderm into neural and non-neural territories evolved at the base of the chordates. This would add fuel to the argument concerning whether or not the CNS of protostomes and chordates evolved independently (Holland, 2003; De Robertis, 2008).
The emerging model for axial patterning is that in the deuterostome ancestor, Nodal/Vg1 signaling specified dorsal identity, and opposition between Nodal/Vg1 and BMP signaling was fundamental to both D/V and A/P patterning. In chordates, head formation requires modulation of these signaling pathways by antagonists that are secreted both anteriorly and dorsally. Thus a fundamental mechanism for D/V and A/P patterning with opposing gradients of BMP and Nodal/Vg1 signaling along both the D/V and A/P axes and of Wnt/β-catenin along the A/P axis was probably present in the embryo of the ancestral chordate (Fig. 8) and has been modified to a greater or lesser extent in various chordate lineages.
Supplementary Material
Acknowledgments
We thank John Lawrence and Susan Bell for space at the University of South Florida, Davin Setiamarga for the phylogenetic analysis in Fig. S1 and Yoshiki Sasai for the xMix2 and xVent reporter plasmids. Trevor Hoffman and Thomas Schilling for pCS2+tdTomato.This research was supported by NSF IOB0416292 to LZH and ND Holland, NSF IOS0743485 to LZH and NIH GM075018 to KWC and IB. TO received fellowships from the Uehara Memorial Foundation, JSPS Postdoctoral fellowship for research abroad, Toyobo Long Term Fellowship, and Mochida Medical Memorial Foundation. JKY received funding from Institute of Cellular and Organismic Biology and a Career Development Award from Academia Sinica, Taiwan.
Footnotes
Appendix A. Supplementary data Supplementary data associated with this article can be found in the online version at doi:xxxxxxxxxxxx
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agius E, Oelgeschlager M, Wessely O, Kemp C, De Robertis EM. Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development. 2000;127:1173–1183. doi: 10.1242/dev.127.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JT, Stickney HL, Choi W-Y, Ciruna B, Talbot WS, Schier AF. Maternal nodal and zebrafish embryogenesis. Nature. 2007;450:E1–E2. doi: 10.1038/nature06314. [DOI] [PubMed] [Google Scholar]
- Birsoy B, Kofron M, Schaible K, Wylie C, Heasman J. Vg1 is an essential signaling molecule in Xenopus development. Development. 2006;133:15–20. doi: 10.1242/dev.02144. [DOI] [PubMed] [Google Scholar]
- Bouwmeester T, Kim S-H, Sasai Y, Lu B, De Robertis EM. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann’s organizer. Nature. 1996;382:595–601. doi: 10.1038/382595a0. [DOI] [PubMed] [Google Scholar]
- Bradham CA, Oikonomou C, Kühn A, Core AB, Modell JW, McClay DR, Poustka AJ. Chordin is required for neural but not axial development in sea urchin embryos. Dev. Biol. 2009;15:221–233. doi: 10.1016/j.ydbio.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camus A, Perea-Gomez A, Moreau A, Collignon J. Absence of Nodal signaling promotes precocious neural differentiation in the mouse embryo. Dev. Biol. 2006;295:743–755. doi: 10.1016/j.ydbio.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Candia AF, Watabe T, Hawley SH, Onichtchouk D, Zhang Y, Derynck R, Niehrs C, Cho KW. Cellular interpretation of multiple TGF-beta signals: intracellular antagonism between activin/BVg1 and BMP-2/4 signaling mediated by Smads. Development. 1997;124:4467–4480. doi: 10.1242/dev.124.22.4467. [DOI] [PubMed] [Google Scholar]
- Chang C, Harland RM. Neural induction requires continued suppression of both Smad1 and Smad2 signals during gastrulation. Development. 2007;134:3861–3872. doi: 10.1242/dev.007179. [DOI] [PubMed] [Google Scholar]
- Christiaen L, Jaszczyszyn Y, Kerfant M, Kano S, Thermes V, Joly JS. Evolutionary modification of mouth position in deuterostomes. Semin. Cell Dev. Biol. 2007;18:502–511. doi: 10.1016/j.semcdb.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Coffman JA. Mitochondria and metazoan epigenesis. Semin. Cell Dev. Biol. 2009;20:21–29. doi: 10.1016/j.semcdb.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman JA, Denegre Mitochondria, redox signaling and axis specification in metazoan embryos. Dev. Biol. 2007;308:266–280. doi: 10.1016/j.ydbio.2007.05.042. [DOI] [PubMed] [Google Scholar]
- Coffman JA, McCarthy JJ, Dickey-Sims C, Robertson AJ. Oral-aboral axis specification in the sea urchin embryo: II. Mitochondrial distribution and redox state contribute to establishing polarity in Strongylocentrotus purpuratus. Dev. Biol. 2004;273:160–171. doi: 10.1016/j.ydbio.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Coffman JA, Coluccio A, Planchart A, Robertson AJ. Oral-aboral axis specification in the sea urchin embryo: III. Role of mitochondrial redox signaling via H2O2. Dev. Biol. 2009;330:123–130. doi: 10.1016/j.ydbio.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byfield S. DaCosta, Major C, Laping NJ, Roberts AB. SB-505124 Is a selective inhibitor of transforming growth factor-beta Type I receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2004;65:744–752. doi: 10.1124/mol.65.3.744. [DOI] [PubMed] [Google Scholar]
- Dale L, Howes G, Price BM, Smith JC. Bone morphogenetic protein 4: a ventralizing factor in early Xenopus development. Development. 1992;115:573–585. doi: 10.1242/dev.115.2.573. [DOI] [PubMed] [Google Scholar]
- De Robertis EM. Evo-devo: Variations on ancestral themes. Cell. 2008;132:185–195. doi: 10.1016/j.cell.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Ann. Rev. Cell Dev. Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza FSJ, Gawantka V, Gomez A. Perea, Delius H, Ang S-L, Niehrs C. The zinc finger gene Xblimp1 controls anterior endomesodermal cell fate in Spemann’s organizer. EMBO J. 1999;18:6062–6072. doi: 10.1093/emboj/18.21.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di-Gregorio A, Sancho M, Stuckey DW, Crompton LA, Godwin J, Mishina Y, Rodriguez TA. BMP signalling inhibits premature neural differentiation in the mouseembryo. Development. 2007;134:3359–3369. doi: 10.1242/dev.005967. [DOI] [PubMed] [Google Scholar]
- Dougan ST, Warga RM, Kane DA, Schier AF, Talbot WS. The role of the zebrafish nodal-related genes squint and cyclops in patterning of mesendoderm. Development. 2003;130:1837–1851. doi: 10.1242/dev.00400. [DOI] [PubMed] [Google Scholar]
- Duboc V, Lepage T. A conserved role for the nodal signaling pathway in the establishment of dorso-ventral and left-right axes in deuterostomes. J. Exp. Zool. B Mol. Dev. Evol. 2008;310:41–53. doi: 10.1002/jez.b.21121. [DOI] [PubMed] [Google Scholar]
- Duboc V, Rottinger E, Besnardeau L, Lepage T. Nodal and BMP2/4 signaling organizes the oral-aboral axis of the sea urchin embryo. Dev. Cell. 2004;6:397–410. doi: 10.1016/s1534-5807(04)00056-5. [DOI] [PubMed] [Google Scholar]
- Duboc V, Lapraz F, Besnardeau L, Lepage T. Lefty acts as an essential modulator of Nodal activity during sea urchin oral-aboral axis formation. Dev. Biol. 2008;320:49–59. doi: 10.1016/j.ydbio.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Duboc V, Lapraz F, Saudemont A, Bessodes N, Mekpoh F, Haillot E, Quirin M, Lepage T. Nodal and BMP2/4 pattern the mesoderm and endoderm during development of the sea urchin embryo. Development. 2010;137:223–235. doi: 10.1242/dev.042531. [DOI] [PubMed] [Google Scholar]
- Feng XH, Derynck R. Specificity and versatility in TGF-beta signaling through Smads. Annu. Rev. Cell Dev. Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- Flowers VL, Courteau GR, Poustka AJ, Weng W, Venuti JM. Nodal/Activin signaling establishes oral-aboral polarity in the early sea urchin embryo. Dev. Dynam. 2004;231:727–740. doi: 10.1002/dvdy.20194. [DOI] [PubMed] [Google Scholar]
- Gazzerro E, Canalis E. Bone morphogenetic proteins and their antagonists. Rev. Endocrine, Metabol. Disorders. 2006;7:51–65. doi: 10.1007/s11154-006-9000-6. [DOI] [PubMed] [Google Scholar]
- Gore AV, Maegawa S, Cheong A, Gilligan PC, Weinberg ES, Sampath K. The zebrafish dorsal axis is apparent at the four-cell stage. Nature. 2005;438:1030–1035. doi: 10.1038/nature04184. [DOI] [PubMed] [Google Scholar]
- Grande C, Patel NH. Nodal signalling is involved in left-right asymmetry in snails. Nature. 2009;457:1007–1011. doi: 10.1038/nature07603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagos EG, Fan X, Dougan ST. The role of maternal Activin-like signals in zebrafish embryos. Dev. Biol. 2007;309:245–258. doi: 10.1016/j.ydbio.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Haramoto Y, Takahashi S, Asashima M. Two distinct domains in pro-region of Nodal-related 3 are essential for BMP inhibition. Biochem. Biophys. Res. Com. 2006;346:470–478. doi: 10.1016/j.bbrc.2006.05.121. [DOI] [PubMed] [Google Scholar]
- Haramoto Y, Tanegashima K, Onuma Y, Takahashi S, Sekizaki H, Asashima M. Xenopus tropicalis nodal-related gene 3 regulates BMP signaling: an essential role for the pro-region. Dev. Biol. 2004;265:155–168. doi: 10.1016/j.ydbio.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Rebagliati M, Ahmad N, Muraoka O, Kurokawa T, Hibi M, Suzuki T. The Cerberus/Dan-family protein charon is a negative regulator of nodal signaling during left-right patterning in zebrafish. Development. 2004;131:1741–1753. doi: 10.1242/dev.01070. [DOI] [PubMed] [Google Scholar]
- Harvey SA, Smith JC. Visualisation and quantification of morphogen gradient formation in the zebrafish. PLoS Biol. 2009;7:e1000101. doi: 10.1371/journal.pbio.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman J. Patterning the early Xenopus embryo. Development. 2006;133:1205–1217. doi: 10.1242/dev.02304. [DOI] [PubMed] [Google Scholar]
- Herpin A, Lelong C, Favrel P. Transforming growth factor-beta-related proteins: an ancestral and widespread superfamily of cytokines in metazoans. Dev. Comp. Immunol. 2004;28:461–485. doi: 10.1016/j.dci.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Holland LZ. Heads or tails? Amphioxus and the evolution of anterior-posterior patterning in deuterostomes. Dev. Biol. 2002;241:209–228. doi: 10.1006/dbio.2001.0503. [DOI] [PubMed] [Google Scholar]
- Holland LZ, Holland ND. Early development in the lancelet (=amphioxus) Branchiostoma floridae from sperm entry through pronuclear fusion: presence of vegetal pole plasm and lack of conspicuous ooplasmic segregation. Biol. Bull. 1992;182:77–96. doi: 10.2307/1542182. [DOI] [PubMed] [Google Scholar]
- Holland LZ, Yu JK. Cephalochordate (amphioxus) embryos: procurement, culture, basic methods. Meth. Cell Biol. 2004;74:195–215. doi: 10.1016/s0091-679x(04)74009-1. [DOI] [PubMed] [Google Scholar]
- Holland LZ, Holland ND. A revised fate map for amphioxus and the evolution of axial patterning in chordates. Integrative Comp. Biol. 2007;47:360–372. doi: 10.1093/icb/icm064. [DOI] [PubMed] [Google Scholar]
- Holland LZ, Holland PWH, Holland ND. Revealing homologies between body parts of distantly related animals by in situ hybridization to developmental genes: amphioxus versus vertebrates. In: Ferraris JD, Palumbi SR, editors. Molecular Zoology: Advances, Strategies, and Protocols. Wiley; New York: 1996. pp. 267–282. [Google Scholar]
- Holland LZ, Panfilio KA, Chastain R, Schubert M, Holland ND. Nuclear β-catenin promotes non-neural ectoderm and posterior cell fates in amphioxus embryos. Dev. Dynam. 2005;233:1430–1443. doi: 10.1002/dvdy.20473. [DOI] [PubMed] [Google Scholar]
- Holland ND. Early central nervous system evolution: an era of skin brains? Nature Rev. Neurosci. 2003;4:617–627. doi: 10.1038/nrn1175. [DOI] [PubMed] [Google Scholar]
- Holland ND, Holland LZ. The fine structure of the growth stage oocytes of a lancelet (= amphioxus), Branchiostoma lanceolatum. Invert. Rep. Dev. 1991;19:107–122. [Google Scholar]
- Holland ND, Panganiban G, Henyey EL, Holland LZ. Sequence and developmental expression of AmphiDll, an amphioxus Distal-less gene transcribed in the ectoderm, epidermis and nervous system: insights into evolution of craniate forebrain and neural crest. Development. 1996;122:2911–2920. doi: 10.1242/dev.122.9.2911. [DOI] [PubMed] [Google Scholar]
- Holland ND, Zhang S-C, Clark M, Panopoulou G, Lehrach H, Holland LZ. Sequence and developmental expression of AmphiTob, an amphioxus homolog of vertebrate Tob in the PC3/BTG1/Tob family of tumor suppressor genes. Dev. Dynam. 1997;210:11–18. doi: 10.1002/(SICI)1097-0177(199709)210:1<11::AID-AJA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hoodless PA, Tsukazaki T, Nishimatsu S.-i., Attisano L, Wrana JL, Thomsen GH. Dominant-negative Smad2 mutants inhibit Activin/Vg1 signaling and disrupt axis formation in Xenopus. Dev. Biol. 1999;207:364–379. doi: 10.1006/dbio.1998.9168. [DOI] [PubMed] [Google Scholar]
- Huang HC, Murtaugh LC, Vize PD, Whitman M. Identification of a potential regulator of early transcriptional responses to mesoderm inducers in the frog embryo. EMBO J. 1995;14:5965–5973. doi: 10.1002/j.1460-2075.1995.tb00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson C, Yasuo H. Patterning across the ascidian neural plate by lateral Nodal signalling sources. Development. 2005;132:1199–1210. doi: 10.1242/dev.01688. [DOI] [PubMed] [Google Scholar]
- John SA, Garrett-Sinha LA. Blimp1: A conserved transcriptional repressor critical for differentiation of many tissues. Exp. Cell Res. 2009;315:1077–1084. doi: 10.1016/j.yexcr.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Jones CM, Lyons KM, Lapan PM, Wright CV, Hogan BL. DVR-4 (bone morphogenetic protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development. 1992;115:639–647. doi: 10.1242/dev.115.2.639. [DOI] [PubMed] [Google Scholar]
- Kelly C, Chin A, Leatherman J, Kozlowski D, Weinberg E. Maternally controlled β-catenin-mediated signaling is required for organizer formation in the zebrafish. Development. 2000;127:3899–3911. doi: 10.1242/dev.127.18.3899. [DOI] [PubMed] [Google Scholar]
- Kofron M, Klein P, Zhang F, Houston DW, Schaible K, Wylie C, Heasman J. The role of maternal axin in patterning the Xenopus embryo. Dev. Biol. 2001;237:183–201. doi: 10.1006/dbio.2001.0371. [DOI] [PubMed] [Google Scholar]
- Lagna G, Hemmati-Brivanlou A. A molecular basis for Smad specificity. Dev. Dynam. 1999;214:269–277. doi: 10.1002/(SICI)1097-0177(199903)214:3<269::AID-AJA10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Laurent MN, Blitz IL, Hashimoto C, Rothbächer U, Cho KW. The Xenopus homeobox gene twin mediates Wnt induction of goosecoid in establishment of Spemann’s organizer. Development. 1997;124:4905–4916. doi: 10.1242/dev.124.23.4905. [DOI] [PubMed] [Google Scholar]
- Lowe CJ, Terasaki M, Wu M, Freeman RM, Runft L, Kwan K, Haigo S, Aronowicz J, Lander E, Gruber C, Smith M, Kirschner M, Gerhart J. Dorsoventral patterning in hemichordates: insights into early chordate evolution. PLoS Biol. 2006;4:e291. doi: 10.1371/journal.pbio.0040291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marikawa Y. Wnt/β-catenin signaling and body plan formation in mouse embryos. Sem. Cell, Dev. Biol. 2006;17:175–184. doi: 10.1016/j.semcdb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Marques S, Borges AC, Silva AC, Freitas S, Cordenonsi M, Belo JA. The activity of the nodal antagonist Cerl-2 in the mouse node is required for correct L/R body axis. Genes Dev. 2004;18:2342–2347. doi: 10.1101/gad.306504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus D, Pang K, Marlow H, Dunn C, Thomsen G, Martindale M. Molecular evidence for deep evolutionary roots of bilaterality in animal development. Proc. Natl. Acad. Sci. USA. 2006a;103:11195–11200. doi: 10.1073/pnas.0601257103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus DQ, Thomsen GH, Martindale MQ. Dorso/ventral genes are asymmetrically expressed and involved in germ-layer demarcation during cnidarian gastrulation. Curr. Biol. 2006b;16:499–505. doi: 10.1016/j.cub.2006.01.052. [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Shinozaki M, Hara T, Furuya T, Miyazono K. Two major Smad pathways in TGF-beta superfamily signalling. Genes to Cells. 2002;7:1191–1204. doi: 10.1046/j.1365-2443.2002.00599.x. [DOI] [PubMed] [Google Scholar]
- Mizutani CM, Bier E. EvoD/Vo: the origins of BMP signalling in the neuroectoderm. Nature Rev. Genet. 2008;9:663–677. doi: 10.1038/nrg2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RT, Kimelman D. From cortical rotation to organizer gene expression: toward a molecular explanation of axis specification in Xenopus. BioEssays. 1998;20:536–546. doi: 10.1002/(SICI)1521-1878(199807)20:7<536::AID-BIES4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Tables of Xenopus larvis. North-Holland, Amsterdam: 1956. [Google Scholar]
- Onai T, Lin H.-cit., Schubert M, Koop D, Osborne PW, Alvarez S, Alvarez R, Holland ND, Holland LZ. Retinoic acid and Wnt/β-catenin have complementary roles in anterior/posterior patterning embryos of the basal chordate amphioxus. Dev. Biol. 2009;332:223–233. doi: 10.1016/j.ydbio.2009.05.571. [DOI] [PubMed] [Google Scholar]
- Osada S, Wright C. Xenopus nodal-related signaling is essential for mesendodermal patterning during early embryogenesis. Development. 1999;126:3229–3240. doi: 10.1242/dev.126.14.3229. [DOI] [PubMed] [Google Scholar]
- Pei W, Williams PH, Clark MD, Stemple DL, Feldman B. Environmental and genetic modifiers of squint penetrance during zebrafish embryogenesis. Dev. Biol. 2007;308:368–378. doi: 10.1016/j.ydbio.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea-Gomez A, Vella FDJ, Shawlot W, Oulad-Abdeighani M, Chazaud C, Meno C, Pfister V, Chen L, Robertson EJ, Hamada H, et al. Nodal antagonists in the anterior rat endoderm prevent the formation of multiple primitive streaks. Dev. Cell. 2002;3:745–756. doi: 10.1016/s1534-5807(02)00321-0. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, De Robertis EM. The head inducer cerberus is a multifunctional antagonist of nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramis JM, Collart C, Smith JC. Xnrs and activin regulate distinct genes during Xenopus development: activin regulates cell division. PLoS One. 2007;2:e213. doi: 10.1371/journal.pone.0000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzsch F, Anton R, Saina M, Hammerschmidt M, Holstein TW, Technau U. Asymmetric expression of the BMP antagonists chordin and gremlin in the sea anemone Nematostella vectensis: Implications for the evolution of axial patterning. Dev. Biol. 2006;296:375–387. doi: 10.1016/j.ydbio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Reissmann E, Jornvall H, Blokzijl A, Andersson O, Chang C, Minchiotti G, Persico MG, Ibanez CF, Brivanlou AH. The orphan receptor ALK7 and the Activin receptor ALK4 mediate signaling by Nodal proteins during vertebrate development. Genes Dev. 2001;15:2010–2022. doi: 10.1101/gad.201801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reversade B, De Robertis EM. Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell. 2005;123:1147–1160. doi: 10.1016/j.cell.2005.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier AF, Talbot WS. Molecular genetics of axis formation in zebrafish. Ann. Rev. Genet. 2005;39:561–613. doi: 10.1146/annurev.genet.37.110801.143752. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Patterson M, Farrel E, Münsterberg A. Dynamic expression of Lef/Tcf family members and β-catenin during chick gastrulation, neurulation, and early limb development. Dev. Dynam. 2004;229:703–707. doi: 10.1002/dvdy.20010. [DOI] [PubMed] [Google Scholar]
- Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nature Rev. Mol. Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- Schneider S, Steinbeisser H, Warga RM, Hausen P. Beta-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech Dev. 1996;57:191–8. doi: 10.1016/0925-4773(96)00546-1. [DOI] [PubMed] [Google Scholar]
- Schubert M, Holland LZ, Panopoulou GD, Lehrach H, Holland ND. Characterization of amphioxus AmphiWnt8: insights into the evolution of patterning of the embryonic dorsoventral axis. Evol. Dev. 2000;2:85–92. doi: 10.1046/j.1525-142x.2000.00047.x. [DOI] [PubMed] [Google Scholar]
- Schubert M, Holland LZ, Stokes MD, Holland ND. Three amphioxus Wnt genes (AmphiWnt3, AmphiWnt5, and AmphiWnt6) associated with the tail bud: the evolution of somitogenesis in chordates. Dev. Biol. 2001;240:262–273. doi: 10.1006/dbio.2001.0460. [DOI] [PubMed] [Google Scholar]
- Schubert M, Holland ND, Laudet V, Holland LZ. A retinoic acid-Hox hierarchy controls both anterior/posterior patterning and neuronal specification in the developing central nervous system of the cephalochordate amphioxus. Dev. Biol. 2006;296:190–202. doi: 10.1016/j.ydbio.2006.04.457. [DOI] [PubMed] [Google Scholar]
- Shaner NC, Lin MZ, Mckeown MR, Steinbach PA, Hazelwood KL, Davidson MW, Tsien RY. Improving the photostability of bright monomeric orange red fluorescent proteins. Nat. Methods. 2008;5:545–551. doi: 10.1038/nmeth.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skromne I, Stern CD. Interactions between Wnt and Vg1 signalling pathways initiate primitive streak formation in the chick embryo. Development. 2001;128:2915–2927. doi: 10.1242/dev.128.15.2915. [DOI] [PubMed] [Google Scholar]
- Smith J, Theodoris C, Davidson EH. A gene regulatory network subcircuit drives a dynamic pattern of gene expression. Science. 2007;318:794–797. doi: 10.1126/science.1146524. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Yokota C, Takano K, Tanegashima K, Onuma Y, Goto J, Asashima M. Two novel nodal-related genes initiate early inductive events in Xenopus Nieuwkoop center. Development. 2000;127:5319–5329. doi: 10.1242/dev.127.24.5319. [DOI] [PubMed] [Google Scholar]
- Tanaka EM, Weidinger G. Heads or tails: can Wnt tell which one is up? Nature Cell Biol. 2008;10:122–124. doi: 10.1038/ncb0208-122. [DOI] [PubMed] [Google Scholar]
- Tian T, Meng A. Nodal signals pattern vertebrate embryos. Cell. Mol. Life Sci. 2006;63:672–685. doi: 10.1007/s00018-005-5503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlet I, Collignon J, Robertson EJ. Nodal expression in the primitive endoderm is required for specification of the anterior axis during mouse gastrulation. Development. 1997;124:1033–1044. doi: 10.1242/dev.124.5.1033. [DOI] [PubMed] [Google Scholar]
- Watabe T, Kim S, Candia A, Rothbächer U, Hashimoto C, Inoue K, Cho KW. Molecular mechanisms of Spemann’s organizer formation: conserved growth factor synergy between Xenopus and mouse. Genes Dev. 1995;9:3038–3050. doi: 10.1101/gad.9.24.3038. [DOI] [PubMed] [Google Scholar]
- White JA, Heasman J. Maternal control of pattern formation in Xenopus laevis. J. Exp. Zool. 2008;310B:73–84. doi: 10.1002/jez.b.21153. [DOI] [PubMed] [Google Scholar]
- Wilm TP, Solnica-Krezel L. Essential roles of a zebrafish prdm1/blimnp1 homolog in embryo patterning and organogenesis. Development. 2005;132:393–404. doi: 10.1242/dev.01572. [DOI] [PubMed] [Google Scholar]
- Wilson EB. Amphioxus, and the mosaic theory of development. J. Morphol. 1893;8:579–639. + pl XXIX-XXXVIII. [Google Scholar]
- Wylie C, Kofron M, Payne C, Anderson R, Hosobuchi M, Joseph E, Heasman J. Maternal β-catenin establishes a ‘dorsal signal’ in early Xenopus embryos. Development. 1996;122:2987–2996. doi: 10.1242/dev.122.10.2987. [DOI] [PubMed] [Google Scholar]
- Yasui K, Saiga H, Wang Y, Zhang PJ, Semba I. Early expressed genes showing a dichotomous developing pattern in the lancelet embryo. Dev. Growth Differ. 2001;43:185–194. doi: 10.1046/j.1440-169x.2001.00566.x. [DOI] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- Yu J-K, Holland LZ, Holland ND. An amphioxus nodal gene (AmphiNodal) with early symmetrical expression in the organizer and mesoderm and later asymmetrical expression associated with left-right axis formation. Evol. Dev. 2002;4:418–425. doi: 10.1046/j.1525-142x.2002.02030.x. [DOI] [PubMed] [Google Scholar]
- Yu J-K, Holland ND, Holland LZ. AmphiFoxQ2, a novel winged helix/forkhead gene, exclusively marks the anterior end of the amphioxus embryos. Dev. Genes Evol. 2003;213:102–105. doi: 10.1007/s00427-003-0302-3. [DOI] [PubMed] [Google Scholar]
- Yu J-K, Satou Y, Holland ND, Shin-I T, Kohara Y, Satoh N, Bronner-Fraser M, Holland LZ. Axial patterning in cephalochordates and the evolution of the organizer. Nature. 2007;445:613–617. doi: 10.1038/nature05472. [DOI] [PubMed] [Google Scholar]
- Yu J-K, Wang MC, Shin-I T, Kohara Y, Holland LZ, Satoh N, Satou Y. A cDNA resource for the cephalochordate amphioxus Branchiostoma floridae. Dev. Genes Evol. 2008;218:723–727. doi: 10.1007/s00427-008-0228-x. [DOI] [PubMed] [Google Scholar]
- Yu X, He F, Zhang T, Espinoza-Lewis RA, Lin L, Yang J, Chen Y. Cerberus functions as a BMP agonist to synergistically induce nodal expression during left-right axis determination in the chick embryo. Dev. Dynam. 2008;237:3613–3623. doi: 10.1002/dvdy.21769. [DOI] [PubMed] [Google Scholar]
- Zhang SC, Holland ND, Holland LZ. Topographic changes in nascent and early mesoderm in amphioxus embryos studied by DiI labeling and by in situ hybridization for a Brachyury gene. Dev. Genes Evol. 1997;206:532–535. doi: 10.1007/s004270050083. [DOI] [PubMed] [Google Scholar]
- Zorn AM, Butler K, Gurdon JB. Anterior endomesoderm specification in Xenopus by Wnt/β-catenin and TGF-β signalling pathways. Dev. Biol. 1999;209:282–297. doi: 10.1006/dbio.1999.9257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.