Abstract
Alterations in executive control and cognitive flexibility, such as attentional set-shifting abilities, are core features of several neuropsychiatric diseases. The most widely used neuropsychological tests for the evaluation of attentional set-shifting in human subjects are the Wisconsin Card Sorting Test (WCST) and the CANTAB Intra-/Extra-dimensional set shift task (ID/ED). These tasks have proven clinical relevance and have been modified and successfully adapted for research in animal models. However, currently available tasks for rodents present several limitations, mainly due to their manual-based testing procedures, which are hampering translational advances in psychiatric medicine. To overcome these limitations and to better mimic the original version in primates, we present the development of a novel operant-based two-chamber ID/ED "Operon" task for rodents. We demonstrated the effectiveness of this novel task to measure different facets of cognitive flexibility in mice including attentional set formation and shifting, and reversal learning. Moreover, we show the high flexibility of this task in which three different perceptual dimensions can be manipulated with a high number of stimuli cues for each dimension. This novel ID/ED Operon task can be an effective preclinical tool for drug testing and/or large genetic screening relevant to the study of executive dysfunction and cognitive symptoms found in psychiatric disorders.
Keywords: Behavior, Issue 107, Executive functions, attentional set-shifting, reversal learning, attentional control, translational medicine, automated tasks, behavior, cognition, flexibility
Introduction
Attentional set-shifting is a measure of cognitive flexibility and executive functions1. It refers to the ability to switch between arbitrary internal rules ("cognitive-attentional sets"). The most widely used neuropsychological tasks for measuring attentional set-shifting and cognitive flexibility in humans are the Wisconsin Card Sorting Test (WCST)2 and a more recent and more refined version: the intra- and extra-dimensional attentional set-shifting (ID/ED) of the Cambridge Neuropsychological Test Automated Battery (CANTAB)3,4. These tasks have been used to identify specific cognitive abnormalities in a wide range of mental disorders including autism5, schizophrenia6, Parkinson's disease7, obsessive compulsive disorders8 and attention-deficit/hyperactive disorders9. The clinical relevance and solid methodological approach of the WCST and the ID/ED tests have attracted interest in the implementation of similar tests in preclinical research10,11. These tasks allow for the selective measurement within the same subject of different cognitive abilities such as discriminative learning, reversal learning, formation of an attentional set, shifting of attention within the same dimension (i.e., intra-dimensional shift: IDS) and between different perceptual dimensions (i.e., extra-dimensional shift: EDS). This is crucial because distinct brain circuits, as well as neuropathology might alter these distinct cognitive functions in different ways. For example, a double dissociation or functional specialization effect has been demonstrated between the lateral (in monkeys and humans) / medial (in rodents) and orbital regions of the PFC in the attentional set-shifting tasks. While the orbitofrontal cortex is more involved in the reversal phases of these tasks, the lateral/medial PFC region governs the extra-dimensional shift stages12-14.
Rodent versions of these attentional set-shifting tests for primates have been successfully generated13-16. However, some aspects of these rodent versions have been limiting their applications and use. For instance, these tasks are manually run and therefore highly labor-intensive and difficult to standardize. Moreover, the presence of food reinforcers inside the stimuli might result in an ambiguous interpretation of animal responses and potential bias in choice-making10. These characteristics have limited the throughput of testing and, more importantly, their application in large-scale genetic and/or drug-screening studies.
To overcome these limitations and to enhance the potential applications of ID/ED shift paradigms in rodents, we present here a novel two-chamber operant-based task to test cognitive flexibility in mice. This novel task closely mimics the ID/ED task used in human and non-human primates and circumvents the problems of earlier rodent versions.
Protocol
All procedures were approved by the Italian Ministry of Health and local Animal Use Committee and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the European Community Council Directives.
Note: Figure 6 shows a timeline of the entire procedure of the protocol to test ID/ED task.
1. Apparatus
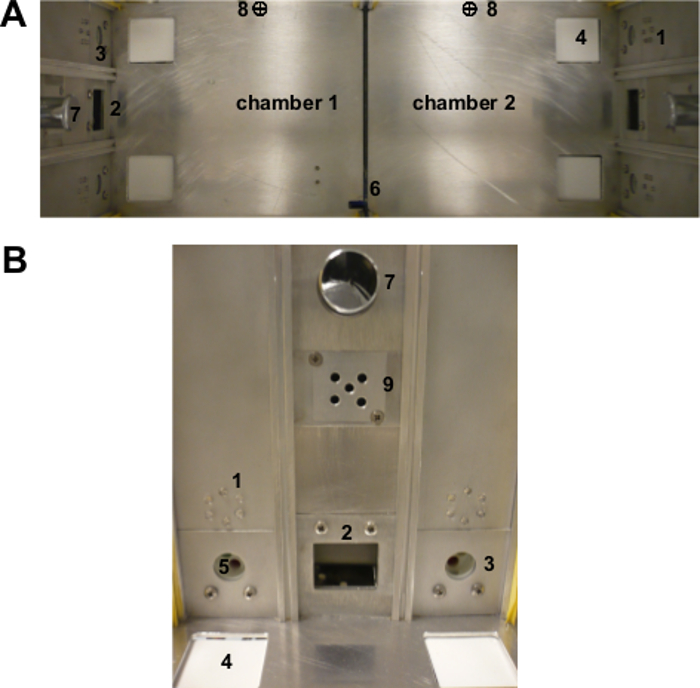
Figure 1. The two-chamber " Operon " apparatus. (A) View from the top of the entire apparatus and (B) view from the front of a single chamber mimicking the mouse point-of-view during the test. 1: visual stimuli (LEDs); 2: food magazine; 3: nose-poke hole; 4: tactile stimulus (texture); 5: olfactory stimulus; 6: automatic sliding door; 7: house-light; 8: infrared photobeam for door control. Chambers (16 x 16 x 16 cm3) are separated by a transparent plastic door (6). Infrared photobeams (8) tracked the animal movements and controlled the opening/closing of the automatic door to allow the mouse to change chambers. Each chamber presents two nose-poke holes (3) with infrared photobeams, and, between them, a food magazine (2) with photobeams where a pellet dispenser delivered the food reinforcement. A house-light (7) is located above each of the two food magazines. Each nose-poke hole is equipped with a series of changeable stimuli that could vary in three different perceptual dimensions (odor, view, tact). Originally published in 'The ultimate Intra- and Extradimensional Attentional Set-Shifting Task for Mice'19. Please click here to view a larger version of this figure.
Note: The Operon apparatus (Figure 1) consists of two identical chambers with Plexiglas walls and an aluminum floor (16 x 16 x 16 cm3 for each chamber). Chambers are separated by a transparent Plexiglas dividing door that can be automatically controlled to allow the mouse to access either chamber. Each chamber has two nose-poke holes with infrared photobeams, and, between them, a food magazine with photobeams where a pellet dispenser delivered the food reinforcement. A fan and a house-light are located above each of the two food magazines.
- Equip each nose-poke hole with a number of changeable stimuli (Table 1) that vary in three different perceptual dimensions (i.e., odor, sight, tactile). Note: The stimuli suggested in Table 1 have been selected in order to avoid any confounding factor and/or bias due to avoidance or preference. Five pairs of exemplars for each dimension allow a within-subject testing of all shifts, with novel stimuli at every shift.
- To deliver olfactory stimuli into the nose-poke holes, use a dilution olfactometer, which condition incoming air to the nose-poke holes by automatically desiccating, filtering, rehydrating and controlling the presentation of pairs of scents.
- Use one olfactometer to control two nose-poke holes. Regulate airflow by an air pump and a vacuum for the removal of the scent in the nose-poke holes. Connect the air pump to the inlet of the olfactometer and the vacuum to the outlets mounted on the nose-poke device. Then connect the outlet for odor delivery to each nose-poke inlet. Adjust the flow rate to 1.5 L per minute.
- Dilute odor scents 1:20 in mineral oil and fill the bottles of the olfactometer.
- For visual stimuli, place light-emitting diodes (LED) on top of each nose-poke hole and secure them in the metal panel of the chamber (see details in Figure 1 and Table 1). Connect them to the output interface.
- For tactile stimuli, mount changeable floor textures in front of each nose poke hole. Mount the different textures on sliding support and move them using frames underneath the floor, so that they are presented in correspondence of a small opening (3 x 3 cm2) on the floor in front of each nose-poke hole.
Control the presentation of the stimuli using software according to manufacturer's instructions in order to automatically change the stimuli of different dimensions during the experiment.
Place a camera on the top of the apparatus in order to record basal alternation and locomotor activity using behavioral monitoring software (e.g., Ethovision, Anymaze), which might help to eliminate animals that have problems unrelated to cognitive functions.
| ODORS | TEXTURES | LIGHTS |
| peach v. sage | velcro v. film | lights on v. lights off |
| vanilla v. lavender | coarse sandpaper v. fine sandpaper | red v. green |
| strawberry v. cinnamon | smooth cardboard v. ridged cardboard | blue v. yellow |
| grapefruit v. oregano | sponge v. smooth plastic | orange v. white |
| lemon v. apricot | honeycomb paper v. aluminium foil | fix lights v. blinking lights |
Table 1. Exemplars used in the ID/ED Operon task. Compound discriminations are based on fixed combinations of pairs of exemplars (see Figure 2 for an example of the sequence of discriminations). The exemplar pairs from different dimensions are presented in random combinations. Neutral stimuli for the different dimensions are: air flux with no scent; white paper; no light stimuli. Table originally published in 'The Ultimate Intra- and Extradimensional Attentional Set-Shifting Task for Mice'19.
2. Animal Preparation
Note: The representative results reported here were obtained from male C57BL/6J mice, 3 to 7 months old, during the light phase.
Weigh the mice, singly-house and then handle for 1 min on three alternate days.
After one week of acclimation to single-housing, record body weight and 24-hr food intake for three consecutive days to determine the baseline weight and food intake.
- Apply a slight food deprivation regimen for 1 week before training for the test. Check the animals' weight every day while food restricted throughout the experiment in order to maintain about 90% of their baseline free-feeding body weight. For three consecutive days before the habituation training phase, give mice in their home cage also ≈20 food reinforcement pellets. These are the food pellets that will be used in subsequent testing. Note: Food restriction is used to increase the animals' motivation to work in the task, however, do not exceed 10% of weight loss during any phase of the whole procedure as it may lead to abnormal behaviors and excessive stress for the mice, which may affect the results.
- Alternatively, in order to avoid single housing, leave the mice group-housed (2 to 4 each cage) and give access to ad libitum food for a specific period of time after testing. Check the animals' weight every day while food restricted throughout the experiment in order to maintain about 90% of their baseline free-feeding body weight.
3. Habituation
Train the mice in a 1-day session of 40 min to move inside the apparatus without the door divider, where a nose-poke in any nose-poke hole results in a pellet delivery in the food receptacle. During this phase, use only neutral stimuli (Table 1) for all different dimensions (Habituation 1).
On the following day, train the animals for 40 min to move from one chamber to the other at the end of each trial (Habituation 2). Use only neutral stimuli for all different dimensions. Also in this phase, a nose-poke in any of the nose-poke holes results in a pellet delivery in the food magazine. When the mouse retrieves the reward in the food magazine, lower the dividing door to give the mouse access to the other chamber for the next trial.
- On the third day (Habituation 3), train the animals to perform two simple discriminations (SD1 and SD2; e.g., velcro vs. film; light cue on vs. light cue off; peach vs. sage) to a criterion of 8 correct responses out of 10 consecutive trials. Do not use these exemplars again during the next phases of the test.
- To start, place the mouse in one chamber with neutral stimuli, while in the other chamber the stimuli cues are turned on; then lower the door to give the mouse access to the chamber with the activated stimuli cues. The mouse has to learn to choose the nose-poke hole where the correct stimulus is presented. Sessions last for 40 min.
- Reward a nose-poke in the correct hole with a pellet delivery and when the mouse enters the food magazine, lower the dividing door to give the mouse access to the other chamber for the next trial. Do not reward a nose-poke in the incorrect hole and turn on the house light for 5 sec. Then lower the door to give the mouse access to the other chamber for the next trial.
- Conduct the first ten trials of each stage as shaping trials: if the mouse selects the incorrect hole, record an error but do not terminate the trial until the mouse pokes also in the correct hole. In subsequent trials, if the mouse pokes in the incorrect hole, record an error and turn off all dimensional stimuli to terminate the trial.
- Terminate each session after 40 min or if a mouse fails to make any response for five consecutive minutes, whichever came first. If the mouse does not reach the criterion in one session, continue the test on the next day.
- If a mouse cannot reach the criterion in the SD1 or SD2 of the Habituation 3 after 5 sessions, stop testing the mouse and eliminate it from the study, because it is not able to reliably perform basic discriminations that could thereafter affect the results of the ID/ED task.
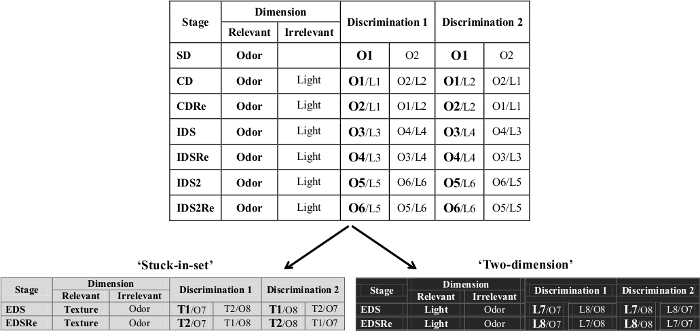
Figure 2. Example of the ID/ED task. An example of the ID/ED 'stuck-in-set' and 'two-dimension' paradigms is shown. Adapted from 'The ultimate Intra- and Extradimensional Attentional Set-Shifting Task for Mice'19.Please click here to view a larger version of this figure.
4. 'Stuck-in-set' ID/ED Paradigm Procedure
Note: For this procedure, previously used in both primates and rodents7,15,16, it is necessary to manipulate three different perceptual dimensions for each single mouse tested. As for the habituation phases, all the testing daily sessions start by placing the mouse in one of the two chambers where all the stimuli are neutral. Before the beginning of a trial, the stimulus cues are switched on in the opposite chamber. Then the dividing door is lowered to give the mouse access to the other chamber where the stimulus cues are on.
- To train the animal to develop a set, or bias, toward a particular perceptual dimension (e.g., odor, light or floor texture), expose mice to the following discriminations presented in this order (see also an example in Figure 2):
- For the Simple discrimination (SD), introduce mice to a dimension (odor, light or texture, see Figure 2) that is relevant in all the tasks until the EDS. That is, the relevant dimension is the one indicating where the correct response is. Following the example of Figure 2, present two odors, such as vanilla (O1) and lavender (O2), and select O1 as the correct response.
- For the Compound discrimination (CD), introduce a second dimension, for example the light, which is irrelevant (i.e., not indicating were the correct response is). Present two lights (L1 and L2) together with the odors (O1 and O2) in order to have two possible discriminations (see example in Figure 2). The correct and incorrect exemplars are the same as in SD.
- For the reversal of the Compound discrimination (CDRe), leave the exemplars and the relevant dimension unchanged, but have the animals learn that the previously correct stimulus is now incorrect. For example, select lavender as the correct response, with vanilla being now the incorrect choice. These same conditions will be found for the other reversal phases (i.e., IDSRe, IDS2Re and EDSRe).
- After that, select an Intra-dimensional shift (IDS) in which new exemplars are used for both the relevant and irrelevant dimensions (a total change design). For example, use strawberry and cinnamon (O3 and O4) as the odors and blue and yellow lights (L3 and L4) as the lights. However, ensure that the testing subjects keep following the same relevant dimension in order to find the correct response. These same conditions will be found for the other intradimensional shift (i.e., IDS2).
- As for the previous discrimination, have the mice perform the reversal of the Intra-dimensional shift (IDSRe). Use the same conditions as in CDRe.
- For Intra-dimensional shift 2 (IDS2), use the same conditions as in IDS.
- Similarly, use the same conditions as in CDRe for the Intra-dimensional shift reversal 2 (IDSRe2).
- In the Extra-dimensional shift (EDS), have the mice choose the correct hole after a newly presented stimulus dimension. Present a pair of stimuli from a new dimension, which is the texture. For example use coarse sandpaper (T1) as the correct response and fine sandpaper (T2) as incorrect, and present them with two new odors, for example lemon and apricot (O7 and O8). The previously relevant dimension has now become irrelevant. Do not present the previously irrelevant dimension anymore.
- For the Extra-dimensional reversal (EDSRe), use the same conditions as in CDRe.
- Measure the performance of the mice until they reach a criterion of 8 correct choices out of 10 consecutive trials to complete each stage. Set the program (see Table of Materials) to automatically move on to the next stage after the criterion is reached. Stop a daily session after 40 min, or if a mouse fails to make any response for 5 consecutive minutes, terminate the session and continue the next day with the mouse on the same stage where it left off.
- For each stage, measure the time to reach the criterion. If a mouse does not reach the criterion in one session, sum up the total time taken in consecutive sessions. For each trial, measure the time from presentation of the stimuli (odors, lights, textures) to a nose-poke response (latency to respond).
At the end of each session, wipe down the apparatus with 70% alcohol.
Use always the same order of the discriminations. However, randomly change the stimulus dimensions and the pairs of exemplars used and equally represent them within experimental groups and counterbalance them between groups.
Make sure to equally counterbalance the perceptual dimensions used within and between each experimental group so that each possible ED shift is represented (i.e., light to odor, light to texture, odor to light, odor to texture, texture to light, texture to odor). The combinations of exemplars are too numerous to permit full counterbalancing; therefore, to reduce the degrees of freedom, always use exemplars in pairs; for example, vanilla stimulus with lavender etc. (see Table 1).
5. 'Two-dimension' ID/ED Paradigm Procedure
Note: Another condition to measure attentional set-shifting ability, used both in primates12,17 and rodents13, is the 'two-dimension' paradigm. In this case, only two perceptual dimensions are used throughout the test.
- For this protocol, use the order of the discriminations and the procedure to follow as described for the 'stuck-in-set' protocol up to the EDS stage (see an example in Figure 2):
- For the SD and the following stages, use the same pairs of the 'stuck-in-set', until the IDS2Re. As shown in the example of Figure 2, use two odors (O1 and O2, use O1 as correct stimulus).
- For the CD, as in the 'stuck-in-set' protocol, introduce a new dimension (for example light, L1 and L2), that is irrelevant and would serve as a confounder.
- The CDRe is similar as in the 'stuck-in-set' protocol, thus leave the exemplars and the relevant dimension unchanged, but have the animals learn that the previously correct stimulus is now incorrect (e.g., O1).
- In the IDS, use always new pairs of stimuli for both dimensions (O3 and O4, L3 and L4, where O3 is the correct stimulus).
- For the IDSRe, have the mice perform the reversal of the IDS. Select the correct exemplar that is the one that was incorrect during the IDS (e.g., O4).
- For the IDS2, introduce new pairs of stimuli for both dimensions (O5 and O6, L5 and L6).
- As in the previous reversal stages, in the IDS2Re, have the animals learn that the previously correct stimulus is now incorrect (e.g., O5).
- For the 'two-dimension' protocol, the EDS, the mice have to choose the correct response after the previously irrelevant dimension now becomes the relevant dimension. Thus, light is the new relevant dimension, which indicates the correct response. In particular, red light (L7) is the correct response. Conversely, the previously relevant dimension (in the example, odor) is now the irrelevant one.
- After the EDS, for the reversal (EDSRe), use same conditions as in CDRe. The relevant dimension is the same as in the EDS but the correct and incorrect exemplars are inverted. Thus, for example, green light (L8) is now the correct response.
6. Data Analysis
Measure the performance by: number of trials to reach the criterion; time to reach the criterion (in minutes); time from presentation of the stimuli to a nose-poke response (latency to respond).
For statistical analysis, use ANOVAs with the different stages (SD, CD, CDRe, IDS, IDSRe, IDS2, IDSRe2, EDS, and EDSRe) as a within-subject factor to examine the number of trials required to reach the criteria, timing needed to complete each stage and the latency to respond. Conduct post-hoc analyses using Newman-Keuls test. Note: The accepted value for significance is p<0.05.
Representative Results
Figure 2 shows an example of the ID/ED task. Pairs of stimuli (either 'Discrimination 1' or 'Discrimination 2') are randomly presented in each stage, and the mouse must choose the correct stimulus in each pair. In this example table, the correct exemplar is reported in bold. In the first stage (SD or simple discrimination), the stimuli presented in the two nose-poke holes differed in one of three dimensions (e.g., O1: Vanilla vs. O2: Lavender)and the mouse is rewarded for choosing the correct exemplar (e.g., O1). Once the subject reaches the criterion in this stage, the next stage (CD or compound discrimination) begins, where the same exemplars of the relevant dimension are presented overlaid at random by exemplars of a second, but irrelevant dimension, introduced as a confounding factor (e.g., L1: blue light vs.L2: yellow light). Two different discriminations are possible in this stage (either 'Discrimination 1' or 'Discrimination 2'). In the next stage (CDRe or compound discrimination reversal), the reward contingencies are reversed but the exemplars and the relevant dimension are unchanged: the mouse has to learn that the previously correct stimulus is now incorrect (e.g., lavender odor is now rewarded). In the next stage (IDS or intra-dimensional shift), new exemplars (both odors and lights) are introduced but the relevant dimension (odor in this example) remains the same (e.g., strawberry is the correct choice). In the next stage (IDR or intra-dimensional reversal), the reward contingencies are reversed. After a second intra-dimensional shift (IDS2 and its reversal), new exemplars are introduced to test the extra-dimensional shift (EDS) in which the relevant dimension is changed. In the 'stuck-in-set' EDS, the mouse has to focus on the new dimension (e.g., the Texture, T1: coarse sandpaper vs. T2: fine sandpaper), while the previously relevant dimension (in this case, odor) is now the irrelevant dimension. In the 'two-dimension' EDS, the previously irrelevant dimension (in this case, light) is now the relevant dimension. In the final stage (EDSRe or extra-dimensional reversal), the reward contingencies are reversed.
In order to obtain reliable results, the stimulus dimensions used in the task should be equally well-learned. As shown in Figure 3, visual, tactile and odor discriminations in this novel apparatus required similar time (F(2,64)=0.36; p=0.69) and similar number of trials (F(2,64)=0.059; p=0.94) to reach the criterion, suggesting that animals are able to perform simple discriminations regardless the dimension presented.
If a reliable attentional set has been developed over the testing phases of the task, the performance of a normal wild-type mouse should be poorer in the EDS stage compared to previous and following stages, as reported in previous studies in rodents and primates12,13. In particular, a robust increase of time and trials needed to reach criteria should be found in the EDS compared to the IDS stages. As illustrated in Figure 4, in our experiment with the 'stuck-in-set' protocol, analysis of performance of the mice revealed a discrimination effect for the number of trials (F(8,168)=9.23; p<0.0001) and time (F(8,168)=8.62; p<0.0001) required to reach criteria. Indeed, mice needed more trials and more time to solve the EDS stage compared to the CD, IDS, IDS2 and EDSRe stages (p<0.05; Figure 4A-B). Similarly, analysis of performance tested with the 'two-dimension' protocol (Figure 5) showed a significant discrimination effect for the number of trials (F(8,72)=3.66; p<0.005) and time (F(8,72)=4.65; p<0.0005) needed to reach criteria. Indeed, we show that mice required more trials (p<0.05) and more time (p<0.05) to solve the EDS compared to CD, IDS, IDS2 and EDSRe (Figure 5A-B). No differences in shifting abilities should be observed between mice tested with different dimensions.
In a normal wild-type mouse, the first reversal learning (i.e., CDRe) is more difficult than the initial discrimination (i.e., CD). In agreement, as evident from Figures 4 and 5, mice needed more trials (p<0.05; Figure 4-5A) and more time (p<0.05; Figure 4-5B) to complete this stage. Moreover, reversal performance should improve from CDRe to IDSRe to IDS2Re, as we show in our experiment with both the 'stuck-in-set' and 'two-dimension' protocols. These results further strengthen the evidence of the formation of an attentional set through the task.
Throughout the task, the mice should improve their speed to respond over consecutive stages. Accordingly, the analysis of the latency to respond showed a significant discrimination effect (F(8,200)=42.59; p<0.0001). In particular, as demonstrated by representative results in Figure 4C the latency to poke in the IDS2 stage is decreased compared to that in the IDS, CD and SD stages (p<0.0005). Moreover, the latency to respond increased during the EDS stage compared to the previous IDS2 and IDS2Re and successive EDSRe stages (p<0.05). In line with these results, the analysis of the latency to respond during the 'two-dimension' task also showed a significant effect of discrimination (F(7,63)=9.98; p<0.0005). As shown in Figure 5C, the latency to make a choice was increased during the EDS compared to previous IDS2 and IDS2Re and successive EDSRe (p<0.05). Since the latency to respond has been considered an index of decisional processing18, these results further suggest that the mice encountered some problems processing the new discriminative rule during the EDS. Based on the behavioral performance of wild-type mice (Figure 4-5), we determined that the minimal number of sample size (by R power analysis) for each experimental group should be 8.
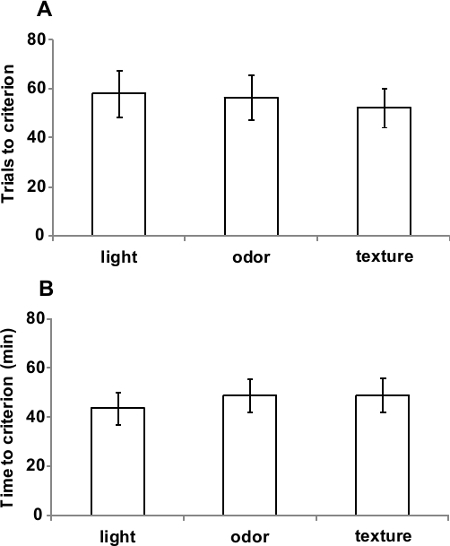 Figure 3. Simple discriminations of light, odor and texture are equivalent. (A) Number of trials and (B) time required to reach the criterion on simple discriminations with only light, odor or texture stimuli. Values represent mean ± SEM throughout Figures 2-4. Data originally published in 'The ultimate Intra- and Extradimensional Attentional Set-Shifting Task for Mice'19.
Figure 3. Simple discriminations of light, odor and texture are equivalent. (A) Number of trials and (B) time required to reach the criterion on simple discriminations with only light, odor or texture stimuli. Values represent mean ± SEM throughout Figures 2-4. Data originally published in 'The ultimate Intra- and Extradimensional Attentional Set-Shifting Task for Mice'19.
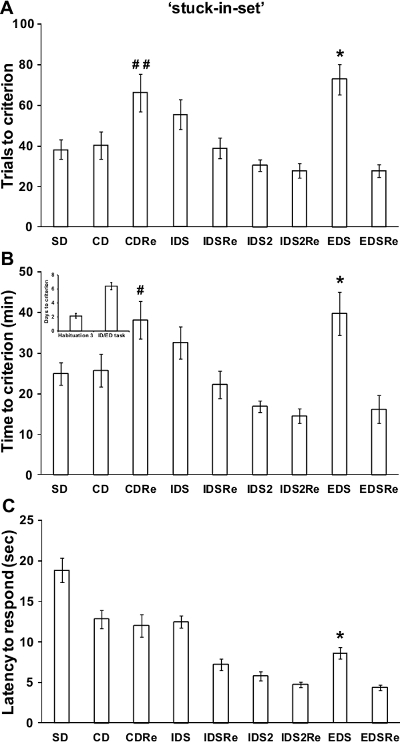 Figure 4. Wild-type C57BL6J male mice performance in the 'stuck-in-set' ID/ED Operon task. (A) Trials, (B) time (in minutes) and days needed to reach the criterion in the different stages of the ID/ED Operon task using a 'stuck-in-set' ID/ED paradigm. (C) Time (in seconds) elapsed between the opening of the divider door and a nose-poke response (latency to respond) during the different stages of the task. A total of 26 mice were tested; 4 mice were excluded because they were not reliably poking to retrieve the food reinforcement during the training or were not able to finish the entire procedure. A: *p <0.05 versus CD, IDS, IDS2, IDS2Re and EDSRe; B: *p <0.05 versus CD, IDS2, IDS2Re and EDSRe. A and B: #p<0.05, ##p<0.005 versus CD, IDSRe and IDS2Re. C: *p <0.05 versus IDS2, IDS2Re and EDSRe. Note that the mice were able to complete the entire task in 5-9 days in all experiments reported in Figures 3-4. Data originally published in 'The ultimate Intra- and Extradimensional Attentional Set-Shifting Task for Mice'19.
Please click here to view a larger version of this figure.
Figure 4. Wild-type C57BL6J male mice performance in the 'stuck-in-set' ID/ED Operon task. (A) Trials, (B) time (in minutes) and days needed to reach the criterion in the different stages of the ID/ED Operon task using a 'stuck-in-set' ID/ED paradigm. (C) Time (in seconds) elapsed between the opening of the divider door and a nose-poke response (latency to respond) during the different stages of the task. A total of 26 mice were tested; 4 mice were excluded because they were not reliably poking to retrieve the food reinforcement during the training or were not able to finish the entire procedure. A: *p <0.05 versus CD, IDS, IDS2, IDS2Re and EDSRe; B: *p <0.05 versus CD, IDS2, IDS2Re and EDSRe. A and B: #p<0.05, ##p<0.005 versus CD, IDSRe and IDS2Re. C: *p <0.05 versus IDS2, IDS2Re and EDSRe. Note that the mice were able to complete the entire task in 5-9 days in all experiments reported in Figures 3-4. Data originally published in 'The ultimate Intra- and Extradimensional Attentional Set-Shifting Task for Mice'19.
Please click here to view a larger version of this figure.
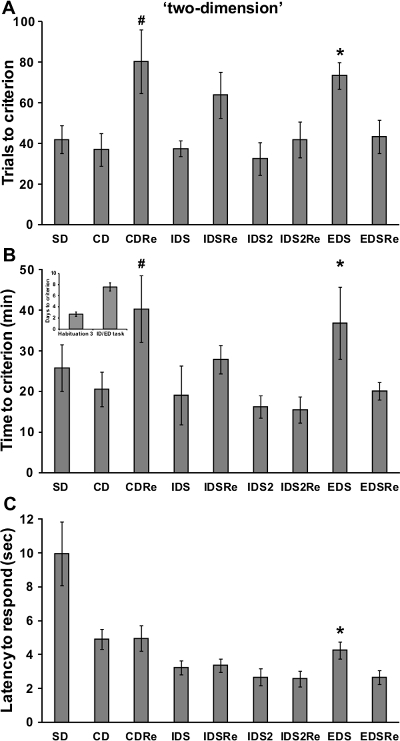
Figure 5. Wild-type C57BL6J male mice performance in the 'two-dimension' ID/ED Operon task. (A) Trials, (B) time (in minutes) and days needed to reach the criterion in the different stages of the ID/ED Operon task using a two-dimension paradigm. (C)Time (in seconds) elapsed between the opening of the divider door and a nose-poke response (latency to respond) during the different stages of the task. A total of 13 mice were tested; 3 mice were excluded because they were not reliably poking to retrieve the food reinforcement during the training or were not able to finish the entire procedure. A and B:#p<0.05 versus CD and IDS2Re, *p <0.05 versus CD, IDS, IDS2, EDSRe. C:*p <0.05 versus IDS2, IDS2Re and EDSRe. Data originally published in 'The ultimate Intra- and Extradimensional Attentional Set-Shifting Task for Mice'19.Please click here to view a larger version of this figure.
 Figure 6. Timeline of the entire procedure of the protocol to test ID/ED task.
Figure 6. Timeline of the entire procedure of the protocol to test ID/ED task.
| Stage | Description | Additional notes | References |
| Simple Discrimination (SD) | |||
| Compound Discrimination (CD) | Stimuli vary in two perceptual dimensions, such as color and shape for visual stimuli in the human task, or between texture and odor stimuli for rodents | ||
| Reversal learning (CDRe – IDSRe – IDS2Re – EDSRe) | Two exemplars within a perceptual dimension have their reinforcement contingencies reversed so that what was previously correct is then incorrect and vice versa) | In serial reversal learning, performance improves with consecutive within-set reversals. Thus, reversal stages (i.e., CDRe, IDSRe, IDS2Re) not only assess function of different cortical areas, but also serve 1) to form the cognitive- attentional set challenged by the EDS stage, and 2) to prevent superstitious conditioning to unintended aspects of the stimulus | - Lesions of the orbitofrontal cortex in mice (Bissonette et al., 2008) and monkyes (Dias et al., 1996) impaired Reversal learning - fMRI during performance on the Reversal learning showed activation of the orbitofrontal cortex (Hampshire and Owen, 2006) |
| Intradimensional shift (IDS – IDS2) | Novel exemplars are introduced, but the same dimension is reinforced | IDS stages serve as a crucial internal control (i.e., EDS should be more difficult than IDS), and also contribute to form the cognitive-attentional set | Dopamine depletion in the PFC impaired attentional set formation (Robbins and Roberts, 2007). 6-OHDA lesioned monkeys did not show the classical reduction the reduction in errors from the first (IDS1) to the last (IDS5) discrimination, which should reflect acquisition of an attentional set |
| Extradimensional shift (EDS) | ‘stuck-in-set’ protocol: previously irrelevant stimulus dimension is replaced by a new stimulus dimension which immediately becomes relevant | failure to shift to the new relevant dimension cannot be attributed to any prior learning about this dimension since it had not been previously experienced. Failure, therefore, reflects perseveration to the previously relevant dimension | - Lesions of the mPFC have been show to impair EDS in mice (Bissonette et al., 2008), and mokeys (Dias et al., 1996) - Frontal lobe patients are impaired in the stuck-in-set 'perseveration' condition but not in the ‘two dimension' condition (Owen et al., 1993) - Dopamine in the mPFC modulated EDS performance in mice (Papaleo et al., 2008; Scheggia et al., 2014) and rats (Tunbridge et al., 2004) |
| ‘two dimensions’ protocol: the previously irrelevant dimension is reinforced | an apparent failure to shift attentional set may arise when a subject is able to shift attention away from a previously relevant dimension (when it becomes irrelevant) but is, nevertheless, unable to refocus attention on the newly relevant dimension | - Lesion of the mPFC in rats (Birrell and Brown, 2000) impaired EDS shift - fMRI during performance on the EDS has been shown activation of the ventro-lateral PFC (Hampshire and Owen, 2006) |
Table 2: Stages of the ID/ED Operon task. Description of the stages of the task, including references to lesion and pharmacological studies addressing the role of brain areas involved in the different constructs tested during the task.
Discussion
In this study we present a novel automated two-chamber ID/ED "Operon" task for mice that is able to reliably measure cognitive flexibility through reversal learning, attentional set formation and shifting. This paradigm is analogous to the WCST and ID/ED tasks commonly used in human and nonhuman primates and overcomes the major limitations of the previous versions for rodents. This Operon paradigm can be used as a new effective tool for large drug and/or genetic screenings relevant to cognitive (dys)functions in mice with high relevance to translational medicine.
This automated task have the following advantages over previously used ID/ED task for rodents: (1) it has less labor-intensive procedures than the manual versions (e.g., the software regulates all the phases of the task, greatly decreasing interventions by the experimenter); (2) it eliminates any source of subjectivity in the measured parameters (e.g., the experimenter is not required to judge whether or not the animal has actually made a choice response); (3) it eliminates any possibility that mice might follow reinforcement-related cues in order to make a response (i.e., the reward is always automatically delivered in the center magazine); (4) it avoids arbitrary environmental conditions (e.g., use of mazes and apparatus of different sizes/materials, use of manually prepared cue stimuli); (5) it allows manipulation of three distinct dimensions with a large range of different stimuli, in accordance to the equivalent human tasks used in the clinical setting. Here we present data only from mice, but similar advantages might be expected also for rats.
There are critical steps in the task, which are internal construct-validity parameters that can be used in order to identify areliable attentional set-shifting performance: i) poorer performance in the EDS compared to previous stages ii) a general improvement from IDS to IDS2; iii) a specific improvement in consecutive reversal stages, as the more an animal is trained in within-set reversals, the better it should perform on subsequent within-set reversals20; iv) better performance in the EDSRe compared to the EDS. This novel ID/ED operon task presents all these features, consistent with previous studies in primates using the CANTAB ID/ED task and in rodents using the manual "digging" version12,13. Moreover, each stage of this automated task was learnt in an equivalent number of trials irrespective of the relevant/irrelevant dimension used (i.e., odor, texture and light)19. This demonstrated that all the stimuli used have similar salience and are suitable for attentional set-formation and/or shifting. Our experiments demonstrate that the difficulty in solving the EDS is also highlighted by the increase in response latencies. Processing time increases to maintain optimal accuracy during harder discriminations21. Thus, considering the latency to respond as an index of speed of information processing, decision-making and problem solving18, the slower latencies during the EDS might reflect a strategy adopted to face the difficulty in solving the set-shifting.
We have shown that our novel apparatus can be effectively used with the more 'classical' attentional set-shifting paradigm employing only two dimensions throughout the test (i.e., the two-dimension) or with a stuck-in-set paradigm, implying the use of three dimensions. Depending on the cognitive domain of interest, it is possible to choose the most appropriate protocol. Both of these paradigms have been previously used in humans, primates and rodents7,12-17. However, the stuck-in-set procedure is able to distinguish between different components of set-shifting and is a more selective measure of frontal lobe functioning in human patients7,22. Furthermore, one problem that might occur in the two-dimension paradigm is the learned irrelevance that might bias the results. Learned irrelevance perseveration refers to the inability to attend to and learn about information that was previously irrelevant7. This situation can occur in the EDS phase of the two-dimension paradigm, as the subject is required to shift the attention to the previously irrelevant dimension. In this case, it is basically impossible to discern whether an EDS deficit is due to the inability to shift attention away from a previously relevant dimension and/or the inability to now shift the attention to a previously irrelevant dimension. Impairment may then just reflect the active inhibition of responding to a dimension previously made irrelevant by its random association with reinforcing feedback. In contrast, in the stuck-in-set condition, failure to shift to the new relevant dimension cannot be attributed to any prior learning about this dimension since it had not been experienced previously. Failure, therefore, reflects perseveration to the previously relevant dimension. In conclusion, even if in normal wild-type mice the 'stuck-in-set' and the 'two-dimension' attentional set-shifting paradigms might produce similar results, we expressly prefer the stuck-in-set perseveration for the reasons discussed above.
Disclosures
The authors declare that they have no financial conflicts of interest.
Acknowledgments
We thank G. Pruzzo for his excellent technical assistance and the IIT mechanical workshop for help in building our new apparatus. This research was supported by the Istituto Italiano di Tecnologia, the Marie Curie FP7-Reintegration-Grant N°268247, and by the Italian Ministry of Health Grants for Young Researchers (GR-2010-2315883).
References
- Keeler JF, Robbins TW. Translating cognition from animals to humans. Biochemical pharmacology. 2011;81(12):1356–1366. doi: 10.1016/j.bcp.2010.12.028. [DOI] [PubMed] [Google Scholar]
- Milner B. Effects of Different Brain Lesions on Card Sorting. Arch Neurol. 1963;9:100–110. [Google Scholar]
- Roberts AC, Robbins TW, Everitt BJ. The effects of intradimensional and extradimensional shifts on visual discrimination learning in humans and non-human primates. The Quarterly journal of experimental psychology. B, Comparative and physiological psychology. 1988;40(4):321–341. [PubMed] [Google Scholar]
- Barnett JH, Robbins TW, Leeson VC, Sahakian BJ, Joyce EM, Blackwell AD. Assessing cognitive function in clinical trials of schizophrenia. Neuroscience and biobehavioral reviews. 2010;34(8):1161–1177. doi: 10.1016/j.neubiorev.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends in Cognitive Sciences. 2004;8(1):26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Ceaser AE, Goldberg TE, Egan MF, McMahon RP, Weinberger DR, Gold JM. Set-shifting ability and schizophrenia: a marker of clinical illness or an intermediate phenotype. Biological psychiatry. 2008;64(9):782–788. doi: 10.1016/j.biopsych.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Hodges JR, Robbins TW. Contrasting mechanisms of impaired attentional set-shifting in patients with frontal lobe damage or Parkinson's disease. Brain. 1993;116(5):1159–1175. doi: 10.1093/brain/116.5.1159. [DOI] [PubMed] [Google Scholar]
- Head D, Bolton D, Hymas N. Deficit in cognitive shifting ability in patients with obsessive-compulsive disorder. Biological Psychiatry. 1989;25(7):929–937. doi: 10.1016/0006-3223(89)90272-2. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, et al. Translational approaches to frontostriatal dysfunction in attention-deficit/hyperactivity disorder using a computerized neuropsychological battery. Biological psychiatry. 2011;69(12):1192–1203. doi: 10.1016/j.biopsych.2010.08.019. [DOI] [PubMed] [Google Scholar]
- Gilmour G, et al. Measuring the construct of executive control in schizophrenia: Defining and validating translational animal paradigms for discovery research. Neuroscience and biobehavioral reviews. 2012. pp. 1–16. [DOI] [PubMed]
- Barch DM, Braver TS, Carter CS, Poldrack RA, Robbins TW. CNTRICS final task selection: executive control. Schizophrenia bulletin. 2009;35(1):115–135. doi: 10.1093/schbul/sbn154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380(6569):69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial Frontal Cortex Mediates Perceptual Attentional Set Shifting in the Rat. The Journal of Neuroscience. 2000;20(11):4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. The Journal of Neuroscience. 2008;28(44):11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, et al. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. The Journal of Neuroscience. 2008;28(35):8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner JP, Thogerson CM, Würbel H, Murray JD, Mench JA. Animal neuropsychology: validation of the Intra-Dimensional Extra-Dimensional set shifting task for mice. Behavioural brain research. 2006;173(1):53–61. doi: 10.1016/j.bbr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologica. 1991;29(10):993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163(3-4):362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Scheggia D, Bebensee A, Weinberger DR, Papaleo F. The ultimate intra-/extra-dimensional attentional set-shifting task for mice. Biological psychiatry. 2014;75(8):660–670. doi: 10.1016/j.biopsych.2013.05.021. [DOI] [PubMed] [Google Scholar]
- Machkintosh NJ. The effects of overtraining on a reversal and a nonreversal shift. Journal of Comparative and Physiological Psychology. 1962;55(4):555–559. [Google Scholar]
- Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron. 2004;44(5):865–876. doi: 10.1016/j.neuron.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Cools R, Rogers R, Barker RA, Robbins TW. Top-down attentional control in Parkinson's disease: salient considerations. Journal of cognitive neuroscience. 2010;22(5):848–859. doi: 10.1162/jocn.2009.21227. [DOI] [PubMed] [Google Scholar]


