Abstract
The enteric nervous system (ENS) plays an important role in regulating gastrointestinal (GI) motility and can function independently of the central nervous system. Changes in ENS function are a major cause of GI symptoms and disease and may contribute to GI symptoms reported in neuropsychiatric disorders including autism. It is well established that isolated colon segments generate spontaneous, rhythmic contractions known as Colonic Migrating Motor Complexes (CMMCs). A procedure to analyze the enteric neural regulation of CMMCs in ex vivo preparations of mouse colon is described. The colon is dissected from the animal and flushed to remove fecal content prior to being cannulated in an organ bath. Data is acquired via a video camera positioned above the organ bath and converted to high-resolution spatiotemporal maps via an in-house software package. Using this technique, baseline contractile patterns and pharmacological effects on ENS function in colon segments can be compared over 3-4 hr. In addition, propagation length and speed of CMMCs can be recorded as well as changes in gut diameter and contraction frequency. This technique is useful for characterizing gastrointestinal motility patterns in transgenic mouse models (and in other species including rat and guinea pig). In this way, pharmacologically induced changes in CMMCs are recorded in wild type mice and in the Neuroligin-3R451C mouse model of autism. Furthermore, this technique can be applied to other regions of the GI tract including the duodenum, jejunum and ileum and at different developmental ages in mice.
Keywords: Neuroscience, Issue 108, Gastrointestinal motility, Colonic migrating motor complexes (CMMCs), spatiotemporal maps, enteric nervous system (ENS), video-imaging, pharmacology, synaptic disorders, mouse, genetic mutation, neuroligin-3
Introduction
The enteric nervous system (ENS) is the intrinsic neuronal network of the gastrointestinal tract and modulates various functions such as digestion of intestinal content, absorption of nutrients and the secretion and reabsorption of fluid. Neurons of the ENS are located in the myenteric and submucosal plexuses. The myenteric plexus plays a major role in regulating gastrointestinal motility1 whereas the submucosal plexus is primarily involved in the control of secretion2,3. The myenteric plexus is situated between the longitudinal and circular muscle layers of the gastrointestinal wall. The contractile activity of the smooth muscle layers of the intestinal wall facilitates the primary functions of the gastrointestinal tract by mixing and propelling intestinal content along the length of the intestine3. Although the extrinsic nerve supply to the gastrointestinal tract from the CNS contributes to gastrointestinal function in vivo, the ENS is capable of regulating gastrointestinal function independently. This unique characteristic enables the functional investigation of enteric neuronal circuits and their contribution to gastrointestinal motility ex vivo.
Colonic migrating motor complexes (CMMCs) are spontaneous, neurogenic events that are the predominant motor pattern observed in isolated mouse colon in the absence of fecal pellets4-9. CMMCs are defined as rhythmic contractions that propagate along a horizontal distance that is at least half the total length of the colon (i.e., from the cecum to the rectum)10. The relationship between CMMCs and the contractile patterns that propel fecal pellets is yet to be clearly established, however some pharmacological differences have been reported11. Nevertheless, the ability of the ENS to function independently of the CNS and the existence of neural-mediated motor patterns in the isolated colon provides an ideal assay system to examine disturbances in motility resulting from underlying ENS dysfunction. The spontaneity of gastrointestinal motor patterns allows functional changes in response to pharmacological stimuli to be evaluated.
The use of video imaging and spatiotemporal mapping was first developed to quantitatively examine small intestinal peristalsis in guinea pigs12. Here, an ex vivo technique is described that enables the study of mouse colonic motility patterns using video imaging and analysis of these recordings to construct high-resolution (~100 µm, 33 msec) maps of colonic diameter as a function of position along the colon and of time (spatiotemporal maps). Using in-house edge detection software (Analyse2; available on request), data from full length colonic segments contracting in real time are processed to generate spatiotemporal maps for each experiment. In this step, video (AVI) files are summarized and converted to spatiotemporal maps using Analyse2. Spatiotemporal maps (Figure 2) depict contractility over time and enable the measurement of multiple parameters including propagation speed, magnitude, length and duration. Gut diameter is also recorded throughout the duration of the experiment as a measure of the overall contractility of the tissue segment. This method can be applied to identify differences in the point of initiation of contractile complexes which could indicate altered enteric neural connectivity.
A similar video imaging protocol designed to assess pellet propulsion in guinea pigs has been reported13 however here we outline the application of the video imaging approach for quantification of spontaneous colonic motility (i.e., in the absence of pellets). We also provide detailed information to assist in the dissection and preparation of gastrointestinal tissue for the video imaging approach. This protocol provides researchers with an accessible and easily replicated tool for analyzing enteric neural control of gastrointestinal function in animal models of disease including genetic mouse models.
The video imaging technique enables the analysis of colonic motility in response to various pharmacological agents. Drugs can be administered via the gut lumen or the organ bath external to the colonic preparation. Different regions of the mouse gastrointestinal tract exhibit specific motility patterns such as small intestinal segmentation and CMMCs in the colon.
This technique has been used to identify strain differences in small intestinal function; differential sensitivity to 5-HT3 and 5-HT4 antagonists were observed in the jejunum of Balb/c and C57/Bl6 mice due to the polymorphic nature of the tph2 gene expressed in the two strains6. The effect of 5-HT inhibition on motility remains controversial, as conflicting data has been reported on the importance of endogenous 5-HT on colonic peristalsis and CMMCs14,15. Alterations in motility pre- and postnatally during development7, and the effects of gene mutations on gastrointestinal motility in animal models of disease10 can also be examined by utilizing video imaging. Here we illustrate use of the method for a study of colonic motility in the NL3R451C mouse model of autism, which expresses a missense mutation in the Nlgn3 gene encoding the synaptic adhesion protein Neuroligin-316. This mutation was first identified in patients diagnosed with Autism spectrum disorder (ASD)17, which is strongly associated with GI dysfunction18-22. We investigated whether the NL3 R451C synaptic mutation affects neural outputs in the ENS using the video imaging technique. We present data characterizing CMMCs at baseline and in response to the serotonergic 5HT3/4 receptor antagonist tropisetronin the NL3R451C mouse model of autism.
Protocol
Animal handling and cervical dislocation of animals prior to all experiments were performed strictly according to protocols approved by the Animal Experimentation Committee for the University of Melbourne (Ethics ID: 1212494.7)
1. Tissue Collection and Dissection
Euthanize adult mice by cervical dislocation. If possible avoid anesthesia to prevent influences on gut function via receptors located on neuronal populations of interest.
Record the animal's total body weight, pin the body (via the four paws, ventral side exposed to experimenter) firmly to a dissection board using hypodermic needles (Size: 20 G).
Using dissecting forceps and scissors; make an incision through the epidermis overlaying the lower abdominal muscle layers. Continue to use the forceps and scissors to open the abdominal cavity along the midline of the abdomen to the sternum.
To prevent the tissue dehydrating, pour physiological saline (Krebs solution: 118 NaCl, 4.6 KCl, 2.5 CaCl2, 1.2 MgSO4, 1 NaH2PO4, 25 NaHCO3, 11 D-glucose in mM - bubbled at RT with carbogen gas; 95% O2 and 5% CO2 for a minimum of 20 min) onto abdominal contents at regular intervals (i.e., every 30-60 sec) during the dissection process.
To isolate the colon while attached to the cecum and rectum, hold the cecum (located adjacent to the proximal end of colon) gently using dissecting forceps approximately 4-5 cm above the body of the animal while trimming the mesentery using fine dissection scissors.
Take care not to handle, stretch or cut the gastrointestinal tissue while removing the adjoining mesentery.
Remove excess tissue (i.e., urinary bladder and testes). Using coarse dissecting scissors, make two vertical incisions (i.e., approximately 0.5 cm from the midline) to cut the pelvic bone on each side of the colon.
Use fine scissors to separate the rectum from adjoining pelvic tissue and trim surrounding muscle from the colon.
Place the full-length colon (from cecum to rectum) into a beaker containing physiological saline (previously oxygenated with carbogen) and continue to oxygenate at RT. Remove the caecum and rectum using fine dissecting scissors and replace in beaker.
- Empty fecal pellets/intestinal content from colonic preparations by applying gentle positive pressure at the oral end using a 5 ml syringe attached to a 200 µl pipette tip filled with physiological saline. In order to orientate the tissue in future, use an insect pin to mark the most proximal end of the colon, where striations in the mucosa are visible.
- Modify the plastic pipette tip to fit the 5 ml syringe by removing approximately 1 cm of the widest/proximal end using a razor blade. To increase the flow rate, widen the distal/smaller end by cutting on an angle (approximately 45 °C) using a razor blade.
Grip the colon tissue gently at its proximal end with dissecting forceps, insert the pipette tip into the lumen of the colon and flush physiological saline through the lumen.
2. Preparation of Colonic Tissue and Experimental Set Up for Video Imaging
Flush physiological saline through all tubing attached to a two chambered organ bath set up (Figure 1 and Figure 3), using a syringe attached to a 200 µl pipette tip. This step removes any debris within the tubing that could block the flow of solution. For tubing specifications refer to the materials table.
Ensure that the chambers of the organ bath are continuously superfused with physiological saline bubbled with carbogen (95% O2 and 5% CO2, flow rate of 5 ml min-1). Using a temperature probe at regular intervals, ensure that the temperature of the bath is maintained between 33 °C -37 °C.
Fill the inflow reservoir connected via a 3-way stopcock to the inlet tube with approximately 20-30 ml physiological saline using a 50 ml syringe.
During the experiment, maintain the pressure in the inflow reservoir using arubber stopper with a glass tube (5 mm inside diameter) inserted through its center. Make sure that the upper opening of the inflow reservoir is securely sealed (with the inner glass tube remaining unsealed).
Place the colon (length 5-7 cm) into the organ bath chamber, making sure that it is fully submerged in physiological saline.
Cannulate each end of the colonic segment to the two submerged inlet/outlet tubes (inlet/outlet tubes of different diameter can be changed according to the size of the tissue lumen eg. postnatal tissue, adult mice and guinea pig) in the bath using standard cotton sewing thread. Attach the proximal end of the colon to the inlet tube and the distal end of the colon to the outlet tube (connected via a 3-way stopcock to a vertical outflow tube; 6 cm in height). Make sure that the tissue is not overstretched or too relaxed after cannulation to avoid interference with future measurements and analysis.
Record the length of the colon by measuring the distance between the proximal and distal cannulations using a 15 cm ruler. This is important for interpreting changes in contraction length, propagation speed and contraction initiation points.
Ensure a stable baseline luminal pressure (cm H2O) is established before beginning the experiment. Calculate luminal pressure by measuring the vertical distance from the tissue to the meniscus of physiological saline within the glass tube of inflow reservoir (oral) and the vertical outflow tube (anal).
Maintain tissue preparations at a constant intraluminal pressure (e.g., 1-2 cm H2O) by ensuring that the inflow stopper seal pressure is kept constant and that no blockages are present in the set up.
Leave the colon to equilibrate in physiological saline for 30 min before recording intestinal movements. Check for any blockages arising in the in- or outflow tubes and remove them by applying pressure through the inflow reservoir or by re-cannulating the oral and anal ends of the colon.
3. Image Capture and Experimental Protocol
Record intestinal movements as video files using a video camera (30 frames/sec, 640 x 840 pixels) positioned 10-15 cm above the organ bath on a standard laboratory retort stand (Figure 1).
- Open Virtual Dub (video capture software) from the icon on the desktop. From the 'File' tab, select 'capture AVI' to view the camera image. On the 'Audio' tab deselect 'enable audio capture' to disable sound.
- In the video tab select 'compression' and 'DivX6.9.2. Codec' to compress video files to minimize usage of storage space. This function will become available upon the installation of the compression software 'DivX'.
- Manually adjust the camera location so that the entire cannulated colon segment is visible (with cannulated regions immediately adjacent to the vertical edges of the video frame) via the video capture software window (see Figure 1). In order to improve image quality and minimize reflections, attach a paper/cardboard shield to the camera support to block extraneous light onto the organ bath. Optimize video quality by adjusting the brightness, contrast and exposure settings using the camera software interface.
- Before recording, set a specific file name; in the 'File' tab, select 'Set capture file' and specify a unique name for the video.
- Begin capturing video by pressing 'Capture file' from the 'Capture' tool bar. To stop recording select the 'Stop capture' button or the 'Esc' key on the keyboard.
- Replace the physiological saline contained within the inflow reservoir with fresh physiological saline every 30 min.
- Record luminal pressure (see 2.9) and using a temperature probe, take note of the organ bath temperature. Repeat these steps throughout the experiment (i.e., every 30 min) to ensure that these variables are constant.
- Record colonic activity in total for 3 hr (including drug application and washout duration). For data storage purposes, record data in 15 min video segments. A full experiment will typically be composed of 12 x 15 min video recordings.
- Record activity for 1 hr under control conditions (physiological saline).
- Apply the drug (either superfused into the bath or administered into the lumen via the inflow reservoir) for 30 min to 1 hr. Note: Different routes of administration can yield different effects23.
- Apply fresh physiological saline to the bath or lumen during a 1 hr washout period.
4. Data Processing and Generation of Spatiotemporal Maps
- Process each video recording offline using in-house software written in MATLAB (R2012a, Version 7.4; available on request) in order to generate spatiotemporal maps illustrating colonic motility.
- Open analysis software from the icon on the desktop.
- In the command window, enter "Analyse2" in order to open the analysis control panel.
Utilize the edge detection function to generate spatiotemporal maps by clicking "Edge detection for .avi files" in the control panel window. This function will enable the analysis software to read the recoded videos in Audio Video Interleaved (AVI) format.
- Carry out the following steps sequentially to generate spatiotemporal maps using an adaptive edge detection algorithm.
- Open recorded video files by selecting the "Add files" tab, select 'Open video' and select the location of the video files.
- From the video files listed within the analysis software, select the file of interest.
- Select a rectangular region of interest within the frame of the video, e.g., the entire length of the colon, from the point at which the colon is cannulated orally to the anal cannulation. Ensure that the proximal and distal cannulation points are adjacent to the vertical boundaries of the region of interest. Edge detection lines (red and green) will automatically appear superimposed on the real time image.
- Ensure that the edge detection threshold lines are immediately adjacent to the colon on both the upper and lower boundaries of the colon tissue (this can be optimized for each video by altering detection threshold values on the control panel). Ensure that the edge detection lines are continuous along the length of the colon image by adjusting the contrast and brightness using the control panel.
- Enter the full length of the colon (mm) into the width dialog box (as recorded in step 2.7). Then select "Generate heat map".
- In the pop up window, select a location for the file to be saved and specify a file name for the spatiotemporal map.
- Select the '.su2' format and select 'Add to queue'. Note: .su2 format compresses data to a smaller file size and multiple video files can be added to the queue.
- Select 'Begin detection' to generate spatiotemporal maps.
5. Analysis of Spatiotemporal Maps
- Utilize spatiotemporal maps to estimate parameters such as frequency, propagation speed, length and duration of CMMCs, gut diameter and the point of initiation and direction of contractions.
- To start analyzing spatiotemporal maps, type 'analyse2' in the command window as described in the previous section. Instead of "Edge detection", select the "Heat map analysis" button.
- Open spatiotemporal map files (.su2 files) by selecting the 'Add files' tab and specifying the location of previously obtained .su2 files.
- Once the spatiotemporal map is visible on the screen, specify a color range on the control panel, ensuring that the minimum is set to 1. Note that the maximum can range from 5-10 according to the contractility of the tissue.
- Select 'Lock color range' to ensure all the maps from a single experiment are analyzed under the same conditions.
- Ensure that the time frame settings are constant for all spatiotemporal maps from a given experiment.
In order to determine CMMC frequency, manually count contractions that traverse more than 50% of the length of the colon. These contractions can be visualized as red/orange streaks (standard HSV color index) on the spatiotemporal map (see Figure 2 for an example). Other contractions that are shorter or do not propagate can also be identified and quantified.
- If desired, conduct further analysis at a higher resolution from these maps. For example, detailed properties including the speed and duration of each contraction can be examined.
- In order to measure the speed and duration, select the "Annotate contraction waves" button on the heat map analysis control panel.
- Next, zoom in to each CMMC by clicking on the "Zoom" button and selecting the area of interest. Then select the "Manually annotate" button to annotate each contraction by using the mouse to draw a line from the most initial position to the end of each contraction. This method can be used to measure the apparent propagation speed and duration of dilations that appear to propagate (for e.g., see anal end of maps in Figure 2A, 2B). Note: The data for speed and duration will appear under the results window. These values can be transferred to a spreadsheet of interest by selecting the "Export data" tab. Unwanted annotations can be removed by clicking on the "Remove selected annotations" button.
If desired, utilize the x and y coordinates of the spatiotemporal maps (time and position along the colon, respectively) to determine the position of initiation of small contractions or CMMCs.
Similarly, in order to determine intervals between contractions, use the "Annotate" function to measure the duration between contractions. As previously, these results can be exported to a spreadsheet by selecting the "Export data" tab.
- In order to measure the resting gut width, select the "Take cross section" button on the Heat map analysis panel.
- Select 'Add' on the temporal cross sections panel. Double click at any location within the map to generate a horizontal line superimposed on the heat map. Gut diameter data will now be displayed in a new window.
- To measure gut diameter, place the cursor on a peak and adjacent trough to obtain average gut width for a specific experimental condition. Changes in gut diameter can be compared between experimental conditions.
Representative Results
Up to 90% of patients with ASD experience an array of gastrointestinal disorders, including diarrhea and constipation18,24,25. However, the underlying causes of these gastrointestinal issues are unknown. Many mutations identified in patients with ASD are associated with synaptic proteins contributing to alterations and disturbances in synaptic transmission or function. One such mutation, in the gene coding for the cell adhesion molecule neuroligin-3 (NL3 R451C), was identified in two brothers with ASD17. This mutation results in an arginine residue at position 451 of the Neuroligin protein being replaced with a Cysteine. NL3R451C mice expressing this mutation show increased GABA mediated transmission in the somatosensory cortex16,26 alongside increased AMPA and NMDA receptor mediated activity within the hippocampus25,27.
Neuroligin proteins are present in enteric neurons28-30. As the enteric nervous system plays a major role in regulating gastrointestinal function, we postulated that the R451C mutation would affect motility. Therefore, in order to investigate possible alterations in gastrointestinal functions due to synaptic abnormalities we sought to examine the effects of the R451C mutation on CMMC frequency in these mice.
Because serotonin (5-HT) acts on the ENS to modulate gastrointestinal function in mice6 we analyzed motility patterns in response to the 5-HT3/4 receptor antagonist tropisetron in colonic preparations from WT and NL3R451C mice.
To assess whether the synaptic mutation alters CMMCs when the enteric nervous system is pharmacologically perturbed, the 5HT3/4 receptor antagonistTropisetron (Trop; 10 µM, which blocks both 5HT3 and 5HT4 receptors) was added to the bath containing the colon preparations (Figure 2). Colonic tissue from nineteen age matched male mice (11 WT and 8 NL3R451C) was used. In the presence of tropisetron, NL3R451C mice showed a decrease in CMMC frequency compared to WT littermates. Representative examples of spatiotemporal maps showing CMMCs and contractile activity in WT and NL3R451C colon preparations are shown in Figures 2A to 2E respectively. Although no difference was observed between WT and NL3R451C during control conditions, tropisetron significantly reduced CMMC frequency in both WT and NL3R451C mice (Figure 2C, 2F). In WT mice, the median number of CMMCs was 23 in control conditions compared to 15 in tropisetron (p = 0.023). In NL3 mice, the median number of CMMCs in control conditions was 19.5 compared with 2 in the presence of tropisetron (p = 0.022). In addition, tropisetron had a larger effect on the frequency of CMMCs in NL3R451C mice compared to WT (p = 0.047).
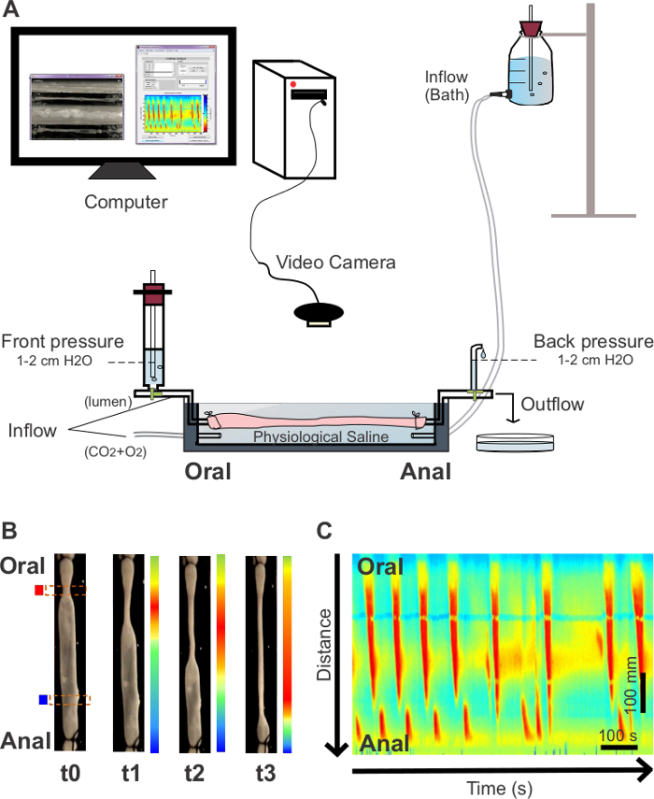 Figure 1. Organ bath set up and generation of spatiotemporal maps. (A) A freshly dissected gastrointestinal segment is placed in an organ bath (cross sectional view) containing physiological saline and cannulated at oral and anal ends. The oral cannula is connected to an inflow reservoir filled with physiological saline and the anal cannula connected to an outflow tube. A video camera is positioned above the organ bath in order to record contractile activity of the colon. (B) motility is converted to high resolution spatiotemporal maps labeling regions of the colon that are dilated in blue and constricted regions in red. (C) A spatiotemporal map showing colonic motility (CMMCs) from an adult WT mouse. Individual CMMCs are indicated as red vertical regions within the map. The X axis shows time increasing (0-15 min). The Y axis represents the spatial location along the colon segment (anal at base, oral at top). Please click here to view a larger version of this figure.
Figure 1. Organ bath set up and generation of spatiotemporal maps. (A) A freshly dissected gastrointestinal segment is placed in an organ bath (cross sectional view) containing physiological saline and cannulated at oral and anal ends. The oral cannula is connected to an inflow reservoir filled with physiological saline and the anal cannula connected to an outflow tube. A video camera is positioned above the organ bath in order to record contractile activity of the colon. (B) motility is converted to high resolution spatiotemporal maps labeling regions of the colon that are dilated in blue and constricted regions in red. (C) A spatiotemporal map showing colonic motility (CMMCs) from an adult WT mouse. Individual CMMCs are indicated as red vertical regions within the map. The X axis shows time increasing (0-15 min). The Y axis represents the spatial location along the colon segment (anal at base, oral at top). Please click here to view a larger version of this figure.
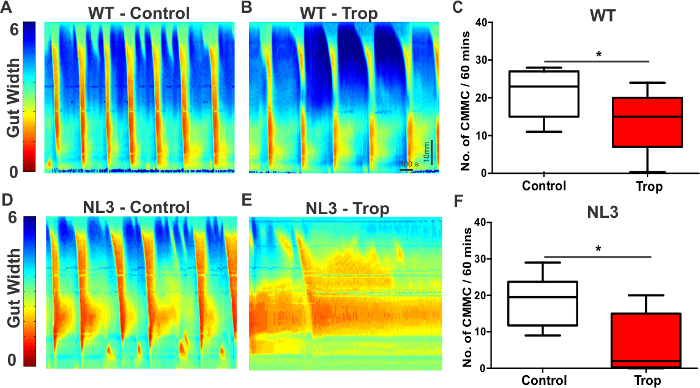 Figure 2. Spatiotemporal maps show increased sensitivity to Tropisetron in NL3R451C mouse colon. Spatiotemporal maps showing CMMC frequency in colon segments from WT controls (A) and in the presence of Tropisetron (Trop; B). Trop reduced CMMC frequency in WT colon (C). Spatiotemporal maps from NL3 colonunder control conditions (D) and in the presence of Trop (E). Trop caused a strong reduction in CMMC frequency in NL3 colon (F). CMMC frequency in response to Trop was significantly reduced in NL3 compared with WT colon (p = 0.047, not shown). Gut width (pixel color) is indicated on the Y axis (arbitrary units, range 1-6). Scale bar in E applies to all maps. Trop; Tropisetron. Please click here to view a larger version of this figure.
Figure 2. Spatiotemporal maps show increased sensitivity to Tropisetron in NL3R451C mouse colon. Spatiotemporal maps showing CMMC frequency in colon segments from WT controls (A) and in the presence of Tropisetron (Trop; B). Trop reduced CMMC frequency in WT colon (C). Spatiotemporal maps from NL3 colonunder control conditions (D) and in the presence of Trop (E). Trop caused a strong reduction in CMMC frequency in NL3 colon (F). CMMC frequency in response to Trop was significantly reduced in NL3 compared with WT colon (p = 0.047, not shown). Gut width (pixel color) is indicated on the Y axis (arbitrary units, range 1-6). Scale bar in E applies to all maps. Trop; Tropisetron. Please click here to view a larger version of this figure.
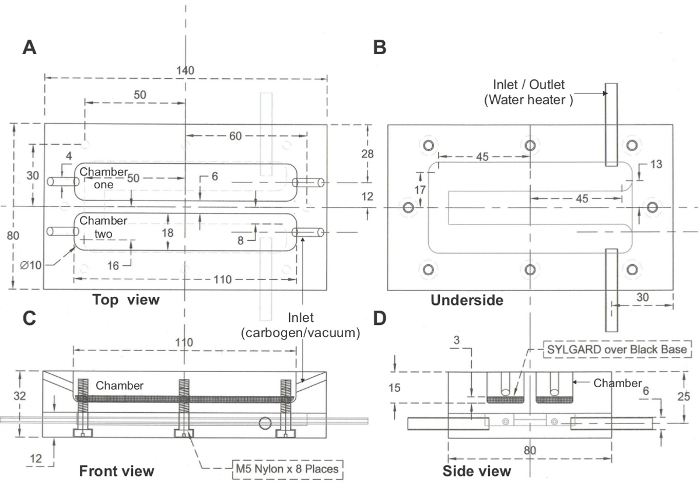 Figure 3. Schematic of organ bath. (A) Top view, (B) Underside, (C) Front view, (D) Side view, of a two chambered organ bath set up. Dimensions in mm. Please click here to view a larger version of this figure.
Figure 3. Schematic of organ bath. (A) Top view, (B) Underside, (C) Front view, (D) Side view, of a two chambered organ bath set up. Dimensions in mm. Please click here to view a larger version of this figure.
Discussion
Using this video imaging technique, CMMC frequency was measured as an indication of colonic motility in wild type and NL3R451C mice, a mouse model of autism spectrum disorder17. Our results indicate a reduction in the number of CMMCs in mutant NL3R451C mice compared to wild type mice in the presence of the 5HT3/4 receptor antagonist Tropisetron suggesting that NL3R451C mice exhibit an increased sensitivity to Tropisetron. Accordingly, we propose that the neuroligin-3 R451C mutation alters the serotonin pathway, potentially by modulating either 5HT3 or 5HT4 receptor function in enteric neurons, the mucosa or both. This highlights the method's value for identification of phenotypic differences between genotypes and for identification of specific targets for subsequent studies.
This method can be modified to enhance spatial resolution by acquiring videos via a stereo-microscope fitted with a camera mount. This approach enables recordings to be made from small preparations of the gastrointestinal tract at embryonic time points as early as E12.531. Neuromodulators can be applied via the lumen or in the organ bath external to the colonic preparation. Furthermore, this method is useful for assessing both large and small gastrointestinal motility in a range of species including mice, rats and guinea pig.
Common troubleshooting steps for this method include verifying the flow of solution via the tubing, viability of the tissue preparation, maintenance of constant luminal pressure and ensuring that colon segments are located away from the walls of the organ bath. Blockages within the tubing can alter luminal pressure and prevent contractions from occurring; therefore all tubes must be cleaned thoroughly to remove salt crystals or debris/fecal matter before cannulation. Air must be removed from tubing lines directly associated with the cannula prior to experiments (i.e., by priming the tubes with saline). In addition, tissue preparations must be handled with care in order to prevent damage resulting in immobility of the colon. To avoid tissue damage, ensure that the colon is firmly (but not tightly) attached to the cannula during the recording process and maintain a constant temperature and a continuous supply of CO2 + O2 to the bath. Also ensure that the luminal pressure is kept constant and that no contractions are manually initiated by adjusting inflow reservoirs during the recording period. Ensure that the colon tissue does not contact the wall of the organ bath during contractions as this will prevent edge detection analysis of the relevant spatiotemporal maps. This can be avoided by monitoring the contractions during the equilibration period and adjusting the position of the colon to prevent this from occurring during the experiment.
Several limitations associated with this technique should be taken in to consideration when analyzing and interpreting the data including the low throughput nature of this approach. While the method is effective in identifying changes in migrating motor patterns, it cannot determine whether dilations occurring during the progression of a CMMC are neurally mediated or simply passive responses to the contractile activity (i.e., resulting from the movement of fluid). The concentration gradients for diffusion across the colonic wall allow the effects of luminally applied drugs to be ascribed to actions within the mucosa, but in prolonged experiments mucosal degeneration may occur thereby altering the sites of action of these drugs over the recording period. Furthermore, whether drugs have distinct effects in the myenteric and submucosal plexuses cannot be determined using this method. In contrast, this approach enables a collective evaluation of effects on the enteric nervous system by measuring an overall change in motility patterns. Further considerations include the need to take into account the nature of the data (i.e., count data for CMMC frequency, requiring non-parametric analysis) and the low frequency of CMMCs, when designing experiments and appropriate data analysis strategies.
Recently, Barnes and colleagues proposed that colonic tissue requires stimulation in order to observe CMMCs32, however published findings from our lab demonstrate that spontaneous CMMCs can be observed by simply pinning the tissue to the organ bath via the mesentery7. The presence of CMMCs under these conditions not only demonstrates the spontaneity of CMMCs, but further elaborates on the usefulness of this technique to establish changes in colonic motility. Although this approach is applicable to extra-colonic regions of the gastrointestinal tract, the complexity of small intestinal motility requires more detailed analysis strategies than those used for quantifying CMMCs33.
This experimental approach has very high spatial and temporal resolution and includes the option of drug delivery both external to and inside the lumen for investigating the effects of varying concentration gradients on the enteric nervous system. Furthermore, this method is suitable for analyzing small intestinal segmentation during the fed state6,23. The ex vivo nature of this method enables the role of the enteric nervous system to be assessed in the absence of central nervous system inputs and is therefore an ideal way to investigate gastrointestinal motility in a variety of models, including genetic models of disease (see Figure 2)6,34.
This method can also be used to compare physiological data to computer simulations of motor activity23,33,35. Such simulations can predict motor patterns in the form of spatiotemporal maps for direct comparisons with physiological experiments33,35. Using Fast Fourier Transform and wavelet analysis36, the contribution of smooth muscle pacemakers (generated by interstitial cells of Cajal) to motility can also be extracted. Furthermore, this video imaging technique can be combined with extracellular recording of electrical activity in the muscle3 to allow contributions of neural and myogenic pattern generators to be distinguished. Note, the extracellular recording method resolves inhibitory junction potentials in the absence of smooth muscle contractions or relaxations.
While this technique is well established for the analysis of gastrointestinal motility in a wide range of preparations and species, it also has the potential to be used in other systems such as the study of vasoconstriction in the mesentery (previously analyzed via a simpler diameter tracking system37) and in skeletal muscle.
Disclosures
The authors have nothing to disclose
Acknowledgments
JCB and ELH-Y were supported by the US Department of Defense CDMRP Autism Research Program (AR11034). NHMRC (1047674) to ELH-Y.The May Stewart Bursary-University of Melbourne trust funded scholarship to MS. We thank Ali Taher, Fátima Ramalhosa and Gracia Seger for technical contributions.
References
- Powell AK, O'Brien SD, Fida R, Bywater RA. Neural integrity is essential for the propagation of colonic migrating motor complexes in the mouse. Neurogastroenterol Motil. 2002;14:495–504. doi: 10.1046/j.1365-2982.2002.00350.x. [DOI] [PubMed] [Google Scholar]
- Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- Gwynne RM, Bornstein JC. Mechanisms underlying nutrient-induced segmentation in isolated guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1162–G1172. doi: 10.1152/ajpgi.00441.2006. [DOI] [PubMed] [Google Scholar]
- Bush TG, Spencer NJ, Watters N, Sanders KM, Smith TK. Spontaneous migrating motor complexes occur in both the terminal ileum and colon of the C57BL/6 mouse in vitro. Auton Neurosci. 2000;84:162–168. doi: 10.1016/S1566-0702(00)00201-0. [DOI] [PubMed] [Google Scholar]
- Fida R, Lyster DJ, Bywater RA, Taylor GS. Colonic migrating motor complexes (CMMCs) in the isolated mouse colon. Neurogastroenterol Motil. 1997;9:99–107. doi: 10.1046/j.1365-2982.1997.d01-25.x. [DOI] [PubMed] [Google Scholar]
- Neal KB, Parry LJ, Bornstein JC. Strain-specific genetics, anatomy and function of enteric neural serotonergic pathways in inbred mice. J Physiol. 2009;587:567–586. doi: 10.1113/jphysiol.2008.160416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RR, Murphy JF, Young HM, Bornstein JC. Development of colonic motility in the neonatal mouse-studies using spatiotemporal maps. Am J Physiol Gastrointest Liver Physiol. 2007;292:G930–G938. doi: 10.1152/ajpgi.00444.2006. [DOI] [PubMed] [Google Scholar]
- Spencer NJ. Control of migrating motor activity in the colon. Curr Opin Pharmacol. 2001;1:604–610. doi: 10.1016/s1471-4892(01)00103-5. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Bywater RA. Enteric nerve stimulation evokes a premature colonic migrating motor complex in mouse. Neurogastroenterol Motil. 2002;14:657–665. doi: 10.1046/j.1365-2982.2002.00367.x. [DOI] [PubMed] [Google Scholar]
- Roberts RR, Bornstein JC, Bergner AJ, Young HM. Disturbances of colonic motility in mouse models of Hirschsprung's disease. Am J Physiol Gastrointest Liver Physiol. 2008;294:G996–G1008. doi: 10.1152/ajpgi.00558.2007. [DOI] [PubMed] [Google Scholar]
- Tough IR, et al. Endogenous peptide YY and neuropeptide Y inhibit colonic ion transport, contractility and transit differentially via Y(1) and Y(2) receptors. Br J Pharmacol. 2011;164:471–484. doi: 10.1111/j.1476-5381.2011.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig GW, Costa M, Chen BN, Brookes SJ. Quantitative analysis of peristalsis in the guinea-pig small intestine using spatio-temporal maps. J Physiol. 1999;517(Pt 2):575–590. doi: 10.1111/j.1469-7793.1999.0575t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JM, Brooks EM, Mawe GM. Gastrointestinal Motility Monitor (GIMM) J Vis Exp. 2010. [DOI] [PMC free article] [PubMed]
- Smith TK, Gershon MD. Rebuttal from Terence K. Smith and Michael D. Gershon. J Physiol. 2015;593:3233. doi: 10.1113/JP270683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Sia TC, Brookes SJ, Costa M, Keating DJ. CrossTalk opposing view: 5-HT is not necessary for peristalsis. J Physiol. 2015;593:3229–3231. doi: 10.1113/JP270183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaidez V, Hansen RL, Hertz-Picciotto I. Gastrointestinal problems in children with autism, developmental delays or typical development. J Autism Dev Disord. 2014;44:1117–1127. doi: 10.1007/s10803-013-1973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim SH, Voigt RG, Katusic SK, Weaver AL, Barbaresi WJ. Incidence of gastrointestinal symptoms in children with autism: a population-based study. Pediatrics. 2009;124:680–686. doi: 10.1542/peds.2008-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohane IS, et al. The co-morbidity burden of children and young adults with autism spectrum disorders. PloS One. 2012;7:e33224. doi: 10.1371/journal.pone.0033224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics. 2014;133:872–883. doi: 10.1542/peds.2013-3995. [DOI] [PubMed] [Google Scholar]
- Peters B, et al. Rigid-compulsive behaviors are associated with mixed bowel symptoms in autism spectrum disorder. J Autism Dev Disord. 2014;44:1425–1432. doi: 10.1007/s10803-013-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis M, Chambers JD, Gwynne RM, Bornstein JC. Serotonin and cholecystokinin mediate nutrient-induced segmentation in guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol. 2013;304:G749–G761. doi: 10.1152/ajpgi.00358.2012. [DOI] [PubMed] [Google Scholar]
- Parracho HM, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol. 2005;54:987–991. doi: 10.1099/jmm.0.46101-0. [DOI] [PubMed] [Google Scholar]
- Buie T, et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;125(Suppl 1):S1–S18. doi: 10.1542/peds.2009-1878C. [DOI] [PubMed] [Google Scholar]
- Etherton M, et al. Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function. Proc Natl Acad Sci U S A. 2011;108:13764–13769. doi: 10.1073/pnas.1111093108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton MR, Tabuchi K, Sharma M, Ko J, Sudhof TC. An autism-associated point mutation in the neuroligin cytoplasmic tail selectively impairs AMPA receptor-mediated synaptic transmission in hippocampus. EMBO J. 2011;30:2908–2919. doi: 10.1038/emboj.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, et al. Expression of neurexin and neuroligin in the enteric nervous system and their down-regulated expression levels in Hirschsprung disease. Mol Biol Rep. 2013;40:2969–2975. doi: 10.1007/s11033-012-2368-3. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. Expression and significance of neuroligins in myenteric cells of Cajal in Hirschsprung's disease. PloS One. 2013;8:e67205. doi: 10.1371/journal.pone.0067205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, et al. The down-regulation of neuroligin-2 and the correlative clinical significance of serum GABA over-expression in Hirschsprung's disease. Neurochem Res. 2014;39:1451–1457. doi: 10.1007/s11064-014-1334-y. [DOI] [PubMed] [Google Scholar]
- Roberts RR, et al. The first intestinal motility patterns in fetal mice are not mediated by neurons or interstitial cells of Cajal. J Physiol. 2010;588:1153–1169. doi: 10.1113/jphysiol.2009.185421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes KJ, Spencer NJ. Can colonic migrating motor complexes occur in mice lacking the endothelin-3 gene? Clin Exp Pharmacol Physiol. 2015;42:485–495. doi: 10.1111/1440-1681.12380. [DOI] [PubMed] [Google Scholar]
- Chambers JD, Bornstein JC, Thomas EA. Multiple neural oscillators and muscle feedback are required for the intestinal fed state motor program. PloS One. 2011;6:e19597. doi: 10.1371/journal.pone.0019597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia DJ, et al. Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. J Physiol. 2013;591:5939–5957. doi: 10.1113/jphysiol.2013.256230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JD, Bornstein JC, Thomas EA. Insights into mechanisms of intestinal segmentation in guinea pigs: a combined computational modeling and in vitro study. Am J Physiol Gastrointest Liver Physiol. 2008;295:G534–G541. doi: 10.1152/ajpgi.90303.2008. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, et al. The origin of segmentation motor activity in the intestine. Nat Commun. 2014;5:3326. doi: 10.1038/ncomms4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neild TO, Shen KZ, Surprenant A. Vasodilatation of arterioles by acetylcholine released from single neurones in the guinea-pig submucosal plexus. J Physiol. 1990;420:247–265. doi: 10.1113/jphysiol.1990.sp017910. [DOI] [PMC free article] [PubMed] [Google Scholar]


