Abstract
The process of mammary epithelial morphogenesis is influenced by hormones. The study of hormone action on the breast epithelium using 2D cultures is limited to cell proliferation and gene expression endpoints. However, in the organism, mammary morphogenesis occurs in a 3D environment. 3D culture systems help bridge the gap between monolayer cell culture (2D) and the complexity of the organism. Herein, we describe a 3D culture model of the human breast epithelium that is suitable to study hormone action. It uses the commercially available hormone-responsive human breast epithelial cell line, T47D, and rat tail collagen type 1 as a matrix. This 3D culture model responds to the main mammotropic hormones: estradiol, progestins and prolactin. The influence of these hormones on epithelial morphogenesis can be observed after 1- or 2-week treatment according to the endpoint. The 3D cultures can be harvested for analysis of epithelial morphogenesis, cell proliferation and gene expression.
Keywords: Bioengineering, Issue 108, T47D cells, mammary gland morphogenesis, collagen fibers, estrogen, progestins, prolactin
Introduction
Unlike standard 2D cultures, 3D cell culture surrogate models allow for the study of epithelial cell behavior in a physiologically relevant context, one resembling a tissue. 3D cultures of the mammary gland have helped elucidate many aspects of mammary gland development and neoplasia. However, most of the 3D culture models currently available are unsuitable to study hormone action because the human epithelial cell lines used for the task lack hormone receptor expression 6,7,9.
Herein, we describe a 3D culture model of the human breast epithelium that is suitable to study hormone action 12. This model uses the commercially available hormone-responsive human breast epithelial cell line, T47D 3,11,13, which were originally derived from a pleural effusion obtained from a 54 year old female patient with an infiltrating ductal carcinoma of the breast. We use rat tail collagen type 1 as a matrix. This 3D culture model is appropriate for the study of the action of the three main mammotropic hormones (estradiol, promegestone (an analogue of progesterone), and prolactin) on human breast epithelial cells. Hormone-induced epithelial morphology can be assessed quantitatively over time by morphometric analysis12.
An appropriate seeding density allows these 3D cultures to be kept for 2 weeks. By this time, the development of structures is sufficient for a robust quantitative assessment of hormone action on epithelial morphology. Gels may also be harvested at earlier time points for cell proliferation and gene expression analyses. Additionally, this model is suitable to test the effects of a sequential hormonal treatment; for example, after treatment with estradiol during the first week and replacement with other hormone/combination of hormones during the following week. The effect of estrogenic compounds and antiestrogens, such as ICI 182,780, can also be studied using this 3D culture model 12.
Protocol
1. Preparation of Reagents
Dissolve the synthetic progestagen promegestone (R5020) and 17-β-estradiol (E2) in ethanol to make 10-3 M stock solutions. Dissolve prolactin in distilled deionized water to make a 1 mg/ml stock solution. Dissolve the antiestrogen ICI 182,780 in DMSO to make a 10-2M stock solution. Store these solutions at -20 ° C for up to 6 months.
- Charcoal dextran (CD) stripped serum and CDFBS medium:
- Heat-inactivate fetal bovine serum (FBS) in a 57 °C water bath for 30 min. Note: Use only acid-washed glassware for the remainder of this protocol.
- Calculate the amount of charcoal needed. Using 10% more volume than the amount of serum to compensate for the displacement caused by the charcoal, weigh 5% wt/vol activated powdered charcoal.
- Wash charcoal twice with cold deionized distilled water using the same volume as the amount of serum. For each wash centrifuge at 50 x g for 2 min to pellet the charcoal. Remove the water between washes. Add 0.5% wt/vol Dextran T-70 to the last charcoal pellet. Re-suspend with distilled, deionized water and centrifuge at 100 x g for 5 min. Aspirate the water.
- Add the appropriate volume of serum to the pellet and re-suspend the charcoal dextran pellet into the serum. Incubate, while rolling (6 RPM), at 37 ºC for 60 min. Centrifuge at 2,000 x g for 20 min. If supernatant appears cloudy, repeat centrifugation; however, serum may not be completely clear at this time but will be cleared during filtration.
- Filter through a 0.80 micron filter and then again through a 0.45 micron filter to remove larger particulate matter. Finally, filter-sterilize with a 0.20 micron filter. Store filtered CDFBS in glass tubes or T-25 culture flasks at -20 ºC.
- Charcoal Dextran Stripped Fetal Bovine-containing media (CDFBS media): mix 375 ml of phenol red-free DMEM and 125 ml of DMEM/F-12. Remove 37.5 ml and replace it with the same volume of Charcoal Dextran Stripped Fetal Bovine serum. Add 106 U/ml penicillin and L-glutamine to a 2 mM final concentration. The resulting media is 75% DMEM, 25% DMEM/F-12, 7.5% Charcoal dextran stripped-FBS, 2mM L-glutamine, and 106 U/ml penicillin.
Prepare 10X PBS, 1N NaOH and distilled deionized water. Prepare enough volume of each according to the volume of collagen solution needed. Sterilize all solutions by filtering. Store the 10x PBS and sterile distilled deionized water at 4 ° C for up to 1 month. Do not store the 1N NaOH solution.
Prepare collagen solution at a 1 mg/ml final concentration according to the protocol for "Alternate Gelation Procedure for Collagen I, Rat tail" provided by the manufacturer. Each gel is made of 1.5 ml of collagen solution. For example, prepare 18.5 ml (0.5 ml to account for loss during pipetting) of collagen solution to prepare 12 gels. Use the collagen solution immediately or hold on ice for up to 2-3 hr.
- Carmine Alum dye:
- Combine 1 g Carmine and 2.5 g Aluminum potassium sulfate in 500 ml distilled water. Boil while stirring for 20 min. Watch carefully to avoid boiling over. Adjust final volume back to 500 ml with additional distilled water.
- Add a crystal of thymol for antimicrobial activity and cover bottle with aluminum foil to protect from light. Filter through a laboratory grade paper filter before each use. Reuse the carmine solution for up to three months. Store at RT, protected from light.
Collagenase solution: dissolve the collagenase in DMEM/F12 medium at a concentration of 0.125% w/v. Do not store this solution.
2. 3D Culture of T47D Cells in Rat Tail Collagen Type 1 Gels and Hormone Treatment
Note: keep sterile serological pipettes and pipette tips at 4 °C.
Prepare a single-cell suspension and perform a cell count. Split T47D cell cultures before they reach 70% confluence. Centrifuge the volume that contains the amount of cells needed. Consider that one gel should contain about 75,000 T47D cells.
Using a cold-pipette, re-suspend the cell pellet in the appropriate volume of collagen. Consider that each gel is made up of 1.5 ml of collagen. Mix the cells and the collagen gently to avoid introducing bubbles.
To prevent the effect of static electricity on cell distribution, brush the borders, top and bottom of the 12-well plate with a static-reducing brush after unwrapping the plate. To avoid static charge buildup during contact with the working surface of the hood, place this plate on top of another 12-well plate (blank plate) that has also been brushed with the static-reducing brush.Gently, pour 1.5 ml of the mix into each well of the 12-well plate.
Place the plate in an incubator at 37 °C for 30 min. Remove the plate from the incubator and place it back in the hood.
Using a p200 sterile tip, carefully detach the gel in a gentle circular motion from the border of the well. The gel should also detach from the bottom of the well.
Dilute hormones in CDFBS media to be added to the wells. Use E2 and R5020 at a 10 -10 M final concentration and prolactin at a at 10-7 M final concentration. As control, use CDFBS media without hormones added but still containing the highest volume of DMSO equal to the hormone stocks used. Ensure that each well contains 1.5 ml of media. Place the 3D cultures inside a 37 °C, 5% CO2, 100%humidity tissue culture incubator. Maintain the cultures for 1 or 2 weeks, changing the media every 2 days.
3. Gel Processing for Whole Mounts
Remove culture media and add 1.5 ml of PBS to each well.
Transfer the gel to a slide. Cut the gel in half using a curved surgical blade. Continue processing with one half and save the other half for histological analysis.
Transfer the half gel for whole mount to a slide. Let gel dry for 5 min and then transfer the slide to a coplin jar containing 10% formalin. Note: all incubations should be done at RT.
Fix gels in a coplin jar on a shaker O/N.
Wash slides twice with PBS for 10 min each on shaker. Transfer slides to 70% EtOH; incubate for 1 hr on shaker. Transfer slides to Carmine Alum O/N.
The next day, dehydrate slides on shaker in 70% EtOH, 95% EtOH and 100% EtOH for 1 hr each. Transfer slides to xylene twice for 1 hr each and keep on shaker. Use toluene-based mounting medium to mount the gels and 1.5 mm thick coverslips.
Examine whole mounts under regular light (microscope or stereoscope). For a more detailed analysis of shape and lumen use confocal microscopy and visualize Carmine dye with HeNE 633 nm laser.
4. Gel Processing for Processing for Histology Analysis
Transfer the remaining half of the gel to a 20 ml glass vial containing 10% formalin and fix on shaker O/N.
Wash gels twice with PBS for 10 min each on shaker. Place gels into processing cassettes for paraffin embedding.
Transfer gels to 70% EtOH, incubate for 1 hr and follow with 95% EtOH for 1 hr, change to fresh 95% EtOH for 1 hr, 100% EtOH for 1 hr, and change to fresh 100% EtOH for 1 hr. At this point, gels may be stored in 100% EtOH O/N at RT or incubated with xylene for 1 hr and then again in fresh xylene for 1 hr.
Transfer cassettes to paraffin for 1 hr at 60 °C in vacuum incubator, repeat with fresh paraffin twice more.
Fill plastic molds with clean paraffin. Open the cassette, remove the gel and place it in the plastic mold containing paraffin. Fill up with paraffin and add top of cassette containing the label on top and let block cool.
Remove the plastic mold and section the block using a microtome. Obtain 5 um thick sections for H-E staining and immunocytochemistry.
5. Extraction of Cells from Cells from Gels Using Collagenase Treatment
Remove gel from the well and transfer it to a petri dish containing PBS. Rinse gel briefly.
Transfer the gel to a 15 ml tube containing 2 ml of 0.125% collagenase. Pipette up and down 4-5 times in order to break the gel into small pieces.
Incubate at 37 °C for 25 min (until gel has dissolved and cells are in suspension)
Add 2 ml of serum supplemented culture medium. Collect cells by centrifugation (1,000 x g, 5 min), discard supernatant, re-suspend cells and count.
Representative Results
Figure 1 summarizes the procedure for preparing the hormone-sensitive 3D cultures. Epithelial structures are observed in whole mounts of gels cultured for 2 weeks in the presence of E2 alone and in combination with other hormones. Only single cells or groups of 2-3 cells are present when no hormones are added to the culture medium (CDFBS medium) (Figure 2). This condition serves as a negative control.
Cells in 3D culture form structures that vary in shape, size, and lumen presence. The distribution of structures in the gel is typically heterogeneous. As shown previously, there are generally more structures along the borders of the gel than seen at the center 4. Because of this spatial heterogeneity, comparisons among specimens should be restricted to corresponding regions across samples.
The species from which collagen type 1 is derived influences the phenotype and quality of the resulting epithelial structures 5. Rat tail collagen type 1, as used here, was chosen since seeding in bovine collagen type 1 resulted in formation of disorganized cell groups (Figure 3).
A detailed analysis of the epithelial structures can be achieved using confocal microscopy (Figure 4). E2 treatment results in rounded and elongated structures (Figure 4A). The presence of E2 and prolactin results in more budding structures (Figure 4B), while the presence of E2 and promegestone results in irregular structures with cellular projections reminiscent of side branching (Figure 4C). E2 alone and E2 plus prolactin thicken tissue structures when measured by growth in the z-direction, and when compared to the ones observed in E2 plus promegestone.
In order to quantify the cell number after hormone treatment, cells are extracted from the gel using collagenase treatment. For example, using this method we can study how the antiestrogen ICI 182,780 inhibits the effect of E2 on cell proliferation in the 3D cultures (Figure 5).
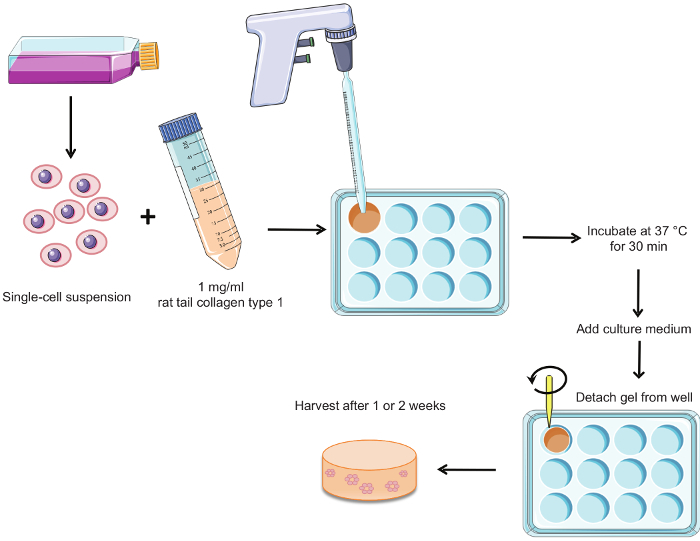 Figure 1. Procedure for the preparation of hormone-sensitive 3D cultures. Briefly, a single-cell suspension of T47D cells is mixed with rat tail collagen type 1 and poured into wells of a 12-well plate. The gels are allowed to congeal for 30 min in a 37° C/5% CO2 incubator. Culture medium is added and the gels are detached from the well. Cultures can be harvested after 1 or 2 weeks according to the end-point. Please click here to view a larger version of this figure.
Figure 1. Procedure for the preparation of hormone-sensitive 3D cultures. Briefly, a single-cell suspension of T47D cells is mixed with rat tail collagen type 1 and poured into wells of a 12-well plate. The gels are allowed to congeal for 30 min in a 37° C/5% CO2 incubator. Culture medium is added and the gels are detached from the well. Cultures can be harvested after 1 or 2 weeks according to the end-point. Please click here to view a larger version of this figure.
 Figure 2. Whole mounts of 3D cultures after staining with Carmine Alum dye.(A) Whole mount of half a gel stained with carmine alum to visualize the epithelial structures, an example of ROI for morphology analysis is indicated by a box; WM of 3D cultures grown for 2 weeks in (B) CDFBS media (no hormones added) and (C) CDFBS media supplemented with E2 and prolactin. Scale bars are 5,000 µm (A) and 500 µm (B&C). Please click here to view a larger version of this figure.
Figure 2. Whole mounts of 3D cultures after staining with Carmine Alum dye.(A) Whole mount of half a gel stained with carmine alum to visualize the epithelial structures, an example of ROI for morphology analysis is indicated by a box; WM of 3D cultures grown for 2 weeks in (B) CDFBS media (no hormones added) and (C) CDFBS media supplemented with E2 and prolactin. Scale bars are 5,000 µm (A) and 500 µm (B&C). Please click here to view a larger version of this figure.
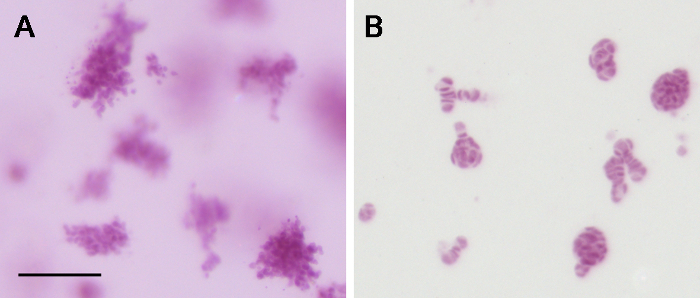 Figure 3. Comparison of matrices used in 3D culture. (A) Bovine collagen type 1 results in disorganized clusters of cells, as opposed to the organized structures (B) observed when using rat tail collagen type 1. Scale bar: 100 µm. Please click here to view a larger version of this figure.
Figure 3. Comparison of matrices used in 3D culture. (A) Bovine collagen type 1 results in disorganized clusters of cells, as opposed to the organized structures (B) observed when using rat tail collagen type 1. Scale bar: 100 µm. Please click here to view a larger version of this figure.
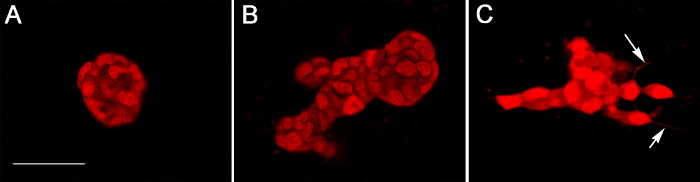 Figure 4. Confocal images of T47D structures observed in 3-D collagen gels after 2 weeks. Treatment with (A) E2, (B) E2 + prolactin and (C) E2 + R5020. Arrows point to cytoplasmic projections. Thickness of epithelial structures was 24, 40 and 20 µm respectively. Scale bar: 40 µm. Please click here to view a larger version of this figure.
Figure 4. Confocal images of T47D structures observed in 3-D collagen gels after 2 weeks. Treatment with (A) E2, (B) E2 + prolactin and (C) E2 + R5020. Arrows point to cytoplasmic projections. Thickness of epithelial structures was 24, 40 and 20 µm respectively. Scale bar: 40 µm. Please click here to view a larger version of this figure.
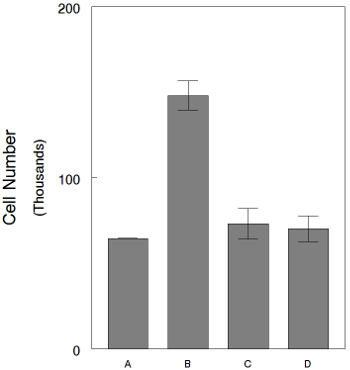 Figure 5. 3D cultures treated with either E2 alone or E2 plus ICI 182,780 for 1 week. Cell counts after cell extraction using collagenase treatment. (A) CDFBS medium, (B) CDFBS medium plus E2 at 1x10-10 M, (C) ICI 182,780 at 1x10-8 M plus E2 at 1x10-10 M and (D) ICI 182,780 at 1x10-7 M plus E2 at 1x10-10 M. Bars: SEM. Please click here to view a larger version of this figure.
Figure 5. 3D cultures treated with either E2 alone or E2 plus ICI 182,780 for 1 week. Cell counts after cell extraction using collagenase treatment. (A) CDFBS medium, (B) CDFBS medium plus E2 at 1x10-10 M, (C) ICI 182,780 at 1x10-8 M plus E2 at 1x10-10 M and (D) ICI 182,780 at 1x10-7 M plus E2 at 1x10-10 M. Bars: SEM. Please click here to view a larger version of this figure.
Discussion
Here, we describe a hormone-sensitive 3D culture model to test the action of hormones on breast epithelium. The response to hormones can be assessed at the tissue morphology, cell proliferation and gene expression levels 12. One limitation of this technique is that visualization during the culture period is restricted to light microscopy since the cultures are grown in a plastic bottom plate. The 3D culture system could be adapted to glass bottom plates to allow for live imaging of the cultures 1.
The way in which cells are maintained appears to be critical for retention of hormone-responsive phenotypes. It is important to frequently check for E2 responsiveness in these cells. We recommend performing an E2 dose-response curve on the fourth passage after thawing a new vial, and routinely after every 10 passages thereafter. An E2 concentration of 10 -10 M will typically yield three times the control cell number in 2D culture and two times in 3D culture when compared to those grown in CDFBS medium for 1 week 12. Phenol red-free media should be used for all experiments involving hormone-responsiveness, due to the proven estrogenicity of phenol red 2. In addition, the brands listed in this protocol are current without hormonal activity. If the estrogenic response in 2D or 3D changes, the plastic materials used for tissue culture should be evaluated for leaching of hormonally-active compounds.
The presence of static electricity during gel preparation may affect cell distribution. This may cause the cells to quickly sink to the bottom of the well during congealing and then grow as a 2D monolayer. In this case, the gel appears mostly empty of cells with them attached directly to the bottom plastic surface. To prevent this effect, it is recommended to brush the borders, top and bottom of the 12-well plate with a static-reducing brush after unwrapping the plate. To avoid static charge buildup during contact with the hood working surface, place this plate on top of another 12-well plate (blank plate) that has also been brushed with the static-reducing brush. These recommendations are especially useful during periods of low ambient humidity.
As opposed to existing "on top" culture models9, the culture system that we describe here allows for the epithelial cells to form 3D structures which are embedded in a matrix. Furthermore, this culture system enables for the study of the influence of extracellular matrix components, such as collagen fibers, and mechanical properties, such as matrix stiffness, in hormone-instructed morphogenesis of 3D epithelial structures 1,12.
The hormone-sensitive 3D culture model described herein also allows for the addition of cell types considered of relevance in breast tissue morphogenesis, such as fibroblasts, adipocytes and circulating blood cells 6,8,10. Finally, testing of hormonal influence on the breast epithelium in this 3D culture model is not limited to endogenous hormones alone. The response to environmental estrogenic compounds and other hormonally-active chemicals can also be tested to uncover their effects and those elicited when tested in the presence of the natural, endogenous steroidal and polypeptidic hormones.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We greatly appreciate the editorial contributions by Cheryl Schaeberle. This research was supported by Avon Grants #02-2009-093 and 02-2011-095, and NIEHS/NIH ES 08314 to AMS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
References
- Barnes C, et al. From single cells to tissues: interactions between the matrix and human breast cells in real time. PLoS ONE. 2014;9:e93325. doi: 10.1371/journal.pone.0093325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red in tissue culture is a weak estrogen: implications concerning the study of estrogen responsive cells in culture. Proc.Nat.Acad.Sci.USA. 1986;83:2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalbos D, Vignon F, Keydar I, Rochefort H. Estrogens stimulate cell proliferation and induce secretory proteins in a human breast cancer cell line (T47D) J.Clin.Endocrinol.Metab. 1982;55:276–283. doi: 10.1210/jcem-55-2-276. [DOI] [PubMed] [Google Scholar]
- Dhimolea E, Maffini MV, Soto AM, Sonnenschein C. The role of collagen reorganization on mammary epithelial morphogenesis in a 3D culture model. Biomaterials. 2010;31:3622–3630. doi: 10.1016/j.biomaterials.2010.01.077. [DOI] [PubMed] [Google Scholar]
- Dhimolea E, Soto AM, Sonnenschein C. Breast epithelial tissue morphology is affected in 3D cultures by species-specific collagen-based extracellular matrix. J.Biomed.Mat.Res.A. 2012;100:2905–2912. doi: 10.1002/jbm.a.34227. [DOI] [PubMed] [Google Scholar]
- Krause S, et al. Dual regulation of breast tubulogenesis using extracellular matrix composition and stromal cells. Tissue Eng Part A. 2012;18:520–532. doi: 10.1089/ten.tea.2011.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause S, Maffini MV, Soto AM, Sonnenschein C. A novel 3D in vitro. culture model to study stromal-epithelial interactions in the mammary gland. Tissue Eng.Part C Methods. 2008;14:261–271. doi: 10.1089/ten.tec.2008.0030. [DOI] [PubMed] [Google Scholar]
- Krause S, Maffini MV, Soto AM, Sonnenschein C. The microenvironment determines the breast cancer cells' phenotype: organization of MCF7 cells in 3D cultures. BMC Cancer. 2010;10:263–275. doi: 10.1186/1471-2407-10-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat.Methods. 2007;4:359–365. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinson HA, Jindal S, Durand-Rougely C, Borges VF, Schedin P. Wound healing-like immune program facilitates postpartum mammary gland involution and tumor progression. Int.J.Cancer. 2014. [DOI] [PMC free article] [PubMed]
- Soto AM, Murai JT, Siiteri PK, Sonnenschein C. Control of cell proliferation: evidence for negative control on estrogen-sensitive T47D human breast cancer cells. Cancer Res. 1986;46:2271–2275. [PubMed] [Google Scholar]
- Speroni L, et al. Hormonal regulation of epithelial organization in a 3D breast tissue culture model. Tissue Eng.Part C Methods. 2014;20:42–51. doi: 10.1089/ten.tec.2013.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignon F, Bardon S, Chalbos D, Rochefort H. Antiestrogenic effect of R5020, a synthetic progestin in human breast cancer cells in culture. J.Clin.Endocrinol.Metab. 1983;56:1124–1130. doi: 10.1210/jcem-56-6-1124. [DOI] [PubMed] [Google Scholar]


