Abstract
More active high-dose regimens are needed for refractory/poor-risk relapsed lymphomas. We previously developed a regimen of infusional gemcitabine/busulfan/melphalan, exploiting the synergistic interaction. Its encouraging activity in refractory lymphomas led us to further enhance its use as a platform for epigenetic modulation. We previously observed increased cytotoxicity in refractory lymphoma cell lines when the histone deacetylase inhibitor vorinostat was added to gemcitabine/busulfan/melphalan, which prompted us to clinically study this four-drug combination. Patients ages 12 to 65 with refractory diffuse large B cell lymphoma (DLCL), Hodgkin (HL), or T lymphoma were eligible. Vorinostat was given at 200 mg/day to 1000 mg/day (days −8 to −3). Gemcitabine was infused continuously at 10 mg/m2/minute over 4.5 hours (days −8 and −3). Busulfan dosing targeted 4000 μM-minute/day (days −8 to −5). Melphalan was infused at 60 mg/m2/day (days −3 and −2). Patients with CD20+ tumors received rituximab (375 mg/m2, days +1 and +8). We enrolled 78 patients: 52 DLCL, 20 HL, and 6 T lymphoma; median age 44 years (range, 15 to 65); median 3 prior chemotherapy lines (range, 2 to 7); and 48% of patients had positron emission tomography–positive tumors at high-dose chemotherapy (29% unresponsive). The vorinostat dose was safely escalated up to 1000 mg/day, with no treatment-related deaths. Toxicities included mucositis and dermatitis. Neutrophils and platelets engrafted promptly. At median follow-up of 25 (range, 16 to 41) months, event-free and overall survival were 61.5% and 73%, respectively (DLCL) and 45% and 80%, respectively (HL). In conclusion, vorinostat/gemcitabine/busulfan/melphalan is safe and highly active in refractory/poor-risk relapsed lymphomas, warranting further evaluation.
Keywords: Vorinostat, Phase 1 trial, High-dose chemotherapy, Autologous stem-cell, transplantation, Lymphoma
Introduction
High-dose chemotherapy (HDC) with regimens such as carmustine/etoposide/cytarabine/melphalan (BEAM) with autologous stem cell transplantation (ASCT) is standard treatment of chemosensitive relapsed lymphomas. Early randomized trials showed benefit from HDC in chemosensitive relapsed diffuse large B cell lymphoma (DLCL) in the pre-rituximab era, as well as in Hodgkin's lymphoma (HL) [1-3]. However, the more recent CORAL trial showed worse outcomes after BEAM ± rituximab in DLCL patients who relapsed after rituximab-cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) than previously seen in patients who relapsed after CHOP alone [4]. Furthermore, particularly poor outcomes were seen in patients with primary refractory or relapsed tumors with poor-risk features, such as a first complete remission (CR) < 12 months, secondary International Prognostic Index (IPI) at relapse > 1, exposure to multiple salvage regimens, or the presence of active tumor at HDC [5-8]. Similar adverse prognostic factors have been described for HL [9,10]. Therefore, there is a clear need to develop more effective HDC regimens for these patient populations.
We previously developed a high-dose combination of gemcitabine/busulfan/melphalan (Gem/Bu/Mel), which showed a synergistic interaction based on gemcitabine inhibition of DNA damage repair [11]. Gemcitabine was infused at a fixed dose rate of 10 mg/m2/minute, optimizing formation of its active intracellular triphosphate metabolite [12,13]. Busulfan was administered intravenously with pharmacokinetic-guided dosing. Gem/Bu/Mel showed encouraging activity in lymphomas. An analysis of concurrent patient cohorts with refractory HL treated with Gem/Bu/Mel, Bu/Mel, or BEAM, showed superior outcomes in the Gem/Bu/Mel cohort despite a higher prevalence of poor-prognosis features [14].
An important factor affecting the activity of DNA-targeting agents is access to DNA. Changes in histone acetylation lead to changes in chromatin configuration. Inhibition of histone deacetylases weakens the histone-DNA bonds and decondenses chromatin [15]. In our prior preclinical experiments in resistant B and T lymphoma cell lines, a synergistic increase in cytotoxicity was observed when the histone deacetylases inhibitor vorinostat (suberanilo hydroxamic acid) was added to gemcitabine/busulfan/melphalan combination [16]. We saw a significant increase in the γ-H2AX level and cleavage of poly-[ADP-ribose]-polymerase-1 (PARP-1), suggesting increased DNA damage response and apoptosis, respectively [17]. Furthermore, there was important sequence specificity, and concurrent treatment was more active than sequential drug exposures.
These intriguing preclinical observations motivated us to test vorinostat/Gem/Bu/Mel clinically. Prior studies have shown that the duration of histone hyperacetylation is proportional to vorinostat dose [18]. We hypothesized that vorinostat can be safely combined with Gem/Bu/Mel with ASCT. We report here the results of our first dose-finding study of vorinostat with Gem/Bu/Mel in patients with refractory/poor-risk relapsed Hodgkin's and non-Hodgkin's lymphomas.
Patients and Methods
Patient Population
The study protocol was approved by the clinical research committee and institutional review board of MD Anderson Cancer Center. Patients provided written informed consent before enrollment. Eligibility included ages 12 to 65 and 1 of the following lymphomas: (1) DLCL with primary refractory disease (less than CR or induction failure after R-CHOP), relapse within 12 months of R-CHOP, secondary IPI >1, less than partial response to first salvage chemotherapy, or prior treatment with ≥ 3 chemotherapy lines; (2) HL with primary refractory disease (progression during or within 3 months of frontline chemotherapy), relapse within 12 months of frontline chemotherapy, within a prior irradiation field, extranodal, or bulky (defined as any lesion > 5 cm) disease, less than metabolic CR to second-line chemotherapy, or second relapse or beyond; and (3) refractory/relapsed T lymphoma. Additional eligibility criteria included adequate renal (creatinine clearance ≥ 50 mL/minute), hepatic (serum glutamic oxaloacetic transaminase [SGOT]/serum glutamic pyruvic transaminase [SGPT]/bili-rubin ≤ 3 × upper normal limit, pulmonary (forced expiratory volume in 1 second [FEV1]/forced vital capacity [FVC]/corrected diffusing lung capacity for carbon monoxide [cDLCO] ≥ 50%) and cardiac function (left ventricular ejection fraction ≥ 40%), performance status 0 to 1, no prior whole brain irradiation or radiation within 1 month of enrollment, no active hepatitis B, and no chronic hepatitis C causing cirrhosis/stage 3 to 4 fibrosis.
Tumors were restaged within 30 days before enrollment, at 1, 3, and 6 months after HDC, and every 6 months thereafter. Responses were assessed before planned post-HDC radiotherapy. Positron emission tomography (PET) scans were interpreted with mediastinal blood pool activity as reference background [19].
HDC
Patients received an intravenous test dose of busulfan of 32 mg/m2 over 60 minutes during the preadmission week (Table 1). Vorinostat was administered from days −8 to −2 at doses ranging from 200 to 1000 mg per oral daily per cohort (Table 2) within 1 hour before the start of chemotherapy. Gemcitabine was administered on days −8 and −3 as a loading bolus of 75 mg/m2 followed by a continuous infusion per cohort, immediately followed by busulfan or melphalan. Busulfan was infused daily over 3 hours from days −8 to −5 targeting an average daily area under the curve of 4000 μM-minute, with the first 2 therapeutic doses calculated from the pharmacokinetics after the test dose. If necessary, the third and fourth doses were readjusted after the first therapeutic dose analysis, targeting an aggregate course area under the curve of 16,000 μM-minute. The sampling and analytical processes have been described previously [20,21]. A fixed busulfan dose of 100 mg/m2/day would be given in cases where pharmacokinetic dosing was not feasible. Melphalan was administered at 60 mg/m2/day over 30 minutes on days −3 and −2.
Table 1. Treatment Schedule.
| Day | … | −9 | −8 | − 7 | −6 | −5 | −4 | −3 | −2 | − 1 | 0 | +1 | +8 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Admission | Х | ||||||||||||
| Vorinostat | Х | Х | Х | Х | Х | Х | Х | ||||||
| Gemcitabine | Х | Х | |||||||||||
| Busulfan | x (test)* | Х | Х | Х | Х | ||||||||
| Melphalan | Х | Х | |||||||||||
| PBPC | Х | ||||||||||||
| Rituximab (CD20+ tumors) | Х | Х |
PBPC indicates peripheral blood progenitor cells.
Busulfan test dose to be administered in the week before admission.
Table 2. Dose Escalation.
| Level | Vorinostat (mg/day) | Gemcitabine (mg/m2/day) | Busulfan (μm-min/day or mg/m2/day) | Melphalan (mg/m2/day) | |
|---|---|---|---|---|---|
|
| |||||
| Loading Dose | Continuous Infusion* | ||||
| 1 | 200 | 75 | 2100 | 4000 or 105 | 60 |
| 2 | 300 | 75 | 2100 | 4000 or 105 | 60 |
| 3 | 400 | 75 | 2100 | 4000 or 105 | 60 |
| 4 | 500 | 75 | 2100 | 4000 or 105 | 60 |
| 5 | 600 | 75 | 2100 | 4000 or 105 | 60 |
| 6 | 600 | 75 | 2400 | 4000 or 105 | 60 |
| 7 | 600 | 75 | 2700 | 4000 or 105 | 60 |
| 8 | 700 | 75 | 2700 | 4000 or 105 | 60 |
| 9 | 800 | 75 | 2700 | 4000 or 105 | 60 |
| 10 | 900 | 75 | 2700 | 4000 or 105 | 60 |
| 11 | 1000 | 75 | 2700 | 4000 or 105 | 60 |
Continuous infusion of gemcitabine administered at 10 mg/m2/minute.
Supportive Care
Acetaminophen, azoles, and metronidazole were avoided from day −10 to −1. Phenytoin 300 mg/day to 600 mg/day was given from day −9 to −4. Dexamethasone 8 mg i.v. was given twice daily from day −9 to −2. Intravenous hydration started on admission until day −1. Oral care with palifermin, glutamine, and supersaturated calcium/phosphate rinses, and oral cryotherapy during melphalan, was performed uniformly as previously described [11]. Infusion of peripheral blood progenitor cells was on day 0. Departmental guidelines for post-transplantation filgrastim, antiemetics, antimicrobials, and blood product transfusions were followed.
Trial Design
The primary endpoint was to determine the maximum tolerated dose of vorinostat, up to a maximum planned dose (MPD) of 1000 mg/day, combined with Gem/Bu/Mel, based on dose-limiting toxicity (DLT), defined as any grade 4 nonhematological and noninfectious toxicity, or any grade 3 mucositis or skin toxicity lasting more than 3 days at peak severity. Secondary endpoints included event-free survival (EFS), overall survival (OS), response, and CR rates and to describe the toxicity profile. Dose finding used the continual reassessment method with a target DLT probability per cohort of 20% [22]. In stage 1 of the trial, doses were chosen adaptively for successive cohorts of minimum size 2, up to dose level 11 with a MPD of vorinostat of 1000 mg/day (Table 2). If the lowest dose level were found to be excessively toxic, the trial would move to stage 2 using a lower dose of gemcitabine. Toxicity scoring followed the NCI Common Toxicity Criteria, v3.0 [23].
Correlative Studies of DNA Damage Repair and Apoptosis
The phosphorylation status of histone 2AX (γ-H2AX) and the level of PARP-1 were determined in peripheral blood mononuclear cells from patients enrolled in this study at the highest dose of suberanilo hydroxamic acid (1000 mg/day) and in separate trials of Gem/Bu/Mel and Bu/Mel, all of which followed a parallel schedule from day −8 to −2 using the same drug doses as in the present studies. Samples were collected at baseline, day −7 (1 hour after busulfan), and day −2 (1 hour after melphalan). The γ-H2AX level was measured by immunofluorescent staining and flow cytometry using anti-γ-H2AX antibody (EMD Millipore, Billerica, MA) [24]. Western blot assay used protein extract separation on polyacrylamide-SDS gels and blotting onto nitrocellulose membranes probed with anti−PARP-1 (Santa Cruz, Dallas, TX) and anti-β-actin (Sigma-Aldrich, St Louis, MO) antibodies. Chemiluminescence immunoblot analysis used Immobilon Western Chemiluminescent HRP Substrate (EMD Millipore). X-ray films were scanned with the EPSON Perfection V750 PRO and analyzed with UN-SCAN-IT software (Silk Scientific, Orem, UT). PARP-1 bands were normalized relative to β-actin. Two-way comparisons of the levels of γ-H2AX and PARP-1 between the groups used the t-test. A sample size of 15 patients in each group offered 80% power to detect differences between the vorinostat/Gem/Bu/Mel, Gem/Bu/Mel, and Bu/Mel groups, with estimated median relative change in levels of γ-H2AX and PARP-1 of 3 fold, 2 fold, and .5 fold, respectively.
Statistical Methods
Overall response (OR) and CR rates were calculated among patients with measurable disease at HDC following the usual criteria [25]. EFS was defined as the time from transplantation to either relapse or death, whichever occurred first, or last contact. OS was defined as the time from transplantation to death or last contact. Kaplan-Meier survival curves estimated unadjusted time-to-event distributions [26]. The log-rank test was used to compare EFS and OS between subgroups [27]. Categorical variables were compared by generalized Fisher's exact test [28]. P values are 2 sided. All calculations used R v2.12.1 and Open BUGS v3.1.2 rev668.
Results
Patient Enrollment
Seventy-eight patients were enrolled between September 2011 and November 2013 (Table 3). Median age was 44 (range,15 to 65). Diagnoses were DLCL (n = 52), HL (n = 20), and T non-Hodgkin lymphoma (T-NHL) (n = 6). Patients had received a median of 3 prior regimens (range, 2 to 7) and had extensive tumor involvement (median, 3 organs). Of 52 patients with DLCL, 26 had primary refractory tumors, 13 were in first relapse (6, 6, and 1 patients had a secondary IPI of 0 to 1, 2 to 3, and >3, respectively), and 13 had >1 prior relapse. Ten patients had double-hit DLCL. In the HL sub-group,13 patients (65%) had a primary refractory tumor, and extranodal disease and bulky tumor at relapse/progressive disease (PD) were present in 12 and 11 patients, respectively; 6 patients had >1 prior relapse. Four of 6 patients with T-NHL had primary refractory disease. At HDC, 48% of all patients had PET-positive tumors and 26% of patients had unresponsive disease (PD in 17% of them).
Table 3. Patient Characteristics (n = 78).
| Characteristic | Value |
|---|---|
| Age: median (range), yr | 44 (15-65) |
| Gender: Male/female | 53/25 |
| Primary refractory tumor/poor-risk or refractory relapse | 57%/43% |
| No. prior chemotherapy lines, median, (range) | 3 (1-7) |
| Prior radiotherapy, % | 43% |
| Disease status at HDC: CR/PR/no response, % | 52%/22%/26% |
| PD at HDC | 17% |
| Diagnoses | |
| DLCL | 52 |
| Variants | |
| NOS | 39 |
| T cell rich | 4 |
| Primary CNS | 2 |
| Anaplastic | 2 |
| Plasmablastic | 2 |
| Transformed | 3 |
| Double hit (% of DLCL) | 10 (19%) |
| Cell of origin | |
| Germinal center | 15 |
| Activated B cell | 12 |
| Primary mediastinal | 8 |
| Not determined | 17 |
| Primary refractory (%) (induction PR/induction failure) | 26 (50%) (14/12) |
| Relapsed (%) | 26 (50%) |
| First relapse (%) | 13 (25%) |
| Length of CR1: <12months/≥12 months | 6/7 |
| Secondary IPI: 0-1/2/>3 | 6/6/1 |
| Second relapse or later (%) | 13 (25%) |
| Hodgkin's | 20 |
| Primary refractory (% of Hodgkin's) | 13 (65%) |
| Poor-risk relapse | 7 |
| First relapse (%) | 1 (CR1 ≤12 months) (5%) |
| ≥Second relapse (%) | 6 (30%) |
| Extranodal disease at relapse/PD | 12 |
| Bulky tumor at relapse/PD | 11 |
| B symptoms at relapse/PD | 2 |
| T-NHL | 6 |
| Histology (PTCL NOS/AITL/ALCL/NK-T) | 2/2/1/1 |
| Primary refractory/relapsed | 4/2 |
PR indicates partial response; NOS, not otherwise specified; CNS, central nervous system; PTCL, peripheral T-cell lymphoma; AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large-cell lymphoma; NK-T, natural killer T cell.
Hematologic Recovery
The stem cell source was peripheral blood. Neutrophil and platelets engrafted promptly at medians of 10 days (range, 8 to 13) and 12 days (range, 8 to 55), respectively.
Regimen-Related Toxicities
Dose escalation of vorinostat proceeded from 200 mg/day (dose level 1) up to its MPD of 1000 mg/day (dose level 11), combined with Gem/Bu/Mel (Table 2). The MPD level was expanded to 22 patients to fully characterize its toxicity profile. No regimen-related deaths or grade 4 toxicities were seen in the study. The side effect profile of vorinostat/Gem/Bu/Mel was as follows.
Mucositis
Grade 2 and 3 mucositis were observed in 45% and 32% of patients, respectively, in levels 1 to 10, and in 48% and 38% of patients, respectively, at level 11. It started at median day +4 (range, day 0 to +7), lasting at maximal severity for a median of 3 (range, 1 to 9) days.
Dermatitis
Grade 1 and 2 erythematous rashes were common. Six patients had a grade 3 rash, 2 of them at level 11. All cases resolved spontaneously or with topical sunburn remedies or topical steroids.
Hepatic effects
Early self-limited transaminase elevation was frequent across all levels (6 patients grade 3, 21 patients grade 2), starting on median day −1 (range, day −7 to 9), peaking at a median 146 (range, 43 to 930) IU/L and resolving within 1 week. Transient hyperbilirubinemia at median 2.1 mg/dL, (range, 1.2 to 9.7) was seen in 51% patients in the first week after transplantation (12 patients grade 3, 19 patients grade 2), with no cases of veno-occlusive disease.
Pulmonary effects
DLCO decreased from before (median, 78.5% of predicted; range, 52% to 152%) to 1 to 3 months after transplantation (median, 71%; range, 31% to 150%) (P = .002). There were 3 symptomatic cases of steroid-responsive grade 2 pneumonitis.
Other toxicities
Diarrhea was mild, with only 12 and 4 cases of grade 2 and grade 3 diarrhea, respectively. Two patients experienced grade 2 renal toxicity. No neurological or cardiac toxicities were observed. Specifically, there was no QTc prolongation from before HDC (median, 462; range 425 to 599 milliseconds) to day −2 (median, 451; range 418 to 497 milliseconds).
There was no correlation of preadmission values of C-reactive protein, B-type natriuretic peptide, ferritin, haptoglobin, or troponin with toxicity (data not shown). Likewise, there was no significant effect of age on toxicity (data not shown).
Infections
Two patients had antibiotic-responsive methicillin-resistant Staphylococcus aureus pneumonia. The following documented infections resolved with antimicrobials: Candidemia (n = 1), Stenotrophomonas bacteremia/urinary tract infection (UTI) (n = 1), S epidermidis bacteremia (n = 1), cytomegalovirus pneumonia (n = 1), respiratory syncytial virus upper (n = 1) and lower (n = 1) respiratory infections, Herpes simplex esophagitis (n = 1), Enterococcus UTI (n = 1), and Pseudomonas aeruginosa UTI (n = 1).
Busulfan Pharmacokinetic Studies
Busulfan pharmacokinetics were calculated in all patients. The overall mean of the variation of the calculated test-to-therapeutic clearance was 6.3% (95% confidence interval, −9.9% to 19.5%). Only 3 patients showed a busulfan clearance > 20% different when the first and test doses were compared. For the remaining 75 patients, the clearance variation between the test and the first therapeutic doses was <20%. The mean (% coefficient of variation) population clearance, volume of distribution, and plasma half-life from the first therapeutic dose were 97 mL/minute/m2 (16.3%), 24.2 L/m2 (16%), and 2.9 hours (14.7%), respectively. These population pharmacokinetics do not differ from those previously estimated with Bu/Mel [21] or Gem/Bu/Mel [11] (data not shown).
Tumor Responses
The OR and CR rate among 28 patients with DLCL and measurable disease were 96% and 73%, respectively. The OR and CR rate among 14 patients in PD at the time of HDC were 100% and 64%, respectively.
Eight of 10 patients with measurable HL (5 of them in PD at HDC) had a CR. Two of 2 patients with measurable T-NHL (both in PD at HDC) experienced a CR.
Post-HDC Treatment
Seventeen patients (9 DLCL, 6 NHL, 1 T-NHL, 1 plasmablastic lymphoma [PL]) with pretransplantation bulky (>5 cm) PET-positive lesions received involved site radiotherapy to 30.6 to 42 Gy starting 1 to 2 months after transplantation, with good tolerance. Irradiated sites were mediastinum (n = 8), axillary/supraclavicular lymph nodes (n = 3), chest wall (n = 2), thigh (n = 2), hip (n = 1), and splenectomy bed (n = 1).
Patient Outcomes
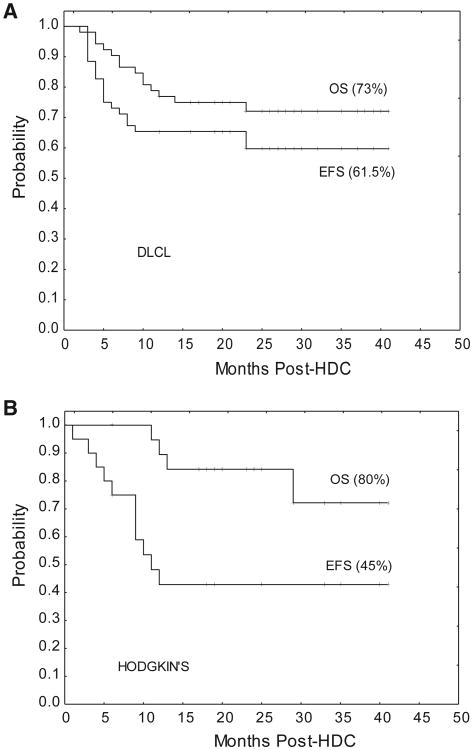
Median follow-up is 25 months (range, 16 to 41). Only 1 relapse has occurred beyond 1 year after HDC. The EFS and OS rates of the DLCL group were 61.5% and 73%, respectively (Figure 1A). The fraction of patients alive in CR within the different DLCL subgroups are 6 of 8 primary mediastinal, 6 of 10 double hit, 4 of 4 T cell rich, 2 of 2 plasmablastic, and 2 of 2 primary central nervous system. As per cell-of-origin type, 10 of 15 patients in the germinal center and 8 of 12 patients in the activated B cell categories remain in CR.
Figure 1.
Event-free (EFS) and overall survival (OS) curves. (A) DLCL subgroup (n = 52). (B) HL subgroup (n = 20).
The EFS and OS rates among HL patients were 45% and 80%, respectively (Figure 1B). Lastly, 5 of 6 patients with T-NHL were alive in CR at 16 to 29 months after HDC.
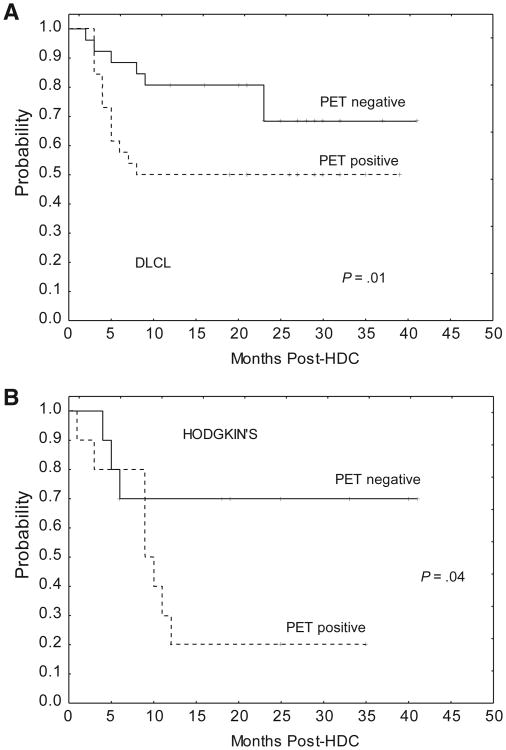
As expected, patients with PET-positive tumors had worse EFS than those with PET-negative tumors at HDC, both in the DLCL (50% versus 81%, P = .01) (Figure 2A) and HL groups (27% versus 73%, P = .04) (Figure 2B).
Figure 2.
Event-free survival according to PET status at transplantation. (A) DLCL subgroup. (B) HL subgroup.
DNA Damage Response and Apoptosis Studies
The levels of γ-H2AX increased after Bu/Mel (n = 19) from baseline to day −2 by a median 1.4-fold (Figure 3A). γ-H2AX increased on day -2 after Gem/Bu/Mel (n = 16) and vorinostat/Gem/Bu/Mel (n = 18) by 5.8-fold and 5.1-fold, respectively (P < .05 for each of these 2 groups compared to Bu/Mel, P = not significant between them). These results suggest activation of DNA damage response in patients who received Gem/Bu/Mel ± vorinostat, consistent with our previous in vitro data.
Figure 3.
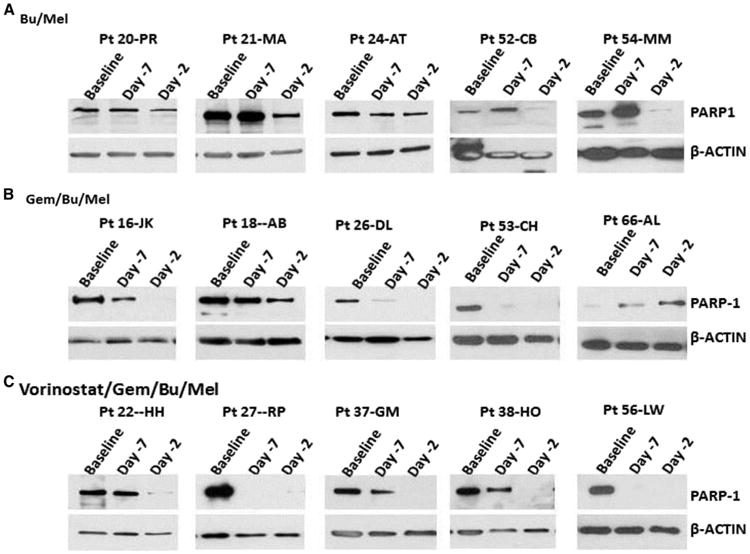
Representative cases of decrease in the level of PARP-1 in patients treated with Bu/Mel (A), Gem/Bu/Mel (B), and vorinostat/Gem/Bu/Mel (C).
The levels of PARP-1 of Bu/Mel patients did not change significantly on day −7 (median, 1.2), and did only slightly on day −2 (median, .5) (Figure 3B). A steeper day −7 drop was observed after Gem/Bu/Mel (median, .5) and vorinostat/Gem/Bu/Mel (median, .4). PARP-1 was undetectable on day −2 in some Gem/Bu/Mel patients (median, .1) and in almost all vorinostat/Gem/Bu/Mel patients (median, .0). The relative baseline/day −2 PARP-1 decrease was more pronounced after vorinostat/Gem/Bu/Mel compared with after Gem/Bu/Mel (P = .05) or Bu/Mel (P = .003).
Discussion
Our study shows that vorinostat can be safely added to Gem/Bu/Mel. The schedule includes a daily dose of vorinostat, at its MPD of 1000 mg/day, preceding full doses of Gem/Bu/Mel.
Confirming our preclinical studies, we saw high activity of vorinostat/Gem/Bu/Mel among patients with heavily pre-treated and refractory lymphoma, mostly DLCL and HL. At a median follow-up of 25 months, the EFS rates in these 2 groups are 64% and 45%, respectively. Although longer follow-up is needed, these outcomes are encouraging, as most relapses in these high-risk populations typically occur early after transplantation [4-10]. Given the limitations of this study, intrinsic to early clinical trials, these results should be considered preliminary. Determining whether vorinostat/Gem/Bu/Mel is superior to BEAM for any type of lymphoma will need a randomized study.
To our knowledge, this is the first report of the combination of an epigenetically active agent with HDC. This study builds on our prior clinical work with Gem/Bu/Mel, a regimen designed to exploit the synergistic inhibition exerted by gemcitabine on DNA damage repair [11]. The use of peripheral blood progenitor cells circumvents the increased myelotoxicity of infusional gemcitabine, allowing us to capitalize on its increased antitumor activity, compared to shorter infusions of this drug. Our prior observations of high activity of Gem/Bu/Mel in lymphoid malignancies prompted us to use this regimen as a platform for epigenetic HDC modulation. We previously observed that vorinostat augmented the in vitro cytotoxicity of gemcitabine, busulfan, and melphalan in resistant lymphoma B cell and Tcell lines [16]. This effect could be, at least in part, mediated through activation of the DNA damage response signaling pathway, as shown by increased γ-H2AX [16]. The present observations in patient samples are also indicative of increased genomic injury that may, among other responses, lead to apoptosis. In our previous preclinical experiments, apoptosis was detected by cleavage of PARP-1. Interestingly, patients enrolled in this study experienced a decrease or disappearance of PARP-1, rather than its cleavage. Because PARP-1 is a DNA-repair protein [29], our present observations indicate a serious compromise of the ability of tumor cells to repair drug-induced damaged DNA. Because vorinostat has multiple effects on protein acetylation besides histone acetylation, other potentiating mechanisms of the effect of Gem/Bu/Mel are also possible.
Previous reports of vorinostat combined with standard-dose chemotherapy in patients with various tumors had shown more prolonged myelosuppression but no significant increase of nonhematological toxicities [30-33]. The side effects of vorinostat/Gem/Bu/Mel in our study were manageable, including mucositis, dermatitis, and clinically silent elevation of liver function tests. This toxicity profile is similar to that we previously described with Gem/Bu/Mel [11]. Buglio et al. reported an in vitro dose response effect of vorinostat in lymphoma cell lines from 1 μM up to 5 μM [34]. By simple extrapolation from this range of in vitro levels, we designed the dose escalation of vorinostat from 200 mg (which results in a level of around 1 μM [18]) up to a MPD of 1000 mg (which approximately corresponds to 5 μM, the upper limit of the range of concentrations where those authors observed an in vitro dose-response effect) [34]. Because a daily dose of 1000 mg was well tolerated, we consider it the dose of vorinostat to be combined with Gem/Bu/Mel in future studies.
We did not observe DLTs at the MPD of vorinostat. It would be useful to identify predictive markers of toxicity. Previous reports have correlated high pretransplantation values of C-reactive protein, ferritin, or brain natriuretic peptide (BNP) with severe transplantation-related toxicity [35-37]. Likewise, haptoglobin levels have been inversely associated with gemcitabine hematological toxicity [38]. In contrast, we could not establish any correlation between those markers, or any patient characteristic, and toxicity. It is possible that polymorphic genetic variation of relevant enzymes involved in the metabolism of Gem/Bu/Mel might predict toxicity.
Finally, busulfan pharmacokinetics were similar to those we previously estimated with Gem/Bu/Mel [11], indicating no pharmacokinetic interaction between busulfan and vorinostat.
In conclusion, vorinostat up to 1000 mg daily can be safely combined with Gem/Bu/Mel with ASCT. This regimen induced high CR rates, promising early outcomes, and encouraging correlative in vitro data from patients with refractory or poor prognosis relapsed Hodgkin's and NHL. Further investigation of this combination is warranted.
Acknowledgments
This trial was registered at ClinicalTrials.gov (NCI-2011-02891).
Financial disclosure: Grant in aid from Otsuka Pharmaceutical Development and Commercialization Inc. (Y.N.) and National Cancer Institutes grant P30 CA016672 (P.T.)
Footnotes
Conflict of interest statement: There are no potential conflicts of interest.
References
- 1.Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin's disease: results of a BNLI randomised trial. Lancet. 1993;341:1051–1054. doi: 10.1016/0140-6736(93)92411-l. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autolo-gous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;359:2065–2071. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 3.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 4.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blay J, Gomez F, Sebban C, et al. The International Prognostic Index correlates to survival in patients with aggressive lymphoma in relapse: analysis of the PARMA trial. Parma Group. Blood. 1998;92:3562–3568. [PubMed] [Google Scholar]
- 6.Guglielmi C, Gomez F, Philip T, et al. Time to relapse has prognostic value in patients with aggressive lymphoma enrolled onto the Parma trial. J Clin Oncol. 1998;16:3264–3269. doi: 10.1200/JCO.1998.16.10.3264. [DOI] [PubMed] [Google Scholar]
- 7.Caballero MD, Pérez-Simón JA, Iriondo A, et al. High-dose therapy in diffuse large cell lymphoma: results and prognostic factors in 452 patients from the GEL-TAMO Spanish Cooperative Group. Ann Oncol. 2003;14:140–151. doi: 10.1093/annonc/mdg008. [DOI] [PubMed] [Google Scholar]
- 8.Vose JM, Zhang MJ, Rowlings PA, et al. Autologous transplantation for diffuse aggressive non-Hodgkin's lymphoma in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol. 2011;19:406–413. doi: 10.1200/JCO.2001.19.2.406. [DOI] [PubMed] [Google Scholar]
- 9.Lazarus HM, Loberiza FR, Jr, Zhang MJ, et al. Autotransplants for Hodgkin's disease in first relapse or second remission: a report from the autologous blood and marrow transplant registry (ABMTR) Bone Marrow Transplant. 2001;27:387–396. doi: 10.1038/sj.bmt.1702796. [DOI] [PubMed] [Google Scholar]
- 10.Sureda A, Constans M, Iriondo A, et al. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin's lymphoma autografted after a first relapse. Ann Oncol. 2005;16:625–633. doi: 10.1093/annonc/mdi119. [DOI] [PubMed] [Google Scholar]
- 11.Nieto Y, Thall P, Valdez B, et al. High-dose infusional gemcitabine combined with busulfan and melphalan with autologous stem-cell transplantation in patients with refractory lymphoid malignancies. Biol Blood Marrow Transplant. 2012;18:1677–1686. doi: 10.1016/j.bbmt.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grunewald R, Kantarjian H, Du M, et al. Gemcitabine in leukemia: a phase I clinical, plasma, and cellular pharmacology study. J Clin Oncol. 1992;10:406–413. doi: 10.1200/JCO.1992.10.3.406. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi V, Plunkett W, Du M, et al. Prolonged infusion of gemcitabine: clinical and pharmacodynamic studies during a phase I trial in relapsed acute myelogenous leukemia. J Clin Oncol. 2002;20:665–673. doi: 10.1200/JCO.2002.20.3.665. [DOI] [PubMed] [Google Scholar]
- 14.Nieto Y, Popat U, Anderlini P, et al. Autologous stem cell transplantation for refractory or poor-risk relapsed Hodgkin's lymphoma: effect of the specific high-dose chemotherapy regimen on outcome. Biol Blood Marrow Transplant. 2013;19:410–417. doi: 10.1016/j.bbmt.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 16.Valdez BC, Nieto Y, Murray D, et al. Epigenetic modifiers the synergistic cytotoxicity of combined nucleoside analog-DNA alkylating agents in lymphoma cell lines. Exp Hematol. 2012;40:800–810. doi: 10.1016/j.exphem.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogakou EP, Pilch DR, Orr AH, et al. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 18.Kelly WK, O'Connor OA, Krug LM, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23:3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–578. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 20.De Lima M, Couriel D, Thall PF, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloa-blative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 21.Kebriaei P, Madden T, Kazerooni R, et al. Intravenous busulfan plus melphalan is a highly effective, well-tolerated preparative regimen for autologous stem cell transplantation in patients with advanced lymphoid malignancies. Biol Blood Marrow Transplant. 2011;17:412–420. doi: 10.1016/j.bbmt.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Quigley J, Pepe M, Fisher L. Continual reassessment method: A practical design for phase I clinical trial in cancer. Biometrics. 1990;46:33–48. [PubMed] [Google Scholar]
- 23.NCI Common Toxicity Critera v 3.0. Available at: http://ctep.cancer.gov/protocoldevelopment/electronic…/ctcaev3.pdf.
- 24.Ewald B, Sampath D, Plunkett W. H2AX phosphorylation marks gemcitabine-induced stalled replication forks and their collapse upon S-phase checkpoint abrogation. Mol Cancer Ther. 2007;6:1239–1248. doi: 10.1158/1535-7163.MCT-06-0633. [DOI] [PubMed] [Google Scholar]
- 25.Cheson BD, Pfistner B, Juweid ME, et al. International Harmonization Project on Lymphoma. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 27.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–176. [PubMed] [Google Scholar]
- 28.Fisher R. On the interpretation of ×2 from contingency tables, and the calculation of P. J Royal Stat Soc. 1922;85:87–94. [Google Scholar]
- 29.D'Amours D, Desnoyers S, D'Silva I, Poirier GG. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem J. 1999;342:249–268. [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Manero G, Tambaro FP, Bekele NB, et al. Phase II trial of vorinostat with idarubicin and cytarabine for patients with newly diagnosed acute myelogenous leukemia or myelodysplastic syndrome. J Clin Oncol. 2012;30:2204–2210. doi: 10.1200/JCO.2011.38.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munster PN, Marchion D, Thomas S, et al. Phase I trial of vorinostat and doxorubicin in solid tumors: Histone deacetylase 2 expression as a predictive marker. Br J Cancer. 2009;101:1044–1050. doi: 10.1038/sj.bjc.6605293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fakih MG, Pendyala L, Fetterly G, et al. A phase I, pharmacokinetic and pharmacodynamic study of vorinostat in combination with 5-fluorouracil, leucovorin, and oxaliplatin in patients with refractory colorectal cancer. Clin Cancer Res. 2009;15:3189–3195. doi: 10.1158/1078-0432.CCR-08-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramalingam SS, Maitland ML, Frankel P, et al. Carboplatin and pacli-taxel in combination with either vorinostat or placebo for first-line therapy of advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:56–62. doi: 10.1200/JCO.2009.24.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buglio D, Georgakis GV, Hanabuchi S, et al. Vorinostat inhibits STAT6-mediated TH2 cytokine and TARC production and induces cell death in Hodgkin lymphoma cell lines. Blood. 2008;112:1424–1433. doi: 10.1182/blood-2008-01-133769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Artz AS, Wickrema A, Dinner S, et al. Pretreatment C-reactive protein is a predictor for outcomes after reduced-intensity allogeneic hemato-poietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:1209–1216. doi: 10.1016/j.bbmt.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim ZY, Fiaccadori V, Gandhi S, et al. Impact of pre-transplant serum ferritin on outcomes of patients with myelodysplastic syndromes or secondary acute myeloid leukaemia receiving reduced intensity conditioning allogeneic haematopoietic stem cell transplantation. Leuk Res. 2010;34:723–727. doi: 10.1016/j.leukres.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 37.Kataoka K, Nannya Y, Iwata H, et al. Plasma brain natriuretic peptide is associated with hepatic veno-occlusive disease and early mortality after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45:1631–1637. doi: 10.1038/bmt.2010.26. [DOI] [PubMed] [Google Scholar]
- 38.Matsubara J, Ono M, Negishi A, et al. Identification of a predictive biomarker for hematologic toxicities of gemcitabine. J Clin Oncol. 2009;27:2261–2268. doi: 10.1200/JCO.2008.19.9745. [DOI] [PubMed] [Google Scholar]