Abstract
Objective
To determine the factors associated with thyroid cancer, focusing on first-degree family history and ultrasonography (US) features, in euthyroid asymptomatic patients with thyroid nodules.
Materials and Methods
This retrospective study included 1310 thyroid nodules of 1254 euthyroid asymptomatic patients who underwent US-guided fine-needle aspiration biopsy between November 2012 and August 2013. Nodule size and clinical risk factors–such as patient age, gender, first-degree family history of thyroid cancer, multiplicity on US and serum thyroid stimulating hormone (TSH) levels–were considered together with US features to compare benign and malignant nodules. Multiple logistic regression analysis was performed to assess the risk of thyroid malignancy according to clinical and US characteristics.
Results
Although all of the clinical factors and US findings were significantly different between patients with benign and malignant nodules, a solitary lesion on US (p = 0.041–0.043), US features and male gender (p < 0.001) were significant independent risk factors for thyroid malignancy in a multivariate analysis. Patient age, a first-degree family history of thyroid cancer and high normal serum TSH levels did not independently significantly increase the risk of thyroid cancer. However, multicollinearity existed between US assessment and patient age, first-degree family history of thyroid cancer and serum TSH values.
Conclusion
Ultrasonography findings should be the primary criterion used to decide the management of euthyroid asymptomatic patients with thyroid nodules. The concept of first-degree family history as a risk factor for thyroid malignancy should be further studied in asymptomatic patients.
Keywords: Thyroid cancer, First-degree family history, Ultrasonography, Risk factor
INTRODUCTION
Some subtypes of thyroid cancer have several well-known hereditary traits. The RET (tyrosine kinase receptor) proto-oncogene mutation, a proven genetic mechanism of medullary thyroid cancer, is found in both patients with multiple endocrine neoplasia type 2 and patients with sporadic cancers. Nonmedullary thyroid cancers (NMTCs) can also arise as thyroid involvement of genetic syndromes that result in multisystemic neoplasms. Causative mutations, such as inactivation of the adenomatous polyposis coli gene in familial adenomatous polyposis or Gardener's syndrome, the phosphate and tensin homolog gene mutation in Cowden's syndrome, and the PRKAR1α mutation in Carney's complex, have been reported (1). If a patient diagnosed as NMTC without the abovementioned genetic syndromes has two or more first-degree relatives with a history of thyroid cancer, he/she corresponds with the definition of familial nonmedullary thyroid cancer (FNMTC) (2), a condition suggested to be a subentity of NMTC that is inherited in an autosomal dominant pattern with incomplete penetrance (3).
Because the causative gene has not been identified, the only way to differentiate sporadic and familial cases is by the patients themselves reporting the number of affected first-degree relatives. However, as thyroid cancer has become one of the most common malignancies (4), a greater number of people will have a family history of thyroid cancer in the future. Today, ultrasonography (US) together with the serum hormone test for thyroid function is considered the gold standard modality for initial evaluation of thyroid nodules (5). Guidelines recommend the assessment of several clinical risk factors for thyroid malignancy, including family history, when planning the management of thyroid nodules (5,6). However, the importance of known clinical risk factors reported before the era of widespread health checkups should be reconsidered. To our knowledge, there has been no study regarding family history as a risk factor for thyroid cancer in the euthyroid asymptomatic population that included US analysis as a confounding factor. Therefore, we investigated the factors associated with the presence of thyroid cancer, focusing on first-degree family history and US features, in euthyroid asymptomatic patients with thyroid nodules.
MATERIALS AND METHODS
The Institutional Review Board of our institution approved this retrospective observational study and required neither patient approval nor informed consent for review of patient images and records. However, written informed consent was obtained from all patients prior to US-guided fine needle aspiration (US-FNA) as part of daily practice.
Patients
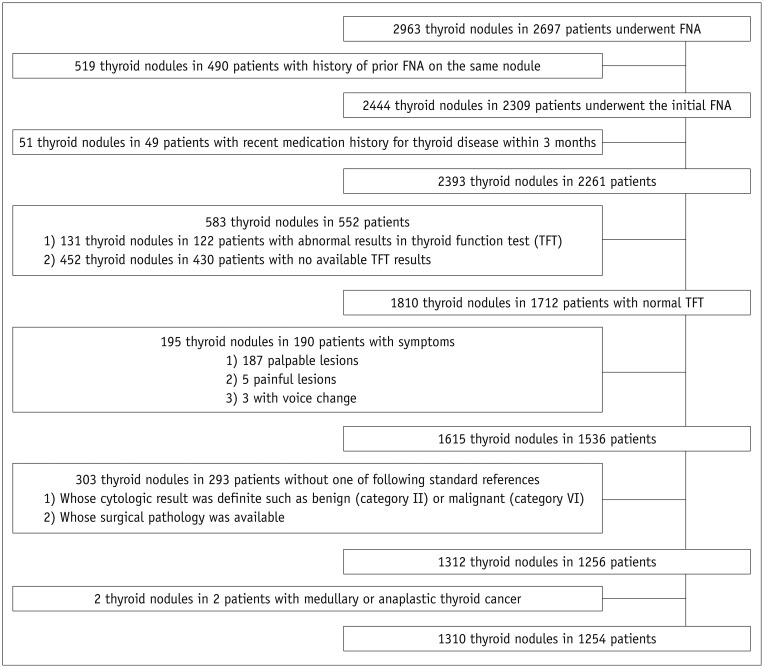
From November 2012 to August 2013, 2697 patients underwent US-FNA for initial evaluation of thyroid nodules at our institution. Among them, a total of 1310 nodules in 1254 patients were included if they satisfied the following criteria: 1) patients who had no recent history of medication for thyroid disease within 3 months; 2) patients in euthyroid status according to a reference serum thyroid stimulating hormone (TSH) level of 0.30–4.99 µIU/mL, triiodothyronine (T3) 0.6–1.8 ng/mL, and free thyroxine (fT4) 0.70–1.48 ng/dL; 3) patients without any symptoms such as palpability, pain, or voice change; and 4) nodules with available standard references such as surgical pathology and Bethesda category II (benign) or VI (malignant) cytologic results, which are highly predictive of surgical pathology. Patients confirmed to have medullary or anaplastic thyroid cancer were excluded (Fig. 1). Information on each patient's family history of thyroid cancer was gathered from electronic medical records. To analyze the risk of malignancy, only family history among first-degree relatives–such as parents, siblings, sons, and daughters–regardless of histologic type was included (5). No patient had more than two direct family members with thyroid cancer. The mean age of the patients was 49.6 ± 12.5 years (range, 17–87 years). The mean lesion size of the thyroid nodules was 13.3 ± 8.9 mm (range, 2–72 mm).
Fig. 1. Flow chart of case enrollment.
FNA = fine-needle aspiration
Imaging Methods and Analysis
A 5–12 MHz linear probe (iU22, Philips Medical Systems) was used for US evaluation of all thyroid glands and cervical lymph nodes. Fourteen board-certified radiologists who were specialized in thyroid imaging with 1–18 years of experience performed the US examinations. US features used to describe the thyroid nodules were prospectively recorded, including internal component, level and homogeneity of echogenicity, margin, calcification, vascularity and shape at the time of US examination. US features considered suspicious for malignancy were: marked hypoechogenicity, microlobulated or irregular margin, microcalcifications, and taller than wider shape (7). If thyroid nodules showed one or more suspicious US features, they were regarded as suspicious malignant nodules. Nodules without any of the suspicious US features described above were classified as probably benign nodules. Nodule size was measured as the longest diameter. Multiplicity regardless of position in the ipsilateral or contralateral lobe of the thyroid gland was also recorded. Simple cysts or colloid cysts were excluded when evaluating multiplicity.
US-Guided Fine-Needle Aspiration
Ultrasonogaphy-FNAs were performed by the same radiologists who performed the US examinations targeting either thyroid nodules with suspicious US features or the largest nodule with probably benign US features in cases in which no nodule showed suspicious US findings. Nodules smaller than 5 mm were not routinely aspirated, but biopsies were performed upon patient request. Each lesion was aspirated at least twice using a free-hand biopsy technique with a 23-gauge needle attached to a 20 mL disposable plastic syringe. The needles were disconnected from the syringes and the obtained materials were expelled onto glass slides, smeared, and fixed in 95% alcohol for Papanicolaou staining. One of seven cytopathologists interpreted the stained slides in the cytopathologists' reading room with optional special staining if necessary. The Bethesda System for Reporting Thyroid Cytopathology was applied to the cytology reports of thyroid aspiration.
Statistical Analysis
We used cytopathological results as the "reference standard." To evaluate the correlation between adolescence and old age with the risk of malignancy, we used the ages of 20 and 60 years as cut-off values (8). We compared clinical characteristics including known risk factors associated with thyroid cancer and US assessment between benign and malignant nodules using the χ2 test for categorical variables and the independent t test for continuous variables. Multiple logistic regression analysis was performed to assess independent associations between thyroid cancer and all clinical factors with adjustment for the factors. Serum TSH levels were classified into three grades (low normal, mid-normal, and high normal) using cut-off values from a previous study (9). Odds ratios (ORs) with relative 95% confidence intervals were also calculated. C-statistics were calculated to compare models with and without US characteristics and their subdivisions. The C-statistics of each model were compared using Delong's method. The χ2 test or independent two-sample t test was used to evaluate the multicollinearity of factors. Correlation between presence of first-degree family history and malignancy risk in each final US assessment group was assessed by the χ2 test or Fisher's exact test. The Breslow-Day test was used to compare the homogeneity of the ORs between groups.
Analysis was performed using SAS (version 9.2, SAS Institute Inc., Cary, NC, USA). Statistical significance was assumed when the two-sided p value was less than 0.05.
RESULTS
Among the total 1310 nodules of the included 1254 patients, 731 (55.8%) nodules were shown to be benign and the remaining 579 (44.2%) nodules malignant on cytopathology. There were 560 (42.7%) nodules that were pathologically confirmed postoperatively (Table 1). The mean age of patients with malignant nodules was significantly younger than that of patients with benign nodules (47.6 ± 12.8 years vs. 51.2 ± 12.0 years; p < 0.001). When the study population was classified according to age, patients between 20 and 60 years of age showed a higher rate of malignancy compared to other age groups (46.1% [485/1051] vs. 36.3% [94/259]; p = 0.004). The rate of malignancy was higher in male patients compared to female patients (50.5% [152/301] vs. 42.3% [427/1009]; p = 0.014). Patients with malignant nodules reported a family history of thyroid cancer more frequently than did patients with benign nodules (10.9% [63/578] vs. 6.6% [48/732]; p = 0.011). The mean size of the malignant nodules was 9.8 ± 6.4 mm, significantly smaller than the benign nodules (16.0 ± 9.6 mm; p < 0.001). Although a majority of malignant nodules were reported as one of synchronous multiple nodules (372/579, 64.2%), solitary nodules were significantly more malignant (48.3% [207/429] vs. 42.2% [372/881]; p = 0.044). The final US assessment was significantly associated with malignancy (p < 0.001). The mean serum TSH level was higher in malignant nodules than benign nodules, albeit not significantly so (1.7 ± 0.9 years vs. 1.6 ± 0.9 years; p = 0.075). The proportion of each TSH grade differed slightly between benign and malignant nodules (p = 0.024) (Table 2).
Table 1. Pathology of 560 Nodules Confirmed Postoperatively.
| Benign (n = 32) | Malignant (n = 528) | ||
|---|---|---|---|
| Adenomatous hyperplasia | 24 | Papillary carcinoma | |
| Lymphocytic thyroiditis with adenomatous hyperplasia | 4 | Conventional | 504 |
| Lymphocytic thyroiditis | 2 | Follicular variant | 17 |
| Follicular adenoma | 2 | Diffuse sclerosiong variant | 2 |
| Warthin like type | 2 | ||
| Follicular carcinoma, minimally invasive | 3 | ||
Table 2. Baseline Characteristics of 1310 Asymptomatic Thyroid Nodules.
| Benign | Malignant | P | |
|---|---|---|---|
| No. of nodules | 731 | 579 | |
| Mean age (years) | 51.2 ± 12.0 | 47.6 ± 12.8 | < 0.001 |
| Age (years) | 0.004 | ||
| < 20 or > 60 | 165 (22.6%) | 94 (16.2%) | |
| 20–60 | 566 (77.4%) | 485 (83.8%) | |
| Gender | 0.014 | ||
| Male | 149 (20.4%) | 152 (26.3%) | |
| Female | 582 (79.6%) | 427 (73.7%) | |
| 1st degree family history | 0.011 | ||
| Yes | 48 (6.6%) | 63 (10.9%) | |
| One | 40 (5.5%) | 57 (9.8%) | |
| Two | 8 (1.1%) | 6 (1.0%) | |
| No | 683 (93.4%) | 516 (89.1%) | |
| Mean nodule size (mm) | 16.0 ± 9.6 | 9.8 ± 6.4 | < 0.001 |
| Multiplicity | 0.044 | ||
| Solitary | 222 (30.4%) | 207 (35.8%) | |
| Multiple | 509 (69.6%) | 372 (64.2%) | |
| Serum TSH value | |||
| Mean TSH level (mIU/L) | 1.6 ± 0.9 | 1.7 ± 0.9 | 0.075 |
| TSH grade | 0.024 | ||
| 0.3–1.39 | 381 (52.1%) | 258 (44.6%) | |
| 1.4–2.49 | 242 (33.1%) | 220 (38.0%) | |
| 2.5–4.99 | 108 (14.8%) | 101 (17.4%) | |
| US final assessment | < 0.001 | ||
| Probably benign | 566 (77.4%) | 49 (8.5%) | |
| Suspicious malignant | 165 (22.6%) | 530 (91.5%) |
1st degree = first-degree, TSH = thyroid stimulating hormone, US = ultrasonography
Multivariate analysis of thyroid cancer according to clinical risk factors showed that patient age, male gender, presence of first-degree family history, and TSH grade increased the risk of malignancy (Table 3). However, on multivariate analysis including both clinical and US findings, only three factors–solitary lesion on US (p = 0.041–0.043), US features, and male gender (p < 0.001)–were independently associated with thyroid cancer. Patient age, a family history of thyroid cancer, and high normal serum TSH levels or grades did not independently significantly increase the risk of malignancy (Table 4). C-statistics in models with US features were higher than in models without US features (0.874 vs. 0.580–0.591). C-statistics did not significantly differ according to how TSH levels were measured or categorized (p = 0.103 in the model including clinical characteristics only, p = 0.925 in the model including clinical characteristics and US assessment). However, there was multicollinearity between the final US assessment and other clinical risk factors, such as first-degree family history, age, and serum TSH levels (Table 5).
Table 3. Multivariable Analysis for ORs with 95% CIs of Thyroid Cancer According to Clinical Characteristics.
| Adjusted OR | P | Adjusted OR* | P | Adjusted OR† | P | |
|---|---|---|---|---|---|---|
| Age < 20 or > 60 | 0.694 (0.522, 0.923) | 0.012 | 0.698 (0.524, 0.928) | 0.013 | 0.695 (0.523, 0.924) | 0.012 |
| Male gender | 1.394 (1.071, 1.814) | 0.013 | 1.382 (1.062, 1.797) | 0.016 | 1.365 (1.050, 1.774) | 0.020 |
| 1st degree family history | 1.618 (1.088, 2.405) | 0.018 | 1.612 (1.084, 2.397) | 0.018 | 1.655 (1.114, 2.458) | 0.013 |
| Solitary lesion on US | 1.182 (0.933, 1.498) | 0.167 | 1.180 (0.931, 1.495) | 0.172 | 1.193 (0.942, 1.512) | 0.143 |
| Serum TSH level | 1.171 (1.033, 1.327) | 0.013 | ||||
| TSH grade | 0.029 | |||||
| 0.3–1.39 | 1 | |||||
| 1.4–2.49 | 1.317 (1.031, 1.681) | 0.027 | ||||
| 2.5–4.99 | 1.415 (1.029, 1.947) | 0.033 | ||||
| TSH grade | ||||||
| 0.3–2.49 | 1 | |||||
| 2.5–4.99 | 1.258 (0.931, 1.699) | 0.135 | ||||
| C-statistics | 0.591 (0.560, 0.622) | 0.586 (0.555, 0.616) | 0.580 (0.550, 0.610) |
Numbers in parentheses are 95% CIs. *TSH levels were categorized into three groups: relatively low in normal range, mid-normal, relatively high in normal range, †TSH levels were categorized into two groups: low and mid normal, relatively high in normal range. 1st degree = first-degree, CIs = confidence intervals, OR = odds ratio, TSH = thyroid stimulating hormone, US = ultrasonography
Table 4. Multivariable Analysis for ORs with 95% CIs of Thyroid Cancer According to Clinical and US Characteristics.
| Adjusted OR | P | Adjusted OR* | P | Adjusted OR† | P | |
|---|---|---|---|---|---|---|
| Age < 20 or > 60 | 0.812 (0.550, 1.199) | 0.295 | 0.810 (0.548, 1.196) | 0.288 | 0.810 (0.548, 1.195) | 0.288 |
| Male gender | 1.994 (1.360, 2.925) | < 0.001 | 1.996 (1.362, 2.925) | < 0.001 | 1.994 (1.361, 2.921) | < 0.001 |
| 1st degree family history | 1.278 (0.742, 2.203) | 0.377 | 1.281 (0.743, 2.209) | 0.373 | 1.283 (0.744, 2.211) | 0.370 |
| Solitary lesion on US | 1.413 (1.014, 1.970) | 0.041 | 1.409 (1.011, 1.965) | 0.043 | 1.410 (1.012, 1.966) | 0.043 |
| Serum TSH level | 1.046 (0.881, 1.242) | 0.605 | ||||
| TSH grade | 0.757 | |||||
| 0.3–1.39 | 1 | |||||
| 1.4–2.49 | 1.020 (0.729, 1.427) | 0.907 | ||||
| 2.5–4.99 | 1.176 (0.762, 1.816) | 0.464 | ||||
| TSH grade | ||||||
| 0.3–2.49 | 1 | |||||
| 2.5–4.99 | 1.166 (0.776, 1.752) | 0.461 | ||||
| US assessment | 39.778 (27.953, 56.606) | < 0.001 | 39.870 (28.003, 56.765) | < 0.001 | 39.927 (28.065, 56.802) | < 0.001 |
| C-statistics | 0.874 (0.855, 0.894) | 0.874 (0.855, 0.894) | 0.874 (0.855, 0.894) |
Numbers in parentheses are 95% CIs. *TSH levels were categorized into three groups: relatively low in normal range, mid-normal, relatively high in normal range, †TSH levels were categorized into two groups: low and mid normal, relatively high in normal range. 1st degree = first-degree, CIs = confidence intervals, OR = odds ratio, TSH = thyroid stimulating hormone, US = ultrasonography
Table 5. Association of Each Clinical Risk Factor with Final US Assessment.
| P | |
|---|---|
| Age < 20 or > 60 | 0.007 |
| Gender | 0.723 |
| 1st degree family history | 0.009 |
| Solitary lesion on US | 0.777 |
| Serum TSH value | |
| Mean TSH level (mIU/L) | 0.001 |
| TSH grade (3 categories) | 0.001 |
| TSH grade (2 categories) | 0.168 |
P values from χ2-square test or independent two sample t test. TSH = thyroid stimulating hormone, US = ultrasonography
There was no significant correlation between presence of first-degree family history and the risk of malignancy in each final US assessment group (p > 0.999 in the probably benign US group, p = 0.208 in the suspicious malignant US group), and the trends did not differ between groups (p = 0.555).
DISCUSSION
Although all of the clinical factors were associated with thyroid cancer when analyzed individually, only two factors, solitary lesion on US and male gender, were found to increase the risk of malignancy, whereas a family history of thyroid cancer did not when US features were included in the analysis. As expected, US features surpassed clinical risk factors with high ORs, suggesting that asymptomatic thyroid nodules should be managed based primarily on US features.
Increased risk of malignancy in a patient with familial history might be due to both a heredity effect and the sharing of a causative environment. Radiation (10), iodine deficiency (11), and some genetic syndromes (12) are widely accepted to be correlated with the risk of thyroid cancer, but their clinical implication in the general population has been limited.
To date, several genes or loci have been reported as possible candidates for the genetic background of NMTC, but the correlation between these mutations and thyroid cancer remains controversial (13). Overall, while a multi-genetic pathway has been hypothesized, it has not been verified. Therefore, the differentiation of FNMTC from sporadic cases simply follows the definition of FNMTC; the presence of two or more already diagnosed first-degree family members (2), theoretically based on calculations by Charkes (14) that demonstrated an ~99% chance of familial disease, with the probability decreasing by half when there were only two patients among first-degree relatives. The probability of thyroid cancer was reported to increase with an increasing number of affected first-degree family members (15). The familial tendency was most clearly demonstrated among sisters (16). Furthermore, other reports suggested the necessity of more aggressive evaluation and treatment including total thyroidectomy regardless of cancer size with routine central compartment neck dissection in familial cases due to the relatively high rate of multifocality/lymph node metastasis (17,18), relatively low reliability of FNA (19), and unfavorable clinical features and adverse outcomes of FNMTC (20,21,22,23,24).
However, as knowledge of thyroid cancer and financial support for health checkups have become available to the general population, a greater number of patients with indolent or early stage thyroid cancer are diagnosed by screening US. This trend will lead to an increase in the prevalence of patients with only a single family member with thyroid cancer history as well as FNMTC. Moreover, even patients who are regarded as sporadic cases at the time of this study might be revealed to be FNMTC when other family members with currently undetected cancers are diagnosed with thyroid cancer at a later date. Mazeh et al. (25) insisted that clinicians not overlook patients with a single family history, a possible subgroup of FNMTC, because these patients had a higher risk of malignancy compared to those without any family history. The next issue of interest is whether all patients with a family history of thyroid cancer should be regarded to be at risk of thyroid cancer. The problem is that the characteristics of the population diagnosed by screening US today might be different from that of the older generation of patients who were diagnosed at more advanced cancer stages based on the symptoms of thyroid cancer. Thus, we should question whether previous conclusions that regarded a first-degree family history of thyroid cancer as a risk factor for thyroid malignancy are valid in an era in which screening US has become commonplace.
In contrast to the inconclusive results regarding the clinical implications of family history as a risk and prognostic factor for thyroid cancer, the diagnostic value of US features is generally accepted. Many studies have shown that the malignancy rate of thyroid nodules is closely correlated with US findings (7,26,27). In our study, despite significant differences in the frequency of family history between patients with benign and malignant thyroid nodules, family history was not a significant risk factor for thyroid cancer in a multivariate analysis that included US features. However, there was a significant correlation between the final US assessment and other clinical risk factors, such as first-degree family history, age, and serum TSH levels (Table 5). Considering the multicollinearity of the factors and overwhelming significance of US features as a predictor of malignancy, we cannot disregard first-degree family history as a risk factor for thyroid cancer. However, we can deduce that additional consideration of the history of one or two first-degree family members with thyroid cancer may not be necessary for patient management after full evaluation of US features.
Although there are conflicting reports about gender as a risk factor for thyroid cancer (28,29), current guidelines recommend that more attention be paid to male patients (6). Similar to other reports of a higher rate of malignancy in male patients with thyroid nodules (30,31,32), we found similar results in a multivariate analysis. Considering that male patients have worse prognosis of thyroid cancer than female patients (33,34), a result confirmed in this study, meticulous US evaluation is needed so that such cases are not misdiagnosed.
It has been suggested that a solitary nodule is more likely to be malignant (30,35,36,37), but other reports have suggested that the number of nodules is not correlated with malignancy (31,38,39). Some authors reported that the likelihood of malignancy per person was independent of multiplicity, but that the rate of malignancy had a negative correlation with the number of nodules in multinodular disease (40). The results of this study suggest that solitary nodules are at somewhat greater risk of malignancy than multiple nodules, but our results cannot support aggressive FNA in all solitary nodules due to the relatively low ORs.
There were several limitations to our study. First, some nodules were excluded due to a lack of determinative cytopathology. This resulted in the possibility of bias in patient selection. Second, we included nodules without follow-up cytology or surgical pathology and used the initial FNA results as a standard reference if the nodules were of a definitive category, such as benignity or malignancy. False-negative or false-positive cytology might have influenced the results. Third, there might be bias derived from the small number of patients with a history of thyroid cancer among first-degree family members. Family history was rarely reported in our study population (8.5%, 111/1310), and FNMTC was found in only 0.9% of cases (12/1310), a percentage lower than previous reports. Fourth, we did not find a possible relationship between patients and relatives with thyroid cancer. A specific kinship, such as sisterhood, might have shown a different correlation with the rate of malignancy compared to other kinships, such as brotherhood or parenthood. Fifth, evaluation for adolescence as a risk factor was limited due to the small number of patients. Sixth, we did not include past patient history of thyroid disease or reproductive status as a factor in the analysis.
In conclusion, US findings should be the primary criterion used to decide the management of asymptomatic thyroid nodules. Clinical factors such as male gender or solitary nodule can be additionally considered. Further studies on whether first-degree family history is a risk factor for thyroid malignancy in the euthyroid asymptomatic group in the era of high-performance US should be performed.
References
- 1.Vriens MR, Suh I, Moses W, Kebebew E. Clinical features and genetic predisposition to hereditary nonmedullary thyroid cancer. Thyroid. 2009;19:1343–1349. doi: 10.1089/thy.2009.1607. [DOI] [PubMed] [Google Scholar]
- 2.Alsanea O, Clark OH. Familial thyroid cancer. Curr Opin Oncol. 2001;13:44–51. doi: 10.1097/00001622-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Malchoff CD, Malchoff DM. Familial nonmedullary thyroid carcinoma. Cancer Control. 2006;13:106–110. doi: 10.1177/107327480601300204. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 5.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 6.Gharib H, Papini E, Valcavi R, Baskin HJ, Crescenzi A, Dottorini ME, et al. American Association of Clinical Endocrinologists and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2006;12:63–102. doi: 10.4158/EP.12.1.63. [DOI] [PubMed] [Google Scholar]
- 7.Kim EK, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002;178:687–691. doi: 10.2214/ajr.178.3.1780687. [DOI] [PubMed] [Google Scholar]
- 8.Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005;237:794–800. doi: 10.1148/radiol.2373050220. [DOI] [PubMed] [Google Scholar]
- 9.Choi JS, Nam CM, Kim EK, Moon HJ, Han KH, Kwak JY. Evaluation of serum thyroid-stimulating hormone as indicator for fine-needle aspiration in patients with thyroid nodules. Head Neck. 2015;37:498–504. doi: 10.1002/hed.23616. [DOI] [PubMed] [Google Scholar]
- 10.Schneider AB, Sarne DH. Long-term risks for thyroid cancer and other neoplasms after exposure to radiation. Nat Clin Pract Endocrinol Metab. 2005;1:82–91. doi: 10.1038/ncpendmet0022. [DOI] [PubMed] [Google Scholar]
- 11.Dal Maso L, Bosetti C, La Vecchia C, Franceschi S. Risk factors for thyroid cancer: an epidemiological review focused on nutritional factors. Cancer Causes Control. 2009;20:75–86. doi: 10.1007/s10552-008-9219-5. [DOI] [PubMed] [Google Scholar]
- 12.Moore FD., Jr Inherited aspects of papillary thyroid carcinoma. J Surg Oncol. 2006;94:719–724. doi: 10.1002/jso.20692. [DOI] [PubMed] [Google Scholar]
- 13.Khan A, Smellie J, Nutting C, Harrington K, Newbold K. Familial nonmedullary thyroid cancer: a review of the genetics. Thyroid. 2010;20:795–801. doi: 10.1089/thy.2009.0216. [DOI] [PubMed] [Google Scholar]
- 14.Charkes ND. On the prevalence of familial nonmedullary thyroid cancer. Thyroid. 1998;8:857–858. doi: 10.1089/thy.1998.8.857. [DOI] [PubMed] [Google Scholar]
- 15.Triponez F, Wong M, Sturgeon C, Caron N, Ginzinger DG, Segal MR, et al. Does familial non-medullary thyroid cancer adversely affect survival? World J Surg. 2006;30:787–793. doi: 10.1007/s00268-005-0398-x. [DOI] [PubMed] [Google Scholar]
- 16.Hemminki K, Eng C, Chen B. Familial risks for nonmedullary thyroid cancer. J Clin Endocrinol Metab. 2005;90:5747–5753. doi: 10.1210/jc.2005-0935. [DOI] [PubMed] [Google Scholar]
- 17.Sippel RS, Caron NR, Clark OH. An evidence-based approach to familial nonmedullary thyroid cancer: screening, clinical management, and follow-up. World J Surg. 2007;31:924–933. doi: 10.1007/s00268-006-0847-1. [DOI] [PubMed] [Google Scholar]
- 18.Mazeh H, Sippel RS. Familial nonmedullary thyroid carcinoma. Thyroid. 2013;23:1049–1056. doi: 10.1089/thy.2013.0079. [DOI] [PubMed] [Google Scholar]
- 19.Vriens MR, Sabanci U, Epstein HD, Ngai S, Duh QY, Siperstein AE, et al. Reliability of fine-needle aspiration in patients with familial nonmedullary thyroid cancer. Thyroid. 1999;9:1011–1016. doi: 10.1089/thy.1999.9.1011. [DOI] [PubMed] [Google Scholar]
- 20.Alsanea O, Wada N, Ain K, Wong M, Taylor K, Ituarte PH, et al. Is familial non-medullary thyroid carcinoma more aggressive than sporadic thyroid cancer? A multicenter series. Surgery. 2000;128:1043–1050. discussion 1050-1051. doi: 10.1067/msy.2000.110848. [DOI] [PubMed] [Google Scholar]
- 21.McDonald TJ, Driedger AA, Garcia BM, Van Uum SH, Rachinsky I, Chevendra V, et al. Familial papillary thyroid carcinoma: a retrospective analysis. J Oncol. 2011;2011:948786. doi: 10.1155/2011/948786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capezzone M, Marchisotta S, Cantara S, Busonero G, Brilli L, Pazaitou-Panayiotou K, et al. Familial non-medullary thyroid carcinoma displays the features of clinical anticipation suggestive of a distinct biological entity. Endocr Relat Cancer. 2008;15:1075–1081. doi: 10.1677/ERC-08-0080. [DOI] [PubMed] [Google Scholar]
- 23.Park YJ, Ahn HY, Choi HS, Kim KW, Park do J, Cho BY. The long-term outcomes of the second generation of familial nonmedullary thyroid carcinoma are more aggressive than sporadic cases. Thyroid. 2012;22:356–362. doi: 10.1089/thy.2011.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hillenbrand A, Varhaug JE, Brauckhoff M, Pandev R, Haufe S, Dotzenrath C, et al. Familial nonmedullary thyroid carcinoma-clinical relevance and prognosis. A European multicenter study. ESES Vienna presentation. Langenbecks Arch Surg. 2010;395:851–858. doi: 10.1007/s00423-010-0696-0. [DOI] [PubMed] [Google Scholar]
- 25.Mazeh H, Benavidez J, Poehls JL, Youngwirth L, Chen H, Sippel RS. In patients with thyroid cancer of follicular cell origin, a family history of nonmedullary thyroid cancer in one first-degree relative is associated with more aggressive disease. Thyroid. 2012;22:3–8. doi: 10.1089/thy.2011.0192. [DOI] [PubMed] [Google Scholar]
- 26.Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study. Radiology. 2008;247:762–770. doi: 10.1148/radiol.2473070944. [DOI] [PubMed] [Google Scholar]
- 27.Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87:1941–1946. doi: 10.1210/jcem.87.5.8504. [DOI] [PubMed] [Google Scholar]
- 28.Amir A, Sands N, Hier M, Payne R. Gender as a risk factor of malignancy in a thyroid nodule. Otolaryngol Head Neck Surg. 2010;143(suppl):P61. [Google Scholar]
- 29.Iribarren C, Haselkorn T, Tekawa IS, Friedman GD. Cohort study of thyroid cancer in a San Francisco Bay area population. Int J Cancer. 2001;93:745–750. doi: 10.1002/ijc.1377. [DOI] [PubMed] [Google Scholar]
- 30.Rago T, Fiore E, Scutari M, Santini F, Di Coscio G, Romani R, et al. Male sex, single nodularity, and young age are associated with the risk of finding a papillary thyroid cancer on fine-needle aspiration cytology in a large series of patients with nodular thyroid disease. Eur J Endocrinol. 2010;162:763–770. doi: 10.1530/EJE-09-0895. [DOI] [PubMed] [Google Scholar]
- 31.Belfiore A, La Rosa GL, La Porta GA, Giuffrida D, Milazzo G, Lupo L, et al. Cancer risk in patients with cold thyroid nodules: relevance of iodine intake, sex, age, and multinodularity. Am J Med. 1992;93:363–369. doi: 10.1016/0002-9343(92)90164-7. [DOI] [PubMed] [Google Scholar]
- 32.Tuttle RM, Lemar H, Burch HB. Clinical features associated with an increased risk of thyroid malignancy in patients with follicular neoplasia by fine-needle aspiration. Thyroid. 1998;8:377–383. doi: 10.1089/thy.1998.8.377. [DOI] [PubMed] [Google Scholar]
- 33.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 34.Jonklaas J, Nogueras-Gonzalez G, Munsell M, Litofsky D, Ain KB, Bigos ST, et al. The impact of age and gender on papillary thyroid cancer survival. J Clin Endocrinol Metab. 2012;97:E878–E887. doi: 10.1210/jc.2011-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barroeta JE, Wang H, Shiina N, Gupta PK, Livolsi VA, Baloch ZW. Is fine-needle aspiration (FNA) of multiple thyroid nodules justified? Endocr Pathol. 2006;17:61–65. doi: 10.1385/ep:17:1:61. [DOI] [PubMed] [Google Scholar]
- 36.Kumar H, Daykin J, Holder R, Watkinson JC, Sheppard MC, Franklyn JA. Gender, clinical findings, and serum thyrotropin measurements in the prediction of thyroid neoplasia in 1005 patients presenting with thyroid enlargement and investigated by fine-needle aspiration cytology. Thyroid. 1999;9:1105–1109. doi: 10.1089/thy.1999.9.1105. [DOI] [PubMed] [Google Scholar]
- 37.Franklyn JA, Daykin J, Young J, Oates GD, Sheppard MC. Fine needle aspiration cytology in diffuse or multinodular goitre compared with solitary thyroid nodules. BMJ. 1993;307:240. doi: 10.1136/bmj.307.6898.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCall A, Jarosz H, Lawrence AM, Paloyan E. The incidence of thyroid carcinoma in solitary cold nodules and in multinodular goiters. Surgery. 1986;100:1128–1132. [PubMed] [Google Scholar]
- 39.Bouhabel S, Payne RJ, Mlynarek A, Hier M, Caglar D, Tamilia M. Are solitary thyroid nodules more likely to be malignant? J Otolaryngol Head Neck Surg. 2012;41:119–123. [PubMed] [Google Scholar]
- 40.Frates MC, Benson CB, Doubilet PM, Kunreuther E, Contreras M, Cibas ES, et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006;91:3411–3417. doi: 10.1210/jc.2006-0690. [DOI] [PubMed] [Google Scholar]