Abstract
Background
Transfusion-transmissible infections have made both blood bankers and health authorities overly cautious. The general public expects and hence reinforces this policy. To obtain a high level of blood product safety, blood and plasma donors have to meet increasingly stringent eligibility criteria; however, it is not known whether this policy translates into improved outcomes for patients. There is a risk that the management of donors does not match the ambition of greater safety for patients. European directives related to the collection process and donor selection will probably be reconsidered in the next few years.
Material and methods
The development of European directives on donor selection and their basis in the literature were reviewed with an emphasis on the background and considerations for eligibility criteria to be included in the directives.
Results
The precautionary principle appears to be the predominant reason behind the set of eligibility criteria. However, the formal eligibility criteria, put into force in 2004, do not balance with the developments of the past decade in laboratory tests and measures that have substantially reduced actual infection risks. In no cases were the effects of eligibility criteria on the donor pool and donor well-being quantified. Regional differences in the epidemiology of transfusion-transmissible infections were not taken into consideration either.
Discussion
First, the Authors promote the collection of epidemiological data on the incidence and prevalence of conditions in the general population and in blood and plasma donors which could pose a risk for transfused patients, in order to use these data as a basis for decision-making in donor-selection policies. Second, the Authors suggest including allowance for differential deferral criteria throughout Europe, based on factual risk levels. There should be an accepted balance between donor and patient welfare, and also between risk to transfusion safety and risk of compromising the blood supply.
Keywords: blood donor, selection criteria, EU directive, evidence-based medicine
Introduction
To protect recipients of blood products, blood centres must operate flawlessly, which puts a heavy responsibility on blood bankers themselves. Mistakes and misjudgements, even small ones, pose potentially great risks and may cause serious harm. This is especially illustrated by episodes related to transfusion-transmissible infections (TTI) such as human immunodeficiency virus (HIV) and hepatitis B virus (HBV) in the 1980s and 1990s and hepatitis C virus (HCV) this century1,2. The biological origin of blood products, which implies a series of specific risks, is the main cause of this situation. However, the real risks may be very different from the perceived risks3. TTI have turned both blood bankers and health authorities into cautious people and the general public expects and supports this attitude3. Indeed, blood products are very safe compared to many other, more traditional pharmaceuticals, such as chemotherapeutics. To reach this high level of blood product safety, blood and plasma donors have to meet increasingly stringent criteria for eligibility. This strategy may carry the risk that proper management of donors does not tally with the ambition of greater safety for patients.
We anticipate that improving safety for patients by means of donor selection has its price; and that this price - including a negative effect on donors, the donor base and the blood supply - can be high.
Donor selection: European Guidelines
In the European Union, the blood collection process is regulated in several Directives4,5 which lay down standards of quality and safety applying to the collection, testing, processing, storage, and distribution of human blood and blood components, in order to ensure a high level of human health protection. Although mainly dealing with regulatory and quality issues, these Directives also regulate, to a varied degree, mandatory requirements on blood and plasma donor eligibility and blood product specifications. Criteria applicable to whole blood donors for the prevention of TTI as a rule well serve the same purpose in plasma donors. This is because processing plasma allows more vigorous steps to eliminate micro-organisms, which would damage cellular components. Moreover, some criteria, such as those related to the preventing the transmission of malaria, are superfluous when selecting plasma donors.
The Directorate for the Quality of Medicines & Health Care of the Council of Europe has produced recommendations relating to the preparation, use and quality assurance of blood components in a guide intending to form the basis for standard operating procedures6. Although non-binding, this guide serves as a template for most member state regulations and contains detailed descriptions of donor eligibility criteria, including questions on donor health, biometrical requirements and laboratory testing requirements. In many cases these requirements reflect expert opinion rather than quantitative, risk-based considerations; particularly where evidence is not available.
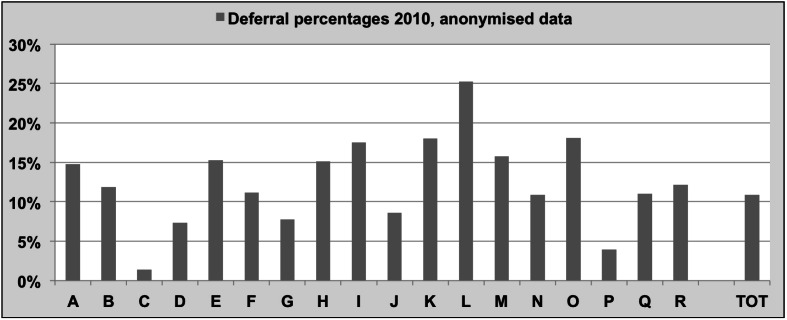
Implementing these directives and guidelines, therefore, may give rise to differing eligibility policies. Subsequently, deferral rates vary widely among blood establishments, as shown in Figure 1, which illustrates that the 2010 deferral percentages of blood establishments in several European countries, the USA and Canada varied between 1.4% and 25% (European Blood Alliance, Workshop on Donor Deferral, Amsterdam, 2012, unpublished survey results). Such a variation cannot readily be explained by epidemiological differences and true risks - such as actual prevalences of TTI and countries’ risk behaviour profiles - and is more likely the result of qualitative risk appraisals. However, differences in education of, and information to donors, resulting in differential self-selection may also have played a role.
Figure 1.
Deferral rates in European and Northern American Blood Establishments participating in the Donor Deferral Workshop, held in Amsterdam, February 2012.
The deferral percentages shown reflect results for 2010. Source: European Blood Alliance; Donor Deferral Workshop 2012.
Risk-based criteria for eligibility
Precautionary principle vs a balanced response
Ideally, to set eligibility criteria for donors, one should consider both material and health risks as well as benefits for donors and recipients7. In such a risk-based assessment of criteria, benefits should outweigh the costs. In setting donor eligibility criteria, the risk analysis is particularly difficult because costs and benefits may not be equally distributed among donors and recipients. Moreover, costs for donors are often hard to assess.
When faced with a potential risk, and in the absence of direct data, intuitive or plausible assumptions may have to be made in order to take preventive measures, because waiting and seeing is generally not an option. The case of variant Creutzfeld-Jacob disease is a good example. However, over-adherence to the concept of “first do no harm”, or a rigid interpretation of the precautionary principle may not be the best guide to reach the maximum health benefit, as discussed in a review on drug regulation8. This may also be true for donor selection and product rejection in the transfusion chain. A recent European Court of Justice judgment, too, referred to the need to evaluate the epidemiological situation in the country and to respect the principle of proportionality in national legislation on donor selection9.
Cost effectiveness of interventions
An important notion often used in cost-effectiveness analyses is the incremental costs effectiveness ratio (ICER). The ICER is the ratio of the (additional) costs of a measure taken to the increased number of quality-adjusted life years (QALY). A non-mandatory World Health Organization (WHO) criterion of the ICER is $ 100,000 (€ 80,000) per QALY10. A lower threshold of $ 30,000–$ 45,000 (€ 20,000–30,000) per QALY has been suggested in the UK11. Of course, it is not always possible to calculate all costs involved exactly, neither is it truly possible to value human lives or quality of life, and different approaches can be valid in such circumstances12,13. Nevertheless, a carefully performed cost-effectiveness analysis, including all available knowledge and information, can be helpful in comparing different measures, or in evaluating measures taken.
Perceived risks vs actual risks
In contrast to what many people believe or feel, most dangers we are confronted with in everyday life are fairly rare. Assuming an equal chance for each inhabitant in Europe, the annual chance of being killed in a traffic accident is about 1 in 18,00014, while the average chance of dying of any cause is about 1 in 10015. The chance of being killed on purpose by someone else (murdered) in Northern, Western and Southern Europe is even smaller, at about 1 in 100,00016. The chance of acquiring an HBV infection in Europe is somewhere in between these two risks: 1 in 65,00017. We must keep in mind that acquiring an infection does not imply dying from it. For example, an HBV infection acquired through transfusion brings about the loss of only 0.29 QALY because most infections do not result in serious morbidity or mortality18.
However, chances are not homogeneously distributed in the population. In criminal circles, for example, the risk of being killed by someone else is much higher16. Likewise, the risk of acquiring an HBV infection is not uniformly distributed and varies with geographical region, socio-economic condition, differences in (sexual) behaviour, vaccination policy, and other preventive actions such as education. In high-prevalence areas or in subpopulations that migrated from high-prevalence areas, there may be a 5- to 10-fold excess risk of acquiring a HBV infection. The historical policy in blood banking has been that when any excess risk is present, where feasible this risk should be avoided: “zero risk”. Clearly this mind-set is changing, as demonstrated by a number of countries revising policies relating to men having sex with men (MSM) over the past few years, supported by their regulators. However this change is not occurring universally, and in some countries MSM remains a permanent deferral. The argument most often put forward to justify the retention of permanent deferral for MSM is precautionary: the possibility that an emerging infectious disease may present in this donor cohort.
The level of risk changes over time
A few decades ago, having a piercing or tattoo implied an increased risk of HBV, HCV or other infections, caused by infected tattoo-ink and inadequate decontamination of the instruments used. A few decades ago there were also regular incidents of infections transmitted via endoscopy instruments. As a result, deferral criteria were set and even included in the European Directive5.
Over time, hygiene measures have improved; national regulations have been implemented, and in many countries of the European Union there is no longer a marked risk increase associated with piercings, tattoos or endoscopy19. Ever more people are being tattooed, while the rate of tattoo-associated HBV/HCV infections has not increased substantially. Not surprisingly, compliance with the deferral policy for tattoos and piercing appears to be poor20. Besides reduced exposure risk, HBV vaccination programmes in some countries significantly reduce the likelihood of donors harbouring HBV and increased levels of community immunity reduces the likelihood of patients acquiring HBV through transfusions. However, the Directive 2004/33/EC has not been adjusted accordingly5. Some changes in practice are occurring. In August 2005, the Canadian Blood Services decreased the deferral period for tattoos and piercing from 12 to 6 months and, moreover, small surgery procedures such as endoscopy are no longer a reason for deferral there. In the USA, AABB standards were amended to permit blood donations if tattooing and piercing were performed in a licensed establishment, with sterile needles and non-reused ink. In contrast, EU rules and guides have not changed in this respect.
Epidemiological pitfalls
A better understanding of some epidemiological mechanisms is helpful when considering setting or changing selection criteria. To this purpose, we first discuss some general pitfalls that can easily become apparent when striving to increase the safety of blood product recipients through setting or changing eligibility criteria.
Donors comprise a random sample of the general population
Some readily assume that risk levels in donors are the same as those in the general population. However, prior frequency of disease differs widely between the general population and the new, first time donor population. The latter is not a random sample of the general population21–23. This reflects the powerful impact of community education regarding risk factors for blood-borne infections as well as the fact that people with serious health problems usually do not apply to become donors. Moreover, whole blood donation in general is non-remunerated, thereby avoiding the potential incentive of financial compensation. Therefore, the prevalence and incidence rates of disease - including infectious disease - in new donors are lower than in the general population, although the phenomenon of test seeking could attenuate the difference to some extent (Zaaijer H, personal communication).
Regular donors are not the same as new donors either. Blood products from regular donors are estimated to be safer for the recipients than those from new donors. The prevalence and incidence of TTI are substantially lower in regular donors than in new donors. To illustrate this: an infectious disease is always preceded by a risk event. The number of risk events can, therefore, be expected to be proportionate to the number of infectious diseases. It was recently observed that the incidence of risk events in regular donors was at least 3-fold lower than that in new donors23. Despite the fact that risk behaviours are more frequent in first-time donors than in repeat donors, the incidence of HIV, reflecting the risk of recent infections, is not necessarily higher in first-time donors. Using the method of nucleic acid amplification test yield cases24 to monitor the presence of HIV and HCV in France in the period from July 2001–2013, no significant difference was found between incidences in first-time donors and repeat donors (total 34.2 million donations; 5.5 million from first-time donors and 28.7 million from repeat donors). During this period, the incidence of HIV was estimated to be 2.64 per 105 person-years (95% CI [confidence interval]: 0.85–7.26) in first-time donors and 2.04 (95% CI: 1.21–3.39) in repeat donors. The incidence of HCV was estimated to be 0.47 per 105 person-years (95% CI: 0.15–1.30) in first time donors and 0.23 (95% CI: 0.12–0.43) in repeat donors25.
Identification of risk carriers is perfect
Commonly applied tools for identifying people at risk are questionnaires and laboratory tests. Questionnaires are as a rule less reliable than laboratory tests. The validity of a laboratory test is expressed in terms of the well-known concepts of sensitivity and specificity. Modern blood banks apply screening tests with a very high accuracy in terms of these specifications. Still, these tests cannot be 100% correct. This implies that two kinds of wrong decisions are made: the result is either false negative or false positive. The former could indeed result in the transmission of an infectious agent. If a false negative result is related to an answer to a questionnaire, this would incur only an undetected chance of a transmission - albeit a higher chance than average, for example, if a donor forgets about a tattoo got during a holiday a few months earlier and wrongly states that he or she did not get one. Lessons learned from anonymous surveys and from monitoring post-donation information indicate that some of the information about the risks remains undetected in the donor selection process26. Although errors in the blood donor qualification process cannot be excluded, some donors do not respond accurately to questions about risk factors: sometimes because of shame and embarrassment, and sometimes for other reasons, such as desire for incentives, or test-seeking. It has been evaluated that 4.3% of first-time donors and 3.4% of repeat donors have undisclosed high-risk sexual behaviour27. False positive results cause donors to be labelled wrongly, which only has negative implications for the donor, while there is no risk at all for the recipient.
Possible effects of these assumptions being ignored are illustrated below.
Bayes’ rule applied to two examples: screening tests and questionnaires
When considering a new laboratory test, quite often it is known how many people with the infection test positive: the sensitivity of the test. However, when implementing the test as a screening tool in donor selection, we are confronted with the opposite question: in how many people (donors) testing positive is the disease truly present? In other words, what is the positive predictive value of the test?
In the 18th century, the Reverend Bayes, who had an interest in statistics, derived his famous rule on how to deal with such a question. The result is, sometimes, counterintuitive.
Screening for transfusion-transmissible infections in donors
Table Ia and Ib shows the screening test results for TTI in The Netherlands and Austria. After a donor is found to be repeatedly reactive in screening tests, confirmatory testing is performed. The tables display the number (percentage) of repeatedly reactive donations and the number (percentage) of confirmed positive donations. Being repeatedly negative with a confirmatory test implies that this donor is not infected, i.e. the donor has a false positive result. The last columns in Table Ia and Ib actually answers the “Bayesian” question: “What is the chance - the positive predictive value - that a donor tested repeatedly reactive is indeed infected with the agent tested for?” We can see that for HIV, HCV and human T-lymphotropic virus (HTLV) this chance in The Netherlands is less than 1 in 50 (being even lower for HIV, at less than 1 in 500) while for HBV and syphilis it is about 1 in 7. In Austria values for the positive predictive value seem to be higher, but again the number of false positive results is substantial: up to 95% in the case of HIV testing and even two-thirds for HBsAg (hepatitis B surface antigen).
Table Ia.
TTI screening results in The Netherlands showing annual averages over the period 2009–2013.
| Agent | N. of tests | N. repeatedly reactive, RR (%) | N. confirmed positive, CP (%) | Positive predictive value, PPV (% = CP*100/RR) |
|---|---|---|---|---|
| HBsAg | 889,297 | 146 (0.02) | 21 (0.0024) | 14.7% |
| Anti-HCVf | 880,942 | 383 (0.04) | 6.2 (0.0007) | 1.6% |
| Anti-HIV | 889,425 | 670 (0.08) | 1.6 (0.0002) | 0.2% |
| Anti-HTLV | 815,934 | 157 (0.02) | 2.5 (0.0003) | 1,6% |
| Syphilis | 889,198 | 111 (0.01) | 15 (0.0017) | 13.5% |
|
| ||||
| Total | - | 1,467 | 47 | 3.2% |
Source: Sanquin Blood Supply, The Netherlands. TTI: transfusion-transmissible infections; HBsAg: hepatitis B surface antigen; HCV: hepatitis C virus; HIV: human immunodeficiency virus; HTLV: human T-lymphotropic virus.
Table Ib.
TTI screening results in Austria showing annual averages over the period 2009–2013.
| Agent | N. of tests | N. repeatedly reactive, RR (%) | N. confirmed positive, CP (%) | Positive predictive value PPV % = CP*100/RR |
|---|---|---|---|---|
| HBsAg | 167,289 | 45 (0.027) | 15 (0.0088) | 33% |
| Anti-HCV | 167,289 | 73 (0.043) | 10 (0.0060) | 13.8% |
| Anti-HIV | 167,289 | 82 (0.049) | 3.6 (0.0022) | 4.4% |
|
| ||||
| Total | - | 200 | 28 | 14.2% |
Source: Austrian Red Cross Blood Transfusion Services. TTI: transfusion-transmissible infections; HBsAg: hepatitis B surface antigen; HCV: hepatitis C virus; HIV: human immunodeficiency virus.
Sensitivity values of the confirmatory tests, as given by the manufacturers, are close to 100% (test manufacturers’ fact sheets), supporting the conclusion that donors with repeat reactive and negative confirmatory test results are not infected and, consequently, nor are their donations. These repeatedly reactive, but false-positive donations are not infectious, but are still rejected at the product release control.
The data in Table I show that, on an annual basis, more than 1,400 units tested false positive in The Netherlands; in the Austrian Red Cross Blood Establishment the 5-year average number was approximately 170. Based on the Guide of the Council of Europe these units were rejected6. However, these units were proven to be non-infectious, since confirmatory testing showed negative results. Our message to donors with a false-positive test result is one of reassurance that they need no medical follow up, because there is nothing wrong with their health condition, nor with their donation. While false-positive donations are not infectious, important consideration still needs to be given to the operational impact on the Blood Service, before accepting them for supply.
Donor health questionnaires
Applying questionnaires for the identification of carriers of infectious disease involves dual uncertainty. First, questionnaires are not 100% valid, giving rise to donors being falsely identified as being TTI-risk-carriers. Second, because they are constructed to identify risk-carriers, not pathogen-carriers, a great majority of the risk-bearing individuals do not carry the pathogen. In particular when the prevalence of the condition sought for is low, the relative number of false-positives rises steeply with decreasing specificity, and the positive predictive value becomes (very) low.
It is extremely difficult, if not impossible, to construct a questionnaire that is 100% accurate and gives an unambiguous decision on the TTI-risk carrier status. Even very high values of sensitivity and especially specificity may result in a substantial number of false-positives.
From Table II we may conclude that if we defer a donor who has answered positively to the question(s) on risky behaviour, there is a chance of approximately 1 in 40,000 that we have indeed prevented the transmission of HBV or syphilis, and a chance of about 1 in 300,000–600,000 that transmission of HCV or HIV has been prevented23. An important reason for a false-positive result is that deferral ensues in the case of doubt, for example, when there is doubt on the time elapsed since the risk event or on the nature of the procedure: e.g. when did the tattoo, piercing take place? What kind of contact with a contaminated surface/needle/sharp object, acupuncture or endoscopic procedure did really take place? Assuming a specificity of 99.7%, we can estimate that about one in three deferred donors is a false-positive case, meaning that this donor did not in fact experience the event deemed risky at all. We must realise that the proportion of false-positives and the value of the true prevalence vary when different values for specificity are used.
Table II.
Number of deferrals to prevent one case of a transfusion-transmitted disease*.
| Agent | N. of deferrals |
|---|---|
| HBsAg | 39,760 |
| Anti-HCV | 564,600 |
| Anti-HIV | 352,875 |
| Anti-HTLV | 42,772 |
| Syphilis | 39,760 |
de Kort et al., 2014.
HBsAg: hepatitis B surface antigen; HCV: hepatitis C virus; HIV: human immunodeficiency virus; HTLV: human T-lymphotropic virus.
Still more eligibility criteria actually have been introduced without proof of effectiveness or an indication of the magnitude of the added value in terms of donor health protection. Examples of these criteria are the age limits imposed in most EU member states and the cardiovascular risk questions for donors above a certain age, 60 years of age in most instances. Just to illustrate another aspect, we mention, without discussing it in detail, the measurement of haemoglobin level. Although this measurement is paramount in avoiding collecting units of blood with insufficient quantities of haemoglobin, haemoglobin is not a good parameter for reflecting the iron status of donors28,29 and it is precisely the iron status of donors that is of key influence to donor health in the long run.
Discussion
Donor position, rejected units
Selecting donors is practising public health care, while transfusion of blood products is practising patient health care. Consequently, the selection of donors follows the rules of group treatment, while treating patients with blood products follows the rules of treating individuals. Still, important individual effects in donors can be expected.
Setting aside the effect on the donor base, being deferred has an often-underestimated psychological effect on donors. Disappointment and even having the feeling of being insulted are important side effects of deferral. Donor return among deferred donors is less than among non-deferred donors30. Deferrals, therefore, have a dual effect on the donor base: for each deferred donor a substitute must be found to meet blood product demand and the decreased donor return implies that additional substitute new donors have to be recruited.
A similar effect is seen for blood components that have been rejected. Rejecting about 0.2% of all successfully donated blood components in the example of false-positive testing results in The Netherlands brings forth a relatively small, but still substantial loss in absolute terms for the blood supply, without, however, any beneficial effect for the recipient. In contrast, these donations did have a negative effect on the donors’ iron status and did bring about adverse reactions in some of them. We point here to the conflicting messages to donors and recipients: donors are informed that testing repeatedly reactive, but negative at confirmation, has no health implications and yet their donations are discarded because of an infection risk to the recipient.
Criteria for screening
Almost five decades ago, a WHO-report written by Wilson & Jungner stated criteria to appraise the validity of a screening programme. Although these criteria were put together for screening people for their own benefit, they are also applicable to screening candidate donors. However, benefits and disadvantages are not evenly distributed. Many selection criteria aim at increasing the benefit for the recipient by excluding risks at the cost of a negative effect on donors, i.e. too many unjustified donor deferrals.
Therefore, different questions must be asked to appraise the validity of donor selection criteria. Concisely put, in setting up a system of (donor) selection, the questions that should be asked are:
- What is the nature and size of the risk to be prevented, to the donor or to the recipient?
- Does the risk presented by the donor, bring incurable morbidity or unavoidable mortality in the recipient?
- What is the prevalence and incidence of the risk in donors?
- What are the validity specifications of the screening test in both new and regular donor populations?
- If there is no screening test, what are the validity specifications of the question(naire) used to identify the corresponding risk in a donor? These validity specifications must also take into account that being a risk-carrier is not synonymous with being a pathogen-carrier.
- What is the anticipated cost-effectiveness of the measure to be taken, expressed, for example, by the ICER, or number of lives saved per annum?
Conclusions
Increasing or grading up selection criteria may be marginally beneficial to recipients, while often resulting in large numbers of extra deferrals and discarded blood and blood components, with at times a substantial impact on the donor base and blood component supply. As a rule, the criteria for preventing TTI that are used in selecting whole blood donors, well serve the same purpose in selecting plasma donors. Although donor screening tests and donor health questionnaires have the same objective - to improve the safety of blood components - there are significant differences in their implementation. Before their routine use, laboratory tests are submitted to numerous checks and validations in which their efficiency should be confirmed and they are constantly improving over time. In contrast, donor selection criteria are often not evidence-based, and they are used routinely for many years without their validity being re-appraised7.
When implementing and changing donor selection criteria, experience from haemovigilance systems, including donor vigilance systems, should be used more widely, especially when assessing effectiveness of different interventions aimed at improving transfusion safety.
In the perspective of a likely revision of the European Directives, enforced 10 years ago or more, and in line with the Council of Europe Resolution CM/Res(2013)3 adopted by 36 countries31, we would like to call for two sets of measures for revising donor selection policies in Europe. The first would promote the collection of epidemiological data on the incidence and prevalence of conditions that could pose a risk to transfused patients (e.g. behaviour-related transmitted infections) in the general population and in donors, for use as a basis for decision-making in donor-selection policies. Such an approach to basing donor deferral criteria on sound scientific evidence, including quantitative assessments of risky conditions, has been widely adopted to review donor selection criteria regarding (sexual) behaviour of donors that have an impact on transfusion safety32–34.
The second measure, to follow the first measure, would be to include allowance for differential deferral criteria throughout Europe, based on factual risk levels. In the Council of Europe Resolution CM/Res(2013)31, health authorities were encouraged to support blood establishments by publically communicating the relationship between available data on the safety of the blood supply and subsequent decisions on donor selection criteria. This should lead to better acceptance that donor deferral criteria could vary as epidemiological data could vary from country to country. Thus, keeping in mind that one rule cannot apply to all, the level of harmonisation on an international level is limited. Consequently, directives may become more effective when being less detailed and therefore leaving scope for national, or even regional, differences in risk avoidance measures. There should be an accepted balance between donor and patient welfare, and also between risk to transfusion safety and risk of compromising the blood and plasma supply. Such an approach would greatly help to improve both donors’ adherence to donor deferral criteria and patients’ acceptance of being transfused when needed.
Acknowledgements
The Authors wish to thank the following people for their support and general comments: Kari Aranko, Rosario Arrieta, Johanna Castren, Veerle Compernolle, Bruno Danic, Beatrice Aspevall Diedrich, Birgit Gasthof, Mary Morgan, William Murphy, Cath O’Brien, Satu Pastilla, Joanne Pink, Primoz Rozman, Rudolf Schwabe, Franz Weinauer and Lorna Williamson.
Footnotes
Authorship contributions
WdK drafted the text. WM and CJ provided the Austrian data. All Authors critically reviewed the text and provided additional thoughts and input.
The Authors declare no conflict of interest.
References
- 1.Alter HJ, Klein HG. The hazards of blood transfusion in historical perspective. Blood. 2008;112:2617–26. doi: 10.1182/blood-2008-07-077370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stramer SL, Dodd RY Subgroup AT-TDEID. Transfusion-transmitted emerging infectious diseases: 30 years of challenges and progress. Transfusion. 2013;53:2375–83. doi: 10.1111/trf.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ngo LT, Bruhn R, Custer B. Risk perception and its role in attitudes toward blood transfusion: a qualitative systematic review. Transfus Med Rev. 2013;27:119–28. doi: 10.1016/j.tmrv.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Directive 2002/98/EC of the European Parliament and of the Council of 27 January 2003 setting standards of quality and safety for the collection, testing, processing, storage and distribution of human blood and blood components and amending Directive 2001/83/EC. OJ, L33, 8/2/2003, p.30
- 5.Commission Directive 2004/33/EC of 22 March 2004 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards certain technical requirements for blood and blood components. OJ, L91, 30/3/2004, p.25
- 6.Council of Europe. Guide to the Preparation, Use and Quality Assurance of blood products. 17th ed. Strasbourg: Council of Europe Publishing; 2013. [Google Scholar]
- 7.Menitove JE, Leach Bennett J, Tomasulo P, Katz LM. How safe is safe enough, who decides and how? From a zero-risk paradigm to risk-based decision making. Transfusion. 2014;54:753–7. doi: 10.1111/trf.12569. [DOI] [PubMed] [Google Scholar]
- 8.Eichler HG, Bloechl-Daum B, Brasseur D, et al. The risks of risk aversion in drug regulation. Nat Rev Drug Discov. 2013;12:907–16. doi: 10.1038/nrd4129. [DOI] [PubMed] [Google Scholar]
- 9.Judgement of the Court (Fourth Chamber); European Court of Justice, editor. Léger v. Établissement Francais du Sang - Case C-528/13. Luxembourg: Apr 29, 2015. [Accessed on 27/09/2015]. Available at: http://curia.europa.eu/juris/document/document.jsf?text=&docid=164021&pageIndex=0&doclang=EN&mode=lst&dir=&occ=first&part=1&cid=357418. [Google Scholar]
- 10.Tan-Torres Edjer T, Baltussen R, Adam T, et al., editors. Making choices in health: WHO guide to cost-effectiveness analysis. World Health Organization; Geneva: 2003. [Google Scholar]
- 11.National Institute for Health and Clinical Excellence. Social Value Judgements: Principles for the development of NICE guidance. NICE; London: 2012. [PubMed] [Google Scholar]
- 12.Murphy W. Managing threats rather than risks in blood transfusion: robust design for a complex system. Transfusion. 2006;46:2011–3. doi: 10.1111/j.1537-2995.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- 13.Murphy WG. Of mad cows and bolted horses: the economics of blood safety. Transfusion. 2012;52:2278–81. doi: 10.1111/j.1537-2995.2012.03931.x. [DOI] [PubMed] [Google Scholar]
- 14.European Commission. Road safety: EU reports lowest ever number of road deaths and takes first step towards an injuries strategy. Brussels. 2013. [Accessed on 27/09/2015]. Available at: http://europa.eu/rapid/press-release_IP-13-236_en.htm.
- 15.Central Intelligence Agency. Death rate. 2015. [Accessed on dd/mm/yyyy]. Available at: http://www.indexmundi.com/european_union/death_rate.html.
- 16.United Nations Office of Drugs and Crime. UNODC Global Study on Homicide 2013. Vienna: 2014. [Accessed on 27/09/2015]. Available at: https://www.unodc.org/documents/gsh/pdfs/2014_GLOBAL_HOMICIDE_BOOK_web.pdf. [Google Scholar]
- 17.World Health Organization Regional office for Europe. Data and statistics. 2015. [Accessed on 27/09/2015]. Available at: http://www.euro.who.int/en/health-topics/communicable-diseases/hepatitis/data-and-statistics.
- 18.Borkent-Raven BA, Janssen MP, van der Poel CL, et al. Cost-effectiveness of additional blood screening tests in the Netherlands. Transfusion. 2012;52:478–88. doi: 10.1111/j.1537-2995.2011.03319.x. [DOI] [PubMed] [Google Scholar]
- 19.Urbanus AT, van den Hoek A, Boonstra A, et al. People with multiple tattoos and/or piercings are not at increased risk for HBV or HCV in The Netherlands. PLoS One. 2011;6:e24736. doi: 10.1371/journal.pone.0024736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Brien SF, Xi G, Fan W, et al. Are donors in Canada compliant with deferral for tattoos and piercing? Blood Transfus. 2014;12:141–2. doi: 10.2450/2014.0233-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atsma F, Veldhuizen I, Verbeek A, et al. Healthy donor effect: its magnitude in health research among blood donors. Transfusion. 2011;51:1820–8. doi: 10.1111/j.1537-2995.2010.03055.x. [DOI] [PubMed] [Google Scholar]
- 22.Golding J, Northstone K, Miller LL, et al. Differences between blood donors and a population sample: implications for case-control studies. Int J Epidemiol. 2013;42:1145–56. doi: 10.1093/ije/dyt095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Kort W, van den Burg P, Geerligs H, et al. Cost-effectiveness of questionnaires in preventing transfusion-transmitted infections. Transfusion. 2014;54:879–88. doi: 10.1111/trf.12349. [DOI] [PubMed] [Google Scholar]
- 24.Busch MP, Glynn SA, Stramer SL, et al. Group N-RNS. A new strategy for estimating risks of transfusion-transmitted viral infections based on rates of detection of recently infected donors. Transfusion. 2005;45:254–64. doi: 10.1111/j.1537-2995.2004.04215.x. [DOI] [PubMed] [Google Scholar]
- 25.Pillonel J, Laperche S. Estimating residual risks of transfusion-transmitted HIV-1 and HVC infection in France. ISBT Congress; London. 2015. [Google Scholar]
- 26.Williams AE, Thomson RA, Schreiber GB, et al. Estimates of infectious disease risk factors in US blood donors. Retrovirus Epidemiology Donor Study. JAMA. 1997;277:967–72. [PubMed] [Google Scholar]
- 27.Goldman M, Yi QL, Ye X, et al. Donor understanding and attitudes about current and potential deferral criteria for high-risk sexual behavior. Transfusion. 2011;51:1829–34. doi: 10.1111/j.1537-2995.2011.03078.x. [DOI] [PubMed] [Google Scholar]
- 28.Gorlin J. Iron man pentathlon or “we have met the enemy and they is us!”. Transfusion. 2014;54:747–9. doi: 10.1111/trf.12568. [DOI] [PubMed] [Google Scholar]
- 29.Baart AM, van Noord PAH, Vergouwe Y, et al. High prevalence of subclinical iron deficiency in whole blood donors not deferred for low hemoglobin. Transfusion. 2013;53:1670–7. doi: 10.1111/j.1537-2995.2012.03956.x. [DOI] [PubMed] [Google Scholar]
- 30.van Dongen A, Abraham C, Ruiter RAC, Veldhuizen IJT. The influence of adverse reactions, subjective distress, and anxiety on retention of first-time blood donors. Transfusion. 2013;53:337–43. doi: 10.1111/j.1537-2995.2012.03810.x. [DOI] [PubMed] [Google Scholar]
- 31.Committee of Ministers - Council of Europe. Resolution CM/Res(2013)3 on sexual behaviours of blood donors that have an impact on transfusion safety. 2013. [Accessed on dd/mm/yyyy]. Available at https://wcd.coe.int/ViewDoc.jsp?id=2051047.
- 32.Offergeld R, Kamp C, Heiden M, et al. Sexual risk behaviour and donor deferral in Europe. Vox Sang. 2014;107:420–7. doi: 10.1111/vox.12179. [DOI] [PubMed] [Google Scholar]
- 33.Epstein J, Ganz PR, Seitz R, et al. A shared regulatory perspective on deferral from blood donation of men who have sex with men (MSM) Vox Sang. 2014;107:416–9. doi: 10.1111/vox.12166. [DOI] [PubMed] [Google Scholar]
- 34.Davison KL, Conti S, Brailsford SR. The risk of transfusion-transmitted HIV from blood donations of men who have sex with men, 12 months after last sex with a man: 2005–2007 estimates from England and Wales. Vox Sang. 2013;105:85–8. doi: 10.1111/vox.12024. [DOI] [PubMed] [Google Scholar]