To transfuse or not to transfuse: that is the question! And this is a longstanding issue that has to be faced if we want to consider allogeneic blood transfusion to be a lifesaving procedure. It does, however, involve several risks, including infectious (viral and bacterial) complications, transfusion-related acute lung injury, ABO- and non-ABO-associated haemolytic transfusion reactions, transfusion-associated Graft-versus-Host disease, and transfusion-associated circulatory overload1. These complications represent the principal causes of allogeneic blood transfusion-related morbidity and mortality. Over the last thirty years, this has led to a number of randomised controlled trials (RCTs) being carried out aimed at comparing the effect on patients of restrictive (haemoglobin concentration 7–8 g/dL) with more liberal (haemoglobin concentration approximately 10 g/dL) blood transfusion strategies in a variety of clinical settings2. In parallel, a number of systematic reviews and meta-analyses3–10 have been conducted with the aim of performing a pooled analysis of the data from these RCTs (see Table I for a summary of results).
Table I.
Main results of the systematic reviews and meta-analyses of trials on blood transfusion strategies in various clinical settings.
| First author, year [reference] | Selection criteria | Studies/patients included | Main findings |
|---|---|---|---|
| Carson, 2002 [3] | RCTs 1970–2001 | 10/1,780 | The literature analysis supported the use of restrictive transfusion triggers in patients without serious cardiac disease. |
| Carson, 2012 [4] | RCTs 1970–2011 | 19/6,264 | Restrictive vs liberal transfusion strategy was associated with a statistically significant reduction in hospital mortality (RR 0.77, 95% CI: 0.62–0.95). |
| Curley, 2014 [5] | RCTs 1950–2013 | 7/1,262 | Restrictive vs liberal transfusion strategy was associated with a decreased transfusion of RBCs (mean difference −0.71, 95% CI: −0.31 to −1.09) without an associated change in adverse events in patients undergoing cardiovascular surgery. |
| Salpeter, 2014 [6] | RCTs 1966–2013 | 3/23,641 | Restrictive vs liberal transfusion strategy significantly reduced cardiac events (RR 0.44; 95% CI: 0.22–0.89), re-bleeding (RR 0.64, 95% CI: 0.45–0.90), bacterial infections (RR 0.86; 95% CI: 0.73–1.00), in-hospital mortality (RR 0.74, 95% CI: 0.60–0.92), and total mortality (RR 0.80; 95% CI: 0.65–0.98). |
| Holst, 2015 [7] | RCTs 1950–2014 | 31/9,813 | Restrictive vs liberal transfusion strategy was associated with a decreased transfusion of RBCs (mean difference −1.43, 95% CI: −2.01 to −0.86) with no effect on overall morbidity and mortality risks. |
| Brunskill, 2015 [8] | RCTs 1946–2014 | 6/2,272 | No difference in mortality, functional recovery or post-operative morbidity between restrictive and liberal thresholds was observed. |
| Ripollés Melchor, 2015 [9] | RCTs 1950–2014 | 6/2,156 | No differences in mortality between the restrictive and liberal groups (RR 0.86, 95% CI: 0.70–1.05; p=0.14) were observed. |
| Fominskiy, 2015 [10] | RCTs 1986–2015 | 27/11,021 | Liberal transfusion strategy compared with restrictive strategy improved survival in perioperative patients (OR 0.81, 95% CI: 0.66–1; p=0.050) |
Only RCTs using a restrictive transfusion trigger <7 g/dL were included. RCTs: randomised controlled trials; RR: risk ratio; CI: confidence interval; RBCs: red blood cells; OR: odds ratio.
A systematic review of papers published up to 2000 identified 10 trials, and the investigators concluded that the evidence supported the use of restrictive transfusion triggers in patients without serious cardiac disease3. A 2012 Cochrane systematic review, including 19 trials with 6,264 patients, showed that patients receiving liberal transfusion had higher in-hospital mortality compared with those transfused with a restrictive strategy4. A 2014 meta-analysis and systematic review by Salpeter et al. focused on the question as to whether the lower 7 g/dL threshold is superior to the higher threshold of 8 g/dL. Their study showed that, in patients with critical illness or bleeding, restricting blood transfusions by using a haemoglobin trigger lower than 7 g/dL significantly reduces negative outcomes, as well as in-hospital and total mortality6.
A more recent systematic review and meta-analysis of 31 RCTs by Holst et al. revealed a reduction in the number of units and of patients transfused in the restrictive group compared with the liberal group, but there was no difference in mortality and morbidity7. From the overall analysis of these systematic reviews (Table I), it is clear that, in terms of morbidity and mortality, a restrictive red blood cell (RBC) transfusion approach is superior3,4,6 or equivalent5,7–9 to a liberal strategy.
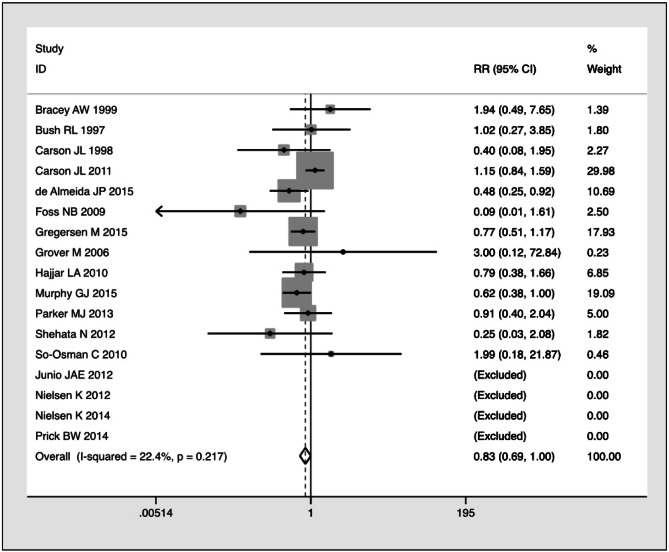
Recently, Fominskiy et al.10 performed yet another meta-analysis, justifying their efforts on the basis of the inclusion of three additional RCTs published in 2015 that had not been included in the previous systematic reviews11–13. The authors claimed that their meta-analysis, which included 27 RCTs with 11,021 patients, demonstrated unequivocally the superiority in terms of overall survival of the liberal transfusion strategy over the conservative approach in peri-operative adult patients (but not in critically ill patients). Besides a number of criticisms that could be raised on the selection criteria and data analysis, such as the inclusion of studies with a wide clinical heterogeneity, the overlaps between restrictive and non-restrictive blood transfusion thresholds, and the choice of the 90-day all-cause mortality as primary outcome (instead of the more reasonable 30-days cutoff chosen by the majority of trials), the main weakness of their work lies in the statistical analysis performed. Indeed, in their meta-analysis the authors should have calculated the risk ratio (RR) rather than the odds ratio (OR). The pooled effect size measured by the RR is a more appropriate tool for a meta-analysis aimed at evaluating the efficacy of a treatment protocol (liberal vs. restrictive transfusion approach) in preventing an adverse event (90-day all-cause mortality). This is corroborated by the fact that all previous meta-analyses published on this topic used an RR-based meta-analytical approach. If we re-analyse the 17 peri-operative studies from the meta-analysis conducted by Fominskiy et al. using the RR10, the already border-line statistical significance regarding a lower all-cause mortality with liberal vs restrictive transfusion strategy observed using the OR (0.81, 95% CI: 0.66–1; p=0.050) disappears (RR 0.83, 95% CI: 0.689–1.001; p=0.051) (Figure 1). Notably, when the same authors performed a sub-analysis of all included trials (i.e. peri-operative and critically ill patients) considering only the high-quality studies with a low risk of bias (Online Supplementary Table II of the meta-analysis10), the OR was not statistically significant (0.88, 95% CI: 0.75–1.04, p=0.13), thus aligning their results with those of previous meta-analyses. Therefore, we believe that the authors should have highlighted the limited evidence provided by their statistical analysis and suggested that an interpretation of their data should be approached with greater caution. In conclusion, it is our view that the meta-analysis by Fominskiy et al.10 adds very little to the existing knowledge in this clinical setting. In addition, as specialists in transfusion medicine, we are well aware that RBC transfusion is a life-saving therapy not without risks14,15 and, consequently, we recommend, in accordance with recent international transfusion medicine and multidisciplinary guidelines, that decisions on transfusion therapy with RBCs be based on both the patient’s haemoglobin values as well as symptoms of anaemia, and a restrictive transfusion approach be adopted with the possible exception of patients with severe ischaemic heart disease16–19.
Figure 1.
Forest-plot of all-cause mortality in peri-operative patients: risk ratio calculation (data extracted from the meta-analysis by Fominskiy et al.10).
Four studies did not record events and were excluded from analysis. Statistical analysis performed using Stata 14.1 (StataCorp, College Station, TX, USA). RR: relative risk; CI: confidence interval.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risk of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113:3406–17. doi: 10.1182/blood-2008-10-167643. [DOI] [PubMed] [Google Scholar]
- 2.Hoghshire L, Carson JL. Red blood cell transfusion: what is the evidence when to transfuse? Curr Opin Hematol. 2013;20:546–51. doi: 10.1097/MOH.0b013e32836508bd. [DOI] [PubMed] [Google Scholar]
- 3.Carson JL, Hill S, Carless P, et al. Transfusion triggers; a systematic review of the literature. Transfus Med Rev. 2002;16:187–99. doi: 10.1053/tmrv.2002.33461. [DOI] [PubMed] [Google Scholar]
- 4.Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;18:CD002042. doi: 10.1002/14651858.CD002042.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curley GF, Shehata N, Mazer CD, et al. Transfusion triggers for guiding RBC transfusion for cardiovascular surgery: a systematic review and meta-analysis. Crit Care Med. 2014;42:2611–24. doi: 10.1097/CCM.0000000000000548. [DOI] [PubMed] [Google Scholar]
- 6.Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a metaanalysis and systematic review. Am J Med. 2014;127:124–31. doi: 10.1016/j.amjmed.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Holst LB, Petersen MW, Haase N, et al. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis. Br Med J. 2015;350:h1354. doi: 10.1136/bmj.h1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunskill SJ, Millette SL, Shokoohi A, et al. Red blood cell transfusion for people undergoing hip fracture surgery. Cochrane Database Syst Rev. 2015;4:CD009699. doi: 10.1002/14651858.CD009699.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ripollés Melchor J, Casans Francés R, Espinosa A, et al. Restrictive versus liberal transfusion strategy for red blood cell transfusion in critically ill patients and in patients with acute coronary syndrome: a systematic review, meta-analysis and trial sequential analysis. Minerva Anestesiol. 2015 Jul 22; Epub ahead of print. [PubMed] [Google Scholar]
- 10.Fominskiy E, Putzu A, Monaco F, et al. Liberal transfusion strategy improves survival in perioperative but not in critically ill patients. A meta-analysis of randomised trials. Br J Anaesth. 2015;115:511–9. doi: 10.1093/bja/aev317. [DOI] [PubMed] [Google Scholar]
- 11.Murphy GJ, Pike K, Rogers CA, et al. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med. 2015;372:997–1008. doi: 10.1056/NEJMoa1403612. [DOI] [PubMed] [Google Scholar]
- 12.de Almeida JP, Vincent JL, Galas FR, et al. Transfusion requirements in surgical oncology patients: a prospective, randomized controlled trial. Anesthesiology. 2015;122:29–38. doi: 10.1097/ALN.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 13.Gregersen M, Borris LC, Damsgaard EM. Postoperative blood transfusion strategy in frail, anemic elderly patients with hip fracture. Acta Orthop. 2015;86:363–72. doi: 10.3109/17453674.2015.1006980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–74. doi: 10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- 15.Patel SV, Kidane B, Klingel M, Parry N. Risks associated with red blood cell transfusion in the trauma population: a meta-analysis. Injury. 2014;45:1522–33. doi: 10.1016/j.injury.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Goodnough LT, Levy JH, Murphy MF. Concepts of blood transfusion in adults. Lancet. 2013;381:1845–54. doi: 10.1016/S0140-6736(13)60650-9. [DOI] [PubMed] [Google Scholar]
- 17.Shander A, Gross I, Hill S, et al. A new perspective on best transfusion practices. Blood Transfus. 2013;11:193–202. doi: 10.2450/2012.0195-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leal-Noval SR, Muñoz M, Asuero M, et al. Spanish Consensus Statement on alternatives to allogeneic blood transfusion: the 2013 update of the “Seville Document”. Blood Transfus. 2013;11:585–610. doi: 10.2450/2013.0029-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaglio S, Prisco D, Biancofiore G, et al. Recommendations for the implementation of a Patient Blood Management programme. Application to elective major orthopaedic surgery in adults. Blood Transfus. 2016;14:23–65. doi: 10.2450/2015.0172-15. [DOI] [PMC free article] [PubMed] [Google Scholar]