Abstract
Background
Epitheliotrophic growth factors (GF) can be supplied topically to patients with severe keratopathy through a variety of blood-derived products. We compared GF content in adult peripheral blood serum (PB-S) and cord blood serum (CB-S) as potential sources of GF. To limit inter-individual variability the assessment was performed in maternal-child pairs at the time of delivery.
Material and methods
The amounts of epidermal GF (EGF), insulin-like GF (IGF), transforming GF-beta (TGF-β), vascular endothelial GF (VEGF) in CB units collected from the umbilical vein and PB from mothers (each group n=30) were estimated by enzyme-linked immunosorbent assays. Obstetric characteristics and haematological data were recorded from the archives of the Emilia Romagna Cord Blood Bank. Statistical evaluations were performed by Wilcoxon’s test and correlations between variables were determined using Spearman’s (ρ) coefficient; p-values <0.05 were considered statistically significant.
Results
EGF, TGF-β and VEGF levels were significantly higher in CB-S than in PB-S (median 1,254.4 vs 646.0 pg/mL, 51.3 vs 38.4 μg/mL and 686.8 vs 30 pg/mL, respectively; all p<0.0001) whereas IGF content was significantly higher in PB-S than in CB-S (159.9 vs 53.5 pg/mL, respectively; p<0.0001). In CB-S, the CD34+ cell concentration appeared to be related to EGF, IGF and TGF-β levels whereas white blood cell count appeared to be related to EGF and TGF-β levels. VEGF levels showed no relation to the haematological parameters considered. Platelet counts were not related to GF level in either CB or PB.
Discussion
The GF content in the two blood sources was different, with CB containing larger amounts. Each GF selectively regulates cellular processes involved in corneal healing, so the use of PB or CB should be targeted to supply specific GF on the basis of the type and severity of the keratopathy.
Keywords: EGF, VEGF, TGF-beta, keratopathy, cord blood
Introduction
Dry eye (DE) syndrome is a very common disorder and is associated with potential damage to the ocular surface resulting in superficial punctate erosions of the cornea as well as corneal and conjunctival epithelial defects. DE may be a sight-threatening disease and a potential cause of blindness, as irreparable ulceration, perforation or scarring can also occur in untreated or uncontrolled, severe cases. In normal subjects tears have lubricating, mechanical, antimicrobial and epitheliotrophic properties, regulating proliferation, differentiation and maturation of the epithelium of the ocular surface1,2; all these functions appear severely impaired in DE syndrome, in which natural tears are poor in quality and/or quantitatively scarce.
Tear substitutes are the first-line treatment for patients with DE syndrome, in order to replace natural tears; however, these substitutes lack the biologically active components that are critical for a healthy epithelium. The Dry Eye WorkShop (DEWS)3 in 2007 staged the severity of DE into four increasing levels and introduced recommendations to treat the disease accordingly. Autologous serum treatment was included among recommended therapeutic strategies at step three.
Eye drops obtained from blood-based preparations have now become a relatively common treatment for severe DE syndrome and the rationale for their use is based on the understanding that they have biochemical similarities with natural tears. This refers in particular to growth factors (GF), substances that play an important role in regulating many of the processes involved in corneal wound healing and are present at very low levels in the tears of patients with DE syndrome. Therefore, supplying GF through blood-based products does appear to be an attractive therapeutic option.
Several different blood derivatives have been proposed for the treatment of various ocular surface disorders4,5. Blood-derived products include eye drops prepared either from patients’ own peripheral blood serum (PB-S), such as autologous serum6–8, platelet-rich plasma9, plasma rich in GF10 and platelet lysate11, or from donors, such as allogeneic umbilical cord blood serum (CB-S)12–17 and allogeneic PB-S18–20. CB-S can be used in the form of eye drops, clots, platelet derivate or isolated factors. In addition to epidermal growth factor (EGF), it contains serum anti-proteases such as α2 macroglobulin with antimicrobial effects21 and other factors such as neuropeptides, vitamin A and fibronectin involved in the maintenance and restoration of the ocular surface and promotion of cell migration, growth and differentiation22–27. CB-S eye drops are prepared in a similar way as autologous serum eye drops but seem to be more effective in decreasing symptoms and signs in patients with severe DE syndrome28,29. Furthermore, a Cochrane metaanalysis recently showed inconsistent efficacy results for autologous serum30. CB-S contains higher levels of GF than PB-S, but potential risks of antigenicity and of transmission of blood-borne infectious diseases from pregnant donors have been postulated19.
To our knowledge, there are a few published papers comparing the content of GF in different blood sources13,31,32, with varying results but with the common finding that significant individual variations in GF concentration exist32.
The purpose of this work was to compare the content of four GF relevant to corneal epithelium healing, EGF, insulin-like growth factor (IGF), transforming growth factor-beta (TGF-β) and vascular endothelial growth factor (VEGF) in two homologous blood sources (PB from adults, CB from a placental umbilical vein). To limit inter-individual variability, the assessment was performed on blood taken from two particular populations, i.e. mothers and their babies, at the time of delivery. Obstetric and haematological parameters recorded at delivery were retrieved, to verify a possible relationship with GF levels.
Materials and methods
This is a retrospective observational study on blood serum samples obtained from the Emilia Romagna Cord Blood Bank between January 2013 and April 2014. The study was performed in respect of the principles in the Declaration of Helsinki. PB from 30 women and CB from their respective babies were collected at the time of delivery, centrifuged at 3,000 g to obtain serum and stored at −80 °C in the Emilia Romagna Cord Blood Bank. CB units stored for banking were included in the study; in these cases, in fact, the mothers’ blood parameters are also required and data could be retrieved. Selected samples were stored at −80 °C until determination, then thawed and processed as described subsequently.
Obstetric data
The following obstetric data were retrieved in anonymous form from the clinical records: parity and gestational age of the mother, sex, birth weight and Apgar score of the neonate, placental weight, duration of labour and the mode of delivery (physiological childbirth with vaginal delivery or primary planned Caesarean delivery prior to initiation of labour). The characteristics of the mothers and their babies are summarised in Table I.
Table I.
Characteristics of the mothers and babies.
| Characteristics | Median [min-max] (%) | |
|---|---|---|
| Group 1: mothers | Number | n=30 |
| Mothers’ age (years) | 31 [21–40] | |
| Gestational age (days) | 281 [259–292] | |
| Mode of delivery | ||
| Physiological vaginal delivery | n=22 (73.3%) | |
| Caesarean section | n=8 (26.7%) | |
| Labour duration (hours) | 6 [2–9] | |
| Parity | ||
| P1 | n=16 (53.3%) | |
| P>1 | n=14 (46.7%) | |
|
| ||
| Group 2: babies | Number | n=30 |
| Sex | ||
| Female | n=15 (50%) | |
| Male | n=15 (50%) | |
| Weight (g) | 3,672 [2,560–4,190] | |
| Female | 3,500 [2,680–3,940] | |
| Male | 3,830 [2,560–4,190] | |
| Apgar (score) | ||
| Apgar I=9 | n=8 (26,6%) | |
| Apgar I=10 | n=22 (73,4%) | |
| Apgar II=9 | n=1 (3.3%) | |
| Apgar II=10 | n=29 (96.7%) | |
Demographic characteristics of the study population (total subjects=60), split between mother and neonates.
P1: nulliparous; P>1: primiparous and multiparous.
Data are expressed as median, minimum and maximum values.
Blood collection
All CB units were collected after compilation of a donor selection questionnaire based on international criteria for CB banking. All steps from the recruitment to the processing and registration of CB were performed according to standard operating procedures and guidelines issued by the Foundation for the Accreditation of Cellular Therapy (FACT). CB was collected when the placenta was still in utero: the umbilical vein was punctured with a sterile system and blood was collected into a bag with 20 mL citrate-phosphate-dextrose (CPD) by gravitational forces (Fresenius-Kabi AG, Bad Homburg, Germany). Moreover blood samples were collected from ex utero placenta vessels with a sterile syringe and transferred into 10 mL Vacutest tubes (Kima srl, Padua, Italy) without any anticoagulant. The mother’s PB was collected into Vacutest tubes without any anticoagulant after delivery. For further processing, the unit and the related samples were sent to the laboratory of the processing facility.
Assessment of cord blood units
Cord blood was collected from spontaneous term births free of complications (≥37th week of pregnancy, n=22) and Caesarean births (n=8) decided by trained and qualified health personnel. Collection took a few minutes and was performed without changing the course of delivery, after the umbilical cord had been cut and once the child had been removed from the delivery site and taken proper care of. The CB units were transported within 1 h inside special containers at a controlled temperature to guarantee product integrity, ensuring that processing of the CB was completed within 48 h. The unit was taken to the processing facility of the CB bank where it went through a series of checks and tests to establish its characteristics and suitability for preservation and therapeutic use. Maternal infectious disease markers were evaluated.
Blood serum samples
The refrigerated bag containing the maternal samples and the test-tubes of placental blood was sent to the processing facility, which is open 24 hours a day, seven days a week. Ex utero placental blood and the mothers’ PB samples were centrifuged at 3,000 g for 10 min and serum samples were transferred into sterile tubes under a laminar flow hood and stored at −80 °C.
The white blood cells and platelets were counted with an auto-analyser (Act5/5dif; Coulter, Milan, Italy). The number of CD34+ cells was measured by flow cytometry with a single-platform technique (Stem Kit, Coulter). The haematological parameters of the mothers and babies are summarised in Table II.
Table II.
Distribution of haematological variables in the mothers and babies.
| Group 1: mothers’ PB | Group 2: babies’ CB | |
|---|---|---|
|
|
||
| Variable | Median [min-max] | Median [min-max] |
| CD34+ cells (number×106/unit) | Not done | 5.3 [1.7–8.3] |
| White blood cells (number×109/L) | 16.3 [11.7–24.5] | 15.6 [11.6–76.8] |
| Platelets (number×109/L) | 329 [249–450] | 278 [198–428] |
PB: peripheral blood; CB: cord blood.
Growth factor dosage
The concentrations of EGF, IGF, TGF-β and VEGF in CB-S and PB-S were determined using a Quantikine Human Immunoassay kit (R&D Systems Inc., Minneapolis, MN, USA) in accordance with the manufacturer’s instructions (http://www.rndsystems.com/, last accessed on 28/05/2015). The absorbance at 450 nm, with correction at 595 nm, was read using a spectrophotometer (Bio-Rad 680, Hercules, CA, USA).
Statistical analysis
The statistical analyses were performed with computer software (SPSS, Version 14.0; SPSS Inc., Chicago, IL, USA) and MedCalc 5.0 (MedCalc Inc, Ostend, Belgium). Descriptive statistics for tests and variables analysed in subjects are reported as median, maximum and minimum values. Spearman’s (ρ) correlation coefficient was calculated; correlations were considered statistically significant at p<0.05 and a correction of p-values for multiple testing was introduced. The strength of correlations ranged from −1 to +1 and was described in absolute values as 0–0.19 “very weak”; 0.20–0.39 “weak”; 0.40–0.59 “moderate”; 0.60–0.79 “strong”; and 0.80–1.0 “very strong”33. The GF levels in the two blood sources were compared with Wilcoxon’s test for paired data; p-values less than 0.05 were considered statistically significant.
Results
GF levels were evaluated in all the mother and baby samples: EGF, VEGF and TGF-β levels were higher in all CB-S than in the corresponding PB-S samples, whereas IGF levels were significantly higher in all PB-S than the corresponding CB-S samples. Data are summarised in Table III, expressed as median, minimum and maximum values; highly statistically significant differences in all GF levels were found between the two blood sources.
Table III.
Growth factor levels in the different blood sources.
| GF | Source | Median | Min | Max | p |
|---|---|---|---|---|---|
| EGF | CB-S | 1,254.4 | 516.8 | 2,824.0 | <0.0001 |
| PB-S | 646.0 | 283.8 | 1,754.0 | ||
|
| |||||
| IGF-1 | CB-S | 53.5 | 40.3 | 81.2 | <0.0001 |
| PB-S | 159.9 | 76.7 | 352.0 | ||
|
| |||||
| TGF-β | CB-S | 51.3 | 30.2 | 75.1 | <0.0001 |
| PB-S | 38.4 | 19.8 | 52.8 | ||
|
| |||||
| VEGF | CB-S | 686.8 | 31.9 | 1,856.0 | <0.0001 |
| PB-S | 30 | 21 | 35 | ||
GF levels are expressed in pg/mL sample, except for TGF-β (μg/mL sample). In all cases, differences between GF content in PB-S from mothers and CB-S from babies were highly statistically significant (Wilcoxon’s test for paired samples). GF: growth factors; EGF: epidermal growth factor; CB-S: cord blood serum; PB-S: peripheral blood serum; IGF-1: insulin-like growth factor 1; TGF-β transforming GF-beta; VEGF: vascular endothelial growth factor.
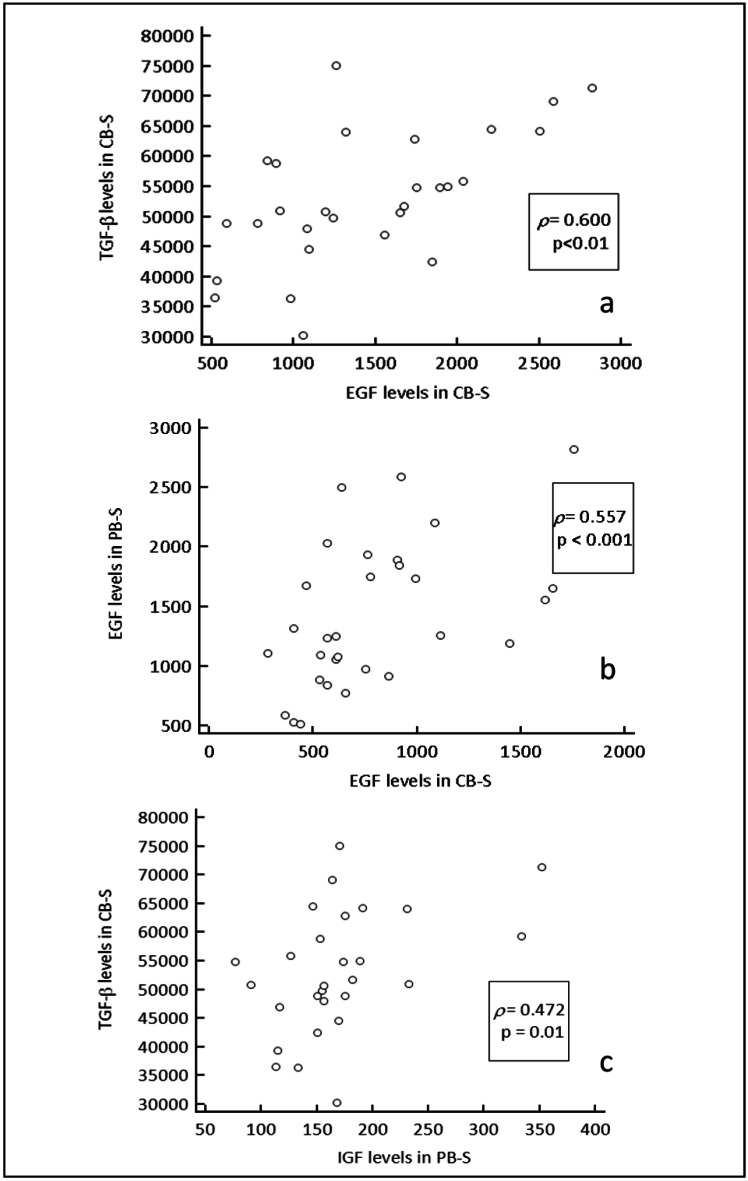
A strong correlation was demonstrated between EGF and TGF-β levels in CB-S (Figure 1a) whereas no correlation among GF was found in PB-S. Correlation coefficients were also calculated comparing each GF level in both blood sources: moderate correlations were found for EGF levels in both sources (Figure 1b) and for TGF-β in CB-S vs IGF-1 in PB-S (Figure 1c).
Figure 1.
A strong correlation was demonstrated between EGF and TGF-β levels in CB-S, (a) whereas no correlation among GF levels was found in PB-S. Moderate correlations were found for EGF levels in both sources (b), and for TGF-β in CB-S vs IGF-1 in PB-S (c). TGF-β: transforming GF-beta; CB-S: cord blood serum; EGF: epidermal growth factors; IGF: insulin-like growth factors; PB-S: peripheral blood serum.
A moderate correlation between duration of labour and EGF levels was found in both CB-S and PB-S (ρ=0.487 and ρ=0.543, respectively; p<0.05). EGF levels in CB-S only showed a weak correlation with mother’s age (ρ=−0.21). No statistically significant correlation was found between parity, neonatal sex, mode of delivery, Apgar score of the neonate and any of the GF levels in either CB-S or PB-S.
Weak correlations between haematological parameters and GF levels were only found in CB-S. CD34+ cell concentration appeared to be related to EGF, IGF and TGF-β levels (ρ=0.30 with p<0.01, and ρ=0.38 and ρ=0.35, respectively, with p<0.05) whereas white blood cell count appeared to be correlated with EGF and TGF-β levels (ρ=0.31 with p <0.01 and ρ=0.39 with p<0.05, respectively). VEGF levels showed no relation with the haematological parameters considered. Platelet counts in both blood sources showed no relation with GF levels.
Discussion
Data from the present study showed that TGF-β, EGF and VEGF levels were significantly higher in all CB-S samples than in the corresponding PB-S samples whereas IGF levels were significantly higher in all PB-S samples than in the corresponding CB-S samples.
To our knowledge, few studies have compared GF levels in CB-S and in PB-S from healthy volunteers. Yoon and co-workers showed that EGF and TGF-β concentrations were higher in CB-S than in autologous serum13. In another work Shen et al. compared EGF, TGF-β1 and IGF-1 levels in CB-S, autologous serum and fresh-frozen plasma31. We hypothesised a theoretical bias in comparing GF levels in blood preparations from different donors due to high inter-individual variability in GF levels17,32.
To limit the inter-individual variability the comparison was designed to analyse mother-baby pairs of samples, i.e. samples from the most closely related people, sharing at least some genes, but still being different organisms. To make the sampling moment coherent, the study was performed in babies (the source of CB) at the time of delivery and their mothers (the source of PB), although we recognise that the stressful state of physiological or Caesarean delivery cannot be considered biologically equivalent to the state of the “average adult”. However this is a borderline situation, as the circulatory system in foetuses is entirely separate from that of the mothers although gases, food and waste are exchanged between the two blood systems through the placenta. The present study showed that the levels of EGF in the two blood samples were directly correlated, although the reason for this cannot be explained on the basis of the data collected and analysed.
Naturally occurring biological fluids are used as a substitute for the natural tears that are lacking in DE syndrome, with the aim of supplying epitheliotrophic substances such as GF. Biological tear substitutes have been shown to maintain the morphology and support the proliferation of in vitro primary human corneal epithelial cells better than pharmaceutical tear substitutes34. Several different blood derivatives have been used for the treatment of various ocular surface disorders. Until recently, the most widely used blood-based eye drops were prepared as autologous serum from the patients’ own blood6–8. According to a proposed, optimised protocol35, autologous serum eye drops were prepared under aseptic conditions by dilution of 20–80% PB-S in a sterile vehicle (usually phosphate-buffered saline), and dispensed in single-dose sterile vials maintained at −20 °C until use. General improvements of the signs and/or symptoms of DE were found after treatment with autologous serum7,8,36–42 in particular in patients with DE associated with immune diseases, such as Sjögren’s syndrome. However, significant differences in patients’ populations, production and storage regimens, and treatment protocols prevent reliable comparisons of the results of the various studies35. A recent meta-analysis concluded that there was lack of evidence on the possible benefits of autologous serum topical treatment, and that well-planned, large, high-quality, randomised clinical trials are still needed, using standardised questionnaires, objective clinical tests as well as objective biomarkers30.
CB-S is a new topical product based on blood preparations effective in the healing of severe corneal epithelial injuries. CB-S offers several advantages over other blood-derived preparations, including a higher content of those GF essential in corneal wound healing, but also a few disadvantages, such as the potential risk of antigenicity and transmission of blood-borne infectious diseases from the pregnant donors. In addition, CB-S preparations appear to be expensive although comparative studies on the cost of the different products have not yet been performed.
A clear statement on GF supply in blood-based products has never been given in published clinical trial data, despite the fundamental role of GF in corneal epithelium healing and their impact on clinical results. Published studies have not described the true GF supply from blood-based eye drops, despite this supply being the fundamental requirement for correct healing and a potential source of associated adverse events. In fact, the GF supply must be in the proper range: “more” does not automatically mean “better”, as data from in vitro models have suggested43.
EGF is a well-known, potent mitogen for epithelial cells44 present in the lachrymal gland and in tears at concentrations of 0.7–9.7 ng/mL45,46. In vitro, EGF was found to increase cell proliferation in a dose-dependent manner at concentrations greater than 0.1 ng/mL47–49. However, in some studies concentrations exceeding approximately 10 ng/mL were found to decrease cell proliferation and cell number47–48. In vivo, it has generally been reported that the topical application of EGF at concentrations ranging from 10 to 20 μg/mL can improve the rates of corneal wound healing44. A recent review on the use of EGF eye drops in human corneal disease treatment concluded that clinical efficacy could not be established because of the marked heterogeneity of data, in particular as far as EGF posology was concerned50.
TGF-β, a pleiotropic molecule of 25 kDa secreted by several types of cells, including epithelial cells51, induces proliferation and migration of corneal stromal fibroblasts52,53, and alters the synthesis of extracellular matrix, which modulates the response to GF after injury. Addition of TGF-β only at a concentration of 1 ng/mL of culture medium was demonstrated to enhance the growth-promoting effects of EGF on keratocytes in vitro by inducing high-affinity EGF receptors47. The present study found a strong correlation between EGF and TGF-β levels only in CB-S; this suggests that CB-S could have combined efficacy in healing keratopathy.
IGF-1 and its receptor were detected in the corneal epithelium and stroma, and in the trabecular meshwork54,55; IGF-1 was shown to support the proliferation of keratinocytes56, enhance the production of the adherens-junction protein N-cadherin57, stimulate the formation of the extracellular matrix58, and increase the synthesis of collagen by keratinocytes59.
VEGF has long been known as a potent GF that stimulates the proliferation of blood vessels and a mediator of vascular permeability60,61. In injured states, VEGF expression in cornea was shown to be upregulated and to contribute to corneal neovascularisation62, a physiological process in wound healing. Recent studies suggested that VEGF may be involved in the physiological repair of corneal nerves63 and play a key role in the recovery from nerve damage occurring in keratopathy. Some studies documented detectable, although widely ranging, levels of VEGF in PB-S64–65 from healthy subjects whereas in the present study the levels of VEGF in PB-S were negligible in all subjects, perhaps in relation to maternal vascular adaptation after delivery66.
A correlation between duration of labour and EGF levels in CB-S was demonstrated in this study, in agreement with previous data from our group and as discussed elsewhere67. A correlation between GF levels and haematological variables was only found in CB-S, in particular regarding the correlation between CD34+ cell concentration and white blood cell content, whereas platelet number did not appear to be related to GF levels in either source. Therefore, CB collected after stressful deliveries, containing a high concentration of CD34+ cells, may be an optimal blood product for preparing topical eye drops rich in GF.
Conclusions
Although CB may have some drawbacks compared to other blood components, this study showed that the EGF and TGF-β content may be significantly higher in CB-S. CB-S is the product of choice for treating corneal nerve damage because the amount of VEGF in PB-S can be negligible. Conversely, PB-S could be indicated as the primary source for products to accelerate keratinocyte metabolism and heal stromal defects, because of its higher IGF content.
Acknowledgements
The Authors thank Mrs Chiara Coslovi for her excellent technical support. This work was supported in part by a grant from the Fondazione Cassa di Risparmio in Bologna to Prof. E.C. Campos and by a grant from Regione Emilia Romagna to the Cord Blood Bank.
Footnotes
Authorship contributions
PV, MB and GG designed the study, AT collected the data, PV and MB analysed the data; all Authors wrote the manuscript.
The Authors declare no conflicts of interest.
References
- 1.Ubels J, Loley K, Rismondo V. Retinol secretion by the lacrimal gland. Invest Ophthalmol Vis Sci. 1986;27:1261–9. [PubMed] [Google Scholar]
- 2.Van Setten G, Tervo T, Tervo K, et al. Epidermal growth factor (EGF) in ocular fluid: presence, origin and therapeutic consideration. Acta Ophthalmol. 1992;202:54–9. doi: 10.1111/j.1755-3768.1992.tb02169.x. [DOI] [PubMed] [Google Scholar]
- 3.Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:163–78. doi: 10.1016/s1542-0124(12)70085-x. [DOI] [PubMed] [Google Scholar]
- 4.Márquez-de-Aracena R, Montero-de-Espinosa I. Subconjunctival application of regenerative factors rich plasma for the treatment of ocular alkali burns. Eur J Ophthalmol. 2009;19:909–15. doi: 10.1177/112067210901900603. [DOI] [PubMed] [Google Scholar]
- 5.Márquez-de-Aracena-del-Cid R, Montero-de-Espinosa I, Muñoz M, Pereira G. Aplicación subconjuntival de concentrado de plaquetas plasmáticas en el tratamiento de quemaduras oculares. Resultados preliminares. Arch Soc Esp Oftalmol. 2007;88:475–82. doi: 10.4321/s0365-66912007000800005. [In Spanish] [DOI] [PubMed] [Google Scholar]
- 6.Fox RI, Chan R, Michelson JB, et al. Beneficial effect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis Rheum. 1984;27:459–61. doi: 10.1002/art.1780270415. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa Y, Okamoto S, Mori T, et al. Autologous serum eye drops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2003;31:579–83. doi: 10.1038/sj.bmt.1703862. [DOI] [PubMed] [Google Scholar]
- 8.Tsubota K, Goto E, Fujita H, et al. Treatment of dry eye by autologous serum application in Sjögren’s syndrome. Br J Ophthalmol. 1999;83:390–5. doi: 10.1136/bjo.83.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alio JL, Arnalich-Montiel F, Rodriguez AE. The role of “eye platelet rich plasma” (E-PRP) for wound healing in ophthalmology. Curr Pharm Biotechnol. 2012;13:1257–65. doi: 10.2174/138920112800624355. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Plandolit S, Morales MC, Freire V, et al. Plasma rich in growth factors as a therapeutic agent for persistent corneal epithelial defects. Cornea. 2010;29:843–8. doi: 10.1097/ICO.0b013e3181a81820. [DOI] [PubMed] [Google Scholar]
- 11.Sandri G, Bonferroni MC, Rossi S, et al. Platelet lysate formulations based on mucoadhesive polymers for the treatment of corneal lesions. J Pharm Pharmacol. 2011;63:189–98. doi: 10.1111/j.2042-7158.2010.01208.x. [DOI] [PubMed] [Google Scholar]
- 12.Yoon KC, Jeong IY, Im SK, et al. Therapeutic effect of umbilical cord serum eyedrops for the treatment of dry eye associated with graft-versus-host disease. Bone Marrow Transplant. 2007;39:231–5. doi: 10.1038/sj.bmt.1705566. [DOI] [PubMed] [Google Scholar]
- 13.Yoon KC, Im SK, Park YG, et al. Application of umbilical cord serum eyedrops for the treatment of dry eye syndrome. Cornea. 2006;25:268–72. doi: 10.1097/01.ico.0000183484.85636.b6. [DOI] [PubMed] [Google Scholar]
- 14.Yoon KC, Choi J, You IC. Application of umbilical cord serum eyedrops for recurrent corneal erosions. Cornea. 2011;30:744–8. doi: 10.1097/ICO.0b013e31820d850f. [DOI] [PubMed] [Google Scholar]
- 15.Sharma N, Goel M, Velpandian T, et al. Evaluation of umbilical cord serum therapy in acute ocular chemical burns. Invest Ophthalmol Vis Sci. 2011;52:1087–92. doi: 10.1167/iovs.09-4170. [DOI] [PubMed] [Google Scholar]
- 16.Yoon KC, You IC, Im SK, et al. Application of umbilical cord serum eyedrops for the treatment of neurotrophic keratitis. Ophthalmology. 2007;114:1637–42. doi: 10.1016/j.ophtha.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Versura P, Profazio V, Buzzi M, et al. Efficacy of standardized and quality-controlled cord blood serum eye drop therapy in the healing of severe corneal epithelial damage in dry eye. Cornea. 2013;32:412–8. doi: 10.1097/ICO.0b013e3182580762. [DOI] [PubMed] [Google Scholar]
- 18.Harritshøj LH, Nielsen C, Ullum H, et al. Ready-made allogeneic ABO-specific serum eye drops: production from regular male blood donors, clinical routine, safety and efficacy. Acta Ophthalmol. 2014;92:783–6. doi: 10.1111/aos.12386. [DOI] [PubMed] [Google Scholar]
- 19.Chiang C-C, Chen W-L, Lin J-M, Tsai Y-Y. Allogenic serum eye drops for the treatment of persistent corneal epithelial defect. Eye. 2009;23:290–3. doi: 10.1038/sj.eye.6703079. [DOI] [PubMed] [Google Scholar]
- 20.Chiang C-C, Lin J-M, Chen W-L, Tsai Y-Y. Allogenic serum eye drops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Cornea. 2007;26:861–3. doi: 10.1097/ICO.0b013e3180645cd7. [DOI] [PubMed] [Google Scholar]
- 21.Geerling G, MacLennan S, Hartwig D. Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol. 2004;88:1467–74. doi: 10.1136/bjo.2004.044347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao J, Xie LX, Zang XJ, Li W. Organ culture for preservation of the cornea: human umbilical cord serum versus fetal bovine serum. Zhonghua Yan Ke Za Zhi. 2004;40:533–8. [In Chinese] [PubMed] [Google Scholar]
- 23.Nakamura M, Kawahara M, Morishige N, et al. Promotion of corneal epithelial wound healling in diabetic rats by the combination of a substance P-derived peptidew (FGLM-NH2) and insulin-like growth factor-1. Diabetologia. 2003;46:839–42. doi: 10.1007/s00125-003-1105-9. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Harwing D, Harloff S, et al. Corneal epitheliotrophic capacity of three different blood-derived preparations. Invest Ophthalmol Vis Sci. 2006;47:2438–44. doi: 10.1167/iovs.05-0876. [DOI] [PubMed] [Google Scholar]
- 25.Gordon JF, Johnson P, Musch DC. Topical fibronectin ophthalmic solution in the treatment of persistent defects of the corneal epithelium. Chiron Vision Fibronectin Study Group. Am J Ophthalmol. 1995;119:281–7. doi: 10.1016/s0002-9394(14)71168-7. [DOI] [PubMed] [Google Scholar]
- 26.Nelson JD, Gordon JF. Topical fibronectin in the treatment of keratoconjunctivitis sicca. Chiron Keratoconjunctivitis Sicca Study Group. Am J Ophthalmol. 1992;114:441–7. doi: 10.1016/s0002-9394(14)71856-2. [DOI] [PubMed] [Google Scholar]
- 27.Nishida T, Nakamura M, Ofuji K, et al. Synergistic effects of substance P with insulin-like growth factor-1 on epithelial migration of the cornea. J Cell Physiol. 1996;169:159–66. doi: 10.1002/(SICI)1097-4652(199610)169:1<159::AID-JCP16>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 28.Yoon KC, Heo H, Im SK, et al. Comparison of autologous serum and umbilical cord serum eye drops for dry eye syndrome. Am J Ophthalmol. 2007;144:86–92. doi: 10.1016/j.ajo.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Vajpayee RB, Mukerji N, Tandon R, et al. Evaluation of umbilical cord serum therapy for persistent corneal epithelial defects. Br J Ophthalmol. 2003;87:1312–16. doi: 10.1136/bjo.87.11.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan Q, Angelina A, Zambrano A, et al. Autologous serum eyedrops for dry eye. Cochrane Database Syst Rev. 2013;27:CD009327. doi: 10.1002/14651858.CD009327.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen EP, Hu FR, Lo SC, et al. Comparison of corneal epitheliotrophic capacity among different human blood-derived preparations. Cornea. 2011;30:208–14. doi: 10.1097/ICO.0b013e3181eadb67. [DOI] [PubMed] [Google Scholar]
- 32.Fréchette JP, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005;84:434–9. doi: 10.1177/154405910508400507. [DOI] [PubMed] [Google Scholar]
- 33.Dancey CP, Reidy J. Statistics without maths for psychology: using SPSS for Windows. 3rd ed. London: Prentice Hall; 2005. [Google Scholar]
- 34.Geerling G, Daniels JT, Dart JK, et al. Toxicity of natural tear substitutes in a fully defined culture model of human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2001;42:948–56. [PubMed] [Google Scholar]
- 35.Liu L, Hartwig D, Harloff S, et al. An optimised protocol for the production of autologous serum eyedrops. Graefes Arch Clin Exp Ophthalmol. 2005;243:706–14. doi: 10.1007/s00417-004-1106-5. [DOI] [PubMed] [Google Scholar]
- 36.Tananuvat N, Daniell M, Sullivan lJ, et al. Controlled study of the use of autologous serum in dry eye patients. Cornea. 2001;20:802–6. doi: 10.1097/00003226-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Kojima T, Ishida R, Dogru M, et al. The effect of autologous serum eye-drops in the treatment of severe dry eye disease: a prospective randomized case-control study. Am J Ophthalmol. 2005;139:242–6. doi: 10.1016/j.ajo.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 38.Urzua CA, Vasquez DH, Huidobro A, et al. Randomized double-blind clinical trial of autologous serum versus artificial tears in dry eye syndrome. Curr Eye Res. 2012;37:684–8. doi: 10.3109/02713683.2012.674609. [DOI] [PubMed] [Google Scholar]
- 39.Noble BA, loh RS, Maclennan S, et al. comparison of autologous serum eye drops with conventional therapy in a randomised controlled crossover trial for ocular surface disease. Br J Ophthalmol. 2004;88:647–52. doi: 10.1136/bjo.2003.026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noda-Tsuruya T, Asano-Kato N, Toda I, Tsubota K. Autologous serum eye drops for dry eye after laSiK. J Refract Surg. 2006;22:61–6. doi: 10.3928/1081-597X-20060101-13. [DOI] [PubMed] [Google Scholar]
- 41.Tsubota K, Goto E, Fujita H, et al. Treatment of persistent corneal epithelial defect by autologous serum application. Ophthalmology. 1999;106:1984–9. doi: 10.1016/S0161-6420(99)90412-8. [DOI] [PubMed] [Google Scholar]
- 42.Schulze SD, Sekundo W, Kroll P. Autologous serum for the treatment of corneal epithelial abrasions in diabetic patients undergoing vitrectomy. Am J Ophthalmol. 2006;142:207–11. doi: 10.1016/j.ajo.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 43.Kruse FE, Tseng SC. Growth factors modulate clonal growth and differentiation of cultured rabbit limbal and corneal epithelium. Invest Ophthalmol Vis Sci. 1993;34:1963–76. [PubMed] [Google Scholar]
- 44.Carpenter G, Cohen S. Epidermal growth factor. Ann Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- 45.Kitazawa T, Kinoshita S, Fujita K, et al. The mechanism of accelerated corneal epithelial healing by human epidermal growth factor. Invest Ophthalmol Vis Sci. 1990;31:1773–8. [PubMed] [Google Scholar]
- 46.Ohashi Y, Motokura M, Kinoshita Y, et al. Presence of epidermal growth factor in human tears. Invest Ophthalmol Vis Sci. 1989;30:1879–82. [PubMed] [Google Scholar]
- 47.Nakamura Y, Sotozono C, Kinoshita S. The epidermal growth factor receptor (EGFR): role in corneal wound healing and homeostasis. Exp Eye Res. 2001;72:511–7. doi: 10.1006/exer.2000.0979. [DOI] [PubMed] [Google Scholar]
- 48.Hongo M, Itoi M, Yamaguchi N, Imanishi J. Distribution of epidermal growth factor (EGF) receptors in rabbit corneal epithelial cells, keratocytes and endothelial cells, and the changes induced by transforming growth factor-b1. Exp Eye Res. 1992;54:9–16. doi: 10.1016/0014-4835(92)90063-x. [DOI] [PubMed] [Google Scholar]
- 49.Imanishi J, Kamiyama K, Iguchi I, et al. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res. 2000;19:113–29. doi: 10.1016/s1350-9462(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 50.Lou-Bonafonte JM, Bonafonte-Marquez E, Bonafonte-Royo S, Martinez-Carpio PA. Posology, efficacy, and safety of epidermal growth factor eye drops in 305 patients: logistic regression and group-wise odds of published data. J Ocul Pharmacol Ther. 2012;28:479–83. doi: 10.1089/jop.2011.0236. [DOI] [PubMed] [Google Scholar]
- 51.Peralta-Zaragoza O, Lagunas-Martinez A, Madrid-Marina V. Transforming growth factor beta-1: structure, function, and regulation mechanisms in cancer. Salud Publica Mex. 2001;43:340–51. [PubMed] [Google Scholar]
- 52.Grant MB, Khaw PT, Schultz GS, et al. Effects of epidermal growth factor, fibroblast growth factor, and transforming growth factor-beta on corneal cell chemotaxis. Invest Ophthalmol Vis Sci. 1992;33:3292–301. [PubMed] [Google Scholar]
- 53.Ohji M, SundarRaj N, Thoft RA. Transforming growth factor-beta stimulates collagen and fibronectin synthesis by human corneal stromal fibroblasts in vitro. Curr Eye Res. 1993;12:703–9. doi: 10.3109/02713689308995765. [DOI] [PubMed] [Google Scholar]
- 54.Saghizadeh M, Chwa M, Aoki A, et al. Altered expression of growth factors and cytokines in keratoconus, bullous keratopathy, and diabetic human corneas. Exp Eye Res. 2001;73:179–89. doi: 10.1006/exer.2001.1028. [DOI] [PubMed] [Google Scholar]
- 55.Robertson DM, Zhu M, Wu Cellular distribution of the IGF-1R in corneal epithelial cells. Exp Eye Res. 2012;94:179–86. doi: 10.1016/j.exer.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jester JV, Ho-Chang J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77:581–92. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- 57.Ko JA, Yanai R, Nishida T. IGF-1 released by corneal epithelial cells induces up-regulation of N-cadherin in corneal fibroblasts. J Cell Physiol. 2009;221:254–61. doi: 10.1002/jcp.21850. [DOI] [PubMed] [Google Scholar]
- 58.Hassell JR, Birk DE. The molecular basis of corneal transparency. Exp Eye Res. 2010;91:326–35. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Etheredge L, Kane BP, Hassell JR. The effect of growth factor signaling on keratocytes in vitro and its relationship to the phases of stromal wound repair. Invest Ophthalmol Vis Sci. 2009;50:3128–36. doi: 10.1167/iovs.08-3077. [DOI] [PubMed] [Google Scholar]
- 60.Ferrara N, Houck KA, Jakeman LB, et al. The vascular endothelial growth factor family of polypeptides. J Cell Biochem. 1991;47:211–8. doi: 10.1002/jcb.240470305. [DOI] [PubMed] [Google Scholar]
- 61.Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001;114:853–65. doi: 10.1242/jcs.114.5.853. [DOI] [PubMed] [Google Scholar]
- 62.Amano S, Rohan R, Kuroki M, et al. Requirement for vascular endothelial growth factor in wound- and inflammation-related corneal neovascularization. Invest Ophthalmol Vis Sci. 1998;39:18–22. [PubMed] [Google Scholar]
- 63.Pan Z, Fukuoka S, Karagianni N, et al. Vascular endothlelial growth factors promotes anatomical and functional recovery of injured peripheral nerves in the avascular cornea. FASEB J. 2013;27:2756–67. doi: 10.1096/fj.12-225185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Larsson A, Sköldenberg E, Ericson H. Serum and plasma levels of FGF-2 and VEGF in healthy blood donors. Angiogenesis. 2002;5:107–10. doi: 10.1023/a:1021588227705. [DOI] [PubMed] [Google Scholar]
- 65.Di Raimondo F, Azzaro MP, Palumbo GA, et al. Elevated vascular endothelial growth factor (VEGF) serum levels in idiopathic myelofibrosis. Leukemia. 2001;15:976–80. doi: 10.1038/sj.leu.2402124. [DOI] [PubMed] [Google Scholar]
- 66.Svedas E, Islam KB, Nisell H, Kublickiene KR. Vascular endothelial growth factor induced functional and morphologic signs of endothelial dysfunction in isolated arteries from normal pregnant women. Am J Obstetr Gynecol. 2003;188:168–76. doi: 10.1067/mob.2003.110. [DOI] [PubMed] [Google Scholar]
- 67.Versura P, Buzzi M, Giannaccare G, et al. Cord blood serum-based eyedrops: the impact of donor haematological and obstetric factors on the variability of epidermal growth factor levels. Blood Transfus. 2014;12:44–50. doi: 10.2450/2013.0115-13. [DOI] [PMC free article] [PubMed] [Google Scholar]