Abstract
Background
Enzymatic conversion of blood group A1B red blood cells (RBC) to group O RBC (ECO) was achieved by combined treatment with α-galactosidase and α-N-acetylgalactosaminidase. The aim of this study was to evaluate the function and safety of these A1B-ECO RBC in vitro.
Materials and methods
A 20% packed volume of A1B RBC was treated with enzymes in 250 mM glycine buffer, pH 6.8. The efficiency of the conversion of A and B antigen was evaluated by traditional typing in test tubes, gel column agglutination technology and fluorescence-activated cell sorting (FACS) analysis. The physiological and metabolic parameters of native and ECO RBC were compared, including osmotic fragility, erythrocyte deformation index, levels of 2,3-diphosphoglycerate, ATP, methaemoglobin, free Na+, and free K+. The morphology of native and ECO RBC was observed by scanning electron microscopy. Residual α-galactosidase or α-N-acetylgalactosaminidase in A1B-ECO RBC was detected by double-antibody sandwich ELISA method. Manual cross-matching was applied to ensure blood compatibility.
Results
The RBC agglutination tests and FACS results showed that A1B RBC were efficiently converted to O RBC. Functional analysis suggested that the conversion process had little impact on the physiological and metabolic parameters of the RBC. The residual amounts of either α-galactosidase or α-N-acetylgalactosaminidase in the A1B-ECO RBC were less than 10 ng/mL of packed RBC. About 18% of group B and 55% of group O sera reacted with the A1B-ECO RBC in a sensitive gel column cross-matching test.
Discussion
The conversion process does not appear to affect the morphological, physiological or metabolic parameters of A1B-ECO RBC. However, the A1B-ECO RBC still reacted with some antigens. More research on group O and B sera, which may partly reflect the complexity of group A1 the safety of A1B-ECO RBC is necessary before the application of these RBC in clinical transfusion.
Keywords: α-N-acetylgalactosaminidase, α-galactosidase, ABO blood group, blood group conversion, universal red blood cells
Introduction
Transfusion of ABO-incompatible units is the major cause of transfusion-induced fatalities1,2. Group O red blood cells (RBC) do not contain either A or B antigens and can be safely transfused from donors into recipients of any ABO blood group; group O RBC are, therefore, designated as universal RBC3–5. Transfusion of universal RBC is an effective way of preventing transfusion errors. The preparation, storage and use of universal RBC are, therefore, of great significance in clinical transfusion, especially in emergencies. Enzymatic removal of blood group A and B antigens to develop universal RBC was a pioneering vision originally proposed more than 30 years ago6–8. Previous studies confirmed that group A, B, and AB RBC can be converted to group O RBC by treatment with α-N-acetylgalactosaminidase or/and α-galactosidase derived from bacteria3,9. The resulting group O RBC were named enzymatically converted group O RBC (ECO RBC). Data from phase I and phase II clinical trials showed that ECO RBC derived from group B RBC (B-ECO RBC) were safe and functional10–13. The first comprehensive report of the preparation of ECO RBC from group A RBC (A-ECO RBC) by bacterial α-N-acetylgalactosaminidase was published in 20073. Thereafter, we successfully prepared A-ECO RBC from both group A1 and A2 RBC using a recombinant α-N-acetylgalactosaminidase from Elizabethkingia meningosepticum isolated from a domestic clinical sample14. Phase I clinical trials of A-ECO RBC, carried out in 2005 by ZymeQuest, indicated that small amounts of A-ECO RBC prepared from a group A donor could be safely re-infused repeatedly into the original donor15. So far, no clinical study on the transfusion of A-ECO RBC into volunteers with other blood groups has been reported.
Enzymatic conversion of AB RBC into group O RBC was also first reported in 20073. We then successfully prepared AB-ECO RBC using a recombinant Bacteroides fragilis α-galactosidase (B-zyme) and Elizabethkingia meningosepticum α-N-acetylgalactosaminidase (A-zyme) in our laboratory9. In the present study, we used A1B RBC because the ratio of the A2B blood type in AB blood donors is far below 1% in China16. The aim of this study was to investigate the effects of the enzymatic conversion process on the structure and function of A1B-ECO RBC. In addition, the compatibility of A1B-ECO RBC with the serum of subjects with other blood groups was evaluated as a preliminary assessment of the possibility application of A1B-ECO RBC in clinical transfusion.
Materials and methods
Materials
The recombinant α-N-acetylgalactosaminidase from Elizabethkingia meningosepticum (A-zyme) with a 6×His tag was expressed in E. coli BL21(DE3) and purified by Ni2+ Sepharose 6 FF methods14. The recombinant α-galactosidase from Bacteroides fragilis (B-zyme) was also expressed in E. coli BL21 (DE3) and purified by cation and anion exchange column chromatography17. Fresh human whole blood, plasma or sera of different types were obtained from the Transfusion Department at the Affiliated Hospital of Academy of Military Medical Sciences (Beijing, China). RBC were the commercial blood bank reagents The A2 from Immucor, Inc. (Norcross, GA, USA).
Enzymatic conversion process and ABO-typing of the A1B-ECO red blood cells
The treatment group were (i) native RBC, (ii) mock-treated control RBC and (iii) enzyme-treated RBC. Briefly, the RBC were divided into three samples of equal volume. RBC in the native group were kept in isotonic saline at 4 °C until the conversion was complete. Meanwhile, the enzymatic reactions were performed in conversion buffer (250 mM glycine, pH 6.8) containing 0.3 mg A-zyme and 0.01 mg B-zyme per mL of packed RBC, with 20% packed RBCs as indicated9. Reactions were incubated for 3 hours with gentle rotation at 26 °C, followed by four repeated washing cycles with 1:4 (v/v) of phosphate-buffered saline (PBS) by centrifugation at 2,700 rpm for 5 minutes. The cells in the mock-treated group were subjected to the same procedure in the absence of A-zyme and B-zyme. The RBC of all three groups were then stored in mono-ammonium phosphate nutrient solution at 4 °C for the functional assays. The washed A1B-ECO RBC were first ABO-typed according to standard blood banking techniques using licensed monoclonal antibody reagents. Murine monoclonal anti-A, anti-B, and anti-A1 lectin were obtained from Shanghai Hemo-pharmaceutical & Biological Co., Ltd. (Shanghai, China). Anti-A,B (clones: ES-15/ES-4) were from Millipore (Livingston, UK). A1B-ECO RBC were also typed by gel column agglutination technology. The DG Gel ABO-CDE, incubator, and centrifuge for DG Gel cards were from Diagnostic Grifols S.A. (Barcelona, Spain).
Flow cytometry
Flow cytometry analyses of A1B RBC and A1B-ECO RBC were performed using a fluorescence activated cell sorting (FACS) flow cytometer (FACSCalibur, BD Biosciences, San Jose, CA, USA) with anti-A, and anti-B blood grouping reagents (Shanghai Hemo-pharmaceutical & Biological Co. Ltd.), anti-A,B blood grouping reagents (Millipore), and Alexa Fluor 488 Goat Anti-Mouse IgM (Molecular Probes, Inc. Eugene, OR, USA). Briefly, 10 μL cells were fixed overnight at room temperature under gentle agitation by the addition of 100 μL 4% paraformaldehyde (w/v, Sigma-Aldrich, St. Louis, MO, USA) in PBS to prevent agglutination of antigen-positive cells. Packed RBC (1 μL) were prewashed with PBS twice and re-suspended in 100 μL PBS. Next, 25 μL of undiluted primary antibody were added and incubated for 3 hours in the dark at 25 °C. After two washes and resuspension in 100 μL PBS, 1.5 μL of undiluted secondary antibody were added and incubated for 1 hour in the dark at 25 °C. Cells were then analysed after another two washes (as above) and re-suspension in 500 μL PBS. A total of 50,000 events were evaluated. The clearance rate of the antigen (%)=[(Geo mean fluorescence intensity of A1B RBC – Geo mean fluorescence intensity of A1B-ECO RBC)/(Geo mean fluorescence intensity of A1B RBC – Geo mean fluorescence intensity of O RBC)]×100. Approximately 600,000 A antigen sites and 700,000 B antigen sites were estimated to be localised on the surface of each A1B RBC18, so the number of residual antigen sites can be calculated as follows:
Functional assays
The physiological and metabolic parameters of RBC, including osmotic fragility, deformability, and levels of 2,3-diphosphoglycerate (2,3-DPG), ATP (adenosine 5′-triphosphate), methaemoglobin, free Na+ and free K+, were measured before and after the ECO process, as described previously19. Erythrocyte deformability was quantified at various shear rate levels using a laser-diffraction ektacytometer system (Model LBY-BX, Precil Company, Beijing, China). Briefly, 50 μL of blood were suspended in 1 mL 15% polyvinylpyrrolidone buffer (molecular weight 30 kDa, 61 mM NaCl, 0.8 mM Na2HPO4, 0.2 mM KH2PO4, pH 7.4, 290 mOsm/kg) and used to measure the erythrocyte deformation index (EI) at shear rates of 100, 400, 600, 800, and 1,000 s−1. The integrated EI value was automatically given by the system when the number for the EI was over 320. ATP was assayed using an adenosine 5′-triphosphate bioluminescent assay kit (Sigma-Aldrich, St. Louis, MO, USA) and 2,3-DPG was assayed using a human 2,3-DPG enzyme-linked immunosorbent assay (ELISA) kit (Rapidbio, West Hills, CA, USA). Methaemoglobin, free Na+, and free K+ were determined with a blood gas analyser (ABL700, Radiometer, Copenhagen, Denmark).
Scanning electron microscopy
The effect of the conversion process on the morphology of the RBC was investigated by examining the native and enzyme-converted RBC by scanning electron microscopy. The samples were fixed with 3% glutaraldehyde in PBS, dried on a glass slide, and postfixed with 1% osmium tetroxide in 0.1 M Na-cacodylate for 90 minutes. The fixed cells were then dehydrated in graded ethanol solutions, and treated with isoamyl acetate for 25 minutes. They were then desiccated in a dryer and gold-plated for imaging with a scanning electron microscope (S-3400N, Hitachi, Tokyo, Japan).
Residual enzyme detection
The residual enzyme in the prepared A1B-ECO RBC was measured with a double-antibody sandwich ELISA method21. The packed A1B-ECO RBC were lysed with sterile water. First, the polyclonal antibodies against A-zyme or B-zyme (10 μg/mL) were coated onto the wells of microplates (Nunc, Roskilde, Denmark) by overnight incubation at 4 °C. After three washes with PBS, the blocking procedure was performed using 250 μL per well of 2% bovine serum albumin (Sigma-Aldrich) in PBS for 2 hours at room temperature in the dark. The microplate wells were washed three times with PBS solution. Aliquots (100 μL) of lysates of A1B-ECO RBC or the standard of A-zyme/B-zyme were added to the microplate wells and incubated for 1 hour at 37 °C in the dark. Then 100 μL of the specific monoclonal antibodies against A-zyme or B-zyme were added to react with the enzyme for 1 hour at 37 °C in the dark. After washing the wells, the secondary horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (immunoglobulin G) antibody (diluted to 1:5,000 in PBS) (ZSGB-BIO, Beijing, China) was added for 30 minutes. Finally, 3,3′,5,5′-tetramethylbenzidine (TMB) (Sigma-Aldrich) at 0.2 mg/mL was added to each well and incubated for 10 minutes at 37 °C in the dark. The reaction was terminated with 50 μL 2M sulphuric acid and the absorbance was measured with a plate reader at 450 nm.
The polyclonal antibodies of A-zyme or B-zyme were made in our laboratory by immunising rabbits with purified recombinant α-N-acetylgalactosaminidase or α-galactosidase and purifying the antibodies on a Hi-Trap rProtein A column. The monoclonal antibody of B-zyme was made by immunising mice with purified recombinant α-galactosidase and the antibody was purified on a Hi- Trap rProtein A column. A murine monoclonal anti-His antibody that reacted with recombinant A-zyme with a His tag was obtained from ZSGB-BIO.
Cross-match tests
The standard procedure for cross-matching was carried out following the manufacturer’s instructions (Diagnostic Grifols S.A., Barcelona, Spain). The relevant reagents, cards, and instruments were also obtained from Grifols. All operations were performed by trained and experienced users. Briefly, 50 μL of the donor’s RBC suspension (1%) and 25 μL of the recipient’s serum or plasma were placed into the microtubes of the DG Gel Coombs cards (which include eight columns with polyspecific anti-human globulin). After incubation for 15 minutes at 37 °C, the DG Gel cards were centrifuged for 9 minutes in the centrifuge for DG Gel cards. The strength of the reaction was given on the following scale: negative (0), weak+ (w+), 1+, 2+, 3+, and 4+. This gel technology is based on size exclusion, so RBC at the bottom of the gel represents a negative reaction while RBC on top of the gel or in the column indicate a positive reaction. About 300 discarded clinical laboratory sera or plasma samples for all four ABO blood groups were used for this test.
Results
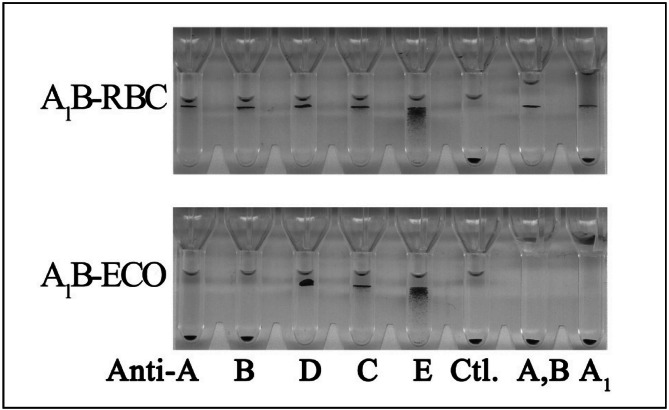
The enzymatic conversion process is efficient. The A1B-ECO RBC were identified as blood group O RBC by different assays used in routine blood-banking practice, including the traditional typing in test tubes using all commercially available anti-A, anti-A1, anti-B, and anti-A,B reagents as well as the gel column agglutination technology that is now widely used in blood banks (Figure 1). FACS analysis also showed that A1B RBC were efficiently converted to O RBC (Table I).
Figure 1.
Blood typing of native red blood cells (A1B RBCs) and enzyme-converted RBCs (A1B-ECO RBCs) by gel column agglutination technology.
The upper and lower gel card sections show reactions with A1B RBC and A1B-ECO RBC, respectively. As the gel technology is based on size exclusion, RBC at the bottom of the gel represent a negative reaction while RBC on top of the gel or in the column indicate a positive reaction. Native RBC (blood group A1B) are tested in parallel with the corresponding enzyme-converted RBC (A1B-ECO RBC).
The ABO-D blood typing card was from Diagnostic Grifols. Anti-A,B (Millipore) and anti-A1 (Shanghai Hemo-pharmaceutical & Biological Co., Ltd.) were added to the right two columns. A1B RBC agglutinate with anti-A, anti-B, anti-D, anti-C, anti-E, anti-A,B, and anti-A1 but show no self-agglutination. A1B-ECO RBC do not agglutinate with anti-A, anti-B, anti-A,B, and anti-A1, but show agglutination with anti-D, anti-C, and anti-E.
Ctl: control, buffered solution without antibodies.
Table I.
FACS analysis of native and enzyme-converted red blood cells (RBC).
| Antigens | Geo mean of the fluorescence intensity | Clearance rate of antigen (%) | Mean of residual antigens (×102) | ||
|---|---|---|---|---|---|
|
| |||||
| O RBC | A1B RBC | A1B-ECO RBC | |||
| B | 5.85 | 4,414.55 | 11.97 | 99.86 | 9.7 |
| A | 6.86 | 6,977.26 | 5.10 | 100.03 | 0 |
| A,B | 7.53 | 8,614.05 | 11.62 | 99.95 | 6.2 |
Effect of the enzymatic conversion process on the structure and function of A1B-ECO red blood cells
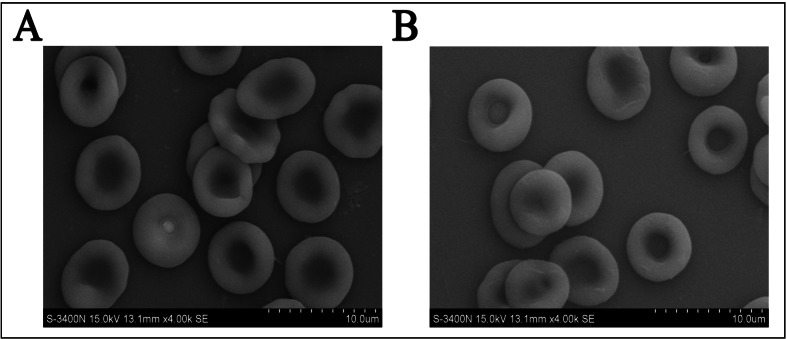
The morphology observed by scanning electron microscopy was the same for both the enzyme-converted RBC and the native RBC (Figure 2), which suggested that the enzymatic conversion process had little effect on the structure of the A1B-ECO RBC. The similarities of the functional assessment results for the two types of RBC, including osmotic fragility, erythrocyte deformation index (integrated EI), and levels of 2,3-DPG, ATP, methaemoglobin, free Na+, and free K+, also indicated that the conversion process did not affect physiological and metabolic parameters of the RBC (Table II).
Figure 2.
The morphology of native (A) and enzyme-converted red blood cells (B) observed by scanning electron microscopy.
Table II.
The physiological and metabolic parameters of RBC before and after undergoing the ECO process.
| Physiological and metabolic parameters of RBCs | A1B-RBC | Mock- treated RBC | A1B-ECO RBC | Reference value |
|---|---|---|---|---|
| Osmotic fragility (NaCl%) | 0.43±0.04 | 0.42±0.03 | 0.41±0.01 | 0.42–0.46 |
| ATP (μmol/gHb) | 0.65±0.19 | 0.71±0.27 | 0.75±0.20 | - |
| 2,3-DPG (μmol/gHb) | 1.38±0.64 | 1.66±0.51 | 1.32±0.08 | - |
| MetHb (%) | 0.45±0.07 | 0.35±0.07 | 0.45±0.21 | 0–1.5% |
| Integrated EI (%) | 21.10±1.53 | 27.23±0.91 | 27.77±0.38 | - |
| Free Na+(mmol/L) | 137.5±17.7 | 121.7±12.0 | 132.0±22.6 | >104 |
| Free K+(mmol/L) | 6.2±3.4 | 14.1±7.2 | 12.5±5.1 | 0.5–25.0 |
RBC: red blood cell; ECO: enzymatically converted group O; ATP: adenosine triphosphate; DPG: diphosphoglycerate; MetHb: methaemoglobin; EI: erythrocyte deformation index.
Residual enzyme detection
A double-antibody sandwich ELISA method was established which was sensitive enough to detect a minimal amount of residual A-zyme or B-zyme at the concentration of 1 ng/mL. After four repeated washing cycles with 1:4 (v/v) PBS, the amount of residual A-zyme or B-zyme associated with A1B-ECO RBC was less than 10 ng/mL of packed RBC for either enzyme.
The results of cross-match tests
Agglutination of A1B-ECO RBC with serum or plasma from clinical samples (n=160) of all four ABO blood groups was also determined by manual cross-match tests using Grifols DG Gel Coombs cards (Table III). All group A and AB sera/plasma samples were compatible with A1B-ECO RBC, but 18% of the sera or plasma from group B (n=40) and 55% from group O (n=40) caused agglutination. A major cross-match test was, therefore, conducted on ECO from A1 RBC, A2 RBC, and B RBC (called A1-ECO, A2-ECO, and B-ECO, respectively) with sera or plasma from all four ABO blood groups using the DG Gel Coombs method. About 13% of group B (n=24) and 48% of group O (n=48) sera/plasma agglutinated A2-ECO RBC, 6% of group O (n=132) sera/plasma agglutinated B-ECO RBC, and 25% of group B (n=48) and 55% of group O (n=56) sera/plasma agglutinated A1-ECO RBC. The cross-match tests with sera/plasma from healthy donors and patients were classified (Table III) and showed that the ratio of agglutination was lower with sera/plasma from healthy donors than from patients.
Table III.
The cross-match tests of enzyme-converted red blood cells (ECO RBC) with sera or plasma from healthy donors or patients.
| ECO RBC | Cross-match tests with sera/plasma from healthy donors | Cross-match tests with sera/plasma from patients | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Blood group | Samples | Results* | Samples | Results* | |||||||||||
|
|
|
||||||||||||||
| 0 | w+ | 1+ | 2+ | 3+ | 4+ | 0 | w+ | 1+ | 2+ | 3+ | 4+ | ||||
|
| |||||||||||||||
| A1B-ECO RBC | AB | 20 | 20 | 20 | 20 | ||||||||||
|
| |||||||||||||||
| B | 20 | 17 | 1 | 2 | 20 | 16 | 1 | 1 | 2 | ||||||
|
| |||||||||||||||
| A | 20 | 20 | 20 | 20 | |||||||||||
|
| |||||||||||||||
| O | 20 | 12 | 1 | 3 | 4 | 20 | 6 | 4 | 1 | 2 | 7 | ||||
|
| |||||||||||||||
| A1-ECO RBC | AB | 16 | 16 | 32 | 32 | ||||||||||
|
| |||||||||||||||
| B | 16 | 14 | 1 | 1 | 32 | 22 | 2 | 6 | 2 | ||||||
|
| |||||||||||||||
| A | 16 | 16 | 32 | 32 | |||||||||||
|
| |||||||||||||||
| O | 24 | 13 | 2 | 3 | 4 | 2 | 32 | 12 | 1 | 3 | 6 | 6 | 4 | ||
|
| |||||||||||||||
| A2-ECO RBC | AB | 8 | 8 | 8 | 8 | ||||||||||
|
| |||||||||||||||
| B | 8 | 8 | 16 | 13 | 1 | 2 | |||||||||
|
| |||||||||||||||
| A | 8 | 8 | 16 | 16 | |||||||||||
|
| |||||||||||||||
| O | 16 | 12 | 4 | 32 | 13 | 3 | 2 | 4 | 7 | 3 | |||||
|
| |||||||||||||||
| B-ECO RBC | AB | 8 | 8 | 8 | 8 | ||||||||||
|
| |||||||||||||||
| B | 8 | 8 | 16 | 16 | |||||||||||
|
| |||||||||||||||
| A | 16 | 16 | 86 | 86 | |||||||||||
|
| |||||||||||||||
| O | 16 | 16 | 116 | 108 | 2 | 3 | 3 | ||||||||
Standard procedure for cross-match tests was carried out following the manufacturer’s (Grifols) instructions using agglutination scores of 0 to 4+. 0: band of red blood cells at the bottom of the column and no visible agglutinations in the rest of the column. w+: scarce, small-sized agglutination in the lower half of the column. 1+: some small-sized agglutinations in the column. 2+: small or medium-sized agglutinations throughout the column. 3+: upper band of medium-sized agglutinations in the upper half of the column. 4+: band of agglutinated RBC in the upper part of the column.
Discussion
The recombinant α-N-acetyl-galactosaminidase from Elizabethkingia meningosepticum (A-zyme) and α-galactosidase from Bacteroides fragilis (B-zyme) are specific glycoside hydrolases for removal of the immunodominant terminal sugars (α1,3GalNAc and α1,3Gal, respectively) on oligosaccharides of blood groups A and B. A previous study revealed that combined treatment with A-zyme and B-zyme converted the glycoproteins and glycolipids of AB RBC to H antigens for group O RBC3,9. In this study, the most powerful commercial monoclonal anti-A, anti-B, or anti-A,B (e.g. ES-15 that detects Ax) antibodies were used to identify the blood group of the converted RBC. The results indicate that group A1B RBC were completely converted to group O RBC. No significant differences were noted in the morphology or ATP and 2,3-DPG levels between native and enzyme-treated RBC, and the osmotic fragility and levels of methemoglobin, free Na+, and free K+ of A1B-ECO RBC remained in the normal range. These findings indicate that the conversion process had very little effect on the oxygen-carrying capability and membrane integrity of the converted RBC. The double-antibody sandwich ELISA results showed that the residual levels of both the A-zyme and B-zyme associated with A1B-ECO RBC can be washed out to less than 10 ng/mL of packed RBC. All these results support successful conversion.
In recent years, commercial kits consisting of column tests for cross-matching have progressively replaced conventional tube tests in most laboratories. Microtube gel column agglutination-based cards provide a more complete test profile and simplify pre-transfusion testing22. In this study, cross-matching was also performed by microtube gel column agglutination-based cards in order to evaluate the safety of A1B-ECO RBC as universal donor blood. The A1B-ECO RBC agglutinated with 18% of group B sera/plasma and 55% of group O sera/plasma. Similar results were also found for A1-ECO and A2-ECO RBC. However, only 6% of group O (n=132) sera/plasma agglutinated the B-ECO RBC. We speculate that the agglutination may have two causes: (i) small amounts of residual antigens could be a major cause. The residual traces of A antigen or B antigen on the surface of ECO RBC may react with anti-A or anti-B antibodies in sera and cause agglutination; (ii) type 3H antigen may be another cause of agglutination of A-ECO RBC and AB-ECO RBC. Since type 3 A antigens [GalNAcα1-3(Fucα1-2) Galβ1-3GalNAcα1-3(Fucα1-2) Galβ1-4GlcNAcβ1-R] only exists in A RBC and AB RBC23–24, treatment with α-N-acetylgalactosaminidase, would convert the type 3A antigens to type 3H antigens [Fucα1-2Galβ1-3GalNAcα1-3(Fucα1-2)Galβ1-4GlcNAcβ1-R]. Because no type 3H antigen exists on the surface of group B and group O RBC, the natural anti-type 3H antibody appears in the sera of group B and group O individuals. Hu ejta et al. reported that anti-type 3H antibodies were present in the blood of 51 of 106 healthy people (no information was reported on blood type)25. Kruskall13 reported that 20% of group A and 40% of group O sera agglutinated B-ECO RBC based on a PEG-enhanced indirect antiglobulin test phase. Despite this, the survival and recovery of B-ECO RBC were comparable with those of ABO-matched RBC in 51Cr-labelling based studies. Olsson and Clausen26 also reported that the new A-ECO and B-ECO RBC reacted with some sera in cross-match tests, mainly with group O sera and to a lesser extent with group B. They believe that the relevance and practical consequence of the remaining cross-match reactivity is one of the main foci for future work, both in experimental studies and clinical trials. The most critical points arising from the present study would be to: (i) develop more sensitive assay(s) to detect any residual A and B antigens following conversion; (ii) address the method of identifying other potential residual antigens (besides A and B, and type 3H) that may be contributing to positive cross-match tests; (iii) specify future directions (using other techniques besides the ones presented) to remove A and B antigens completely from ABO-incompatible RBC; and (iv) confirm that the enzyme-conversion technique does not alter the 3D structure of surface antigens still present on RBC (which could perhaps cause non-specific cross-matching). This could potentially be achieved using ABO-compatible RBC.
Conclusions
The conversion process does not appear to affect physiological or metabolic parameters of A1B-ECO RBC. However, the A1B-ECO RBC still react with some group O and B sera, which may reflect the complexity of group A antigens. Next, we will focus on the consequences of the remaining cross-match reactivity in transfusion practice and safety of A-ECO and AB-ECO RBC as universal donor blood.
Acknowledgements
The Authors would like to thank Bo Dong for his excellent technical assistance with the FACS.
Footnotes
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81300447).
Authorship contributions
H-WG and H-LZ: conception and design of the study; provision of study material, cross-match tests, functional assays; collection, assembly, analysis and interpretation of data; manuscript writing and final approval of the manuscript.
XZ: data collection, production of ECO RBC, cross-match tests, functional assays, and flow cytometry. S-BL, Z-MY: preparation of antibodies and residual enzyme detection. HX, Y-JJ: provision of study material.
S-PJ, Y-XT, Q-FW, QL, FG: manuscript writing, manuscript revision for important intellectual content, and final approval of the manuscript.
H-WG and H-LZ contributed equally to the work and share first authorship.
The Authors declare no conflicts of interest.
References
- 1.Linden JV, Kaplan HS. Transfusion errors: causes and effects. Transfus Med Rev. 1994;8:169–83. doi: 10.1016/s0887-7963(94)70109-7. [DOI] [PubMed] [Google Scholar]
- 2.Williamson L, Cohen H, Love E, et al. The serious hazards of transfusion (SHOT) initiative: The UK approach to haemovigilance. Vox Sang. 2000;78:291–5. [PubMed] [Google Scholar]
- 3.Liu QP, Sulzenbacher G, Yuan H, et al. Bacterial glycosidases for the production of universal red blood cells. Nat Biotechnol. 2007;25:454–64. doi: 10.1038/nbt1298. [DOI] [PubMed] [Google Scholar]
- 4.Garratty G. Progress in modulating the RBC membrane to produce transfusable universal/stealth donor RBCs. Transfus Med Rev. 2004;18:245–56. doi: 10.1016/j.tmrv.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Garratty G. Modulating the red cell membrane to produce universal/stealth donor red cells suitable for transfusion. Vox Sang. 2008;94:87–95. doi: 10.1111/j.1423-0410.2007.01003.x. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein J, Siviglia G, Hurst R, et al. Group B erythrocytes enzymatically converted to group O survive normally in A, B, and O individuals. Science. 1982;215:168–70. doi: 10.1126/science.6274021. [DOI] [PubMed] [Google Scholar]
- 7.Hoskins LC, Boulding ET, Larson G. Puri cation and characterization of blood group A-degrading isoforms of α-N-acetylgalactosaminidase from Ruminococcus torques strain IX-70. J Biol Chem. 1997;272:7932–9. doi: 10.1074/jbc.272.12.7932. [DOI] [PubMed] [Google Scholar]
- 8.Zhu A, Leng L, Monahan C, et al. Characterization of recombinant α-galactosidase for use in seroconversion from blood group B to O of human erythrocytes. Arch Biochem Biophys. 1996;327:324–9. doi: 10.1006/abbi.1996.0129. [DOI] [PubMed] [Google Scholar]
- 9.Gao HW, Li SB, Tan YX, et al. Application of α-N-acetylgalactosaminidase and α-galactosidase in AB to O red blood cells conversion. Artif Cells Nanomed Biotechnol. 2013;41:32–6. doi: 10.3109/10731199.2012.724422. [DOI] [PubMed] [Google Scholar]
- 10.Lenny LL, Hurst R, Goldstein J, et al. Single-unit transfusions of RBC enzymatically converted from group B to group O to A and O normal volunteers. Blood. 1991;77:1383–8. [PubMed] [Google Scholar]
- 11.Lenny LL, Hurst R, Goldstein J, Galbraith RA. Transfusions to group O subjects of 2 units of red cells enzymatically converted from group B to group O. Transfusion. 1994;34:209–14. doi: 10.1046/j.1537-2995.1994.34394196617.x. [DOI] [PubMed] [Google Scholar]
- 12.Lenny LL, Hurst R, Zhu A, et al. Multiple-unit and second transfusions of red cells enzymatically converted from group B to group O: report on the end of phase 1 trials. Transfusion. 1995;35:899–902. doi: 10.1046/j.1537-2995.1995.351196110892.x. [DOI] [PubMed] [Google Scholar]
- 13.Kruskall MS, AuBuchon JP, Anthony KY, et al. Transfusion to blood group A and O patients of group B RBCs that have been enzymatically converted to group O. Transfusion. 2000;40:1290–8. doi: 10.1046/j.1537-2995.2000.40111290.x. [DOI] [PubMed] [Google Scholar]
- 14.Yu CY, Xu H, Wang LS, et al. Human RBCs blood group conversion from A to O using a novel α-N-acetylgalactosaminidase of high specific activity. Chinese Science Bulletin. 2008;53:2008–16. [Google Scholar]
- 15.ZymeQuest. Safety Study of ECO Conversion System For Red Blood Cells; NCT00261274. 2009. [Accessed on 30/11/2005]. Available at: http://clinicaltrials.gov.
- 16.Gao HW, Wu DZ, Li SB, et al. Preparation of A2 reverse grouping cells from A2B red blood cells by alpha-galactosidase. Blood Transfus. 2013;11:305–7. doi: 10.2450/2012.0020-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao HW, Li SB, Bao GQ, et al. A reconstructed B. Fragilis-derived recombinant α-galactosidase developed for human blood type B→O conversion. J Exp Hematol. 2011;19:503–7. [PubMed] [Google Scholar]
- 18.Quinley ED. The ABO blood group system. In: Quinley ED, editor. Immunohematology: Principles and Practice. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 1998. p. 96. [Google Scholar]
- 19.Gao HW, Li SB, Bao GQ, et al. Properties of a novel α-galactosidase from B. fragilis and its potential for human blood-type B to O conversion. Scientia Sinica Vitae. 2011;41:1030–6. [Google Scholar]
- 20.Zhao L, Wang B, You GX, et al. Effects of different resuscitation fluids on the rheologic behavior of red blood cells, blood viscosity and plasma viscosity in experimental hemorrhagic shock. Resuscitation. 2009;80:253–8. doi: 10.1016/j.resuscitation.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Gao HW, Li SB, Bao GQ, et al. Detection of minimal amount of residual α-N-acetylgalactosaminidase involved in conversion of blood type A to O. Lett Biotechnol. 2013;24:101–3. [Google Scholar]
- 22.Chang C, Brown M, Davies L, et al. Evaluation of Erytra® fully automated analyser for routine use in transfusion laboratory. Transfus Med. 2014;24:33–8. doi: 10.1111/tme.12073. [DOI] [PubMed] [Google Scholar]
- 23.Clausen H, Levery SB, Kannagi R, Hakomori S. Novel blood group H glycolipid antigens exclusively expressed in blood group A and AB erythrocytes (type 3 chain H). I. Isolation and chemical characterization. J Biol Chem. 1986;261:1380–7. [PubMed] [Google Scholar]
- 24.Clausen H, Holmes E, Hakomori S. Novel blood group H glycolipid antigens exclusively expressed in blood group A and AB erythrocytes (type 3 chain H). II. Differential conversion of different H substrates by A1 and A2 enzymes, and type 3 chain H expression in relation to secretor status. J Biol Chem. 1986;261:1388–92. [PubMed] [Google Scholar]
- 25.Huflejta ME, Vuskovicb M, Vasiliuc D, et al. Anti-carbohydrate antibodies of normal sera: Findings, surprises and challenges. Mol Immunol. 2009;46:3037–49. doi: 10.1016/j.molimm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Olsson ML, Clausen H. Modifying the red cell surface: towards an ABO-universal blood supply. Br J Haematol. 2008;140:3–12. doi: 10.1111/j.1365-2141.2007.06839.x. [DOI] [PubMed] [Google Scholar]