Abstract
Arterial and venous thromboembolism are leading causes of morbidity and mortality around the world. For almost 70 years, heparins (unfractionated heparin and low molecular weight heparins) and vitamin K antagonists have been the leading therapeutic medical options for the treatment and prevention of thromboembolic disorders. Nevertheless, the many limitations of these traditional anticoagulants have fuelled the search for novel agents over the past 15 years, and a new class of oral anticoagulants that specifically target activated factor X and thrombin has been developed and is now commercially available. In this narrative review, the evolution of anticoagulant therapy is summarised, with a focus on newer oral anticoagulants.
Keywords: unfractionated heparin, low molecular weight heparin, vitamin K antagonists, warfarin, new oral anticoagulants
Introduction
Thromboembolic diseases are the leading cause of death and disability in high-income countries, and their incidence is also dramatically increasing in middle- and low-income countries1. While arterial clots (platelet-rich and fibrin-poor, the so-called white clots) are usually generated at sites of vascular injury under high shear rates and are responsible for myocardial infarction and stroke, venous clots (fibrin- and red blood cell-rich and platelet-poor, the so-called red clots) cause venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE)2,3.
The burden of thromboembolic diseases imposes primary prevention as the most important community goal, through lifestyle interventions on a number of modifiable risk factors such as smoking, hypertension, abdominal obesity, physical inactivity and inappropriate dietary habits. Despite the positive outcome associated with these measures, many people still develop clinical manifestations of thromboembolism, so that a pharmacological approach to prevention and treatment is necessary to reduce morbidity and mortality. Since blood hypercoagulability plays a pivotal role in thrombogenesis, it is reasonable that anticoagulant agents should be regarded as an essential therapeutic tool in the management of these patients4.
Heparins (unfractionated heparin [UFH] first, and low molecular weight heparins [LMWH] subsequently) and vitamin K antagonists (VKA: warfarin, phenprocoumon, acenocoumarol) have been used for decades for the treatment and prevention of thromboembolism5,6. Over the past 15 years, however, the interest in anticoagulants has grown dramatically, as shown by the increasing number of drugs in both preclinical and clinical development as well as by the vast array of anticoagulants currently licensed (Table I). In particular, investigators are concentrating research on the so-called new direct oral anticoagulants (DOAC), which selectively target specific steps of the coagulation cascade7–10. This narrative review summarises the evolution of anticoagulant therapy, focusing on advantages and disadvantages of traditional and newer anticoagulants.
Table I.
Currently available anticoagulant agents.
| Anticoagulants | |
|---|---|
|
| |
| Parenteral | Oral |
| Heparin (UFH, LMWH) | VKA |
| Fondaparinux | Thrombin inhibitors (dabigatran) |
| Thrombin inhibitors (bivalirudin, argatroban) | Factor Xa inhibitors (rivaroxaban, apixaban, edoxaban) |
UFH: unfractionated heparin; LMWH: low molecular weight heparin; VKA: vitamin K antagonists.
Search methods
We reviewed the medical literature for published clinical trials evaluating the efficacy and safety of anticoagulants for the prevention and treatment of thromboembolism. The MEDLINE® electronic database was searched without temporal limits using an English language restriction. The Medical Subject Heading and keywords used were the following: “anticoagulants”, “heparin”, “unfractionated heparin”, “low molecular weight heparins”, “vitamin K antagonists”, “warfarin”, “new oral anticoagulants”, “novel oral anticoagulants”, “direct oral anticoagulants”, “target specific oral anticoagulants”, “non-vitamin K antagonist oral anticoagulants”, “dabigratan”, “rivaroxaban”, “apixaban”, “edoxaban”, “myocardial infarction”, “stroke”, “atrial fibrillation”, “venous thromboembolism”, “pulmonary embolism”, “deep vein thrombosis”, “primary prophylaxis”, “secondary prophylaxis”, “therapy”, “bleeding”, “survival”, “death”. We also screened the reference lists of the most relevant reviews for further eligible studies not captured in our initial literature search. Search terms were also applied to abstracts from the latest international haematology congresses on haemostasis and thrombosis.
Conventional anticoagulant agents
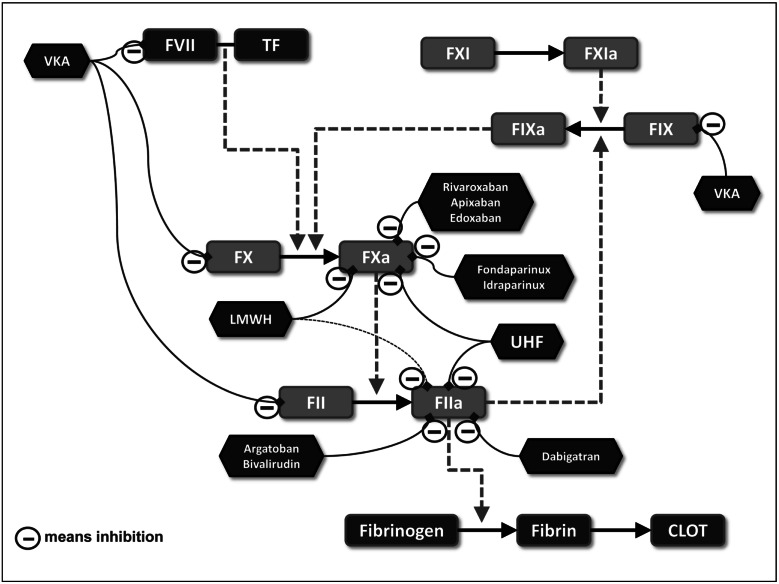
Conventional anticoagulant agents are mainly heparins (UFH and LMWH) and VKA (Figure 1 for their mechanisms of action).
Figure 1.
Biological targets of anticoagulant agents.
FVII: factor VII; TF: tissue factor; FXI: factor XI; FXIa: activated factor XI; FIX: factor IX; FIXa: activated factor IX; FX: factor X; FXa: activated factor X; VKA: vitamin K antagonist; F: factor; LMWH: low molecular weight heparin; TF: tissue factor; UFH: unfractionated heparin; FII: factor II; FIIa: activated factor II.
Unfractionated heparin and low molecular weight heparins
UFH was discovered a century ago, in 1914, by the medical student Jay McLean at John Hopkins in Baltimore, and was then introduced in clinical practice in the 1940s (Table II). UFH is a glycosaminoglycan that requires a cofactor to produce anticoagulant activity (indirect-acting anticoagulant)11,12. UFH, which is administered subcutaneously or intravenously, binds to antithrombin and increases this latter’s ability to inactivate thrombin, factor Xa and factor IXa to a minor extent11. Only UFH chains of at least 18 saccharide units (corresponding to a molecular weight of about 5,400 Daltons) can facilitate the interaction between antithrombin and thrombin. The activated partial thromboplastin time must be monitored in order to assess the anticoagulant effect of UFH, which is associated with an increased risk of developing heparin-induced thrombocytopenia and osteoporosis in patients undergoing long-term therapy13. LMWH are derived from UFH by various chemical or enzymatic depolymerisation processes, and have a mean molecular weight of about one-third that of UFH14. The currently licensed LMWH, which are administered subcutaneously, include enoxaparin, dalteparin, nadroparin, tinzaparin, certoparin, reviparin, ardeparin and bemiparin15. UFH and LMWH have been consistently found to reduce VTE complications in hip or knee arthroplasty and in the setting of high-risk medical conditions (heart failure, acute inflammatory diseases, prolonged immobilisation in bed) by approximately 60%. They are also administered, along with dual antiplatelet therapy (e.g., aspirin and clopidogrel) in patients with acute coronary syndromes, whether or not managed with revascularisation treatment. Since their introduction in the 1980s, LMWH have gradually replaced UFH for most clinical indications due to several advantages over other anticoagulant agents (see below)15. In addition, thanks to their high efficacy and safety profile, LMWH are currently recommended as the treatment of choice for acute and long-term management of cancer-associated VTE16,17. Indeed, besides reducing mortality and morbidity related to VTE in cancer patients, accumulating experimental and clinical data suggest that LMWH significantly improve overall survival by a direct effect on the development and metastatisation of cancer itself18,19.
Table II.
The history of evolution of anticoagulant therapy.
| Year | Anticoagulant drug |
|---|---|
| 1940s | Unfractionated heparin |
| 1950s | Warfarin |
| 1980s | Low molecular weight heparins |
| 1990s | Parenteral direct thrombin inhibitors |
| 2002 | Fondaparinux |
| 2010 | Dabigatran |
| 2011 | Rivaroxaban |
| 2012 | Apixaban |
| 2014 | Edoxaban |
Vitamin K antagonists
Warfarin, the most commonly used VKA, is an oral drug that exerts its anticoagulant activity by interfering with a post-translational modification of several coagulation (factors II, VII, IX, X) and anticoagulation proteins (protein C and S)20,21. VKA have a narrow therapeutic range, frequently interact with food and other drugs, and their metabolism is genetically determined (by two gene polymorphisms: VKORC1, involved in the vitamin K cycle, and CYP2C9, involved in warfarin metabolism)21. Frequent laboratory monitoring of the international normalised ratio (INR) and dose adjustments are, therefore, needed when using these drugs. The benefits of warfarin therapy in a wide array of thromboembolic disorders have been well established. VKA, which were clinically developed more than 60 years ago, are effective at reducing the recurrence of VTE by more than 90%, and cardioembolic stroke in non-valvular atrial fibrillation by approximately 60%21,22. In addition, a recent systematic review and meta-analysis reported that genotype-guided initial VKA dosing is effective at decreasing the risk of major bleeding events, the main drawback of VKA therapy, in approximately 50% of cases23.
Other anticoagulants
Fondaparinux, originally developed in 2002, is a synthetic analogue of the antithrombin-binding pentasaccharide found in UFH and LMWH which selectively inhibits factor Xa in an antithrombin-dependent manner due to is small size24,25. This highly effective drug with a good safety profile is currently licensed for the prophylaxis and treatment of VTE. Thanks to its complete bioavailability after subcutaneous injection and a plasma half-life of approximately 17 hours, fondaparinux is administered once daily at a fixed dose with no need for laboratory monitoring10,25. Parenterally administered direct thrombin inhibitors, which do not require antithrombin for inhibition since they directly inactivate both free and fibrin-bound thrombin, include argatroban and bivalirudin26. Argatroban, a small competitive inhibitor of thrombin, has been approved for treatment of patients with heparin-induced thrombocytopenia, whereas bivalirudin, an analogue of hirudin, has been licensed as an alternative to UFH in patients undergoing percutaneous coronary intervention11,27.
Limitations of conventional anticoagulants
In spite of the excellent clinical results obtained with traditional anticoagulants, there is some wriggle room for the improvement of thromboembolism therapy. The advantages of LMWH over UH include a longer biological half-life and more predictable dose-response, which allows weight-adjusted fixed dosages, less strict requirements for routine coagulation monitoring, and lower binding to platelet factor 4 and bone cells, which results in decreased risks of heparin-induced thrombocytopenia and osteoporosis28. Due to the combination of similar (or even greater) efficacy and safety coupled with a number of other advantages, LMWH have progressively replaced UFH in clinical practice. Nonetheless, the use of LMWH is still associated with a risk of heparin-induced thrombocytopenia (albeit lower than that seen with UFH), and the need for parenteral administration limits their long-term use in the outpatient setting29. Similarly, although the benefits of VKA are well established in a wide spectrum of thromboembolic disorders4, their use is hampered by several drawbacks, such as delayed onset and offset of action, suboptimal adherence to therapy, narrow therapeutic range of clinical effectiveness, genetic variations of metabolism plus food and drug interactions, which necessitate frequent monitoring and dose adjustment30.
New oral anticoagulant drugs
The limitations of heparins and warfarin led to the development of new anticoagulant agents selectively targeting specific steps in the coagulation cascade which, besides having a high efficacy and safety profile, have the advantage of being orally administered at fixed dosages with a lower need for laboratory monitoring. Two types of DOAC are currently licensed for use in thromboembolic disorders: factor Xa inhibitors (apixaban, edoxaban and rivaroxaban) and the thrombin inhibitor dabigatran31. The characteristics and mechanisms of action of these agents are described in Table III and Figure 1, respectively. The main results of phase III randomised trials with DOAC are reported in Table IV.
Table III.
Characteristics of novel oral anticoagulants.
| Characteristics | Direct thrombin inhibitor | Factor Xa inhibitors | |||
|---|---|---|---|---|---|
| Dabigratan | Apixaban | Edoxaban | Rivaroxaban | ||
| Bioavailability (%) | 3–7 | 50 | 62 | 80 | |
| Time to peak concentration (hours) | 1–3 | 1–3 | 1–3 | 2–4 | |
| Half-life (hours) | 12–17 | 8–15 | 8–10 | 7–13 | |
| Renal clearance (%) | 80 | 25 | 35 | 33 | |
| Dosing regimen | 110–150 mg twice daily | 2.5–5 mg twice daily | 15–30 mg once daily | 10–20 mg once daily; 15 mg once or twice daily | |
| Metabolism | P-glycoprotein | P-glycoprotein, CYP3A4 | P-glycoprotein, CYP3A4 | P-glycoprotein, CYP3A4 | |
| Approved indications | Non-valvular AF | North America, Europe | North America, Europe | United States | North America, Europe |
| VTE treatment | United States, Europe | United States, Europe | Unites States | North America, Europe | |
| VTE prevention | Canada, Europe | Canada, Europe | Japan | North America, Europe | |
AF: atrial fibrillation; VTE: venous thromboembolism.
Table IV.
Main results of the phase III trials with new oral anticoagulants.
| Drug | Studyref | Indication | Pts | Study arms (drug vs comparator) | Primary outcome | |
|---|---|---|---|---|---|---|
|
| ||||||
| Efficacy | Safetya | |||||
| Dabigatran | RE-MODEL34 | VTE prophylaxis after TKR | 2,076 | DAB 150 mg od or 220 mg od, 6–10 days | DAB 150 mg 40.5% (p=0.017)b,c | DAB 150 mg 1.3% (p=1.0) |
| ENX 40 mg od, 6–10 days | DAB 220 mg 36.4% (p=0.0003)b | DAB 220 mg 1.5% (p=0.82) | ||||
| ENX 37.7% | ENX 1.3% | |||||
|
| ||||||
| RE-NOVATE35 | VTE prophylaxis after THR | 3,494 | DAB 150 mg od or 220 mg od, 28–35 days | DAB 150 mg 8.6% (p<0.0001)b,c | DAB 150 mg 1.3% (p=0.60) | |
| ENX 40 mg od, 28–35 days | DAB 220 mg 6.0% (p<0.0001)b | DAB 220 mg 2.0% (p=0.44) | ||||
| ENX 6.7% | ENX 1.6% | |||||
|
| ||||||
| RE-MOBILIZE36 | VTE prophylaxis after TKR | 2,715 | DAB 150 mg od or 220 mg od, 28–35 days | DAB 150 mg 33.7% (p=0.0009)c,d | DAB 150 mg 0.6% (p=NS) | |
| ENX 30 mg td, 12–15 days | DAB 220 mg 31.7% (p=0.002)d | DAB 220 mg 0.6% (p=NS) | ||||
| ENX 25.3% | ENX 1.4% | |||||
|
| ||||||
| RE-COVER37 | VTE treatment | 2,564 | DAB 150 mg td, 6 months | DAB 2.4% (HR: 1.10, CI: 0.65–0.84)b,e | DAB 1.6% (HR: 0.83, CI: 0.45–1.48) | |
| WARf, 6 months | WAR 2.1% | WAR 1.9% | ||||
|
| ||||||
| RE-COVER II38 | VTE treatment | 2,568 | DAB 150 mg td, 6 months | DAB 2.3% (HR: 1.08, CI: 0.64–1.8)b,e | DAB 1.2% (HR: 0.69, CI: 0.36–1.32) | |
| WARf, 6 months | WAR 2.2% | WAR 1.7% | ||||
|
| ||||||
| RE-MEDY39 | VTE treatment | 2,856 | DAB 150 mg td, 36 months | DAB 1.8% (p=0.03)b,e | DAB 0.9% (p=0.06) | |
| WARf, 36 months | WAR 1.3% | WAR 1.8% | ||||
|
| ||||||
| RE-SONATE39 | VTE treatment | 1,343 | DAB 150 mg td, 6 months | DAB 0.4% (p<0.0001)e | DAB 0.3% (p=0.996) | |
| Placebo, 6 months | Placebo 5.6% | Placebo 0% | ||||
|
| ||||||
| RE-LY40 | Stroke prophylaxis NAF | 18,113 | DAB 110 mg td or 150 mg td, 2 years | DAB 110 mg 1.5%/year (p<0.001)b,g | DAB 110 mg 2.7%/year (p=0.003) | |
| WARf, 2 years | DAB 150 mg 1.1%/year (p<0.001)h | DAB 150 mg 3.1%/year (p=0.31) | ||||
| WAR 1.7%/year | WAR 3.4%/year | |||||
|
| ||||||
| RE-LYABLE43 | Stroke prophylaxis NAF | 5,851 | DAB 110 mg td, 2.3 years | DAB 110 mg 1.6%/year (HR: 0.91, CI: 0.69–1.20)g | DAB 110 mg 3.0%/year (HR: 1.26, CI: 1.04–1.53) | |
| DAB 150 mg td, 2.3 years | DAB 150 mg 1.5%/year | DAB 150 mg 3.7%/year | ||||
|
| ||||||
| Apixaban | ADVANCE-146 | VTE prophylaxis after TKR | 3,195 | APX 2.5 mg td, 10–14 days | APX 9.0% (p=0.06)b,c | APX 0.7% (p=0.03) |
| ENX 30 mg td, 10–14 days | ENX 8.8% | ENX 1.4% | ||||
|
| ||||||
| ADVANCE-247 | VTE prophylaxis after TKR | 3,057 | APX 2.5 mg td, 10–14 days | APX 15.0% (p<0.0001)h | APX 0.6% (p=0.3014) | |
| ENX 40 mg od, 10–14 days | ENX 24.0% | ENX 0.9% | ||||
|
| ||||||
| ADVANCE-348 | VTE prophylaxis after THR | 5,407 | APX 2.5 mg td, 32–38 days | APX 1.4% (p<0.001)h | APX 0.8% (p=0.54) | |
| ENX 40 mg od, 32–38 days | ENX 3.9% | ENX 0.7% | ||||
|
| ||||||
| AMPLIFY50 | VTE treatment | 5,385 | APX 10 mg td (7 d), 5 mg td (6 months) | APX 2.3% (p<0.001)b,e | APX 0.6% (p<0.001) | |
| ENX 1 mg/kg td (>5 d), WAR (6 months)f | ENX/WAR 2.7% | ENX/WAR 1.8% | ||||
|
| ||||||
| AMPLIFY-EXT51 | VTE treatment | 2,486 | APX 2.5 mg td or 5 mg td (12 months) | APX 1.7% (p<0.001)e,h | APX 2.5 mg 0.2%, APX 5 mg 0.1% | |
| Placebo | Placebo 8.8% | Placebo 0.5% (p=NS) | ||||
|
| ||||||
| ARISTOTLE52 | Stroke prophylaxis NAF | 18,201 | APX 5 mg td, 1.8 years | APX 1.3%/year (p=0.01)g,h | APX 2.1%/year (p<0.001) | |
| WARf, 1.8 years | WAR 1.6%/year | WAR 3.1%/year | ||||
|
| ||||||
| AVERROES53 | Stroke prophylaxis NAF | 5,599 | APX 5 mg td, 1.1 years | APX 1.6%/year (p=0.001)g,h | APX 1.4%/year (p=0.57) | |
| Aspirin (81–324 mg/d), 1.1 years | WAR 3.7%/year | WAR 1.2%/year | ||||
|
| ||||||
| Edoxaban | STARS-E355 | VTE prophylaxis after TKR | 716 | EDX 30 mg od, 11–14 days | EDX 7.4% (p=0.010)c,h | EDX 1.1% (p=0.373) |
| ENX 20 mg td, 11–14 dats | ENX 13.9% | ENX 0.3% | ||||
|
| ||||||
| STARS-J556 | VTE prophylaxis after THR | 264 | EDX 15 mg od or 30 mg od, 11–14 days | EDX 15 mg 3.8% (p=1.000)c | EDX 15 mg 2.2% (p=1.000) | |
| ENX 20 mg td, 11–14 days | EDX 30 mg 2.8% | EDX 30 mg 1.2% | ||||
| ENX 4.1% | ENX 2.3% | |||||
|
| ||||||
| Hokusai-VTE57 | VTE treatment | 8,292 | EDX 60 mg od or 30 mg od, 3–12 months | EDX 3.2% (p<0.001)b,e | EDX 8.5% (p=0.004)h | |
| WARf, 3–12 months | WAR 3.5% | WAR 10.3% | ||||
|
| ||||||
| ENGAGE-AF-TIMI 4858 | Stroke prophylaxis NAF | 21,105 | EDX 60 mg od or 30 mg od, 2.8 years | EDX 60 mg 1.6%/year (p<0.001)b,g | EDX 60 mg 2.7%/year (p<0.001) | |
| WARf, 2.8 years | EDX 30 mg 1.2%/year (p=0.005)b | EDX 30 mg 1.6%/year (p<0.001) | ||||
| WAR 1.5%/year | WAR 3.4%/year | |||||
|
| ||||||
| Rivaroxaban | RECORD-1 59 | VTE prophylaxis after THR | 4,541 | RVX 10 mg od, 35 days | RVX 1.1% (p<0.001)c,h | RVX 0.3% (p=0.18) |
| ENX 40 mg od, 35 days | ENX 3.7% | ENX 0.1% | ||||
|
| ||||||
| RECORD-2 60 | VTE prophylaxis after THR | 2,509 | RVX 10 mg od, 35 days | RVX 2.0% (p<0.001)c,h | RVX 0.08% (p=NS) | |
| ENX 40 mg od, 10–14 days | ENX 9.3% | ENX 0.08% | ||||
|
| ||||||
| RECORD-3 61 | VTE prophylaxis after TKR | 2,531 | RVX 10 mg od, 10–14 days | RVX 9.6% (p<0.001)c,h | RVX 0.6% (p=0.77) | |
| ENX 40 mg od, 10–14 days | ENX 18.9% | ENX 0.5% | ||||
|
| ||||||
| RECORD-4 62 | VTE prophylaxis after TKR | 3,148 | RVX 10 mg od, 10–14 days | RVX 6.9% (p=0.012)c,h | RVX 0.7% (p=0.11) | |
| ENX 30 mg td, 10–14 days | ENX 10.1% | ENX 0.3% | ||||
|
| ||||||
| EINSTEIN-DVT64 | VTE treatment | 3,449 | RVX 15 mg td (3 weeks), 20 mg od (3.6 or 12 months) | RVX 2.1% (p<0.001)b,e | RVX 8.1% (p=0.77) | |
| ENX 1 mg/kg td (≥5 days), WAR (3.6 or 12 months)f | ENX/WAR 3.0% | ENX/WAR 8.1% | ||||
|
| ||||||
| EINSTEIN-PE65 | VTE treatment | 4,832 | RVX 15 mg td (3 weeks), 20 mg od (3.6 or 12 months) | RVX 2.1% (p=0.003)b,e | RVX 1.1% (p=0.003) | |
| ENX 1 mg/kg td (>5 days), WAR (3.6 or12 months)f | ENX/WAR 1.8% | ENX/WAR 2.2% | ||||
|
| ||||||
| EINSTEIN-Extension64 | VTE treatment | 1,197 | RVX 20 mg od, 6–12 months | RVX 1.3% (p<0.001)c,h | RVX 0.7% (p=0.11) | |
| Placebo, 6–12 months | Placebo 7.1% | Placebo 0% | ||||
|
| ||||||
| ROCKET-AF67 | Stroke prophylaxis NAF | 14,264 | RVX 20 mg od, 20 months | RVX 1.7%/year (p<0.001)b,g | RVX 5.6%/year (p=0.576) | |
| WARf, 20 months | WAR 2.2%/year | WAR 5.4%/year | ||||
VTE: venous thromboembolism; od: once daily; td: twice daily; NS: not significant; TKR: total knee replacement; THR: total hip replacement; DAB: dabigatran; ENX: enoxaparin; WAR: warfarin; HR: hazard ratio; CI: confidence interval; NAF: non-valvular atrial fibrillation; APX: apixaban; EDX: edoxaban; HFR: hip fracture surgery; RVX: rivaroxaban.
major or clinically relevant non-major bleeding;
non-inferiority;
VTE or related death;
inferiority;
recurrent VTE;
target INR 2.0–3.0;
stroke or systemic embolism;
superiority.
Direct thrombin inhibitors
Dabigatran etexilate, the only oral direct thrombin inhibitor currently licensed, is a prodrug that is rapidly converted into the active form dabigatran once absorbed from the gastrointestinal tract32. A pooled analysis of three trials33, RE-MOBILIZE, RE-MODEL and RENOVATE34–36, showed that dabigatran was at least as effective as enoxaparin for thromboprophylaxis after hip and knee replacement, and was associated with a similar incidence of major bleeding (1.4% in the enoxaparin group vs 1.4% in the dabigatran 220 mg group and 1.1% in the dabigatran 150 mg group)33. The administration of this drug also produced similar effects as warfarin for acute management (RE-COVER and RE-COVER II trials)37,38 and extended maintenance therapy of VTE (RE-MEDY trial)38. A pooled analysis of the RE-COVER and RE-COVER II trials showed that the incidence of any bleeding, as well as that of major or clinically relevant non-major bleeding, was significantly lower in the dabigatran group than in the warfarin group38. In the RE-SONATE trial39, which assessed safety and efficacy of dabigatran vs placebo for extended treatment of VTE, a 92% relative risk (RR) reduction of recurrent VTE was observed in favour of dabigatran, with a similarly low bleeding risk. Dabigatran was non-inferior (110 mg twice daily) or superior (150 mg twice daily) to warfarin for stroke prevention in atrial fibrillation (RE-LY trial)40. These four randomised trials (i.e., RE-COVER, RE-COVER II, RE-MEDY and RE-LY) along with the PETRO trial41 (which evaluated the efficacy of dabigatran with or without aspirin vs warfarin alone in patients with non-valvular atrial fibrillation) were included in a recent meta-analysis42, which reported that the risk of any bleeding with dabigatran was lower than with warfarin across all the five randomised trials, with a pooled RR of 0.77 (95% confidence interval [95% CI]: 0.64–0.93). A long-term, multicentre extension of dabigatran treatment in patients who completed RE-LY (RELY-ABLE) reported no significant difference in stroke or mortality with the two dabigatran doses (150 mg twice daily vs 110 mg twice daily), although a higher rate of major bleeding was found with the higher dabigatran dose during the additional 2.3 years of treatment43. Finally, a Cochrane systematic review and meta-analysis including eight randomised controlled trial involving a total of 27,557 patients with non-valvular atrial fibrillation reported that dabigatran was non-inferior or superior (150 mg twice daily) with regards to the composite outcome of vascular mortality and ischaemic events with fewer major haemorrhagic events44.
Factor Xa inhibitor
Apixaban acts by reversibly blocking factor X at the active site (Table III)45.
A meta-analysis of three large phase III trials on the prevention of VTE after orthopaedic surgery (ADVANCE-1, ADVANCE-2 and ADVANCE-3)46–48 showed that apixaban 2.5 mg twice daily was associated with a significant reduction in the rate of total VTE, all-cause mortality and a significantly lower risk of clinically relevant bleeding compared to enoxaparin49. Apixaban (10 mg twice daily for 7 days followed by 5 mg twice daily for 6 months) was also non-inferior to conventional therapy with enoxaparin/warfarin for the treatment of acute VTE in the AMPLIFY trial50, and was associated with a significant reduction in major bleeding. One-year extended anticoagulation with apixaban (2.5 mg and 5 mg twice daily) lowered the risk of recurrent VTE compared with placebo, without increasing the incidence of major bleeding (AMPLIFY-EXT)51. A phase III trial (ARISTOTLE) compared apixaban (5 mg twice daily) with warfarin for cardioembolic prophylaxis in patients with atrial fibrillation52, showing that the former drug was superior to warfarin for the prevention of stroke or systemic embolism, caused less bleeding and was ultimately associated with lower mortality. In the AVERROES trial, patients with atrial fibrillation who had failed or were unsuitable for VKA treatment were randomised to aspirin or apixaban (5 mg twice daily)53. Apixaban was associated with a greater reduction of stroke, whereas the rate of major bleeding was similar for the two groups. Edoxaban inhibits factor Xa activity following rapid absorption from the gastrointestinal tract (Table III)54. Two recently published phase III randomised trials comparing edoxaban vs enoxaparin for thromboprophylaxis after total knee (STARS-E3)55 or hip (STARS-J5)56 replacement surgery demonstrated that edoxaban had a similar (STARS-J5) or superior (STARS-E3) efficacy to enoxaparin, while displaying a comparable safety profile. The Hokusai-VTE was the largest phase III study ever conducted in acute symptomatic VTE, with 8,292 patients randomised to receive edoxaban (60 or 30 mg once daily) or warfarin57. The study showed that edoxaban, administered once daily after initial heparin was non-inferior to standard therapy with warfarin after initial heparin treatment, with significantly less major and clinically relevant non-major bleeding. Finally, the antithrombotic effect of edoxaban (30 mg and 60 mg once daily) vs warfarin was explored in the phase III trial ENGAGE AF-TIMI 4858. Both once-daily regimens of edoxaban were non-inferior to warfarin for the prevention of stroke or systemic embolism, and were also associated with significantly lower rates of bleeding and death from cardiovascular causes.
Rivaroxaban is a selective oral direct factor Xa inhibitor partially excreted (33%) by the kidneys (Table III). Four phase III randomised studies compared oral rivaroxaban (10 mg once daily) with enoxaparin (40 mg once daily or 30 mg twice daily) for the prevention of VTE after total hip (RECORD-1 and RECORD-2)59,60 or knee (RECORD-3 and RECORD-4)61,62 arthroplasty. A pooled analysis of these four trials showed that rivaroxaban significantly reduced the incidence of the composite end-point of VTE and all-cause mortality compared to enoxaparin-based regimens, with no statistical evidence for differences in bleeding events63. As regards the role of rivaroxaban in acute VTE treatment, a pooled analysis of EINSTEIN-DVT64 and EINSTEIN-PE65 trials, which compared rivaroxaban to enoxaparin/warfarin for prevention of recurrent VTE, showed the non-inferiority of rivaroxaban to enoxaparin/warfarin and a 41% reduction in the RR of major bleeding66. Extended prophylaxis with rivaroxaban (EINSTEIN-Extension)64 reduced the incidence of symptomatic recurrent VTE to a greater extent than placebo, with a non-significant increase in the incidence of clinically relevant bleeding. In the ROCKET-AF trial, patients with atrial fibrillation and additional risk factors for stroke were randomised to receive either rivaroxaban or dose-adjusted warfarin67. Rivaroxaban was non-inferior to warfarin for the prevention of stroke or systemic embolism, with no significant differences in rates of major and clinically relevant non-major bleeding between the two study groups.
Several systematic reviews and meta-analyses have investigated the primary outcomes of DOAC recipients in various clinical settings by pooling the results from the different trials. In a recent meta-analysis (54,875 patients in 12 phase II and phase III studies)68 of the safety and efficacy of DOAC for stroke prevention in non-valvular atrial fibrillation, novel anticoagulants were associated with reduced total mortality (5.61 vs 6.02%; RR: 0.89, 95% CI: 0.83–0.96) and stroke/systemic embolism (2.40 vs 3.13%; RR: 0.77, 95% CI: 0.70–0.86) in comparison to VKA. A trend toward reduced major bleeding was also noticed (RR: 0.86, 95% CI: 0.72–1.02), with a significant reduction in intracranial haemorrhage (RR: 0.46, 95% CI: 0.39–0.56) in patients receiving DOAC. Another recent meta-analysis conducted in acute VTE patients (24,455 patients in 5 phase III randomised trials) found a similar risk of recurrent VTE (RR: 0.88, 95% CI: 0.74–1.05) and overall mortality (RR: 0.97, 95% CI: 0.83–1.14) in patients treated with DOAC or VKA69. The DOAC were, however, associated with a significantly lower risk of major bleeding (RR: 0.60, 95% CI: 0.41–0.88). The risks of fatal bleeding (RR: 0.60, 95% CI: 0.46–0.77) and major bleeding (RR: 0.80, 95% CI: 0.63–1.01) bleeding were lower in DOAC-treated patients than in VKA recipients in a systematic review pooling data from the six most important randomised controlled trials on the management of atrial fibrillation and VTE70. These results were replicated in a more recent systematic review of 12 randomised controlled trials involving 102,607 patients with VTE or atrial fibrillation71. There is, however, some evidence from post-marketing studies that some DOAC (particularly dabigatran, but also rivaroxaban) increase the rate of gastrointestinal bleeding in comparison with VKA72–74, probably because they remain unchanged in the gastrointestinal tract. Caution should, therefore, be used when prescribing such novel oral anticoagulants to patients, particularly elderly ones, at increased risk of gastrointestinal bleeding.
Conclusions
After several decades without novelty in the armamentarium for long-term anticoagulation management of venous and selected arterial thromboembolic diseases, new classes of oral anticoagulants have emerged during the last decade, and have been subjected to extensive study of clinical effectiveness. Besides the undeniable advantages of oral administration at fixed dosages with less stringent need for laboratory monitoring, the results of the large phase III licensing trials provide solid evidence that DOAC are at least as effective as VKA for the prevention and treatment of thromboembolism and it is, therefore, easily predictable that these novel anticoagulant agents will be increasingly used in the near future in clinical practice worldwide. However, before recommending widespread use of DOAC, there are some important issues that should be addressed, such as their use in very elderly patients (i.e., aged 80 years or older), in those with impaired renal function, in cancer-associated thrombosis, as well as in patients at the extremes of body weight or under dual anti-platelet therapy75–77. There are very few data from published phase III trials regarding these categories of patients. In addition, the lack of specific antidotes suggests the need for particular caution, especially in patients at increased risk of bleeding. Thus, although results from the first real-life studies are encouraging78, further post-marketing long-term trials are needed for definitive assessment of their safety and efficacy with respect to VKA therapy. A final issue regards laboratory monitoring. Although DOAC require less extensive therapeutic monitoring, specific testing may be still required under certain circumstances, such as in the case of acute impairment of renal or liver function, unexpected bleeding or thrombosis, uncertain compliance to therapy, as well as combined administration with certain drugs such as antibiotics79,80. In these circumstances, an appropriate combination of routine and second-line haemostasis testing may still be required to establish the anticoagulant effects of DOAC and adjust or optimise the therapeutic regimen81.
Footnotes
Disclosure of conflicts of interest
The Authors declare that they have no conflicts of interest regarding this manuscript.
References
- 1.Mannucci PM, Franchini M. Old and new anticoagulant drugs. Ann Med. 2011;43:116–23. doi: 10.3109/07853890.2010.539250. [DOI] [PubMed] [Google Scholar]
- 2.Lippi G, Franchini M, Targher G. Arterial thrombus formation in cardiovascular disease. Nat Rev Cardiol. 2011;8:502–12. doi: 10.1038/nrcardio.2011.91. [DOI] [PubMed] [Google Scholar]
- 3.Lippi G, Franchini M. Pathogenesis of venous thromboembolism: when the cup runneth over. Semin Thromb Hemost. 2008;34:747–61. doi: 10.1055/s-0029-1145257. [DOI] [PubMed] [Google Scholar]
- 4.Eikelboom JW, Weitz JI. Update on antithrombotic therapy. New anticoagulants. Circulation. 2010;121:1523–32. doi: 10.1161/CIRCULATIONAHA.109.853119. [DOI] [PubMed] [Google Scholar]
- 5.Hirsh J. Heparin. N Engl J Med. 1991;324:1565–74. doi: 10.1056/NEJM199105303242206. [DOI] [PubMed] [Google Scholar]
- 6.Hirsh J. Oral anticoagulant drugs. N Engl J Med. 1991;324:1865–75. doi: 10.1056/NEJM199106273242606. [DOI] [PubMed] [Google Scholar]
- 7.Jackson LR, 2nd, Becker RC. Novel oral anticoagulants: pharmacology, coagulation measures, and considerations for reversal. J Thromb Thrombolysis. 2014;37:380–91. doi: 10.1007/s11239-013-0958-0. [DOI] [PubMed] [Google Scholar]
- 8.Undas A, Pasierski T, Windyga J, Crowther M. Practical aspects of new oral anticoagulant use in atrial fibrillation. Pol Arch Med Wewn. 2014;124:124–35. doi: 10.20452/pamw.2138. [DOI] [PubMed] [Google Scholar]
- 9.Kornej J, Potpara T, Lip GY. Anticoagulation management in nonvalvular atrial fibrillation: current and future directions. Pol Arch Med Wewn. 2013;123:623–34. doi: 10.20452/pamw.1978. [DOI] [PubMed] [Google Scholar]
- 10.Franchini M, Mannucci PM. A new era for anticoagulants. Eur J Intern Med. 2009;20:562–68. doi: 10.1016/j.ejim.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Garcia DA, Baglin TP, Weitz JI, Samama MM. Parenteral anticoagulants: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e24S–43S. doi: 10.1378/chest.11-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S–94S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linkins LA, Dans AL, Moores LK, et al. Treatment and prevention of heparin-induced thrombocytopenia: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e495S–530S. doi: 10.1378/chest.11-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linhardt RJ, Gunay NS. Production and chemical processing of low molecular weight heparins. Semin Thromb Hemost. 1999;25(Suppl 3):5–16. [PubMed] [Google Scholar]
- 15.Walenga JM, Lyman GH. Evolution of heparin anticoagulants to ultra-low-molecular-weight heparins: A review of pharmacologic and clinical differences and applications in patients with cancer. Crit Rev Oncol Hematol. 2013;88:1–18. doi: 10.1016/j.critrevonc.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Lyman GH, Khorana AA, Kuderer NM, et al. American Society of Clinical Oncology Clinical Practice: Venous thromboembolism prophylaxis and treatment in patients with cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2013;31:2189–204. doi: 10.1200/JCO.2013.49.1118. [DOI] [PubMed] [Google Scholar]
- 17.Farge D, Debourdeau P, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J Thromb Haemost. 2013;11:56–70. doi: 10.1111/jth.12070. [DOI] [PubMed] [Google Scholar]
- 18.Franchini M, Montagnana M, Favaloro EJ, Lippi G. The bidirectional relationship of cancer and hemostasis and the potential role of anticoagulant therapy in moderating thrombosis and cancer spread. Semin Thromb Hemost. 2009;35:644–53. doi: 10.1055/s-0029-1242718. [DOI] [PubMed] [Google Scholar]
- 19.Franchini M, Mannucci PM. Low molecular weight heparins and cancer: focus on antitumoral effect. Ann Med. 2015;47:116–21. doi: 10.3109/07853890.2015.1004361. [DOI] [PubMed] [Google Scholar]
- 20.Guyatt GH, Akl EA, Crowther M, et al. American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):7S–47S. doi: 10.1378/chest.1412S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ageno W, Gallus AS, Wittkowsky A, et al. American College of Chest Physicians. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2912;141(2 Suppl):e44S–88S. doi: 10.1378/chest.11-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131:492–501. doi: 10.7326/0003-4819-131-7-199910050-00003. [DOI] [PubMed] [Google Scholar]
- 23.Franchini M, Mengoli C, Cruciani M, et al. Effects on bleeding complications of pharmacogenetic testing for initial dosing of vitamin K antagonists: a systematic review and meta-analysis. J Thromb Haemost. 2014;12:1480–7. doi: 10.1111/jth.12647. [DOI] [PubMed] [Google Scholar]
- 24.Hirsh J, O’Donnell M, Weitz JI. New anticoagulants. Blood. 2005;105:453–63. doi: 10.1182/blood-2003-12-4195. [DOI] [PubMed] [Google Scholar]
- 25.Petitou M, Duchaussoy P, Herbert JM, et al. The synthetic pentasaccharide fondaparinux: first in the class of antithrombotic agents that selectively inhibit coagulation factor Xa. Semin Thromb Haemost. 2002;28:393–402. doi: 10.1055/s-2002-34309. [DOI] [PubMed] [Google Scholar]
- 26.Harenberg J, Wehling M. Current and future prospects for anticoagulant therapy: inhibitors of factor Xa and factor IIa. Semin Thromb Haemost. 2008;34:39–57. doi: 10.1055/s-2008-1066023. [DOI] [PubMed] [Google Scholar]
- 27.Bittl JA, Strony J, Brinker JA, et al. Treatment with bivalirudin (Hirulog) as compared with heparin during coronary angioplasty for unstable or postinfarction angina. Hirulog Angioplasty Study Investigators. N Engl J Med. 1995;333:764–9. doi: 10.1056/NEJM199509213331204. [DOI] [PubMed] [Google Scholar]
- 28.Bates SM, Weitz JI. New anticoagulants: beyond heparin, low-molecular-weight heparin and warfarin. Br J Pharmacol. 2005;14:1017–28. doi: 10.1038/sj.bjp.0706153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fareed J, Hoppensteadt DA, Fareed D, et al. Survival of heparins, oral anticoagulants, and aspirin after the year 2010. Semin Thromb Hemost. 2008;34:58–73. doi: 10.1055/s-2008-1066025. [DOI] [PubMed] [Google Scholar]
- 30.Hirsh J, O’Donnell M, Eikelboom JW. Beyond unfractionated heparin and warfarin: current and future advances. Circulation. 2007;116:552–60. doi: 10.1161/CIRCULATIONAHA.106.685974. [DOI] [PubMed] [Google Scholar]
- 31.Weitz JI, Eikelboom JW, Samama MM American College of Chest Physicians. New antithrombotic drugs: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e120S–51S. doi: 10.1378/chest.11-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisert WG, Hauel N, Stangier J, et al. Dabigatran: an oral novel potent reversible nonpeptide inhibitor of thrombin. Arterioscler Thromb Vasc Biol. 2010;30:1885–9. doi: 10.1161/ATVBAHA.110.203604. [DOI] [PubMed] [Google Scholar]
- 33.Friedman RJ, Dahl OE, Rosencher N, et al. RE-MOBILIZE, RE-MODEL, RE-NOVATE Steering Committees. Dabigatran versus enoxaparin for prevention of venous thromboembolism after hip or knee arthroplasty: a pooled analysis of three trials. Thromb Res. 2010;126:175–82. doi: 10.1016/j.thromres.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 34.Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the REMODEL randomized trial. J Thromb Haemost. 2007;5:2178–85. doi: 10.1111/j.1538-7836.2007.02748.x. [DOI] [PubMed] [Google Scholar]
- 35.Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 2007;370:949–56. doi: 10.1016/S0140-6736(07)61445-7. [DOI] [PubMed] [Google Scholar]
- 36.Ginsberg JS, Davidson BL, Comp PC, et al. RE-MOBILIZE Writing Committee. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty. 2009;24:1–9. doi: 10.1016/j.arth.2008.01.132. [DOI] [PubMed] [Google Scholar]
- 37.Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–52. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 38.Schulman S, Kakkar AK, Goldhaber SZ, et al. RECOVER II Trial Investigators. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129:764–72. doi: 10.1161/CIRCULATIONAHA.113.004450. [DOI] [PubMed] [Google Scholar]
- 39.Schulman S, Kearon C, Kakkar AJ, et al. Extended use of dabigatran, warfarin or placebo in venous thromboembolism. N Engl J Med. 2013;368:709–18. doi: 10.1056/NEJMoa1113697. [DOI] [PubMed] [Google Scholar]
- 40.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 41.Ezekowitz MD, Reilly PA, Nehmiz G, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study) Am J Cardiol. 2007;100:1419–26. doi: 10.1016/j.amjcard.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 42.Bloom BJ, Filion KB, Atallah R, Eisenberg MJ. Meta-analysis of randomized controlled trials on the risk of bleeding with dabigatran. Am J Cardiol. 2014;113:1066–74. doi: 10.1016/j.amjcard.2013.11.049. [DOI] [PubMed] [Google Scholar]
- 43.Connolly SJ, Wallentin L, Ezekowitz MD, et al. The long-term multicenter observational study of dabigatran treatment in patients with atrial fibrillation (RELY-ABLE) study. Circulation. 2013;128:237–43. doi: 10.1161/CIRCULATIONAHA.112.001139. [DOI] [PubMed] [Google Scholar]
- 44.Salazar CA, del Aquila D, Cordova EG. Direct thrombin inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in people with non-valvular atrial fibrillation. Cochrane Database Syst Rev. 2014;3:CD009893. doi: 10.1002/14651858.CD009893.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raghavan N, Frost CE, Yu Z, et al. Apixaban metabolismand pharmacokinetics after oral administration to humans. DrugMetab Dispos. 2009;37:74–81. doi: 10.1124/dmd.108.023143. [DOI] [PubMed] [Google Scholar]
- 46.Lassen MR, Raskob GE, Gallus A, et al. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361:594–604. doi: 10.1056/NEJMoa0810773. [DOI] [PubMed] [Google Scholar]
- 47.Lassen MR, Raskob GE, Gallus A, et al. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375:807–15. doi: 10.1016/S0140-6736(09)62125-5. [DOI] [PubMed] [Google Scholar]
- 48.Lassen MR, Gallus A, Raskob GE, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363:2487–98. doi: 10.1056/NEJMoa1006885. [DOI] [PubMed] [Google Scholar]
- 49.Eriksson BI, Quinlan DJ, Eikelboom JW. Novel oral factor Xa and thrombin inhibitors in the management of thromboembolism. Annu Rev Med. 2011;62:41–57. doi: 10.1146/annurev-med-062209-095159. [DOI] [PubMed] [Google Scholar]
- 50.Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808. doi: 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- 51.Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368:699–708. doi: 10.1056/NEJMoa1207541. [DOI] [PubMed] [Google Scholar]
- 52.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 53.Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–17. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 54.Bounameaux H, Camm AJ. Edoxaban: an update on the new oral direct factor Xa inhibitor. Drugs. 2014;74:1209–31. doi: 10.1007/s40265-014-0261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fuji T, Wang CJ, Fujita S, et al. Safety and efficacy of edoxaban, an oral factor Xa inhibitor, versus enoxaparin for thromboprophylaxis after total knee arthroplasty: the STARS E-3 trial. Thromb Res. 2014;134:1198–204. doi: 10.1016/j.thromres.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 56.Fuji T, Wang CJ, Fujita S, et al. Safety and Efficacy of edoxaban, an oral factor Xa inhibitor, for thromboprophylaxis after total hip arthroplasty in Japan and Taiwan. J Arthroplasty. 2014;29:2439–46. doi: 10.1016/j.arth.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 57.Büller HR, Décousus H, Grosso MA, et al. Hokusai-VTE Investigators. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406–15. doi: 10.1056/NEJMoa1306638. [DOI] [PubMed] [Google Scholar]
- 58.Giugliano RP, Ruff CT, Braunwald E, et al. ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 59.Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–75. doi: 10.1056/NEJMoa0800374. [DOI] [PubMed] [Google Scholar]
- 60.Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet. 2008;372:31–9. doi: 10.1016/S0140-6736(08)60880-6. [DOI] [PubMed] [Google Scholar]
- 61.Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358:2776–86. doi: 10.1056/NEJMoa076016. [DOI] [PubMed] [Google Scholar]
- 62.Turpie AG, Lassen MR, Davidson BL, et al. RECORD4 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet. 2009;373:1673–80. doi: 10.1016/S0140-6736(09)60734-0. [DOI] [PubMed] [Google Scholar]
- 63.Turpie AG, Lassen MR, Eriksson BI, et al. Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty. Pooled analysis of four studies. Thromb Haemost. 2011;105:444–53. doi: 10.1160/TH10-09-0601. [DOI] [PubMed] [Google Scholar]
- 64.The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 65.The EINSTEIN-PE Investigators. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–97. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- 66.Prins MH, Lensing AW, Bauersachs R, et al. EINSTEIN Investigators. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thromb J. 2013;11:21. doi: 10.1186/1477-9560-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patel MR, Mahaffey KW, Garg J, et al. ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 68.Dentali F, Riva N, Crowther M, et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation. 2012;126:2381–91. doi: 10.1161/CIRCULATIONAHA.112.115410. [DOI] [PubMed] [Google Scholar]
- 69.van der Hulle T, Kooiman J, den Exter PL, et al. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2014;12:320–8. doi: 10.1111/jth.12485. [DOI] [PubMed] [Google Scholar]
- 70.Adam SS, McDuffie JR, Ortel TL, Williams JW., Jr Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism: a systematic review. Ann Intern Med. 2012;157:796–807. doi: 10.7326/0003-4819-157-10-201211200-00532. [DOI] [PubMed] [Google Scholar]
- 71.Chai-Adisaksopha C, Crowther M, Isayama T, Lim W. The impact of bleeding complications in patients receiving target-specific oral anticoagulants: a systematic review and meta-analysis. Blood. 2014;124:2450–8. doi: 10.1182/blood-2014-07-590323. [DOI] [PubMed] [Google Scholar]
- 72.Hernandez I, Baik SH, Piñera A, Zhang Y. Risk of bleeding with dabigatran in atrial fibrillation. JAMA Intern Med. 2015;175:18–24. doi: 10.1001/jamainternmed.2014.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang HY, Zhou M, Tang W, et al. Risk of gastrointestinal bleeding associated with oral anticoagulants: population based retrospective cohort study. BMJ. 2015;350:h1585. doi: 10.1136/bmj.h1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abraham NS, Singh S, Alexander GC, et al. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ. 2015;350:h1857. doi: 10.1136/bmj.h1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Franchini M, Velati C. The use of novel oral anticoagulants: the debate continues! Blood Transfus. 2015;13:170–1. doi: 10.2450/2015.0059-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prandoni P. The treatment of venous thromboembolism with novel oral anticoagulants: warnings and limitations. Blood Transfus. 2015;13:178–80. doi: 10.2450/2015.0002-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Riva N, Ageno W. Which patients with venous thromboembolism should receive non-vitamin K antagonist oral anticoagulants? The majority. Blood Transfus. 2015;13:181–3. doi: 10.2450/2015.0057-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beyer-Westendorf J, Förster K, Pannach S, et al. Rates, management, and outcome of rivaroxaban bleeding in daily care: results from the Dresden NOAC registry. Blood. 2014;124:955–62. doi: 10.1182/blood-2014-03-563577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lippi G, Favaloro EJ. Recent guidelines and recommendations for laboratory assessment of the direct oral anticoagulants (DOACs): is there consensus? Clin Chem Lab. 2015;53:185–97. doi: 10.1515/cclm-2014-0767. [DOI] [PubMed] [Google Scholar]
- 80.Lippi G, Favaloro EJ, Mattiuzzi C. Combined administration of antibiotics and direct oral anticoagulants: a renewed indication for laboratory monitoring? Semin Thromb Hemost. 2014;40:756–65. doi: 10.1055/s-0034-1381233. [DOI] [PubMed] [Google Scholar]
- 81.Lippi G, Ardissino D, Quintavalla R, Cervellin G. Urgent monitoring of direct oral anticoagulants in patients with atrial fibrillation: a tentative approach based on routine laboratory tests. J Thromb Thrombolysis. 2014;38:269–74. doi: 10.1007/s11239-014-1082-5. [DOI] [PubMed] [Google Scholar]