Abstract
Naltrexone is a semi-synthetic opioid with competitive antagonist activity at mu opioid receptors. Its efficacy has been demonstrated in the treatment of alcohol and opioid dependence, but adherence to daily dosing has been recognized as a factor limiting long-term effectiveness. Recently, a long-acting injectable formulation of naltrexone has received FDA-approval for treating alcohol and opioid dependence. This article reviews the pharmacology of naltrexone, the current evidence supporting the use of extended-release naltrexone, and the clinical challenges in the induction of patients to this medication.
Keywords: Naltrexone, Addiction, Toxicology, Alcohol dependence, Opioid dependence
Introduction
Opioid use disorders are increasingly prevalent in the USA and have reached epidemic proportions. The 2013 National Survey of Drug Use and Health reported that almost 5 million individuals were current (past month) nonmedical users of prescription opioids [1]. Longitudinal data from this same survey also found a strong association between nonmedical use of prescription opioids and initiation of heroin use.
On a wider scale, alcohol use disorders remain among the most prevalent substance use disorders throughout the world, affecting an estimated 3.6 % of the global population and 8.5 % of Americans [2]. Considerable research has been done over the years to identify evidence-based therapies to treat these substance use disorders and prevent their associated morbidity and mortality. This research has resulted in the development and FDA-approval of pharmacotherapies that can reduce drug and alcohol cravings and help support recovery.
Naltrexone has been FDA-approved for medication-assisted therapy for alcohol (since 1984), as well as opioid use disorders (since 1994). Medication-assisted therapy for these conditions, however, is currently underutilized. A study of privately funded addiction medicine centers reported that only about one-third used FDA-approved pharmacotherapies [3]. Adherence with daily medication regimens has been a recognized factor affecting outcomes in long-term effectiveness studies.
Advances in drug delivery systems have resulted in the development and FDA-approval of an extended-release formulation of naltrexone. The purpose of this article is to review the pharmacology of naltrexone, to define the pharmacokinetics of a newer extended-release formulation, and summarize the current evidence of effectiveness in the treatment of alcohol and opioid use disorders. Challenges in patient selection, and induction and maintenance on naltrexone therapy will also be addressed.
Naltrexone: Chemistry and Pharmacology
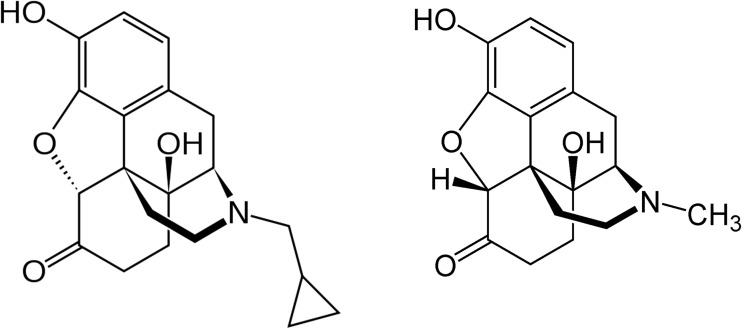
Naltrexone is a semi-synthetic opioid with structural similarity to other opioid agonists. The substitution of a methylcyclopropyl group for the N-methyl group of oxymorphone yields naltrexone (Fig. 1). Naltrexone is a competitive antagonist at mu opioid receptors in the central nervous system. It has similar affinity but partial agonist activity at kappa receptors in the brain and spinal cord, and little to no activity at delta receptors in the spinal cord and peripheral nervous system [4]. After a standard oral dose of 50 mg naltrexone in humans, brain-imaging studies have demonstrated that 95 % of cerebral mu opioid receptors are occupied [5].
Fig. 1.
Structure of naltrexone (left) and oxymorphone (right)
The oral pharmacokinetics of naltrexone has been well-studied. Naltrexone is rapidly absorbed, with peak serum levels occurring in about 60 min. Naltrexone undergoes first pass metabolism in the liver, independent of cytochrome P450 enzymes, to yield an active metabolite, 6-beta-naltrexol [6]. After repeated daily administration for greater than 7 days, the half-life of naltrexone is about 10 h and it primarily undergoes renal excretion. The minimum effective internal dose level for naltrexone, in terms of antagonist effects at the opioid mu receptor, is approximately 1 ng/ml in serum.
Naltrexone and Alcohol Dependence
Interest in the use of naltrexone to treat alcohol use disorders was initially based on the results of animal research studies suggesting that antagonists at the opioid receptor could block the positive reinforcing effects of ethanol. Experimental animal studies reported that blockade of opioid receptors could reduce ethanol drinking behaviors and attenuate self-administration in different species [7, 8]. This led to clinical efficacy studies using naltrexone in humans with alcohol use disorders. The results of two placebo-controlled trials demonstrated reduced cravings, lower relapse rates, and less frequent drinking in alcoholics who received naltrexone [9, 10].
The mechanistic basis for the use of naltrexone in alcohol dependence is through its effects on the reward circuitry for alcohol in the brain. The reinforcing activities of ethanol on the brain are based, in part, on activation of the mesolimbic dopamine pathway. The endogenous opioid system is an important part of the reward system for ethanol in the brain. Alcohol increases the release of endogenous opioid peptides, which activate brain reward circuitry through the disinhibition of GABAergic neurons in the mesolimbic pathway, leading to higher dopamine output. Naltrexone effectively blocks the effects of endogenous opioid release by ethanol, which translates into an attenuation of the euphoria produced by ethanol consumption [11]. Some clinical studies have reported that a single nucleotide polymorphism in the mu opioid receptor OPRM1 gene may have an enhanced response to the effects of naltrexone [12], although a recently published study has raised some uncertainty about this [13].
Extended Release Naltrexone (XR-NTX)
While the clinical efficacy of oral naltrexone has been consistently demonstrated in multiple studies of alcohol and opioid dependence, adherence with daily dosing has been a detriment to the long-term effectiveness of this medication [14]. This factor has led to interest in the development of a long-acting formulation of naltrexone. Currently, one extended-release pharmaceutical product (Vivitrol, Alkermes) is available. It was FDA-approved in 2006 for alcohol and opioid dependence. This product contains naltrexone embedded in a microsphere matrix composed of a biodegradable polyglycolide that has been used in several different applications, including dissolvable sutures and other extended-release pharmaceuticals [15]. The microspheres are mixed with a diluent and administered as a deep intramuscular (gluteal) injection as a single 380 mg dose of naltrexone. The administration of XR-NTX yields plasma levels that effectively antagonize mu opioid receptors for at least 28 days, and the medication can be administered in an outpatient setting as part of a standard office visit.
The pharmacokinetics of XR-NTX has been studied in human clinical studies [16]. The intramuscular injection of XR-NTX results in a Cmax that is similar to oral administration of 50 mg naltrexone. The tmax of XR-NTX is 7 days, much longer than a single dose of oral naltrexone, and results in a greater area under the curve. Intramuscular injection of XR-NTX avoids first-pass hepatic metabolism, resulting in a lower cumulative dose of naltrexone. While XR-NTX has some specific pharmacokinetic advantages over oral naltrexone, the cost of a single dose is approximately $1300, which is much higher than a month supply of the oral formulation (approximately $50).
XR-NTX and Alcohol Dependence
A 6-month, randomized, double-blind placebo-controlled multi-center trial provided support for the FDA-approval of XR-NTX for alcohol dependence [17]. In this study, over 600 subjects were randomized to receive monthly intramuscular injections of XR-NTX (380 or 190 mg) or placebo. All subjects were adults with current diagnoses of alcohol dependence (as defined by DSM-IV). A subset of enrolled subjects was abstinent at the start of the trial (no drinking during the previous 7 days), while most were actively using alcohol at the time of enrollment. The primary endpoints to assess efficacy were the frequency and pattern of heavy drinking (>5 drinks per day for men, >4 drinks per day for women) and risky drinking days (>2 drinks per day for men, >1 drink per day for women).
The results of this study demonstrated that the group receiving the higher dose of XR-NTX had a statistically significant (25 %) decrease in heavy drinking. This treatment effect was more pronounced among subjects who were abstinent at the start of the trial. The majority of subjects (64 %) received all six injections. Adverse events were reported in 14 % of subjects, the most common being symptoms of nausea, headache, and injection site pain. There was no difference in liver function tests (including AST, ALT, and bilirubin) between treatment groups, or compared with placebo.
Since the time of this study, other investigators have reported effectiveness of XR-NTX in the treatment of alcohol dependence. A more recent randomized, double-blind, placebo-controlled study evaluated XR-NTX on quality of life (QOL) endpoints among alcohol dependent patients [18]. The results of this study showed similar rates of medication adherence to previous studies. When compared to placebo, the XR-NTX group had statistically significant improvements from baseline in mental health, general health, physical, and social functioning. These improvements were statistically correlated with reductions from baseline in heavy drinking days.
A recently published functional MRI study provides some additional mechanistic insights towards the effects that naltrexone has on the brain among individuals with alcohol dependence [19]. In this double-blind, placebo-controlled study, adults with alcohol dependence were randomized to receive XR-NTX versus placebo. Prior to the intervention, baseline functional MRI measurements were obtained in an experimental procedure where subjects were exposed to visual and olfactory cues relating to alcohol and nonalcoholic beverages. Subjects were presented these stimuli and instructed to rate their cravings using a visual analog scale. This experimental procedure was repeated in each subject after they had received the XR-NTX or placebo.
The results of this study found that at baseline, visual and olfactory cues relating to alcohol resulted in statistically significant increases in fMRI signaling in orbital and cingulate gyri and areas of the frontal gyri. After the intervention, subjects receiving XR-NTX were found to have statistically significant decreases in brain activation, compared to placebo. Subjectively reported cravings were also statistically lower among the group receiving XR-NTX. The study demonstrated that XR-NTX reduced brain reactivity when alcohol-dependent individuals were exposed to visual and olfactory cues that are known triggers of cravings among individuals with alcohol use disorders.
XR-NTX and Opioid Dependence
Several randomized multi-center, placebo-controlled studies have been published reporting the efficacy of XR-NTX in the treatment of opioid dependence [20]. A recently published study assessed the long-term safety and effectiveness of XR-NTX in a 1-year, open-label investigation [21]. All subjects were adults with opioid dependence, who received monthly injections of 380 mg XR-NTX, as well as counseling. Outcome indicators included results of monthly urine drug tests, reports of craving and social function, and adverse events.
The results of this study found that 62 % of subjects remained in treatment at 1 year, and 51 % remained abstinent at 1 year. An adverse event rate of 21 % was reported, with pain at the injection site being most common. There were no clinically significant elevations of liver function tests, and no deaths or overdoses. Similar to what has been reported in alcohol dependence, a recently published fMRI study found that in opioid dependent individuals receiving XR-NTX, there was a statistically significant reduction in brain activity when presented with visual cues relating to heroin use [22].
Patient Induction on Naltrexone
In addition to cost and insurance coverage, the initiation of naltrexone therapy can present clinical challenges. For patients with alcohol dependence, this is not as much an issue because administration of naltrexone does not induce alcohol withdrawal. Treatment with oral or extended-release naltrexone should ideally be initiated with the patient is engaged in ongoing psychosocial treatment, and not in acute alcohol withdrawal. The assessment of baseline liver function tests is recommended, and the currently available evidence supports that XR-NTX is well tolerated among high-risk subpopulations, including individuals with hepatitis C and HIV [23].
Naltrexone induction in patients with opioid dependence is more challenging because treatment initiation has to occur after a period of abstinence from opioids to avoid the precipitation of acute withdrawal. The selection of patients who will benefit most from naltrexone is also an important consideration. Although evidence-based data is not available to inform these decisions, guidelines based upon collective clinical experience with naltrexone have been published to assist clinicians [24].
Clinical observations suggest that patients who are older and have fewer co-occurring psychological conditions respond better to naltrexone therapy [25]. In addition, patients who have failed other treatment methods and have had longer opioid-free (abstinent) periods may be better candidates for naltrexone. Patients who have completed a hospital-based or residential treatment program and who are currently abstinent from opioids would be considered good candidates for naltrexone.
The FDA-approved prescribing information for XR-NTX advises that patients be abstinent from opioids for a minimum of 7–10 days before taking their first dose of naltrexone. Some clinicians have developed guidelines that attempt to shorten this interval to minimize the withdrawal period and facilitate earlier administration of opioid receptor blockade, with the goal of minimizing the risk of relapse and treatment failure [24].
While the specific clinical guidelines are beyond the scope of this review article, the treatment regimens generally take into consideration the anticipated severity of withdrawal based upon dose and potency of recent opioid use. In addition, these guidelines describe the aggressive use of withdrawal management medications including anxiolytics, antiemetics, and partial agonists (buprenorphine) that can minimize symptoms and facilitate earlier transition on to oral naltrexone or XR-NTX. A summary of suggested treatment algorithms, adapted from these clinical guidelines [24], appears in Table 1. All patients who take naltrexone should carry an identifying bracelet or necklace, to inform health care providers and emergency responders of their treatment status.
Table 1.
Treating algorithms for opioid detoxification and naltrexone induction (adapted from [24])
| Severity of anticipated withdrawal | ||||
|---|---|---|---|---|
| None | Mild | Moderate | Severe | |
| Already abstinent for several days | 1–2 bags heroin/day; <50 mg oxycodone/day | 3–6 bags heroin/day; 50–100 mg oxycodone/day; finishing buprenorphine taper | >6 bags heroin/day; >100 mg oxycodone/day; significant co-occurring medical conditions | |
| Treatment setting | Outpatient | Outpatient or partial hospital | Partial hospital with inpatient backup | Inpatient |
| Buprenorphine | None | None or 4 mg, day 1 | 4–8 mg, day 1 or 2 | 8 mg, day 1 or 2 |
| Clonidine | None | 0.1–0.2 mg tid to qid | 0.2 mg tid to qid | 0.2–0.3 mg qid |
| Clonazepam | None | 0.5 mg bid | 0.5–1.0 mg tid to qid | 1–2 mg qid |
| Time to first oral dose (naltrexone) | Day 1 | Day 3 | Day 3–4 | Day 4–5 |
| Initial oral dose | 25–50 mg qd | 12.5 mg qd | 6 mg bid | 3–6 mg bid |
| Time to injection with XR-NRT | Day 1 or 2 | Day 4; or day 5–6 after titrating up to naltrexone 25–50 mg/day | Days 4–5; or days 5–7 after titrating oral naltrexone to 25–50 mg/day | Days 5–6; or days 6–7 after titrating oral naltrexone to 25–50 mg/day |
Naltrexone: Future Research Needs
As evidence accumulates supporting the effectiveness of XR-NTX for alcohol and opioid dependence [26], there remain several important questions and clinical challenges. The optimum duration of therapy with naltrexone has not been determined, and additional research on the long-term safety and effectiveness is needed. Another issue for which there is very little information in the published literature is the management of acute pain among individuals who are taking naltrexone, particularly XR-NTX [27].
Conclusions
Naltrexone can positively impact outcomes in patients with opioid and alcohol dependence. The development and FDA-approval of XR-NTX addresses some of the challenges of medication adherence, which has been a barrier in the effectiveness of naltrexone in the past. As the evidence for effectiveness of naltrexone accumulates, there is an ongoing need for evidence-based data to guide clinical decision-making. These needs include the identification of patients who are best candidates for this therapy and the successful initiation of treatment with naltrexone among individuals with opioid and alcohol dependence. With increased awareness and confidence with use of naltrexone, clinicians may be able to reduce morbidity and improve the quality of life among patients with these prevalent substance use disorders.
References
- 1.Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD 2014. [PubMed]
- 2.Roerecke M, Rehm J. Alcohol use disorders and mortality: a systematic review and meta-analysis. Addiction. 2013;108(9):1562–78. doi: 10.1111/add.12231. [DOI] [PubMed] [Google Scholar]
- 3.Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addiction Med. 2011;5(1):21–7. doi: 10.1097/ADM.0b013e3181d41ddb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wentland MP, Lou R, Lu Q, Bu Y, Denhardt C, Jin J, et al. Syntheses of novel high affinity ligands for opioid receptors. Bioorg Med Chem Lett. 2009;19(8):2289–94. doi: 10.1016/j.bmcl.2009.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weerts EM, Kim YK, Wand GS, Dannals RF, Lee JS, Frost JJ, et al. Differences in delta- and mu-opioid receptor blockade measured by positron emission tomography in naltrexone-treated recently abstinent alcohol-dependent subjects. Neuropsychopharmacol Off Pub Am College of Neuropsychopharmacol. 2008;33(3):653–65. doi: 10.1038/sj.npp.1301440. [DOI] [PubMed] [Google Scholar]
- 6.Wall ME, Brine DR, Perez-Reyes M. The metabolism of naltrexone in man. NIDA Res Monogr. 1981;28:105–31. [PubMed] [Google Scholar]
- 7.Altshuler HL, Phillips PE, Feinhandler DA. Alteration of ethanol self-administration by naltrexone. Life Sci. 1980;26(9):679–88. doi: 10.1016/0024-3205(80)90257-X. [DOI] [PubMed] [Google Scholar]
- 8.Samson HH, Doyle TF. Oral ethanol self-administration in the rat: effect of naloxone. Pharmacol Biochem Behav. 1985;22(1):91–9. doi: 10.1016/0091-3057(85)90491-5. [DOI] [PubMed] [Google Scholar]
- 9.Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49(11):876–80. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- 10.O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry. 1992;49(11):881–7. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- 11.Unterwald EM. Naltrexone in the treatment of alcohol dependence. J Addiction Med. 2008;2(3):121–7. doi: 10.1097/ADM.0b013e318182b20f. [DOI] [PubMed] [Google Scholar]
- 12.Ray LA, Bujarski S, Chin PF, Miotto K. Pharmacogenetics of naltrexone in asian americans: a randomized placebo-controlled laboratory study. Neuropsychopharmacol Off Pub Am College of Neuropsychopharmacol. 2012;37(2):445–55. doi: 10.1038/npp.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oslin DW, Leong SH, Lynch KG, Berrettini W, O’Brien CP, Gordon AJ, et al. Naltrexone vs placebo for the treatment of alcohol dependence: a randomized clinical trial. JAMA Psychiat. 2015 doi: 10.1001/jamapsychiatry.2014.3053. [DOI] [PubMed] [Google Scholar]
- 14.Swift R, Oslin DW, Alexander M, Forman R. Adherence monitoring in naltrexone pharmacotherapy trials: a systematic review. J. Stud. Alcohol Drugs. 2011;72(6):1012–8. doi: 10.15288/jsad.2011.72.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gastfriend DR. Intramuscular extended-release naltrexone: current evidence. Ann N Y Acad Sci. 2011;1216:144–66. doi: 10.1111/j.1749-6632.2010.05900.x. [DOI] [PubMed] [Google Scholar]
- 16.Dunbar JL, Turncliff RZ, Dong Q, Silverman BL, Ehrich EW, Lasseter KC. Single- and multiple-dose pharmacokinetics of long-acting injectable naltrexone. Alcohol Clin Exp Res. 2006;30(3):480–90. doi: 10.1111/j.1530-0277.2006.00052.x. [DOI] [PubMed] [Google Scholar]
- 17.Garbutt JC. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence. JAMA J. Am. Med. Assoc. 2005;293(13):1617–28. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- 18.Pettinati HM, Gastfriend DR, Dong Q, Kranzler HR, O’Malley SS. Effect of extended-release naltrexone (XR-NTX) on quality of life in alcohol-dependent patients. Alcohol Clin Exp Res. 2009;33(2):350–6. doi: 10.1111/j.1530-0277.2008.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lukas SE, Lowen SB, Lindsey KP, Conn N, Tartarini W, Rodolico J, et al. Extended-release naltrexone (XR-NTX) attenuates brain responses to alcohol cues in alcohol-dependent volunteers: a bold FMRI study. Neuroimage. 2013;78:176–85. doi: 10.1016/j.neuroimage.2013.03.055. [DOI] [PubMed] [Google Scholar]
- 20.Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377(9776):1506–13. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- 21.Krupitsky E, Nunes EV, Ling W, Gastfriend DR, Memisoglu A, Silverman BL. Injectable extended-release naltrexone (XR-NTX) for opioid dependence: long-term safety and effectiveness. Addiction. 2013;108(9):1628–37. doi: 10.1111/add.12208. [DOI] [PubMed] [Google Scholar]
- 22.Langleben DD, Ruparel K, Elman I, Loughead JW, Busch EL, Cornish J, et al. Extended-release naltrexone modulates brain response to drug cues in abstinent heroin-dependent patients. Addict Biol. 2014;19(2):262–71. doi: 10.1111/j.1369-1600.2012.00462.x. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell MC, Memisoglu A, Silverman BL. Hepatic safety of injectable extended-release naltrexone in patients with chronic hepatitis C and HIV infection. J. Stud. Alcohol Drugs. 2012;73(6):991–7. doi: 10.15288/jsad.2012.73.991. [DOI] [PubMed] [Google Scholar]
- 24.Sigmon SC, Bisaga A, Nunes EV, O’Connor PG, Kosten T, Woody G. Opioid detoxification and naltrexone induction strategies: recommendations for clinical practice. Am. J. Drug Alcohol Abuse. 2012;38(3):187–99. doi: 10.3109/00952990.2011.653426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Resnick RB, Washton AM, Thomas MA, Kestenbaum RS. Naltrexone in the treatment of opiate dependence. NIDA Res Monogr. 1978;19:321–32. [PubMed] [Google Scholar]
- 26.Hartung DM, McCarty D, Fu R, Wiest K, Chalk M, Gastfriend DR. Extended-release naltrexone for alcohol and opioid dependence: a meta-analysis of healthcare utilization studies. J Subst Abuse Treat. 2014. [DOI] [PMC free article] [PubMed]
- 27.Curatolo C, Trinh M. Challenges in the perioperative management of the patient receiving extended-release naltrexone. A & A Ccase Rrep. 2014;3(11):142–4. doi: 10.1213/XAA.0000000000000069. [DOI] [PubMed] [Google Scholar]