Abstract
Background
Increase in IgE-antibodies to inhalant allergens is associated with an increased likelihood of wheezing. The role of allergen-specific IgG and IgG4 in relation to wheezing is yet to be determined.
Objective
To investigate whether Fel d 1-specific IgG and IgG4 antibodies modify the association between cat allergen-specific IgE and childhood wheezing.
Methods
We used data from two population-based birth cohorts (UK, n=473 and Australia, n=1336). Current wheeze was defined as wheezing in the previous 12 months at age 5 (UK) and 14 years (Australia). We determined cat allergen-specific IgE (whole extract), and IgG and IgG4 antibodies (purified rFel d 1). We used logistic regression to estimate the relationship between wheeze and the quantitative allergen antibody levels.
Results
In the univariate analysis risk of wheezing increased significantly with increasing cat-specific IgE (UK: OR 1.56, 95%CI 1.28–1.90, Australia 1.29, 1.19–1.40). rFel d1-specific IgG or IgG4 had no significant effect on wheeze in either population. However, a different pattern of the relationship between antibody levels and wheezing emerged in the multivariate analysis. In the UK, cat-specific IgE increased the risk of wheeze (2.01, 1.29–3.12, p=0.002), whilst rFel d 1-specific IgG decreased the risk (0.46, 0.21–0.99, p=0.05). This finding was replicated in Australia (IgE: 1.46, 1.28–1.68, p<0.001; IgG: 0.66, 0.44–0.99, p=0.049). There was no significant association between IgG4 antibodies and wheezing in either population.
Conclusions
rFel d 1-specific IgG, but not IgG4 antibodies significantly modify the association between cat specific IgE and childhood wheezing, with the risk of symptoms decreasing with increasing IgG.
Keywords: asthma, IgE, IgG, IgG4, birth cohorts
BACKGROUND
The presence of allergen-specific IgE antibodies is associated with increased risk of wheezing in children1 and adults2, and with increasing severity of asthma and diminished lung function when the individual is exposed to sensitizing allergen3–5. We have previously demonstrated that the absolute specific IgE antibody levels offer more information about the relationship between IgE-mediated sensitization and respiratory symptoms than just the presence of specific IgE, and found total IgE to be a poorer predictor of wheeze than the sum of specific IgEs6, 7. These data suggested that labeling subjects as sensitized or not based on an arbitrary cut-off is an oversimplification of a trait that is not dichotomous in its relationship with the symptoms of allergic disease8.
Allergen exposure is associated with increasing risk of IgE-mediated sensitization9, 10. However, several studies have shown that at very high levels of exposure (in particular to allergens associated with furry animals) the risk of clinically relevant specific sensitization appears to decrease11–14. Explanations for this observation include the possibility that very high exposures may produce an IgG and IgG4 antibody responses without concomitant IgE-mediated sensitization (a modified T-helper-2 cell response11), and potential blocking effects of IgG4 (which is co-produced with IgE) on IgE-mediated effector mechanisms14. Other studies by contrast have found no evidence of a protective effect of cat ownership or high levels of allergen-specific IgG or IgG4 against IgE sensitization or ensuing respiratory symptoms15, 16. However interpretation of these latter studies is limited respectively by relatively low sample size15 and by the fact that IgG measurements were made against mixtures16 which results in a dominant contribution from low affinity antibodies to the resulting titres, potentially masking biologically relevant high affinity IgG17.
We have readdressed these issues of relationships between IgE and IgG responses in the study on cat allergy and risk for wheeze. We focus exclusively on school children in the age range in which the association between sensitization to inhalant allergens and wheezing illness is strongest18. We have utilized two large population-based birth cohorts studied independently in two geographical areas (United Kingdom and Australia), amounting to ~1900 subjects in whom high affinity IgG responses to Fel d 1 allergen has been measured in parallel with cat-specific IgE.
METHODS
Study design, setting and participants
Two population samples were studied (Manchester and Perth): the Manchester Asthma and Allergy Study (MAAS)19, 20 and The Western Australia Pregnancy Cohort (RAINE) Study18 are unselected population-based birth cohort studies described in detail elsewhere. Both studies were approved by local research ethics committees. Informed consent was obtained from all parents, and children gave their assent if appropriate.
Manchester, UK
Subjects were recruited from the antenatal clinics when all pregnant women were screened for eligibility during the first trimester of pregnancy19. Children were followed prospectively and attended review clinic at age five years (± four weeks)21.
Perth, Western Australia
The Western Australia Pregnancy Cohort Study is a prospective birth cohort established between 1989–1992. Participants were recruited from public antenatal clinics at King Edward Memorial Hospital and nearby private practices. At the time of enrollment the parents completed a questionnaire about their own respiratory illness, smoking behavior and general health. Children were followed prospectively with a clinical assessment and blood collection at age 14 years.
Definitions of variables
Primary outcome measure - Current wheeze
Identical validated questionnaires were administered in both cohorts to collect information on parentally reported symptoms. Current wheeze was defined as a positive response to the question “Has your child had wheezing or whistling in the chest in the last 12 months?” at age 5 years (UK) and age 14 years (Australia).
Antibody measurement
Allergen specific antibody levels were determined using ImmunoCAP™ assay (Phadia AB, Uppsala, Sweden). For the IgE antibody determinations the commercially available reagents of whole allergen extract for cat were used. For the allergen-specific IgG antibody measurements purified recombinant Fel d 1 (rFel d 1) allergen ImmunoCAP was used. Recombinant Fel d1 was produced by Phadia AB and subsequently coupled to the solid phase of flexible, hydrophilic, cellulose sponge polymer matrix activated by CNBr chemistry. The purified components were covalently coupled to the activated solid phase via the amino groups of the proteins according to standard ImmunoCAP methodology as previously described.
In a sub-samples from the UK (n=280) we determined the level of specific IgE antibodies to rFel d 1.
Statistical methods
Logistic regression was used to estimate the relationship between the outcome variables and the quantitative allergen specific antibody level. Odds Ratios (OR) were estimated using the regression model and tests of significance and 95% confidence intervals were according to Wald, using a p-value of 0.05 as significant. Fitted predicted probabilities were plotted using the results from the logistic regression.
The levels of specific antibodies were subject to a logarithmic transformation prior to analysis; OR are presented for different antibody levels expressing the increased or decreased risk associated with increasing antibody levels. Since a logarithmic transformation was used all calculations were done on the logarithmic scale, i.e. the OR was estimated as exp(r*b) and r, the distance between a certain antibody level and the level indicating the absence of antibody, was defined as r=ln(x2)–ln(x1). Computerized statistical analysis was carried out using SAS System V8.01.
RESULTS
Participants
Of 1085 children born into the study in the UK who gave consent to a further follow-up, 128 were prenatally randomized to environmental control22 and excluded from this analysis; 957 children were followed in the observational cohort. Of the children in the observational cohort, 876 (91.5%) attended the 5 year clinic follow-up of whom 534 (62%) agreed to provide blood sample. Due to availability of the sample, IgE was measures in 534, IgG in 453 and IgG4 in 461 children; all three specific antibodies were available in 453 children (243 male). Of those, 91(20.1%) reported current wheezing at age 5 years. Children who were excluded did not differ from those included in terms of family history, parental smoking, maternal age, socioeconomic status, gestational age, birth weight, history of wheeze and skin test results.
1336 adolescents in Australia (687 male) had a complete data comprising questionnaires and blood test results. Of those, 179 (13.4%) reported current wheezing at age 14.
Allergen specific IgE, IgG and IgG4 antibody levels and wheezing
Univariate analysis
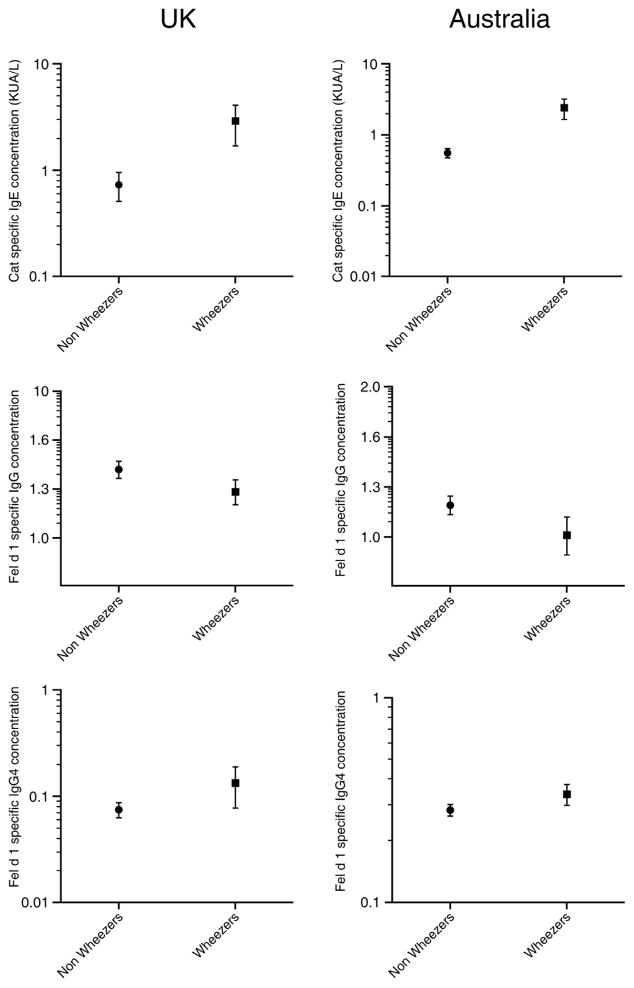
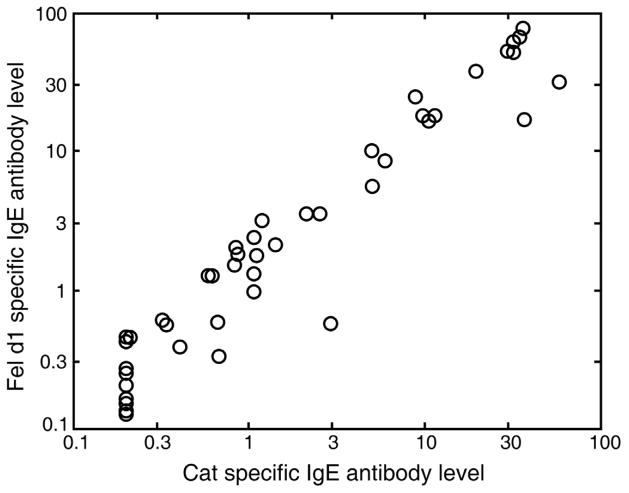
Allergen specific IgE, IgG and IgG4 antibody levels amongst children with current wheezing and those without in the two populations are presented in Figure 1, and in relation to cat ownership in Table 1. In the UK cohort, there was an excellent correlation between the cat extract-specific and rFel d 1-specific IgE antibodies (r=0.88, Figure 2).
Figure 1.
Cat allergen specific IgE, and rFel d 1-specific IgG and IgG4 antibody levels amongst children with current wheezing and those without in the UK and Australian cohort
Table 1.
Cat allergen specific IgE, and rFel d 1-specific IgG and IgG4 antibody levels amongst children currently living in homes with without cat(s) in the UK and Australian cohort
| Whole population | Cat owner | Not cat owner | p-value (cat owners vs. Non-cat owners) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95%CI | Mean | 95% CI | Mean | 95% CI | |||
|
| ||||||||
| UK | Cat-specific IgE | 0.26 | 0.24–0.28 | 0.31 | 0.25–0.38 | 0.24 | 0.22–0.26 | 0.01 |
| rFel d 1-specific IgG | 1.19 | 1.15–1.24 | 1.46 | 1.30–1.64 | 1.13 | 1.09–1.17 | < 0.0001 | |
| rFel d 1-specific IgG4 | 0.12 | 0.11–0.13 | 0.13 | 0.11–0.15 | 0.12 | 0.11–0.13 | 0.30 | |
|
| ||||||||
| Australia | Cat-specific IgE | 0.30 | 0.28–0.31 | 0.30 | 0.27–0.33 | 0.29 | 0.27–0-31 | 0.33 |
| rFel d 1-specific IgG | 1.19 | 1.16–1.23 | 1.28 | 1.22–1.35 | 1.13 | 1.10–1.16 | < 0.0001 | |
| rFel d 1-specific IgG4 | 0.11 | 0.10–0.13 | 0.15 | 0.12–0.18 | 0.09 | 0.08–0.10 | < 0.0001 | |
Figure 2.
Excellent correlation between IgE antibodies of whole allergen extract for cat and IgE antibodies to rFel d1
As expected, cat-specific IgE antibodies were higher amongst children with current wheeze in both populations. In the UK cohort, 22% of the children with current wheeze had cat-specific IgE antibody levels >0.35kU/L compared to only 5% amongst non-wheezers (p<0.0001). In Australian cohort, cat-specific IgE exceeded 0.35kU/L in 28% of wheezers and 14% of non-wheezers (p<0.001). There were no differences in rFel d 1-specific IgG antibody levels between wheezers and non-wheezers in either population (Figure 1).
In the univariate logistic regression analyses, the predicted risk of current wheeze in both populations increased significantly with increasing cat-specific IgE antibody levels (Table 2); the results indicated 1.56 and 1.29-fold increase in risk per logarithmic unit increase in cat IgE antibody level in the UK and Australia respectively. There was no comparable significant effect of rFel d 1-specific IgG or IgG4 antibody levels on current wheeze in either population (Table 2).
Table 2.
Univariate analysis: Relationship between current wheezing and allergen-specific antibodies in two populations
| UK | Australia | |||
|---|---|---|---|---|
| OR | 95%CI | OR | 95%CI | |
| Cat-specific IgE | 1.56 | 1.28–1.90 | 1.29 | 1.19–1.40 |
| rFel d 1-specific IgG | 1.05 | 0.68–1.64 | 0.92 | 0.77–1.10 |
| rFel d 1-specific IgG4 | 1.13 | 0.98–1.29 | 1.08 | 0.97–1.19 |
Multivariate analysis
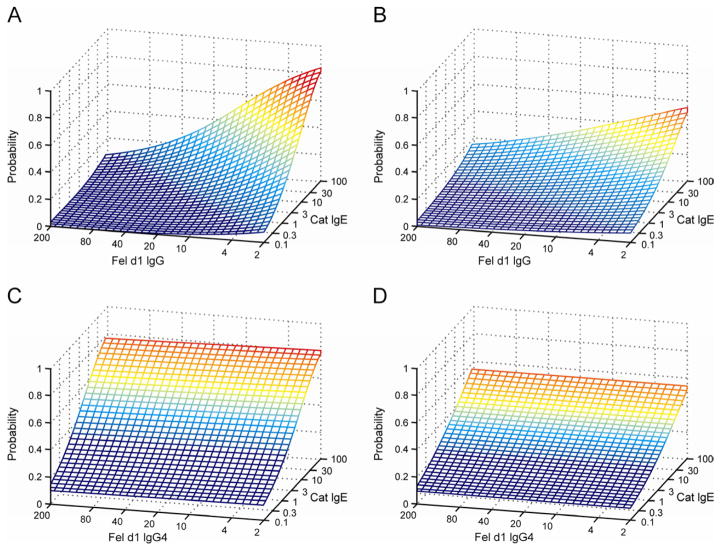
A markedly different relationship was observed when rFel d1 specific total IgG antibody levels and cat-specific IgE were employed as covariates in the multivariate logistic regression analyses (Figure 3). In the UK cohort, cat-specific IgE antibodies increased the risk of current wheeze (OR 2.01, 95% CI 1.29–3.12, p=0.002), whilst rFel d 1-specific IgG antibody levels decreased the risk of current wheezing (0.46, 0.21–0.99, p=0.05). This finding was replicated in the Australian cohort (IgE: OR 1.46, 95% CI 1.28–1.68, p<0.001; IgG: OR 0.66, 95% CI 0.44–0.99, p=0.049 Figure 3, panels A and B). These results indicated 2-fold and 1.33-fold increase in the risk of wheezing per logarithmic unit increase in cat IgE antibody level, with 2.17-fold and 1.28-fold decrease in the risk of wheezing per logarithmic unit increase in rFel d 1-specific IgG antibody levels in the UK and Australia respectively. In contrast, we found no significant association between rFel d 1-specific IgG4 antibodies and current wheezing (UK: 1.08, 0.91–1.28, p=0.37; Australia: 1.04, 0.96–1.14, p=0.35, Figure 3, panels C and D). In addition, we found no statistically significant interaction between IgE and IgG or IgG4 antibody levels in either population.
Figure 3.
Panels A and B: Fitted predicted probability for current wheeze at a given cat-specific IgE value and rFel d 1-specific IgG value derived from the multivariate logistic regression analysis, for children in the UK (A) and Australia (B).
Panels C and D: Fitted predicted probability for current wheeze at a given cat-specific IgE value and rFel d 1-specific IgG4 value derived from the multivariate logistic regression analysis, for children in the UK (C) and Australia (D).
DISCUSSION
Principal findings
In two independent unselected birth cohorts from distinct geographic areas (United Kingdom and Australia), we demonstrated that the association between cat-specific IgE antibodies and childhood wheezing is significantly modified by rFel d 1-specific IgG antibodies. The risk of current wheezing increased significantly with increasing cat-specific IgE, with rFel d 1-specific IgG antibody levels significantly decreasing the risk. In contrast, there was no association between rFel d 1-specific IgG4 and wheezing. We postulate that the IgG-antibody associated effects observed here are due to IgG1 which is the most abundant IgG subclass accounting for 5–20 % of total IgG antibody in most subjects17, 23–26, although we cannot rule out contributions from other minor subclasses.
Limitations and strengths
Although retention in both birth cohorts was excellent, in the UK sample we analyzed data from approximately half of the subjects (mostly because the child refused venepuncture). We emphasize that there was no difference between children excluded or included in the analysis in any relevant parameter. Furthermore, the prevalence of allergic sensitization amongst the parents of the children is similar to that of young adults in the UK21, suggesting the subjects are representative of the general population.
We made every effort to minimize false positive results due to multiple testing. The analysis was hypothesis-driven, and limited to one carefully defined phenotype which was ascertained based on the answer to the identical question in both populations.
The assay technique we used has been shown to accurately quantify IgE antibodies to a variety of allergens including cat in a superior manner compared with other IgE antibody methods27, 28. The IgE antibody determinations using ImmunoCAP have also been shown to be better correlated with clinical disease than for other IgE antibody assays29. For IgG and IgG4 antibodies this type of information is much more scarce, and no literature on comparison of different commercially available methods is available. However, Aalberse et al have concluded that for IgG1, IgG2 and IgG3 purified allergen components like the Fel d 1 should be used17. This is necessary because while allergen extracts contained several components that bind to specific IgE and IgG4 antibodies, extracts from domestic animals and mite also contain antigens similar to and sometimes cross-reactive with bacterial structures. Both allergic and non-allergic individuals produce IgG antibodies as part of their defense against such microbes, and this makes it difficult to evaluate results using a full extract containing both allergens and such antigens17. Accordingly, in this study recombinant Fel d 1 was used to determine the IgG antibody responses and to distinguish those from a general antibody response to antigens from microbes.
We acknowledge that most study participants who were sensitized to cat were also sensitized and exposed to multiple other allergens (e.g. dust mite). However, if anything this would dilute rather than strengthen the associations we report.
Interpretation
IgG4 antibodies comprise <5% of IgG, and the putative role of IgG4 has been reviewed in detail recently17. The spectrum of functions ascribed to this antibody are diverse and include both reaginic activity30, 31 and interference with IgE-mediated effector mechanisms. For example, IgG4 has been postulated to block IgE-dependent resistance to schistosomiasis32 and filariasis33, 34 and very high levels of specific IgG4 antibody are common in both diseases. A protective effect of both IgG and IgG4 antibodies has been suggested in modification of allergic reactions. For example, the blocking of the Prausnitz–Küstner reaction by naturally occurring factors in serum was described as early as 193535. It was subsequently demonstrated that naturally occurring IgG antibodies to Fel d 1 blocked skin test reactions36. More recently, in specific allergen immunotherapy the increase in IgG4 antibodies has been shown to correlate significantly with clinical improvement23, 24, 37. However, it is as yet unclear whether allergen-specific IgG4 has a causal relationship or is just a marker of the protective effect.
It is noteworthy that the immunological scenarios in which negative associations between serum levels of allergen-specific IgG4 and the expression of IgE-associated immunoinflammatory responses appears most consistent (notably parasitism32–34, specific immunotherapy23, 24, 37 and occupational exposures to aeroallergen14) share as a common feature ultra-intense chronic immune stimulation. The very high levels of specific IgG4 attained in these situations suggest that this Th2-dependent IgG subclass is selectively expanded under these circumstances (it may even then represent up to 80% of total IgG antibodies17), which is not surprising given that initial (and sometimes persistent) boosting of specific IgE commonly occurs in parallel.
In contrast the immune response to cat allergen which is driven by normal domestic exposure involves much lower levels of immune stimulation, and in these circumstances IgG4 is a less prominent feature of the overall specific immune response. Our finding that IgG (putative IgG1) and not IgG4 is associated in cat-exposed children with blocking of the clinical effects of cat-specific IgE may reflect this differing balance.
Our findings have potential implications in relation to design of therapeutic strategies in established atopic asthma amongst cat allergic subjects. The currently favoured targets for design of more effective SIT are T-regulatory cells and specific IgG4 antibody, but the successful design of effective therapies to achieve these aims has not yet been achieved. However, if our conclusions from this study prove to be correct, i.e. if a major component of the Fel d 1 specific IgG which we demonstrated here to interfere with the expression of IgE-associated symptoms is indeed IgG1, then selective boosting of this antibody via administration of allergen/Th1-adjuvant could be considered as an alternative therapeutic strategy. An attraction of this alternative approach is the ready availability of clinically proven Th1 adjuvants for human use, which may facilitate more rapid development of new therapeutic vaccines for clinical testing.
The degree to which this rationale may be applicable to other types of allergens needs to be determined in further studies.
Conclusions
In two independent population-based birth cohorts from distinct geographic areas, we have demonstrated that rFel d 1-specific IgG, but not IgG4 antibodies significantly modify the association between cat-specific IgE and childhood wheezing. The risk of symptoms increases significantly with increasing cat-specific IgE, while rFel d 1-specific IgG antibody levels significantly decreased the risk. Our data suggest that Fel d 1-specific IgG interferes with the expression of IgE-associated respiratory symptoms. Thus, measurement of allergen-specific IgG antibodies may improve the diagnostic accuracy of specific IgE antibodies in childhood wheezing illness.
Clinical Implications.
Fel d 1-specific IgG interferes with the expression of IgE-associated respiratory symptoms. Therefore, measurement of allergen-specific IgG antibodies may improve the diagnostic accuracy of specific IgE antibody measurement in childhood wheezing illness.
Acknowledgments
Funding: MAAS was supported by Asthma UK Grant No 04/014 and Moulton Charitable Foundation, and is currently supported by MRC Grant G0601361; The Western Australia Pregnancy Cohort (RAINE) Study is funded by the National Health and Medical Research Council of Australia and the RAINE Foundation
Abbreviations
- IgE
Immunoglobulin E
- IgG
Immunoglobulin G
- Fel d 1
Felis Domesticus allergen 1
- rFel d 1
recombinant Felis Domesticus allergen 1
- MAAS
Manchester Asthma and Allergy Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–7. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 2.Simpson BM, Custovic A, Simpson A, Hallam CL, Walsh D, Marolia H, et al. NAC Manchester Asthma and Allergy Study (NACMAAS): risk factors for asthma and allergic disorders in adults. Clin Exp Allergy. 2001;31:391–9. doi: 10.1046/j.1365-2222.2001.01050.x. [DOI] [PubMed] [Google Scholar]
- 3.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 4.Lowe LA, Woodcock A, Murray CS, Morris J, Simpson A, Custovic A. Lung function at age 3 years: effect of pet ownership and exposure to indoor allergens. Arch Pediatr Adolesc Med. 2004;158:996–1001. doi: 10.1001/archpedi.158.10.996. [DOI] [PubMed] [Google Scholar]
- 5.Langley SJ, Goldthorpe S, Craven M, Morris J, Woodcock A, Custovic A. Exposure and sensitization to indoor allergens: association with lung function, bronchial reactivity, and exhaled nitric oxide measures in asthma. J Allergy Clin Immunol. 2003;112:362–8. doi: 10.1067/mai.2003.1654. [DOI] [PubMed] [Google Scholar]
- 6.Simpson A, Soderstrom L, Ahlstedt S, Murray CS, Woodcock A, Custovic A. IgE antibody quantification and the probability of wheeze in preschool children. J Allergy Clin Immunol. 2005;116:744–9. doi: 10.1016/j.jaci.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 7.Marinho S, Simpson A, Soderstrom L, Woodcock A, Ahlstedt S, Custovic A. Quantification of atopy and the probability of rhinitis in preschool children: a population-based birth cohort study. Allergy. 2007;62:1379–86. doi: 10.1111/j.1398-9995.2007.01502.x. [DOI] [PubMed] [Google Scholar]
- 8.Simpson A, Tan VY, Winn J, Svensen M, Bishop CM, Heckerman DE, et al. Beyond atopy: multiple patterns of sensitization in relation to asthma in a birth cohort study. Am J Respir Crit Care Med. 2010;181:1200–6. doi: 10.1164/rccm.200907-1101OC. [DOI] [PubMed] [Google Scholar]
- 9.Lau S, Illi S, Sommerfeld C, Niggemann B, Bergmann R, von Mutius E, et al. Early exposure to house-dust mite and cat allergens and development of childhood asthma: a cohort study. Multicentre Allergy Study Group. Lancet. 2000;356:1392–7. doi: 10.1016/s0140-6736(00)02842-7. [DOI] [PubMed] [Google Scholar]
- 10.Peat JK, Tovey E, Toelle BG, Haby MM, Gray EJ, Mahmic A, et al. House dust mite allergens. A major risk factor for childhood asthma in Australia. Am J Respir Crit Care Med. 1996;153:141–6. doi: 10.1164/ajrccm.153.1.8542107. [DOI] [PubMed] [Google Scholar]
- 11.Platts-Mills T, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. 2001;357:752–6. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- 12.Custovic A, Simpson BM, Simpson A, Hallam CL, Marolia H, Walsh D, et al. Current mite, cat, and dog allergen exposure, pet ownership, and sensitization to inhalant allergens in adults. J Allergy Clin Immunol. 2003;111:402–7. doi: 10.1067/mai.2003.55. [DOI] [PubMed] [Google Scholar]
- 13.Custovic A, Hallam CL, Simpson BM, Craven M, Simpson A, Woodcock A. Decreased prevalence of sensitization to cats with high exposure to cat allergen. J Allergy Clin Immunol. 2001;108:537–9. doi: 10.1067/mai.2001.118599. [DOI] [PubMed] [Google Scholar]
- 14.Jeal H, Draper A, Harris J, Taylor AN, Cullinan P, Jones M. Modified Th2 responses at high-dose exposures to allergen: using an occupational model. Am J Respir Crit Care Med. 2006;174:21–5. doi: 10.1164/rccm.200506-964OC. [DOI] [PubMed] [Google Scholar]
- 15.Jarvis D, Zock JP, Heinrich J, Svanes C, Verlato G, Olivieri M, et al. Cat and dust mite allergen levels, specific IgG and IgG4, and respiratory symptoms in adults. J Allergy Clin Immunol. 2007;119:697–704. doi: 10.1016/j.jaci.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 16.Lau S, Illi S, Platts-Mills TA, Riposo D, Nickel R, Gruber C, et al. Longitudinal study on the relationship between cat allergen and endotoxin exposure, sensitization, cat-specific IgG and development of asthma in childhood--report of the German Multicentre Allergy Study (MAS 90) Allergy. 2005;60:766–73. doi: 10.1111/j.1398-9995.2005.00781.x. [DOI] [PubMed] [Google Scholar]
- 17.Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy. 2009;39:469–77. doi: 10.1111/j.1365-2222.2009.03207.x. [DOI] [PubMed] [Google Scholar]
- 18.Hollams EM, Deverell M, Serralha M, Suriyaarachchi D, Parsons F, Zhang G, et al. Elucidation of asthma phenotypes in atopic teenagers through parallel immunophenotypic and clinical profiling. J Allergy Clin Immunol. 2009;124:463–70. 70 e1–16. doi: 10.1016/j.jaci.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 19.Custovic A, Simpson BM, Murray CS, Lowe L, Woodcock A. The National Asthma Campaign Manchester Asthma and Allergy Study. Pediatr Allergy Immunol. 2002;13(Suppl 15):32–7. doi: 10.1034/j.1399-3038.13.s.15.3.x. [DOI] [PubMed] [Google Scholar]
- 20.Lowe L, Murray CS, Martin L, Deas J, Cashin E, Poletti G, et al. Reported versus confirmed wheeze and lung function in early life. Arch Dis Child. 2004;89:540–3. doi: 10.1136/adc.2003.038539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowe LA, Simpson A, Woodcock A, Morris J, Murray CS, Custovic A. Wheeze phenotypes and lung function in preschool children. Am J Respir Crit Care Med. 2005;171:231–7. doi: 10.1164/rccm.200406-695OC. [DOI] [PubMed] [Google Scholar]
- 22.Simpson A, Simpson B, Custovic A, Craven M, Woodcock A. Stringent environmental control in pregnancy and early life: the long-term effects on mite, cat and dog allergen. Clin Exp Allergy. 2003;33:1183–9. doi: 10.1046/j.1365-2745.2003.01679.x. [DOI] [PubMed] [Google Scholar]
- 23.Nouri-Aria KT, Wachholz PA, Francis JN, Jacobson MR, Walker SM, Wilcock LK, et al. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J Immunol. 2004;172:3252–9. doi: 10.4049/jimmunol.172.5.3252. [DOI] [PubMed] [Google Scholar]
- 24.Wachholz PA, Durham SR. Mechanisms of immunotherapy: IgG revisited. Curr Opin Allergy Clin Immunol. 2004;4:313–8. doi: 10.1097/01.all.0000136753.35948.c0. [DOI] [PubMed] [Google Scholar]
- 25.Francis JN, Jacobson MR, Lloyd CM, Sabroe I, Durham SR, Till SJ. CXCR1+CD4+ T cells in human allergic disease. J Immunol. 2004;172:268–73. doi: 10.4049/jimmunol.172.1.268. [DOI] [PubMed] [Google Scholar]
- 26.Aalberse RC, Dieges PH, Knul-Bretlova V, Vooren P, Aalbers M, van Leeuwen J. IgG4 as a blocking antibody. Clin Rev Allergy. 1983;1:289–302. doi: 10.1007/BF02991163. [DOI] [PubMed] [Google Scholar]
- 27.Williams PB, Barnes JH, Szeinbach SL, Sullivan TJ. Analytic precision and accuracy of commercial immunoassays for specific IgE: establishing a standard. J Allergy Clin Immunol. 2000;105:1221–30. doi: 10.1067/mai.2000.105219. [DOI] [PubMed] [Google Scholar]
- 28.Wood RA, Segall N, Ahlstedt S, Williams PB. Accuracy of IgE antibody laboratory results. Ann Allergy Asthma Immunol. 2007;99:34–41. doi: 10.1016/S1081-1206(10)60618-7. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Godbold JH, Sampson HA. Correlation of serum allergy (IgE) tests performed by different assay systems. J Allergy Clin Immunol. 2008;121:1219–24. doi: 10.1016/j.jaci.2007.12.1150. [DOI] [PubMed] [Google Scholar]
- 30.Devey ME, Panzani R. The IgG subclasses of antibodies to castor bean allergen in patients with allergic asthma: detection of a high incidence of antibodies of the IgG4 subclass. Clin Allergy. 1975;5:353–61. doi: 10.1111/j.1365-2222.1975.tb01874.x. [DOI] [PubMed] [Google Scholar]
- 31.Parish WE. The clinical relevance of heat-stable, short-term sensitizing anaphylactic IgG antibodies (IgG S-TS) and of related activities of IgG4 and IgG2. Br J Dermatol. 1981;105:223–31. doi: 10.1111/j.1365-2133.1981.tb01210.x. [DOI] [PubMed] [Google Scholar]
- 32.Iskander R, Das PK, Aalberse RC. IgG4 antibodies in Egyptian patients with schistosomiasis. Int Arch Allergy Appl Immunol. 1981;66:200–7. doi: 10.1159/000232819. [DOI] [PubMed] [Google Scholar]
- 33.Ottesen EA, Kumaraswami V, Paranjape R, Poindexter RW, Tripathy SP. Naturally occurring blocking antibodies modulate immediate hypersensitivity responses in human filariasis. J Immunol. 1981;127:2014–20. [PubMed] [Google Scholar]
- 34.Ottesen EA, Skvaril F, Tripathy SP, Poindexter RW, Hussain R. Prominence of IgG4 in the IgG antibody response to human filariasis. J Immunol. 1985;134:2707–12. [PubMed] [Google Scholar]
- 35.Cooke RA, Barnard JH, Hebald S, Stull A. Serological Evidence of Immunity with Coexisting Sensitization in a Type of Human Allergy (Hay Fever) J Exp Med. 1935;62:733–50. doi: 10.1084/jem.62.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Witteman AM, Stapel SO, Sjamsoedin DH, Jansen HM, Aalberse RC, van der Zee JS. Fel d 1-specific IgG antibodies induced by natural exposure have blocking activity in skin tests. Int Arch Allergy Immunol. 1996;109:369–75. doi: 10.1159/000237265. [DOI] [PubMed] [Google Scholar]
- 37.Gehlhar K, Schlaak M, Becker W, Bufe A. Monitoring allergen immunotherapy of pollen-allergic patients: the ratio of allergen-specific IgG4 to IgG1 correlates with clinical outcome. Clin Exp Allergy. 1999;29:497–506. doi: 10.1046/j.1365-2222.1999.00525.x. [DOI] [PubMed] [Google Scholar]