Abstract
Fingolimod, a structural analog of sphingosine derived from fungal metabolites, is a functional antagonist of the G-protein-coupled sphingosine 1-phosphate (S1P) receptors S1P1,3,4,5. In the treatment of relapsing forms of multiple sclerosis (RMS), fingolimod acts by reversibly retaining central memory T cells and naïve T cells in lymph nodes, thereby reducing the recirculation of autoreactive lymphocytes to the central nervous system (CNS). Fingolimod also has differential effects on the trafficking and function of B-cell subtypes and natural killer (NK) cells in peripheral blood and the CNS. Fingolimod also crosses the blood–brain barrier (BBB) and accumulates in the CNS. Experimental evidence increasingly supports a direct action of fingolimod within the CNS on brain cells, providing protection against the neurodegenerative component of RMS. We review the direct influence of this compound on CNS pathogenesis in RMS, including the central effects of fingolimod in animal models of MS and on neural cell types that express S1P receptors, such as astrocytes, BBB endothelial cells, microglia, neurones, and oligodendrocytes, which are all involved in RMS pathology.
Key Points
| Fingolimod can cross the blood–brain barrier and directly influence central nervous system (CNS) pathogenesis in relapsing forms of multiple sclerosis (RMS). |
| In animal models of RMS, fingolimod directly promotes myelin integrity. |
| Fingolimod exerts effects on each of the key brain cells involved in RMS pathology, and sphingosine 1-phosphate modulation of CNS cells may affect clinical outcomes. |
Introduction
Fingolimod (Gilenya®, FTY720 [Novartis Pharma AG]) is a structural analog of sphingosine, derived from fungal metabolites, and affects the G-protein-coupled sphingosine 1-phosphate (S1P) receptors S1P1,3,4,5 [1, 2]. These S1P-receptor subtypes undergo differential G-protein coupling, and their associated downstream signaling pathways affect cell proliferation, survival, migration, and neural cell communication [3].
Fingolimod was approved as the first oral disease-modifying therapy (DMT) for relapsing forms of multiple sclerosis (MS) by the US FDA in 2010, and subsequently by the European Medicines Agency in 2011 [4, 5]. The clinical effects of this agent, demonstrated in several large, randomized, controlled clinical trials of patients with relapsing–remitting MS (RRMS) [6–11], include an early and sustained impact on brain atrophy [6, 7, 9, 12].
In the treatment of patients with relapsing forms of MS, fingolimod acts primarily by reversibly retaining circulating central memory T cells and naïve T cells in lymph nodes, thereby reducing the recirculation of autoreactive lymphocytes to the central nervous system (CNS) [1, 13–15]. There are also differential effects on B cells and natural killer (NK) cells [16–19]. Direct actions of fingolimod on cells within the CNS are likely to provide neuroprotection against the pathologic effects of MS and/or promote endogenous repair mechanisms. Fingolimod may be cytoprotective for neural cells (mediated by signals such as neurotrophins) or act via the blockade of the deleterious actions of inflammatory mediators that contribute to CNS pathology. Sphingolipid signaling systems include the breakdown of the myelin and plasma membrane sphingomyelin pool (via sphingomyelinases and ceramidases) to produce sphingosine, the molecule for which fingolimod is the pharmacologic analog. The central signaling pathway is potentially as important as the peripheral pathway owing to CNS enrichment of these lipids and sphingolipid mobilization during inflammatory demyelination. Evidence supports elaboration of these sphingolipid mediators, in part by inflammatory cytokines and myelin destruction, during the course of demyelinating insults. The detail of these biochemical pathways is reviewed elsewhere [20].
The underlying pathophysiology of MS is attributed to an autoimmune attack on the CNS, mediated by peripheral lymphocytes that are able to cross the blood–brain barrier (BBB) [21]. Focal white-matter lesions are a pathologic hallmark of MS [22–24] and can evolve into ‘black holes’, which are indicative of persistent tissue loss [25]. Focal cortical gray matter lesions, which are not visualized by conventional imaging, correlate with disability status [26, 27]. Diffuse damage, which is the result of widespread irreversible demyelination and neuroaxonal loss, also occurs in grey matter and in normal-appearing white matter (NAWM) [2, 24, 28–31]. Together, focal and diffuse damage contribute to neurodegeneration, which manifests as brain atrophy [22–24]. Spinal cord volume loss correlates with clinical disability in MS (reviewed by De Stefano et al. [32]). The loss of brain volume occurs more rapidly in patients with MS than in healthy individuals without MS and begins early in the course of the disease [28]. It continues throughout the course of MS, at a rate largely independent of MS subtype [33, 34]. Early in the course of MS, CNS neuronal plasticity and myelin repair limit the clinical expression of pathology [24]. Eventually, however, repair mechanisms are insufficient, and neurodegenerative damage accumulates, leading to irreversible disability [35].
A key objective of DMT is to diminish the immune system’s attack on the nervous system. Redressing the balance between damage and repair, two key factors determining MS disability, is also important.
This review was conducted to consolidate the available pharmacologic evidence for direct effects of fingolimod in the CNS. In order to evaluate all relevant literature, we conducted searches (in August 2014) in PubMed, Ovid EMBASE, and Ovid MEDLINE. The searches used the terms ‘fingolimod OR Gilenya OR FTY720 OR FTY720P OR sphingosine’ and ‘cytoprotec* OR neuroprotec* OR CNS OR “central nervous system” OR “brain-derived neurotrophic factor” OR remyelination OR neurone* OR microglia OR astrocytes OR oligodendrocytes’.
Multiple Sclerosis (MS) Pathogenesis: Demyelination and Neurodegeneration
Inflammatory autoimmune reactions play a role in the pathologic mechanisms involved in neurodegeneration and the formation of lesions in MS [24]. Inflammation within the CNS occurs when autoreactive T cells that have crossed the BBB are activated locally by microglia and astrocytes [36]. The resulting secretion of pro-inflammatory molecules from these T cells, which include cytokines, chemokines, and adhesion molecules, increases the permeability of the BBB, leading to increased recruitment of other immune cells (monocytes, macrophages, and B cells) into the CNS [37]. Ultimately, the result is myelin destruction, demyelinated plaques, axonal loss, and injury to clinically eloquent and clinically silent regions of the CNS [38].
Remyelination and Neuronal Regeneration are Impaired in MS
The CNS has an intrinsic capacity to repair itself. However, as MS disease progresses, normal CNS repair and adaptation processes, including remyelination and neuroaxonal regeneration, are increasingly unable to compensate for the damage caused by immune-mediated injury and ongoing neurodegeneration [24, 35].
In the early stages of MS, inflammatory lesions can undergo some degree of myelin repair as peripherally derived macrophages at the site of myelin destruction stimulate the proliferation of oligodendrocyte precursor cells (OPCs) [24, 39]. Remyelination occurs as OPCs proliferate, migrate to the lesion, and differentiate into mature oligodendrocytes, forming compact myelin sheaths [39, 40]. The new myelin is recognizable histologically and is thinner and paler than normal CNS myelin [24, 39]. Following demyelination, astrocytes produce increased levels of endogenous neurotrophic factors such as brain-derived neurotrophic factor (BDNF), which plays a key role in neuronal and axonal survival and mediates axonal protection [41]. In addition to providing support, astrocytes may also contribute to disease pathogenesis, the different roles depending on lesion stage [42, 43]. Astrogliosis and glial scar formation can also limit existing damage [44]; the glial scar forms a barrier that isolates the damage, protects healthy tissue from a cascading wave of uncontrolled tissue damage, and stimulates revascularization, which increases the nutritional, metabolic, and neurotrophic support to the nervous tissue around the site of injury [44].
Remyelination can fail over time. In chronic demyelinated lesions, significant myelin repair is lacking, despite an abundance of OPCs in the region; this may be caused by arrested oligodendroglial differentiation [40, 45–47]. Inflammatory cytokines (such as interleukin [IL]-2 and transforming growth factor-β) have the potential to inhibit the differentiation of OPCs into oligodendrocytes [48]. Repeated bouts of demyelination may exhaust the limited pool of OPCs [49], and areas of remyelination may be more vulnerable to new demyelination compared with NAWM [24]. Nonetheless, OPCs that persist in a chronic non-permissive lesion have the capacity to myelinate, in the appropriate local environment [46]. As well as acting as a physical barrier to OPCs [42], the cells of glial scars secrete several molecules that have a potent inhibitory effect on axon growth, including chondroitin sulfate proteoglycans and semaphorins [44]. The gradual and sustained accumulation of these inhibitory factors and loss of OPC may explain why remyelination is more likely to fail with increased lesion age.
Fingolimod Influences Central Nervous System (CNS) Pathogenesis in MS Through Differential Effects on Lymphocyte Trafficking and Function
Fingolimod affects the trafficking of T-cell subsets between lymph nodes and the blood, with a specific effect on chemokine receptor (CCR)-7-positive lymphocytes [1]. Migration is dependent on S1P1, expressed on the surface of CCR7-positive cells [2]. Specifically, by inducing internalization of S1P1, functionally blocking the S1P signaling pathway, fingolimod reduces the numbers of CCR7-positive naïve T cells and central memory T cells in blood. In contrast, CCR7-negative effector and effector memory T-cell subsets do not regularly recirculate between lymphoid tissues and therefore remain in blood. Upon restimulation in vitro, these blood T cells display a reduced potential to proliferate [1, 2, 50].
Analysis of circulating cluster of differentiation (CD)-8+ T cells in fingolimod-treated patients shows persistence of late effector memory cells, with reduced expression of CCR2 compared with early effector memory cells [51]. The late effector memory cells have a reduced capacity to respond to chemokine signals that would lead to recruitment of these cells into tissues, including the CNS.
Importantly, a complex inter-relationship exists between immune cells in MS, particularly T- and B-cell subtypes. A 12-month study in patients treated with fingolimod demonstrated a relative change in the proportions of T and B cells and their expression of functional molecules [16, 17]. These data were consistent with earlier observations of fingolimod effects on T-cell subtypes, showing elevated proportions of memory and regulatory T cells and reductions of naïve conventional and regulatory T cells in peripheral blood. The proportion of memory B cells in the peripheral blood was decreased, while the proportion of naive B cells was increased during fingolimod treatment [16].
Fingolimod treatment increases the proportion of IL-10-producing regulatory B cells in the blood of MS patients [17], and these changes correlate with disease stability during treatment. This is an important observation because regulatory B cells may have protective functions in autoimmune diseases. Fingolimod also enhanced regulatory B-cell migration across brain endothelium in an in vitro model of the BBB and reduced naïve and memory B cell counts in the cerebrospinal fluid (CSF) to control levels while retaining regulatory B cell numbers [17].
NK cells may also be involved in immunoregulation in MS. Changes during treatment are of particular interest from a therapeutic point of view. Fingolimod can increase NK-cell numbers in the CSF, but inconsistent results have been reported on total NK cells that are in circulation [18, 19]. However, fingolimod decreases the proportion of circulating CD56brightCD62L+ NK cells, without changing production of interferon-γ, tumor necrosis factor (TNF)-α, and IL-10 [19]. A large proportion of NK cells resident in lymph nodes display an immature CD56brightCD62L+ phenotype, suggesting that fingolimod blocks egress of immature NK cells from the lymph nodes [52, 53].
Fingolimod Enters the CNS and Directly Influences CNS Pathogenesis in MS
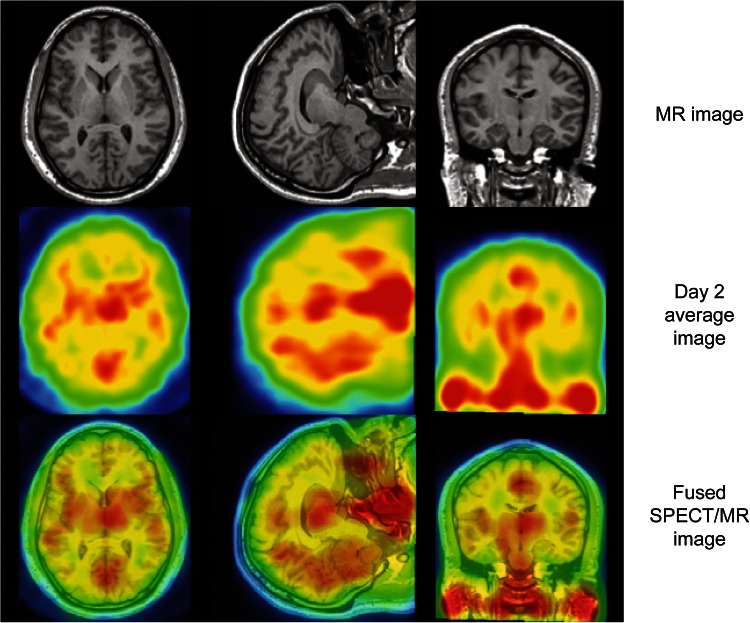
Radiolabeled fingolimod crosses the BBB and accumulates in the white matter of the CNS [54]. In humans, following intravenous injection, a radiolabeled fingolimod analog entered the brain, with uptake steadily increasing up to 26 h after dosing (Fig. 1) [48, 55, 56].
Fig. 1.
Distribution of the fingolimod analog, iodine-123-labelled BZM055, in the human brain. MRI, mean SPECT image on day 2, and fused SPECT/MRI for a representative patient, depicting the brain uptake of the fingolimod analog [123I]BZM055. Chromatic changes indicate the range of no or low BZM055 uptake (blue/green), to moderate or high (yellow/red) uptake. Note that deep nuclei appear to exhibit the highest BZM055 uptake, however, there was no evidence of higher uptake in white matter relative to grey matter. Reproduced from Tamagnan et al. [55], with permission. MRI magnetic resonance imaging, SPECT single-photon emission computed tomography
Fingolimod itself is lipophilic; activated fingolimod phosphate is a charged ester and is not lipophilic. These two forms are in equilibrium [1, 57]. Fingolimod phosphate can be formed from fingolimod in the CNS [54], consistent with evidence that the brain contains endogenous sphingosine kinase 2, the primary enzyme that phosphorylates fingolimod [58].
S1P receptors are widely distributed across multiple human organs and systems [59]. They are expressed on most CNS cells, particularly glia and neurones [2, 3]. S1P promotes neuronal growth and function and has been linked with enhanced neurone proliferation, neurite outgrowth and survival, as well as excitability induced by nerve growth factor [60–64]. Various lines of evidence support a neuroprotective role for receptor-mediated S1P signaling. In vivo, neural stem/progenitor cells migrate towards a site of CNS injury in response to locally elevated S1P concentrations [2, 65]. In vitro, S1P induces activation and proliferation of astrocytes, which parallels S1P-induced astrogliosis in vivo [2, 66].
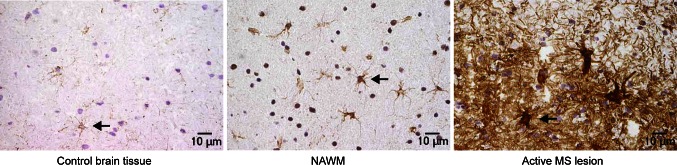
The endogenous activities of S1P in the CNS and the therapeutic effects of fingolimod suggest that S1P signaling could be involved in the pathology of MS. Alterations in S1P biology do occur in the CNS of people with MS. In a study of 40 treatment-naïve patients with MS and 26 control subjects, mean S1P concentrations in CSF were significantly higher in those with MS than in controls (2.2 ± 2.7 nM vs. 0.69 ± 1.1 nM; p < 0.01); this difference was not observed in blood samples from the two groups [67]. Furthermore, a surprisingly high and significant positive correlation was found between CSF S1P levels and disability, measured by the Expanded Disability Status Scale (r = 0.5867; p < 0.001) [67]. Immunohistochemical studies have shown that S1P receptor expression levels and downstream signaling pathways are disrupted in MS. Levels of S1P1, S1P3, and S1P5 are altered in MS lesions compared with NAWM (Fig. 2) [68, 69]. These markers could simply reflect CNS damage. More telling is the increase in S1P1 expression on astrocytes in active and inactive MS lesions [68]. S1P1 on astrocytes may therefore have an early and persistent involvement in MS pathology. Blockade of S1P1 on reactive astrocytes by fingolimod could minimize damage by reducing the secretion of pro-inflammatory chemokines such as monocyte chemoattractant protein (MCP-1) [68].
Fig. 2.
Immunohistochemical staining for S1P1. Compared with control brain tissue, S1P1 staining on astrocytes was upregulated in NAWM in MS, and to the greatest extent in reactive astrocytes in active MS lesions. MS multiple sclerosis, NAWM normal-appearing white matter, S1P sphingosine 1-phosphate. Reproduced from Van Doorn et al. [68], with permission
S1P5 expression levels on cells are also altered in MS. S1P5 is localized to myelinated areas in the CNS. In demyelinated MS lesions, global reductions in S1P5 immunoreactivity are similar to the levels of myelin loss [69]. S1P5 plays a complex role in the regulation of myelinating processes, mediating signaling via G-protein-coupled pathways to regulate oligodendrocyte cell survival or process retraction, depending on the developmental stage of the cell [70]. In vitro, fingolimod can enhance remyelination primarily via S1P5 signaling [71].
Fingolimod Acts Centrally in Animal Models
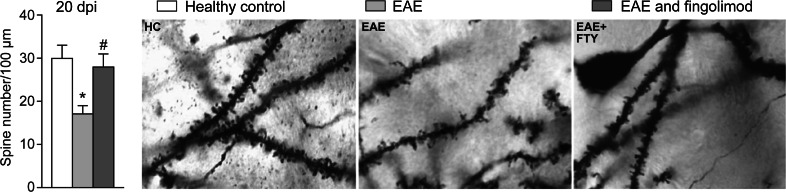
The therapeutic effects of fingolimod have been investigated using experimental autoimmune encephalomyelitis (EAE), an animal model for MS, and some of the findings support direct central activity. In a study using the Dark Agouti rat model, rescue therapy with fingolimod within the first 4–6 weeks of EAE onset was effective, with reversal of BBB permeability, reduced demyelination, decreased paralysis, and normalization of neurologic function [72, 73]. The glutamate hypothesis of damage in MS suggests excessive signaling at glutamate synapses is an intermediate step between inflammation and neurodegeneration in EAE and in MS [74, 75]. In murine EAE, fingolimod prevented and reversed pathological pre- and postsynaptic alterations in glutamate transmission, with a significant reduction in neuronal dendritic pathology (Fig. 3) [74]. These changes were associated with amelioration of the clinical deterioration normally observed in these mice.
Fig. 3.
Effect of fingolimod on dendritic spine loss in a mouse model of EAE. Prophylactic fingolimod treatment reduced the dendritic spine loss observed during the acute phase of EAE (expressed as spine density: number per 100 μm). Examples of single-section Golgi preparations from healthy control mice (n = 5), untreated EAE mice (n = 5), and prophylactic fingolimod-treated EAE mice (n = 5) at 20 dpi. dpi dots per inch, EAE experimental autoimmune encephalomyelitis, FTY fingolimod, HC healthy control. Reproduced from Rossi et al. [74], with permission. *p < 0.05 vs. control; # p < 0.05 vs. untreated EAE
Data generated from EAE animals are of particular interest owing to the ability to separate central mechanisms from effects that are dependent on lymphocyte trafficking. Choi et al. [76] examined conditional null mouse mutants lacking S1P1 in CNS cell lineages. In all mice with the CNS mutation, wild-type lymphocyte trafficking was normal and responded normally to fingolimod. However, EAE was attenuated in mouse mutants lacking S1P1 on astrocytes, with reduced levels of astrogliosis, demyelination, and axonal damage compared with wild-type EAE mice. Fingolimod efficacy was also lost in these mutant animals. Thus, the benefit of fingolimod treatment in EAE may partly rely on direct S1P1-mediated effects on astrocytes in the CNS and cannot be fully accounted for by its peripheral immunologic effects. In support, direct infusion of fingolimod into the brains of rats with EAE decreased disease severity in the absence of peripheral lymphocyte depletion [77]. Improvement that is independent of a reduction in circulating lymphocytes suggests involvement of additional mechanisms of action within the CNS [77–79].
Fingolimod Promotes Myelin Integrity
Treatment with fingolimod supports a normal state of myelination by protecting against demyelination and promoting remyelination during in vivo and in vitro animal studies.
Protection Against Demyelination
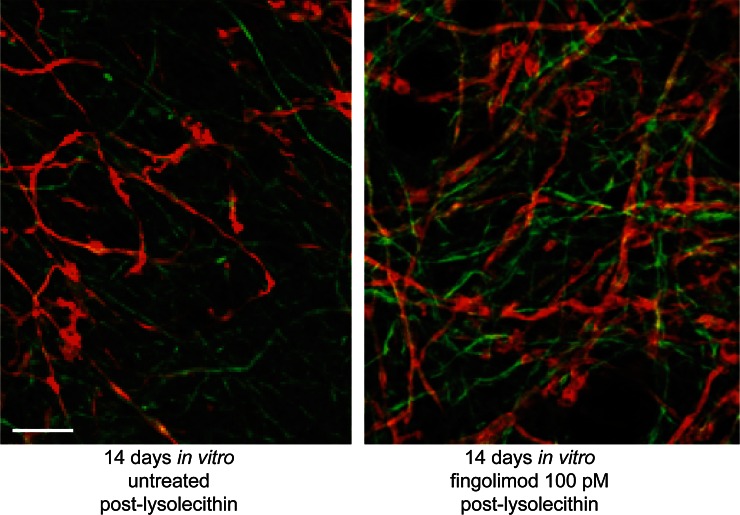
A study using an established focal model of MS in rats—the delayed-type hypersensitivity (DTH) model—showed that fingolimod protected against demyelination behind an intact BBB independent of lymphocyte infiltration [80]. In the cuprizone model of non-inflammatory demyelination in mice, fingolimod protected against demyelination independent of T-cell activity [81] and also attenuated splenocyte-induced demyelination and pro-inflammatory cytokine release [82]. Finally, in rat organotypic cerebellar slice cultures, fingolimod inhibited lysolecithin (LPC)-induced (non-immune-mediated) demyelination (Fig. 4) [71, 83].
Fig. 4.
Recovery of myelin in non-immune-mediated demyelinated cultures. Representative images of demyelinated slices, control or treated with fingolimod (100 pM) for 14 days in vitro post-lysolecithin, immunostained against myelin (MBP; red) and axons (NFM; green). Fingolimod increases the amount of myelin associated with axons compared with untreated control. Scale bar 20 µm. MBP myelin basic protein, NFM neurofilament. Reproduced from Miron et al. [71], with permission
Enhancement of Remyelination
Fingolimod stimulated remyelination and reduced axonal loss in the cuprizone model of chronic demyelination in mice [84]. Fingolimod also enhanced myelin recovery in two in vitro studies utilizing the LPC-induced model of demyelination, suggesting that fingolimod may at least directly affect the speed at which remyelination occurs (Fig. 4) [71, 84, 85]. Physiological doses of fingolimod phosphate applied to demyelinated organotypic cerebellar slice cultures led to enhanced remyelination and increased process extension of OPC and mature oligodendrocytes, mediated via S1P5 [71]. However, the effects of fingolimod on remyelination are not consistent across all studies [86, 87], perhaps because of methodological differences between the studies, such as the duration of cuprizone administration, which may have induced either acute or chronic demyelination, treatment duration, and use of supratherapeutic doses of fingolimod known to have deleterious effects on cell survival [26, 75, 87, 88].
Overall, the effects of fingolimod on demyelination and putative promotion of endogenous remyelination suggest a protective effect of fingolimod that is independent of effects on peripheral lymphocytes.
Fingolimod Exerts Effects on Each of the Key Neural Cells Involved in MS Pathology
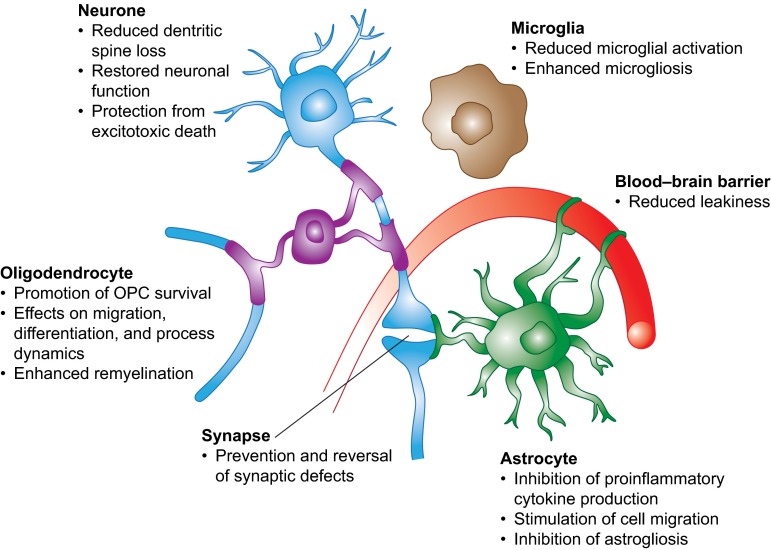
S1P receptors are widely distributed within the CNS, and S1P-mediated signaling has been reported for astrocytes, neurones, oligodendrocytes, and microglia. Fingolimod affects each of these cells in ways relevant to MS pathology. These effects are summarized in Fig. 5.
Fig. 5.
Summary of the effects of fingolimod treatment on different cells in the central nervous system. OPC oligodendrocyte precursor cell. Reproduced from Groves et al. [60], with permission
Astrocytes
Astrocytes are involved in many structural, metabolic, and immunoregulatory functions within the CNS [89]. Fingolimod has effects on astrocytes both in vivo and in vitro [27, 72, 76, 80, 90–93], and their activation is associated with IL-17, an important mediator in MS pathology [42, 43, 94]. In post mortem brain tissue of patients with MS, reactive astrocytes are the primary source of elevated ceramide production in lesions [27]. Fingolimod treatment reduced generation of this pro-inflammatory lipid, which can induce BBB dysfunction and lead to infiltration of immune cells into the brain. Consistent with this, supernatants derived from reactive astrocytes treated with fingolimod significantly reduced transendothelial monocyte migration [27]. This ability of fingolimod to reduce the pro-inflammatory state of astrocytes is supported by other in vitro data. Phosphorylated fingolimod has an anti-inflammatory action on human astrocytes via restriction of the secretion of pro-inflammatory cytokines, and reactive astrocytes—either in MS lesions or cultured under pro-inflammatory conditions—also strongly enhance the expression of S1P1 [68]. A second in vitro study reported down-regulation of pro-inflammatory, pro-apoptotic, and B-cell-promoting proteins in astrocytes in response to fingolimod, as well as up-regulation of factors with neurotrophic potential, such as BDNF and nerve growth factor [95].
The EAE model gives the astrocyte a central role in CNS pathology as well as identifying these cells as the likely proximate cellular target of fingolimod action [72, 76, 92]. In a transgenic mouse study, astrocytes were the main cell type activated during EAE, and this activation was blocked by fingolimod [91]. In a separate study, fingolimod at different times significantly ameliorated murine EAE and significantly reduced astrogliosis [92].
A prominent, though still poorly understood, feature of MS is reactive astrogliosis [96]. Mouse mutants with targeted deletion of astrocyte S1P1 had markedly reduced levels of astrogliosis compared with wild-type EAE animals [76]. Fingolimod treatment and deleting S1P1 on astrocytes had similar effects. Astrocytes are activated by IL-17 [94]. By reducing pro-inflammatory T cells (Th17) that produce IL-17 [97], fingolimod may also contribute to less activation of astrocytes. Fingolimod also reduced astrogliosis in a mouse model of thrombotic stroke [90, 94].
Endothelial cells are also a major component of the BBB, and penetration of immune cells into the CNS across endothelial cells of the BBB is a critical event in the pathogenesis of MS [60, 98]. Cellular interactions between endothelial cells and astrocytes are essential in maintaining normal BBB function [98]. Both astrocytes and endothelial cells express S1P receptors and so fingolimod may have effects on endothelial cells and on astrocytes to influence BBB integrity and function. Fingolimod may increase the ability of endothelial cells to survive cytokine challenge via S1P1 receptors, by a mechanism that is still to be fully elucidated, while inducing granulocyte macrophage colony-stimulating factor (GM-CSF) release from astrocytes to further increase BBB stability [98].
Neurones
S1P receptors appear to have a role in neurogenesis; it would therefore be reasonable to expect fingolimod to promote this process. Animal evidence also supports the action of fingolimod in improving neuronal function and neuroprotection. When investigating the effect of fingolimod on hippocampal neurogenesis in vivo in adult and aging mice, fingolimod treatment significantly increased the number of neural stem cells (NSCs) in the dentate gyrus region, possibly via BDNF [99]. This finding is consistent with the observation that, in non-MS models, fingolimod increased levels of BDNF in cultured cortical neurones in vitro [100, 101] and in rat and mouse hippocampus, cortex, and striatum in vivo [100, 102]. Evidence suggests that fingolimod also protects neurones against excitotoxic cell death [103].
It is noteworthy that in the study described above of conditional null mouse mutants lacking S1P1 in CNS cell lineages, the effects upon EAE of eliminating S1P1 from astrocytes were not seen when S1P1 was removed from neurones [76]. This supports direct CNS neuroprotection, inhibiting demyelination through modulation of astrocyte S1P1.
Oligodendrocytes
Mature oligodendrocytes, the myelin-producing cells of the CNS, together with their migratory OPC precursors, are additional therapeutic targets for fingolimod. Demyelination and the failure of remyelination by oligodendrocytes contribute to progression of disease in MS. Cells of oligodendrocyte lineage can respond to fingolimod owing to expression of S1P1,3,5 [71, 104, 105]. In vitro analysis of oligodendrocytes derived from adult human brain tissue revealed that membrane dynamics and survival responses are affected by fingolimod in a dose- and treatment duration-dependent manner [104]. Chronic treatment with fingolimod at micromolar levels induced process extension in OPCs, mediated via the ERK1/2 pathway [105]. In vitro daily treatment with fingolimod enhanced the differentiation and the axonal ensheathment capacity of OPCs co-cultured with rat dorsal root ganglia neurones, predominantly via effects on S1P1 and possibly S1P5 [106, 107]. Combined with the aforementioned diverse effects of fingolimod on reducing demyelination and enhancing remyelination, this suggests that enhancing the myelinating potential of OPCs may potentially promote tissue repair and functional recovery in MS.
Microglia
Microglia are resident immunocompetent and weak antigen-presenting CNS cells with the capacity to transform from a ‘resting’ ramified phenotype to an ‘activated’ macrophage phenotype following appropriate stimulation [108]. Their primary role is to maintain cellular, synaptic, and myelin homeostasis during the normal function of the CNS and in response to CNS injury [109]. Pro-inflammatory activated microglia may be involved in the development and expansion of MS [110]. They can regulate T-cell functions by promoting T-cell activation and differentiation towards Th1 and Th17 pathogenic subtypes, release pro-inflammatory cytokines such as TNF-α, impair the function of OPCs and NSCs, and also induce synaptic deficits, both of the latter processes reportedly via TNF-α [110]. Microglia have a substantial role in MS pathogenesis. In vivo, fingolimod reduces microglial activation in the DTH model of MS [80, 111, 112]. In vitro, fingolimod down-regulates activated microglial production of pro-inflammatory cytokines (such as TNF-α, IL-1β, and IL-6), and up-regulates microglial production of BDNF and glial cell-derived neurotrophic factor [113]. However, there are differences in the effects of fingolimod on brain-resident microglia and blood-derived monocytes [114]. Microglia in human CNS tissue express a pattern of S1P receptors similar to mature dendritic cells and macrophages but distinct from that seen in circulating monocytes. The myeloid cell populations present in the human CNS exhibit varying responses and intracellular signaling responses to fingolimod under inflammatory conditions [114]. Such varied effects on infiltrating and endogenous myeloid cells might be able to modify the inflammatory environment within the CNS.
Clinical Use of Fingolimod: Immunosurveillance and Considerations when Switching Treatments
Immunocompetent regulatory and memory cells are free to circulate throughout the body during fingolimod treatment, so differential effects on immune cell subtypes are also relevant to the maintenance of immunosurveillance and for patient safety monitoring [4]. The relative sparing of effector memory cells and the preserved function of cells sequestered in lymph nodes suggest that key features of the immune system may be maintained during fingolimod therapy [115, 116]. Careful observation for signs and symptoms of serious infections is certainly necessary during fingolimod treatment [4, 115, 116].
The temporal changes in relative immune cell number and function should be considered when switching between fingolimod and other DMTs [4]. The mode of action and duration of both fingolimod and prior or subsequently prescribed immune-modulating or immunosuppressive drugs need to be considered to avoid unintended additive effects. Recent studies have focused specifically on the appropriate washout period in patients switching from natalizumab to fingolimod [117–119]. These have recommended 8–12 weeks or even shorter intervals (4–8 weeks) to reduce the likelihood of magnetic resonance imaging (MRI) or clinical disease reactivation, with careful consideration given to the risk of additive effects on the immune system in the first months following treatment change because of the long elimination half-life of natalizumab [117–119].
Vigilance for serious infections, including progressive multifocal leukoencephalopathy (PML), is warranted, and should not be restricted only to patients who have previously undergone treatment with natalizumab [4]. The emergence of PML, after 5 years of post-marketing surveillance, suggests that under rare circumstances, the immune effects of fingolimod may be associated with reduced immunosurveillance towards the JC virus. US label guidelines state that, at the first sign or symptom suggestive of PML, fingolimod should be withheld and an appropriate diagnostic evaluation should be conducted [4]. Particular attention should be given to those patients with risk factors for opportunistic infection, such as advanced age, and previous or concomitant use of immunosuppressant drugs.
Conclusions
Taken together, three fundamental factors support direct activity of fingolimod within the CNS of patients with MS. First, the lipophilic fingolimod molecule readily crosses the BBB into the CNS and accumulates in myelin [54, 55]. Second, S1P1,3,4,5 are expressed throughout the CNS, in neurones, astrocytes, oligodendrocytes, and microglia [2]. Third, the endogenous processes relevant to MS pathology, such as astrogliosis and demyelination, are mediated by S1P signaling in the CNS.
Fingolimod phosphate, the active metabolite of fingolimod, regulates signaling in second messenger pathways for both pro-inflammatory and trophic factors, which are downstream of plasma membrane and myelin sphingomyelin (and ceramide) breakdown. This pathway may unify, via a common mechanism, the central and peripheral actions of fingolimod, for example, the peripheral (S1PR-mediated lymphocyte egress) as well as CNS trophic signaling and decreased astro- and microglial activation [20]. Given the widespread distribution of sphingomyelin and its enrichment in the CNS, an important action of fingolimod directly in the CNS would not be unexpected and is supported by the evidence described in this review.
Molecular and animal evidence support a direct S1P1-mediated, favorable action of fingolimod in the CNS. In vivo and in vitro studies indicate that fingolimod can help preserve the integrity and function of multiple neural cells involved in MS. Fingolimod protects against demyelination, axonal loss, dendritic loss, and astrogliosis, may enhance remyelination, and can enhance the proliferation and survival of neural cells.
Clinical studies suggest that the benefits of fingolimod may be in part due to a direct action on the CNS. Fingolimod has an early and sustained impact on brain atrophy, suggesting an effect on diffuse as well as focal damage [6–11, 26]. In the FREEDOMS study, fingolimod had beneficial effects on brain atrophy within 6 months of treatment versus placebo [7] that were maintained over the 2-year study, irrespective of the presence of Gd-enhancing inflammatory lesions [9]. Fingolimod had a sustained impact on atrophy for up to 4 years in the FREEDOMS extension study and for up to 7 years in a phase II study [46, 120]. Brain atrophy occurs early in the course of MS [33] and ranges from 0.5 to 1.35 % per year in patients with RRMS, compared with the normal age-related deterioration in healthy individuals of 0.1–0.3 % per year [32]. Brain atrophy correlates with clinical impairment, leading to suggestions that irreversible tissue loss may prove to be more closely associated with disease progression than conventional MRI assessments of lesion activity and load [28]. Therefore, the rate of brain volume loss (BVL) may be a useful clinical assessment of neurodegeneration in MS [34, 121–123].
In the placebo-controlled study of fingolimod in patients with primary progressive MS (PPMS, INFORMS), there was no effect of treatment on the risk of disability progression confirmed at 3 months or on the rate of BVL [124, 125]. Mean percent brain volume changes from baseline to final MRI were (±standard deviation [SD]) 1.34 ± 1.22 for fingolimod versus 1.42 ± 1.27 for placebo (p = 0.707). Although the overall level of inflammatory activity in the PPMS population was low, fingolimod reduced the number of Gd-enhancing T1 lesions by 78 % and of new/newly enlarging T2 lesions by 73 % (both p < 0.001) [124, 125]. The implications of these data in the context of the CNS effects of fingolimod are not yet clear, but differences in underlying disease processes between RRMS and PPMS mean that distinct, targeted therapeutic strategies may be required for the progressive and relapsing forms of MS.
There is a failure of the CNS repair mechanisms to compensate for worsening neurodegeneration, and this failure is superimposed on inflammatory damage. Evidence summarized here shows that fingolimod has the potential to reduce inflammation both within the periphery as well as in the CNS and to promote neuroprotection and repair of the glio-axonal unit directly. It may redress the balance between damage and repair, two key factors determining MS disability.
Acknowledgments
This article represents the opinion of the authors in their respective domain of expertise. The authors interpreted the literature, critically revised content for important intellectual aspects, and approved the content for publication. Oxford Pharmagenesis conducted literature searches and provided technical writing and editorial support. Funding support and review of the accuracy of scientific data were provided by Novartis Pharmaceuticals Corporation, USA.
Compliance with Ethical Standards
Funding
The authors received no funding for the preparation of this review. Funding for medical writing support and review of the accuracy of scientific data was provided by Novartis Pharmaceuticals Corporation, USA. The open access fees were paid by Novartis Pharmaceuticals Corporation, USA.
Conflict of interest
Samuel F. Hunter has received consulting fees and/or research support from and/or served on speakers’ bureaus for Acorda, Avanir, Bayer, Biogen Idec, Eli Lilly, Genzyme, Novartis, Osmotica, Roche, and Teva.
James D. Bowen has received consulting fees, speaker fees, and/or research support from Acorda, Bayer, Biogen Idec, Genzyme Corporation, GlaxoSmithKline, Medimmune, Novartis, Osmotica, Pfizer/EMD Serono, Roche, SanofiAventis, Synthon, Teva, Vaccinex, and Xenoport, and is an Amgen shareholder.
Anthony T. Reder has received consulting fees from or has been an advisory board member for Abbott Laboratories, Accorda, Astra/Merck, Athena Neurosciences, Bayer, BioMS Medical Corp, Biogen Idec, Blue Cross Blue Shield, Boehringer Ingelheim, Caremark, Cephalon, Connetics (Connective Therapeutics), Elan, Eli Lilly, Genentech, Genzyme, GlaxoSmithKline, Hoffmann-La Roche, Immunex, Neurocrine Biosciences, Novartis, Parke-Davis, Pfizer, Pharmacia & Upjohn, Questcor, Quintiles, Serono, Takeda Pharmaceuticals, and Teva Marion.
Contributor Information
Samuel F. Hunter, Phone: +1 615 791 5470, Email: sfhunter@neurosci.us
James D. Bowen, Email: James.Bowen@swedish.org
Anthony T. Reder, Email: areder@neurology.bsd.uchicago.edu
References
- 1.Brinkmann V. FTY720 (fingolimod) in multiple sclerosis: therapeutic effects in the immune and the central nervous system. Br J Pharmacol. 2009;158(5):1173–1182. doi: 10.1111/j.1476-5381.2009.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol. 2010;33(2):91–101. doi: 10.1097/WNF.0b013e3181cbf825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115(1):84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Food and Drug Administration. Gilenya US prescribing information. Revised August 2015. 2010 [cited 29 September 2015]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022527s019lbl.pdf.
- 5.European Medicines Agency. Gilenya EU summary of product characteristics, latest update of May 2015. 2011 [cited 16 June 2015]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002202/WC500104528.pdf.
- 6.Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 7.Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 8.Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(6):545–556. doi: 10.1016/S1474-4422(14)70049-3. [DOI] [PubMed] [Google Scholar]
- 9.Radue EW, O’Connor P, Polman CH, Hohlfeld R, Calabresi P, Selmaj K, et al. Impact of fingolimod therapy on magnetic resonance imaging outcomes in patients with multiple sclerosis. Arch Neurol. 2012;69(10):1259–1269. doi: 10.1001/archneurol.2012.1051. [DOI] [PubMed] [Google Scholar]
- 10.Barkhof F, de Jong R, Sfikas N, de Vera A, Francis G, Cohen J. The influence of patient demographics, disease characteristics and treatment on brain volume loss in Trial Assessing Injectable Interferon vs FTY720 Oral in Relapsing-Remitting Multiple Sclerosis (TRANSFORMS), a phase 3 study of fingolimod in multiple sclerosis. Mult Scler. 2014;20(13):1704–1713. doi: 10.1177/1352458514532317. [DOI] [PubMed] [Google Scholar]
- 11.Kappos L, Antel J, Comi G, Montalban X, O’Connor P, Polman CH, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355(11):1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 12.Radue EW, Barkhof F, Kappos L, Sprenger T, Haring DA, de Vera A, et al. Correlation between brain volume loss and clinical and MRI outcomes in multiple sclerosis. Neurology. 2015;84(8):784–793. doi: 10.1212/WNL.0000000000001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277(24):21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 14.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 15.Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol. 2012;30:69–94. doi: 10.1146/annurev-immunol-020711-075011. [DOI] [PubMed] [Google Scholar]
- 16.Claes N, Dhaeze T, Fraussen J, Broux B, Van Wijmeersch B, Stinissen P, et al. Compositional changes of B and T cell subtypes during fingolimod treatment in multiple sclerosis patients: a 12-month follow-up study. PLoS One. 2014;9(10):e111115. doi: 10.1371/journal.pone.0111115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grutzke B, Hucke S, Gross CC, Herold MV, Posevitz-Fejfar A, Wildemann BT, et al. Fingolimod treatment promotes regulatory phenotype and function of B cells. Ann Clin Transl Neurol. 2015;2(2):119–130. doi: 10.1002/acn3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kowarik MC, Pellkofer HL, Cepok S, Korn T, Kumpfel T, Buck D, et al. Differential effects of fingolimod (FTY720) on immune cells in the CSF and blood of patients with MS. Neurology. 2011;76(14):1214–1221. doi: 10.1212/WNL.0b013e3182143564. [DOI] [PubMed] [Google Scholar]
- 19.Chanvillard C, Jacolik RF, Infante-Duarte C, Nayak RC. The role of natural killer cells in multiple sclerosis and their therapeutic implications. Front Immunol. 2013;4:63. doi: 10.3389/fimmu.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jana A, Pahan K. Sphingolipids in multiple sclerosis. Neuromol Med. 2010;12(4):351–361. doi: 10.1007/s12017-010-8128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duffy SS, Lees JG, Moalem-Taylor G. The contribution of immune and glial cell types in experimental autoimmune encephalomyelitis and multiple sclerosis. Mult Scler Int. 2014;2014:285245. doi: 10.1155/2014/285245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smirniotopoulos JG, Murphy FM, Rushing EJ, Rees JH, Schroeder JW. Patterns of contrast enhancement in the brain and meninges. Radiographics. 2007;27(2):525–551. doi: 10.1148/rg.272065155. [DOI] [PubMed] [Google Scholar]
- 23.Markovic-Plese S, McFarland HF. Immunopathogenesis of the multiple sclerosis lesion. Curr Neurol Neurosci Rep. 2001;1(3):257–262. doi: 10.1007/s11910-001-0028-4. [DOI] [PubMed] [Google Scholar]
- 24.Kutzelnigg A, Lassmann H. Pathology of multiple sclerosis and related inflammatory demyelinating diseases. Handb Clin Neurol. 2014;122:15–58. doi: 10.1016/B978-0-444-52001-2.00002-9. [DOI] [PubMed] [Google Scholar]
- 25.Bruck W, Bitsch A, Kolenda H, Bruck Y, Stiefel M, Lassmann H. Inflammatory central nervous system demyelination: correlation of magnetic resonance imaging findings with lesion pathology. Ann Neurol. 1997;42(5):783–793. doi: 10.1002/ana.410420515. [DOI] [PubMed] [Google Scholar]
- 26.Sabo JK, Aumann TD, Merlo D, Kilpatrick TJ, Cate HS. Remyelination is altered by bone morphogenic protein signaling in demyelinated lesions. J Neurosci. 2011;31(12):4504–4510. doi: 10.1523/JNEUROSCI.5859-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Doorn R, Nijland PG, Dekker N, Witte ME, Lopes-Pinheiro MA, van het Hof B, et al. Fingolimod attenuates ceramide-induced blood-brain barrier dysfunction in multiple sclerosis by targeting reactive astrocytes. Acta Neuropathol. 2012;124(3):397–410. doi: 10.1007/s00401-012-1014-4. [DOI] [PubMed] [Google Scholar]
- 28.Bermel RA, Bakshi R. The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol. 2006;5(2):158–170. doi: 10.1016/S1474-4422(06)70349-0. [DOI] [PubMed] [Google Scholar]
- 29.Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128(Pt 11):2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- 30.Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132(Pt 5):1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lassmann H. Mechanisms of white matter damage in multiple sclerosis. Glia. 2014;62(11):1816–1830. doi: 10.1002/glia.22597. [DOI] [PubMed] [Google Scholar]
- 32.De Stefano N, Airas L, Grigoriadis N, Mattle HP, O’Riordan J, Oreja-Guevara C, et al. Clinical relevance of brain volume measures in multiple sclerosis. CNS Drugs. 2014;28(2):147–156. doi: 10.1007/s40263-014-0140-z. [DOI] [PubMed] [Google Scholar]
- 33.De Stefano N, Giorgio A, Battaglini M, Rovaris M, Sormani MP, Barkhof F, et al. Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology. 2010;74(23):1868–1876. doi: 10.1212/WNL.0b013e3181e24136. [DOI] [PubMed] [Google Scholar]
- 34.De Stefano N, Stromillo ML, Giorgio A, Bartolozzi ML, Battaglini M, Baldini M, et al. Establishing pathological cut-offs of brain atrophy rates in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2015 [Epub ahead of print]. doi:10.1136/jnnp-2014-309903. [DOI] [PMC free article] [PubMed]
- 35.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 36.Zeinstra E, Wilczak N, Streefland C, De Keyser J. Astrocytes in chronic active multiple sclerosis plaques express MHC class II molecules. Neuroreport. 2000;11(1):89–91. doi: 10.1097/00001756-200001170-00018. [DOI] [PubMed] [Google Scholar]
- 37.McQualter JL, Bernard CC. Multiple sclerosis: a battle between destruction and repair. J Neurochem. 2007;100(2):295–306. doi: 10.1111/j.1471-4159.2006.04232.x. [DOI] [PubMed] [Google Scholar]
- 38.Bruck W, Kuhlmann T, Stadelmann C. Remyelination in multiple sclerosis. J Neurol Sci. 2003;206(2):181–185. doi: 10.1016/S0022-510X(02)00191-0. [DOI] [PubMed] [Google Scholar]
- 39.Miron VE, Kuhlmann T, Antel JP. Cells of the oligodendroglial lineage, myelination, and remyelination. Biochim Biophys Acta. 2011;1812(2):184–193. doi: 10.1016/j.bbadis.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Boyd A, Zhang H, Williams A. Insufficient OPC migration into demyelinated lesions is a cause of poor remyelination in MS and mouse models. Acta Neuropathol. 2013;125(6):841–859. doi: 10.1007/s00401-013-1112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linker RA, Lee DH, Demir S, Wiese S, Kruse N, Siglienti I, et al. Functional role of brain-derived neurotrophic factor in neuroprotective autoimmunity: therapeutic implications in a model of multiple sclerosis. Brain. 2010;133(Pt 8):2248–2263. doi: 10.1093/brain/awq179. [DOI] [PubMed] [Google Scholar]
- 42.Nair A, Frederick TJ, Miller SD. Astrocytes in multiple sclerosis: a product of their environment. Cell Mol Life Sci. 2008;65(17):2702–2720. doi: 10.1007/s00018-008-8059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brosnan CF, Raine CS. The astrocyte in multiple sclerosis revisited. Glia. 2013;61(4):453–465. doi: 10.1002/glia.22443. [DOI] [PubMed] [Google Scholar]
- 44.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 45.Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20(17):6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ludwin SK. Oligodendrocytes from optic nerves subjected to long term Wallerian degeneration retain the capacity to myelinate. Acta Neuropathol. 1992;84(5):530–537. doi: 10.1007/BF00304472. [DOI] [PubMed] [Google Scholar]
- 47.Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346(3):165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- 48.Hunter SF, Bottenstein JE. Growth factor responses of enriched bipotential glial progenitors. Brain Res Dev Brain Res. 1990;54(2):235–248. doi: 10.1016/0165-3806(90)90146-P. [DOI] [PubMed] [Google Scholar]
- 49.Keough MB, Yong VW. Remyelination therapy for multiple sclerosis. Neurotherapeutics. 2013;10(1):44–54. doi: 10.1007/s13311-012-0152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mehling M, Brinkmann V, Antel J, Bar-Or A, Goebels N, Vedrine C, et al. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology. 2008;71(16):1261–1267. doi: 10.1212/01.wnl.0000327609.57688.ea. [DOI] [PubMed] [Google Scholar]
- 51.Johnson TA, Lapierre Y, Bar-Or A, Antel JP. Distinct properties of circulating CD8+ T cells in FTY720-treated patients with multiple sclerosis. Arch Neurol. 2010;67(12):1449–1455. doi: 10.1001/archneurol.2010.312. [DOI] [PubMed] [Google Scholar]
- 52.Mehling M, Burgener AV, Brinkmann V, Bantug GR, Dimeloe S, Hoenger G, et al. Tissue distribution dynamics of human NK cells inferred from peripheral blood depletion kinetics after sphingosine 1-phosphate receptor blockade. Scand J Immunol. 2015;82(5):460–466. doi: 10.1111/sji.12347. [DOI] [PubMed] [Google Scholar]
- 53.Johnson TA, Evans BL, Durafourt BA, Blain M, Lapierre Y, Bar-Or A, et al. Reduction of the peripheral blood CD56(bright) NK lymphocyte subset in FTY720-treated multiple sclerosis patients. J Immunol. 2011;187(1):570–579. doi: 10.4049/jimmunol.1003823. [DOI] [PubMed] [Google Scholar]
- 54.Foster CA, Howard LM, Schweitzer A, Persohn E, Hiestand PC, Balatoni B, et al. Brain penetration of the oral immunomodulatory drug FTY720 and its phosphorylation in the central nervous system during experimental autoimmune encephalomyelitis: consequences for mode of action in multiple sclerosis. J Pharmacol Exp Ther. 2007;323(2):469–475. doi: 10.1124/jpet.107.127183. [DOI] [PubMed] [Google Scholar]
- 55.Tamagnan G, Tavares A, Barret O, Alagille D, Seibyl J, Marek K, et al. Brain distribution of BZM055, an analog of fingolimod (FTY720), in human. Mult Scler. 2012;18(10 S4):[Abstract P839].
- 56.Briard E, Orain D, Beerli C, Billich A, Streiff M, Bigaud M, et al. BZM055, an iodinated radiotracer candidate for PET and SPECT imaging of myelin and FTY720 brain distribution. Chem Med Chem. 2011;6(4):667–677. doi: 10.1002/cmdc.201000477. [DOI] [PubMed] [Google Scholar]
- 57.David OJ, Kovarik JM, Schmouder RL. Clinical pharmacokinetics of fingolimod. Clin Pharmacokinet. 2012;51(1):15–28. doi: 10.2165/11596550-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 58.Billich A, Bornancin F, Devay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem. 2003;278(48):47408–47415. doi: 10.1074/jbc.M307687200. [DOI] [PubMed] [Google Scholar]
- 59.Wang C, Mao J, Redfield S, Mo Y, Lage JM, Zhou X. Systemic distribution, subcellular localization and differential expression of sphingosine-1-phosphate receptors in benign and malignant human tissues. Exp Mol Pathol. 2014;97(2):259–265. doi: 10.1016/j.yexmp.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Groves A, Kihara Y, Chun J. Fingolimod: direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J Neurol Sci. 2013;328(1–2):9–18. doi: 10.1016/j.jns.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harada J, Foley M, Moskowitz MA, Waeber C. Sphingosine-1-phosphate induces proliferation and morphological changes of neural progenitor cells. J Neurochem. 2004;88(4):1026–1039. doi: 10.1046/j.1471-4159.2003.02219.x. [DOI] [PubMed] [Google Scholar]
- 62.Edsall LC, Pirianov GG, Spiegel S. Involvement of sphingosine 1-phosphate in nerve growth factor-mediated neuronal survival and differentiation. J Neurosci. 1997;17(18):6952–6960. doi: 10.1523/JNEUROSCI.17-18-06952.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toman RE, Payne SG, Watterson KR, Maceyka M, Lee NH, Milstien S, et al. Differential transactivation of sphingosine-1-phosphate receptors modulates NGF-induced neurite extension. J Cell Biol. 2004;166(3):381–392. doi: 10.1083/jcb.200402016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang YH, Vasko MR, Nicol GD. Intracellular sphingosine 1-phosphate mediates the increased excitability produced by nerve growth factor in rat sensory neurons. J Physiol. 2006;575(Pt 1):101–113. doi: 10.1113/jphysiol.2006.111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kimura A, Ohmori T, Ohkawa R, Madoiwa S, Mimuro J, Murakami T, et al. Essential roles of sphingosine 1-phosphate/S1P1 receptor axis in the migration of neural stem cells toward a site of spinal cord injury. Stem Cells. 2007;25(1):115–124. doi: 10.1634/stemcells.2006-0223. [DOI] [PubMed] [Google Scholar]
- 66.Sorensen SD, Nicole O, Peavy RD, Montoya LM, Lee CJ, Murphy TJ, et al. Common signaling pathways link activation of murine PAR-1, LPA, and S1P receptors to proliferation of astrocytes. Mol Pharmacol. 2003;64(5):1199–1209. doi: 10.1124/mol.64.5.1199. [DOI] [PubMed] [Google Scholar]
- 67.Kulakowska A, Zendzian-Piotrowska M, Baranowski M, Kononczuk T, Drozdowski W, Gorski J, et al. Intrathecal increase of sphingosine 1-phosphate at early stage multiple sclerosis. Neurosci Lett. 2010;477(3):149–152. doi: 10.1016/j.neulet.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 68.Van Doorn R, Van Horssen J, Verzijl D, Witte M, Ronken E, Van Het Hof B, et al. Sphingosine 1-phosphate receptor 1 and 3 are upregulated in multiple sclerosis lesions. Glia. 2010;58(12):1465–1476. doi: 10.1002/glia.21021. [DOI] [PubMed] [Google Scholar]
- 69.Brana C, Frossard MJ, Pescini Gobert R, Martinier N, Boschert U, Seabrook TJ. Immunohistochemical detection of sphingosine-1-phosphate receptor 1 and 5 in human multiple sclerosis lesions. Neuropathol Appl Neurobiol. 2014;40(5):564–578. doi: 10.1111/nan.12048. [DOI] [PubMed] [Google Scholar]
- 70.Jaillard C, Harrison S, Stankoff B, Aigrot MS, Calver AR, Duddy G, et al. Edg8/S1P5: an oligodendroglial receptor with dual function on process retraction and cell survival. J Neurosci. 2005;25(6):1459–1469. doi: 10.1523/JNEUROSCI.4645-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miron VE, Ludwin SK, Darlington PJ, Jarjour AA, Soliven B, Kennedy TE, et al. Fingolimod (FTY720) enhances remyelination following demyelination of organotypic cerebellar slices. Am J Pathol. 2010;176(6):2682–2694. doi: 10.2353/ajpath.2010.091234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Foster CA, Mechtcheriakova D, Storch MK, Balatoni B, Howard LM, Bornancin F, et al. FTY720 rescue therapy in the dark agouti rat model of experimental autoimmune encephalomyelitis: expression of central nervous system genes and reversal of blood-brain-barrier damage. Brain Pathol. 2009;19(2):254–266. doi: 10.1111/j.1750-3639.2008.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Balatoni B, Storch MK, Swoboda EM, Schonborn V, Koziel A, Lambrou GN, et al. FTY720 sustains and restores neuronal function in the DA rat model of MOG-induced experimental autoimmune encephalomyelitis. Brain Res Bull. 2007;74(5):307–316. doi: 10.1016/j.brainresbull.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 74.Rossi S, Lo Giudice T, De Chiara V, Musella A, Studer V, Motta C, et al. Oral fingolimod rescues the functional deficits of synapses in experimental autoimmune encephalomyelitis. Br J Pharmacol. 2012;165(4):861–869. doi: 10.1111/j.1476-5381.2011.01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kipp M, Clarner T, Dang J, Copray S, Beyer C. The cuprizone animal model: new insights into an old story. Acta Neuropathol. 2009;118(6):723–736. doi: 10.1007/s00401-009-0591-3. [DOI] [PubMed] [Google Scholar]
- 76.Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K, et al. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci USA. 2011;108(2):751–756. doi: 10.1073/pnas.1014154108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schubart A, Seabrook T, Rausch M, Gemayel J, Hoyer D, Dev KK, et al. CNS mediated effects of FTY720 (fingolimod) in EAE. AAN Annual Meeting. 2007:Poster P07.101.
- 78.Herr DR, Chun J. Effects of LPA and S1P on the nervous system and implications for their involvement in disease. Curr Drug Targets. 2007;8(1):155–167. doi: 10.2174/138945007779315669. [DOI] [PubMed] [Google Scholar]
- 79.Webb M, Tham CS, Lin FF, Lariosa-Willingham K, Yu N, Hale J, et al. Sphingosine 1-phosphate receptor agonists attenuate relapsing-remitting experimental autoimmune encephalitis in SJL mice. J Neuroimmunol. 2004;153(1–2):108–121. doi: 10.1016/j.jneuroim.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 80.Anthony DC, Sibson NR, Losey P, Meier DP, Leppert D. Investigation of immune and CNS-mediated effects of fingolimod in the focal delayed-type hypersensitivity multiple sclerosis model. Neuropharmacology. 2014;79:534–541. doi: 10.1016/j.neuropharm.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 81.Kim HJ, Miron VE, Dukala D, Proia RL, Ludwin SK, Traka M, et al. Neurobiological effects of sphingosine 1-phosphate receptor modulation in the cuprizone model. FASEB J. 2011;25(5):1509–1518. doi: 10.1096/fj.10-173203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pritchard AJ, Mir AK, Dev KK. Fingolimod attenuates splenocyte-induced demyelination in cerebellar slice cultures. PLoS One. 2014;9(6):e99444. doi: 10.1371/journal.pone.0099444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sheridan GK, Dev KK. S1P1 receptor subtype inhibits demyelination and regulates chemokine release in cerebellar slice cultures. Glia. 2012;60(3):382–392. doi: 10.1002/glia.22272. [DOI] [PubMed] [Google Scholar]
- 84.Slowik A, Schmidt T, Beyer C, Amor S, Clarner T, Kipp M. The sphingosine 1-phosphate receptor agonist FTY720 is neuroprotective after cuprizone-induced CNS demyelination. Br J Pharmacol. 2015;172(1):80–92. doi: 10.1111/bph.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jackson SJ, Giovannoni G, Baker D. Fingolimod modulates microglial activation to augment markers of remyelination. J Neuroinflamm. 2011;8:76. doi: 10.1186/1742-2094-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Soares de Souza A, Savinainen A, Chang R, Crandall T, Quattrini A, Podini P, et al. ONO-4641 significantly increased myelin content in the cuprizone model of demyelination. Mult Scler. 2013;19(11 S1):[Abstract P1030].
- 87.Hu Y, Lee X, Ji B, Guckian K, Apicco D, Pepinsky RB, et al. Sphingosine 1-phosphate receptor modulator fingolimod (FTY720) does not promote remyelination in vivo. Mol Cell Neurosci. 2011;48(1):72–81. doi: 10.1016/j.mcn.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 88.Acs P, Kipp M, Norkute A, Johann S, Clarner T, Braun A, et al. 17beta-estradiol and progesterone prevent cuprizone provoked demyelination of corpus callosum in male mice. Glia. 2009;57(8):807–814. doi: 10.1002/glia.20806. [DOI] [PubMed] [Google Scholar]
- 89.Markiewicz I, Lukomska B. The role of astrocytes in the physiology and pathology of the central nervous system. Acta Neurobiol Exp (Wars) 2006;66(4):343–358. doi: 10.55782/ane-2006-1623. [DOI] [PubMed] [Google Scholar]
- 90.Brunkhorst R, Kanaan N, Koch A, Ferreiros N, Mirceska A, Zeiner P, et al. FTY720 treatment in the convalescence period improves functional recovery and reduces reactive astrogliosis in photothrombotic stroke. PLoS One. 2013;8(7):e70124. doi: 10.1371/journal.pone.0070124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Groves A, Kihara Y, Rivera R, Mayford M, Chun J. A new reporter mouse for CNS cellular activity selectively identifies astrocyte activation produced by experimental autoimmune encephalomyelitis. Mult Scler. 2013;19(11 S1):[Abstract P576].
- 92.Huhn K, Brecht L, Linker R, Lee D. Fingolimod modulates astroglial glutamate metabolism in autoimmune demyelination. Mult Scler. 2013;19 (11 S1):[Abstract P817].
- 93.Wu C, Leong SY, Moore CS, Cui QL, Gris P, Bernier LP, et al. Dual effects of daily FTY720 on human astrocytes in vitro: relevance for neuroinflammation. J Neuroinflamm. 2013;10:41. doi: 10.1186/1742-2094-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kang Z, Altuntas CZ, Gulen MF, Liu C, Giltiay N, Qin H, et al. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity. 2010;32(3):414–425. doi: 10.1016/j.immuni.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hoffmann F, Hofereiter J, Rübsamen H, Faber H, Weber P, Pütz B, et al. Effects of fingolimod treatment on astrocyte functions. Mult Scler. 2013;19(11 S1):[Abstract P575].
- 96.Moreno M, Guo F, Mills Ko E, Bannerman P, Soulika A, Pleasure D. Origins and significance of astrogliosis in the multiple sclerosis model, MOG peptide EAE. J Neurol Sci. 2013;333(1–2):55–59. doi: 10.1016/j.jns.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9(11):883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 98.Spampinato SF, Obermeier B, Cotleur A, Love A, Takeshita Y, Sano Y, et al. Sphingosine 1-phosphate at the blood–brain barrier: can the modulation of S1P receptor 1 influence the response of endothelial cells and astrocytes to inflammatory stimuli? PLoS One. 2015;10(7):e0133392. doi: 10.1371/journal.pone.0133392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Efstathopoulos P, Charalampopoulos I, Gravanis A. Neurogenic effects of fingolimod in mice. Mult Scler. 2012;18(10 S4):[Abstract P501].
- 100.Deogracias R, Yazdani M, Dekkers MP, Guy J, Ionescu MC, Vogt KE, et al. Fingolimod, a sphingosine-1 phosphate receptor modulator, increases BDNF levels and improves symptoms of a mouse model of Rett syndrome. Proc Natl Acad Sci USA. 2012;109(35):14230–14235. doi: 10.1073/pnas.1206093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Doi Y, Takeuchi H, Horiuchi H, Hanyu T, Kawanokuchi J, Jin S, et al. Fingolimod phosphate attenuates oligomeric amyloid beta-induced neurotoxicity via increased brain-derived neurotrophic factor expression in neurons. PLoS One. 2013;8(4):e61988. doi: 10.1371/journal.pone.0061988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fukumoto K, Mizoguchi H, Takeuchi H, Horiuchi H, Kawanokuchi J, Jin S, et al. Fingolimod increases brain-derived neurotrophic factor levels and ameliorates amyloid beta-induced memory impairment. Behav Brain Res. 2014;268:88–93. doi: 10.1016/j.bbr.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 103.Di Menna L, Molinaro G, Di Nuzzo L, Riozzi B, Zappulla C, Pozzilli C, et al. Fingolimod protects cultured cortical neurons against excitotoxic death. Pharmacol Res. 2013;67(1):1–9. doi: 10.1016/j.phrs.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 104.Miron VE, Hall JA, Kennedy TE, Soliven B, Antel JP. Cyclical and dose-dependent responses of adult human mature oligodendrocytes to fingolimod. Am J Pathol. 2008;173(4):1143–1152. doi: 10.2353/ajpath.2008.080478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miron VE, Jung CG, Kim HJ, Kennedy TE, Soliven B, Antel JP. FTY720 modulates human oligodendrocyte progenitor process extension and survival. Ann Neurol. 2008;63(1):61–71. doi: 10.1002/ana.21227. [DOI] [PubMed] [Google Scholar]
- 106.Cui QL, Fang J, Kennedy T, Almazan G, Antel J. Effects of S1P receptor modulator FTY720 on human oligodendrocyte progenitor cell differentiation. Neurology. 2013;80(1):[Abstract P05.150].
- 107.Cui QL, Fang J, Almazan G, Antel J. Sphingosine 1-phosphate receptor agonists promote axonal ensheathment by human fetal oligodendrocyte progenitors. Mult Scler. 2011;17(10 S1):[Abstract P823].
- 108.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91(2):461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 109.Benarroch EE. Microglia: multiple roles in surveillance, circuit shaping, and response to injury. Neurology. 2013;81(12):1079–1088. doi: 10.1212/WNL.0b013e3182a4a577. [DOI] [PubMed] [Google Scholar]
- 110.Gao Z, Tsirka SE. Animal models of MS reveal multiple roles of microglia in disease pathogenesis. Neurol Res Int. 2011;2011:383087. doi: 10.1155/2011/383087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Leppert D, Campbell S, Seneca N, Warren E, Balazs M, Anthony D. Anti-CD20 therapy reduces microglial activation and lesion volume in focal models of pattern I and pattern II multiple sclerosis. Neurology. 2012;78(1):[Abstract P02.085].
- 112.Airas L, Dickens A, Haaparanta-Solin M, Anthony D, Rinne J. In vivo micro-PET imaging demonstrates diminished microglial activation after FTY720 treatment in an animal model of multiple sclerosis. Neurology. 2013;80(1):[Abstract P05.151]. [DOI] [PubMed]
- 113.Noda H, Takeuchi H, Mizuno T, Suzumura A. Fingolimod phosphate promotes the neuroprotective effects of microglia. J Neuroimmunol. 2013;256(1–2):13–18. doi: 10.1016/j.jneuroim.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 114.Durafourt BA, Lambert C, Johnson TA, Blain M, Bar-Or A, Antel JP. Differential responses of human microglia and blood-derived myeloid cells to FTY720. J Neuroimmunol. 2011;230(1–2):10–16. doi: 10.1016/j.jneuroim.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 115.Francis G, Kappos L, O’Connor P, Collins W, Tang D, Mercier F, et al. Temporal profile of lymphocyte counts and relationship with infections with fingolimod therapy. Mult Scler. 2014;20(4):471–480. doi: 10.1177/1352458513500551. [DOI] [PubMed] [Google Scholar]
- 116.Kappos L, Cohen J, Collins W, de Vera A, Zhang-Auberson L, Ritter S, et al. Fingolimod in relapsing multiple sclerosis: an integrated analysis of safety findings. Mult Scler Rel Dis. 2014;3:494–504. doi: 10.1016/j.msard.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 117.Kappos L, Radue EW, Comi G, Montalban X, Butzkueven H, Wiendl H, et al. Switching from natalizumab to fingolimod: a randomized, placebo-controlled study in RRMS. Neurology. 2015;85(1):29–39. doi: 10.1212/WNL.0000000000001706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jokubaitis VG, Li V, Kalincik T, Izquierdo G, Hodgkinson S, Alroughani R, et al. Fingolimod after natalizumab and the risk of short-term relapse. Neurology. 2014;82(14):1204–1211. doi: 10.1212/WNL.0000000000000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cohen M, Maillart E, Tourbah A, De Seze J, Vukusic S, Brassat D, et al. Switching from natalizumab to fingolimod in multiple sclerosis: a French prospective study. JAMA Neurol. 2014;71(4):436–441. doi: 10.1001/jamaneurol.2013.6240. [DOI] [PubMed] [Google Scholar]
- 120.Kappos L, O’Connor P, Radue EW, Polman C, Hohlfeld R, Selmaj K, et al. Long-term effects of fingolimod in multiple sclerosis: the randomized FREEDOMS extension trial. Neurology. 2015;84(15):1582–1591. doi: 10.1212/WNL.0000000000001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mehta LR, Schwid SR, Arnold DL, Cutter GR, Aradhye S, Balcer LJ, et al. Proof of concept studies for tissue-protective agents in multiple sclerosis. Mult Scler. 2009;15(5):542–546. doi: 10.1177/1352458508101939. [DOI] [PubMed] [Google Scholar]
- 122.Sormani MP, Arnold DL, De Stefano N. Treatment effect on brain atrophy correlates with treatment effect on disability in multiple sclerosis. Ann Neurol. 2014;75(1):43–49. doi: 10.1002/ana.24018. [DOI] [PubMed] [Google Scholar]
- 123.Anderson VM, Fox NC, Miller DH. Magnetic resonance imaging measures of brain atrophy in multiple sclerosis. J Magn Reson Imaging. 2006;23(5):605–618. doi: 10.1002/jmri.20550. [DOI] [PubMed] [Google Scholar]
- 124.Lublin FD, Miller D, Freedman M, Cree B, Wolinsky J, Weiner H, et al. Oral fingolimod versus placebo in patients with primary progressive multiple sclerosis (PPMS): results of the INFORMS phase III trial. Neurology. 2015;85(4):EM006. [Google Scholar]
- 125.Yaldizli Ö, MacManus D, Stutters J, Häring DA, Lublin F, Freedman MS, et al. Brain and cervical spinal cord atrophy in primary progressive multiple sclerosis: results from a placebo-controlled phase III trial (INFORMS). Mult Scler J. 2015;21(11):[Abstract 110].