Abstract
Certain cells of the human retina are extremely sensitive to loss of function of the retinoblastoma tumor suppressor gene RB. Retinoblastomas develop early in life and at high frequency in individuals heterozygous for a germ-line RB mutation, and sporadic retinoblastomas invariably have somatic mutation in the RB gene. In contrast, retinoblastomas do not develop in Rb+/- mice. Although retinoblastoma is thought to have developmental origins, the function of Rb in retinal development has not been fully characterized. Here we studied the role of Rb in normal retinal development and in retinoblastoma using conditional Rb mutations in the mouse. In late embryogenesis, Rb-deficient retinas exhibited ectopic S-phase and high levels of p53-independent apoptosis, particularly in the differentiating retinal ganglion cell layer. During postnatal retinal development, loss of Rb led to more widespread retinal apoptosis, and adults showed loss of photoreceptors and bipolar cells. Conditional Rb mutation in the retina did not result in retinoblastoma formation even in a p53-mutant background. However, on a p107- or p130-deficient background, Rb mutation in the retina caused retinal dysplasia or retinoblastoma.
Keywords: Retinoblastoma, p130, p107, p53, apoptosis
The RB gene was the first known tumor suppressor gene identified (Friend et al. 1986; Fung et al. 1987; Lee et al. 1987). In humans, inheritance of a mutant allele of RB leads to retinoblastoma at about a 90% frequency, with loss of the remaining wild-type allele thought to be a rate-limiting step in tumorigenesis. RB is also mutated in sporadic cancers of the brain, breast, bladder, lung, and bone (Horowitz et al. 1990). Moreover, mutation of some component of the “RB pathway,” such as loss of p16INK4a or amplification of upstream RB regulators cyclin D or CDK4/6 is thought to occur in the majority of human cancers. Germ-line RB+/- humans are at some increased risk for tumors other than retinoblastoma, such as osteosarcomas (Eng et al. 1993); however, no tissue is as exquisitely sensitive to RB loss as the retina. The reason for this tissue sensitivity is unknown.
The product of the RB gene, pRB, is a nuclear phosphoprotein that is an important regulator of the cell cycle (Weinberg 1995). pRB forms complexes with members of the E2F family of transcription factors to repress transcription of genes important for S-phase entry and progression. Interaction of pRB with chromatin remodeling enzymes such as HDAC appears to contribute to gene repression. Phosphorylation of pRB by cyclin-dependent kinases leads to dissociation of pRB from E2Fs and release of pRB-mediated repression. In addition to regulating S-phase entry, RB also regulates apoptosis and terminal differentiation and has been implicated in still other cellular processes (Lipinski and Jacks 1999; DiCiommo et al. 2000; Classon and Harlow 2002; Chau and Wang 2003).
In contrast to most human tumors, retinoblastomas usually arise during the first few years of life. Retinoblastomas have also been found in the developing human fetus in utero (Salim et al. 1998). Therefore, elucidating the role of RB in normal retinal development should help to explain the origins of this tumor. The adult retina consists of seven cell types, including six neuronal cell types and one type of glial cell, arranged in three nuclear layers. The outer nuclear layer contains rod and cone photoreceptor cell bodies. The inner nuclear layer includes bipolar, amacrine, and horizontal neuron cell bodies as well as the cell bodies of the single glial cell type, the Muller glia. The innermost retinal ganglion cell layer contains both ganglion cells and displaced amacrine cells. The cells in the adult retina develop from multi-potent progenitor cells in a birth order that changes as development proceeds (Turner and Cepko 1987; Turner et al. 1990). Both intrinsic properties of progenitor cells and responses to extrinsic cues dictate retinal cell fate (Cepko et al. 1996; Marquardt and Gruss 2002). Retinoblastomas have been proposed to derive from a primitive neuroectodermal cell (Kyritsis et al. 1984), and evidence of photoreceptor differentiation has been described in some tumors (Bogenmann et al. 1988; Vrabec et al. 1989; Tajima et al. 1994). However, the cell of origin of retinoblastomas has been controversial, and there has been limited examination of markers specific to neuronal retinal cell types other than photoreceptors in human tumor material.
Early attempts to model retinoblastoma in the mouse through germ-line mutation of Rb were not successful. Germ-line Rb+/- animals never develop retinoblastomas, although they do develop pituitary and thyroid tumors at high frequency (Jacks et al. 1992). Germ-line Rb mutant homozygous embryos die in midgestation with defects in erythropoiesis and placental development (Clarke et al. 1992; Jacks et al. 1992; Lee et al. 1992; Wu et al. 2003). Chimeric Rb++;/-- mice survive to adulthood, but these mice also do not develop retinoblastomas (Maandag et al. 1994; Williams et al. 1994b). An examination of retinas from chimeric embryos revealed increased apoptosis (Maandag et al. 1994), and retinas from adult chimeras showed a relatively low contribution from Rb-/- cells (Maandag et al. 1994; Williams et al. 1994b).
Rb is the founding member of a gene family containing two other members, p107 and p130. These proteins have structural similarities in the so-called A/B pocket region that is important for binding of pRB to E2F family members and to DNA tumor virus oncoproteins such as SV40 T Antigen, human papillomavirus E7, and adenovirus E1A (Weinberg 1995). The three “pocket protein” family members have both overlapping and distinct cellular and developmental functions (Classon and Harlow 2002; Trimarchi and Lees 2002). For example, in contrast to the essential role of Rb during development, p130-/- and p107-/- mice on a similar genetic background survive to adulthood without clear phenotypes (Cobrinik et al. 1996; Lee et al. 1996).
Human retinoblastomas have not been found to carry mutations in p107 or p130, although absence of p130 expression has been found in a subset of tumors (Bellan et al. 2002). Also, the chromosomal arm harboring RB2/p130 undergoes frequent alteration in human retinoblastomas (Mairal et al. 2000; Chen et al. 2001; Herzog et al. 2001; Lillington et al. 2003). In the mouse, whereas Rb-/-;+/+ chimeras do not develop retinoblastoma, chimeras composed of cells lacking both Rb and p107 developed retinoblastomas with characteristics of amacrine cells (Robanus-Maandag et al. 1998). Embryonic retinas from these chimeras exhibited dysplasia with high levels of apoptosis. Loss of both copies of p107 in an Rb+/- background also causes retinal dysplasia in adults (Lee et al. 1996). These data indicate that Rb-deficient retinas have tumorigenic potential that is realized with p107 mutation and suggest that p107 can compensate for Rb loss in the murine retina. A potential role for p130 in compensating for retinal Rb loss has not been reported.
Understanding the role of Rb in the development of the different retinal cell types is critical to understanding both the cause of retinoblastoma and the retinal cell type susceptible to transformation. Loss of Rb in chimeric embryo retinas has been associated with increased apoptosis, and it has been proposed that mutation to evade apoptosis may be required for retinoblastoma development (Gallie et al. 1999). However, the response of specific cell types in the retina to RB/Rb deletion has not been fully examined. Here we use conditional mutation to delete Rb in early retinal progenitors in the mouse. Here we characterize the cell type-specific effects of Rb loss in the development of the retina, and we report the cell-type characteristics of a novel murine model of retinoblastoma that develops in the absence of retinal Rb and p130.
Results
We previously described the use of a conditional loss-of-function allele of Rb (Rblox) combined with the expression of Cre-recombinase from the nestin promoter (NesCre1; MacPherson et al. 2003). In this system, Rb deletion occurs in the developing nervous system and other tissues; the resulting embryos survive gestation and die at birth (MacPherson et al. 2003). We and others have observed a different degree of Cre-mediated recombination from the NesCre1 transgene depending on whether the transgene is inherited from the mother or the father, presumably due to imprinting effects (Fan et al. 2001). Paternally inherited NesCre1 gives significantly more extensive and complete Cre-mediated recombination. The initial experiments described here were performed using Rb conditional embryos with paternally inherited NesCre1 (designated NesCre1(p)) in order to address the effects of Rb deficiency on retinal development. To characterize NesCre1(p) transgene activity in the retina, these animals were crossed to a reporter strain that expresses the LacZ gene following Cre-mediated deletion of a lox-STOP-lox cassette (Soriano 1999). Widespread NesCre1(p)-driven transgene activity was evident in the optic vesicle from which the retina derives at embryonic day 9.5 (E9.5; Supplementary Fig. 1A). X-gal staining of adult retinas revealed complete recombination throughout the retina when NesCre1 is paternally inherited (Supplementary Fig. 1B,C). Consistent with evidence of early and widespread retinal transgene activity from LacZ reporter gene analyses, paternal transmission of NesCre1 resulted in complete absence of pRB on a Rblox/lox background in the retina at E13.5 (Fig. 1A).
Figure 1.
Cell-cycle defects in Rb-deficient retina. Retinas obtained from paternally inherited NesCre1(p) Rb-null or control retinas. (A) Western blot for pRB expression at E13.5 in control or NesCre1(p) Rb mutants. (B) BrdU analysis for S-phase activity at E18.5 in controls or NesCre1(p) Rb mutants. Arrows denote ectopic BrdU-positive cells. (C) Phospho-histone H3 (PH3) immunostaining for evidence of mitotic activity at E18.5. Arrows show ectopic mitotic cells in inner layer of NesCre1(p) Rb mutants. (D) Quantification of overall BrdU-positive cells, or ectopic BrdU-positive cells. The ratio of BrdU-positive to all retinal cells was quantified from the central retina at the level of the optic nerve. Ectopic BrdU-positive cells were those that extend beyond the normal zone of S phase to the inner ganglion cell layer (*, p < 0.01; Student's t-test; n = 6). (E) Quantification of mitoses along neurepithelium immediately adjacent to RPE (ventricular) or ectopic mitoses outside of the ventricular zone along the entire retina at level of optic nerve (*, p = 0.01; Student's t-test; n = 6). (n) Neuroblastic layer; (g) ganglion cell layer.
Cell-cycle defects in the Rb-deficient retina
Loss of Rb in the nervous system has been reported to cause cell-cycle defects (Macleod et al. 1996; Ferguson et al. 2002; MacPherson et al. 2003). Therefore, we investigated cell-cycle control in NesCre1(p) conditional Rb mutant retinas. In control retinas, mitosis occurs in the neurepithelium immediately adjacent to the retinal pigment epithelium (RPE). Migration away from this zone occurs during G1, S, and G2, and nuclei return to the outer surface for the next mitosis. pRB is highly expressed in the differentiating retinal ganglion cell layer at E18.5 (Jiang et al. 1997). S-phase activity in E18.5 Rb-deficient or control retinas was analyzed by BrdU incorporation. In control embryos there is a clear zone of cells that incorporate BrdU, and no BrdU-positive cells were seen in the innermost, retinal ganglion cell layer (Fig. 1B,D). In NesCre1(p) conditional Rb mutants, however, ectopic S-phase activity was prevalent in the retinal ganglion cell layer (Fig. 1B,D). The overall proportion of cells in the retina that were BrdU-positive did not differ significantly between Rb mutants and controls; however, ectopic S-phase entry was greatly increased in Rb mutants (Fig. 1B,D). To examine whether cells undergoing inappropriate S-phase proceeded to mitosis, we stained for the mitotic marker phospho-histone H3 (PH3). In contrast to controls, where strong PH3 staining was restricted to the retinal ventricular zone, in Rb mutants ectopic PH3 staining was observed (Fig. 1C,E). Increased PH3 staining was particularly high in the Rb mutant retinal ganglion cell layer (Fig. 1C). Cells in this layer normally should have undergone a permanent exit from the cell cycle. Thus, the inappropriate S-phase and mitosis suggest that Rb deficiency causes impairment of cell-cycle exit.
Rb-/- retina undergoes p53-independent apoptosis
Histological examination of NesCre1(p) conditional Rb mutant retinas at E18.5 revealed significant numbers of pyknotic nuclei indicative of apoptosis (data not shown). Apoptosis was confirmed using immunostaining for active caspase3 (Fig. 2A,B). Apoptosis was prevalent throughout the retina but was notably higher in the retinal ganglion cell layer. The morphology of cells in the retinal ganglion layer at E18.5 was abnormal, with both apoptotic and morphologically aberrant cells throughout. Combined with the data suggesting that Rb is required for proper cell-cycle exit, the increased apoptosis in this cell population would indicate that defects in the coordination of the cell-cycle withdrawal and onset of differentiation lead to cell death. However, Rb might also inhibit apoptosis more directly (Chau and Wang 2003).
Figure 2.
p53-independent apoptosis in Rb-deficient retina. (A) Immunostaining for active caspase3 in E18.5 retinas of control, paternally inherited NesCre1(p) Rb and NesCre1(p) Rb;p53-/- embryos. (B) Quantification of Caspase3-positive cells in sections at level of optic nerve (n = 6). (C) Hematoxylin and eosin (H&E) staining of adult NesCre1 Rb mosaics and p53-/- NesCre1 Rb mosaic adults showing reduced photoreceptor layer. Cre was maternally inherited. (D) Quantification of thickness of photoreceptor layer in control (n = 4), Rb NesCre1 (n = 8), and RbNesCre1 p53-/- adult mosaics (n = 4; *, p < 0.01 vs. controls; Student's t-test). (n) Neuroblastic layer; (g) ganglion cell layer; (i) inner nuclear layer; (o) outer nuclear layer.
Previous studies have demonstrated p53-dependent apoptosis in the CNS of Rb-/- embryos, and that this death was due to primary defects in the placenta leading to hypoxia (Macleod et al. 1996; Ferguson et al. 2002; de Bruin et al. 2003; MacPherson et al. 2003; Wu et al. 2003). p53 mutation can also cooperate with Rb pathway inhibition to contribute to tumorigenesis by suppressing apoptosis (Howes et al. 1994; Symonds et al. 1994). To determine whether the retinal apoptosis was p53-dependent, we bred the Rb Nes-Cre1(p) mutant animals onto a p53-/- background and analyzed retinas at E18.5, as shown in Figure 2. Levels of apoptosis were similar in the Rb mutants with and without p53. Thus, in contrast to previous findings from midgestation Rb-/- CNS, we found that retinal apoptosis at E18.5 in conditional Rb mutants is a more direct consequence of Rb deficiency in that it occurs in embryos with normal placentas and normal erythropoiesis and is not dependent on p53. Although most retinas with p53 and Rb deficiency appeared identical to Rb mutants, we observed one compound NesCre1(p) conditional Rb;p53-/- mutant that had high levels of apoptosis and also exhibited focal dysplasia in both eyes (data not shown). This suggests that effects of p53 deficiency may vary depending on the presence of modifier genes that may differ between embryos on a mixed 129:Bl6 genetic background.
Rb mosaic retinas have reduced photoreceptor layer
Use of the paternally inherited NesCre1 transgene led to complete deletion of Rb in the retina, but it also causes perinatal lethality (MacPherson et al. 2003). However, due to imprinting effects, a lower degree of Cre-mediated excision occurs with maternal NesCre1 inheritance (Fan et al. 2001). Reporter analyses revealed that maternal NesCre1 inheritance leads to a mosaic expression of Cre through all layers of the retina that was highly variable from animal to animal (Supplementary Fig. 1B,C). Use of maternal inheritance of the NesCre1 transgene allowed us to produce viable offspring with a mosaic pattern of Rb deletion. These animals were produced at below Mendelian frequency (∼41% of the expected frequency); we refer to them below as “NesCre1 Rb mosaics”. Histological analyses of adult NesCre1 Rb mosaic retinas revealed a dramatic reduction in the number of cells in the photoreceptor-containing outer nuclear layer (Fig. 2C,D). The outer segments of the photoreceptors also appeared diminished. The thickness of the NesCre1 Rb mosaic outer nuclear layer was half the thickness of controls (Fig. 2C,D). This phenotype was similar in Rb mosaic mice that were 3 wk old and those near 4 mo old, suggesting that most photoreceptor loss occurred in the first weeks of life. When Rb mosaics were examined at postnatal day 3, a time of high levels of photoreceptor generation, we observed high levels of apoptosis (data not shown; see similar data in Fig. 5, below). In addition to the loss of photoreceptors in adults, we found subtle defects in the inner nuclear cells in the Rb mosaics. We observed some disorganization in the inner nuclear layer, with clusters of ectopic inner nuclear layer cells in the outer plexiform layer approaching the photoreceptors (Fig. 2C).
Figure 5.
Loss of bipolar cells and decreased photoreceptor layer in Rbloxlox α-Cre distal retina. (A,B) H&E staining of 3-wk control (A) and Rbloxlox α-Cre (B) retinas showing reduced photoreceptor layer and inner nuclear layer (100×). Proximal and distal retina is labeled. Cre expressed in the distal retina deletes Rb in B. (Inset) Distal retina (400×). (C,D) Immunostaining with anti-PKCα, labeling bipolar cells in control (C) and Rbloxlox α-Cre (D) 3-wk retina (100×). (Inset) Distal retina (400×). (E,F) Active caspase3 immunostaining at P4 in control (E) and Rbloxlox α-Cre (F) distal retina. (G,H) Active Caspase3 immunostaining at P12 in control (G) and Rbloxlox α-Cre (H) distal retina. (I,J) BrdU analysis at P12 in control (I) and Rbloxlox α-Cre (J) distal retina. Note the complete absence of BrdU incorporation in controls, with many positive cells in Rbloxlox α-Cre mutants at this time. BrdU was injected 1 h prior to animal sacrifice. (g) Ganglion cell layer; (i) inner nuclear layer; (o) outer nuclear layer.
Rb mosaics were aged to determine whether animals would develop retinal tumors or early lesions. Rb mosaics had an average lifespan of 107 d (S.D. = ±19 d, n = 12), and in all cases became moribund due to pituitary tumor development. No retinoblastomas were detected in Rb mosaic adults, and we did not observe histological evidence of early retinal tumors. To determine whether loss of Rb could cooperate with p53 mutation in apoptosis suppression and tumor development, we bred NesCre1 Rb mosaics onto a p53-/- background. The photoreceptor layer was still depleted in the absence of p53 (Fig. 2C,D), and, consistent with the findings that p53 is not mutated in human retinoblastomas, retinal tumors did not occur in p53-/-; NesCre1 Rb mosaic compound mutant animals (n = 5). However, focal dysplasia was found in some retinas, reminiscent of the retinal dysplasia observed previously in p53-/-Rb+/- animals (Williams et al. 1994a). p53-/-; NesCre1 Rb mosaic animals were sacrificed due to tumor burden from pituitary and other nonretinal tumors at an average lifespan of 92 ± 15 d. Thus, we conclude that p53 mutation does not suppress apoptosis or cooperate with Rb loss in retinoblastoma formation in the mouse.
Increased severity of retinal phenotypes upon compound mutation of Rb family members
We were interested in examining the possible role of the Rb family members p107 and p130 in compensating for Rb deletion in retinal progenitors. Therefore, we bred NesCre1(p) Rb mutants onto a p107- or p130-deficient background to generate embryos with retinas completely lacking Rb and either p107 or p130. We observed massive retinal dysplasia late in embryonic development (E18.5) in the absence of both Rb and p107 (Fig. 3A). Active Caspase3 immunostaining revealed extensive apoptosis throughout the compound Rb/p107 mutant retinas (Supplementary Fig. 2A) This phenotype of retinal dysplasia and high levels of apoptosis is similar to the embryonic retinal phenotype in Rb-/-;p107-/- chimeras (Robanus-Maandag et al. 1998). Interestingly, we did not observe extensive retinal disorganization at this time in development in embryos lacking both retinal Rb and p130 (Fig. 3A), and the levels of apoptosis in Rb/p130 mutant retinas were similar to levels with Rb mutation alone (Supplementary Fig. 2B). Homozygous mutation of either p107 or p130 alone did not lead to retinal phenotypes (data not shown).
Figure 3.
Expression of retinoblastoma family members and cyclin D1 in response to Rb loss. (A) H&E staining reveals p107 deficiency leads to massive dysplasia in NesCre1(p) Rb mutant E18.5 retinas. (B) pRB expression in wild-type, p107-/-, and p130-/- E18.5 retinas. (C) Biochemistry of NesCre1(p) Rb-deficient retinas at E18.5. Note p107 shift in mobility and decreased cyclin D1 expression with either Rb or Rb and p130 deletion. (D) Treatment of E18.5 control retinas with calf-intestine- and λ-phosphatases (Ppase) leads to shift from hyperphosphorylated to hypophosphorylated p107 that comigrates with mobility-shifted p107 from Rb-deficient or Rb and p130-deficient E18.5 retina extracts. (E) Western blot showing cyclin D1 levels decrease in absence of Rb in retinas collected at E13.5, E16.5, and E18.5. (F) Northern blot showing cyclin D1 down-regulation at RNA level in E18.5 Rb-deficient retina. Note that for D-F, “con” refers to Rblox/+ NesCre1(p) controls, and “Rb-/-” refers to Rblox/lox NesCre1(p) mutants. (n) Neuroblastic layer; (g) ganglion cell layer.
The NesCre1(p) Rb deletion strategy also allowed us determine whether compensatory up-regulation of Rb family members occurs in Rb-deficient embryonic retinas. Extracts of microdissected embryonic retinas from Rb mutant, Rb/p107, and Rb/p130 compound mutants and controls were subjected to Western blot analysis. Mutation of p107 or p130 alone did not lead to alterations in the levels of pRB (Fig. 3B) and in contrast to earlier data from other systems (Hurford et al. 1997), p107 was not substantially up-regulated at the protein level in response to Rb loss (Fig. 3C). We did find a slight increase in p130 levels with combined Rb and p107 deletion, but not with Rb deletion alone (Fig. 3C). Interestingly, in Rb-deficient retinas there was a partial mobility shift to a faster migrating form of p107 compared to controls (Fig. 3C). An even greater p107 mobility shift to the faster migrating form was found in retinas lacking both Rb and p130 (Fig. 3C). To determine whether the altered mobility of p107 with Rb and p130 mutation corresponded to a change in p107 phosphorylation, we treated control retina samples with phosphatases. Phosphatase treatment resulted in a complete shift from the slower migrating (hyperphosphorylated) form to the faster migrating (hypophosphorylated) form of p107 (Fig. 3D). These data suggest that compensation in response to Rb loss or Rb and p130 loss may involve regulation of an upstream mediator of p107 phosphorylation and increased p107 activity.
Down-regulation of cyclin D1 in the absence of Rb and family members
Cyclin D1 is expressed at extremely high levels in the developing retina in the mouse (Sicinski et al. 1995). Because cyclin D1 can regulate p107 phosphorylation (Beijersbergen et al. 1995), we examined the levels of cyclin D1 in retinas lacking Rb. As shown in Figure 3, levels of cyclin D1 decreased with Rb mutation, and further decreased with additional mutation of p130 (Fig. 3C). p107 mutation led to cyclin D1 down-regulation even with an intact Rb allele present (Fig. 3C, last lane). Down-regulation of cyclin D1 in response to Rb loss occurred at each time point in retinal development examined between E13.5 and E18.5 (Fig. 3E). Northern blot analysis on embryonic retinal RNA revealed that the decrease in cyclin D1 expression in Rb-mutant tissue occurred at the level of RNA (Fig. 3F). This decrease in cyclin D1 levels correlated with p107 hypophosphorylation in the absence of pRB or pRB and p130. Thus, we hypothesize that a feedback mechanism involving cyclin D1 may act to dampen the effects of Rb mutation. This mechanism could help prevent retinoblastoma development following Rb mutation in the mouse.
Bilateral retinoblastomas in p130-/- NesCre1 Rb mosaics
In order to further pursue the role of p107 and/or p130 in compensating for Rb loss, we attempted to generate NesCre1 Rb mosaics lacking p107 or p130. However, through breeding strategies from which 22 compound mutant p107-/- NesCre1 Rb mosaics would be expected based on Mendelian ratios, no adult double mutant mosaics were produced. Thus, we conclude that p107-/- NesCre1 Rb mosaics die prior to weaning age. In contrast, we were able to generate viable NesCre1 Rb mosaics on a p130-/- background. Five viable p130-/- NesCre1 Rb mosaics (out of an expected Mendelian frequency of 11 animals) were generated and followed for tumor development. Strikingly, all five of the double mutant animals developed retinoblastomas, four of these being bilateral. Mice were sacrificed when body condition was poor, or if gross retinal tumor burden was apparent; the average tumor-free lifespan in these animals was 79 d (S.D. = ±38 d).
Histological analyses of retinoblastomas revealed histology similar to that of the human tumor (Fig. 4A-F). The tumors were sometimes aggressive, invading surrounding muscle (Fig. 4B). All of the retinoblastomas examined had rosettes similar to Homer-Wright rosettes present in human retinoblastomas (Fig. 4D,F) and had foci with high levels of apoptosis and mitoses. The mouse tumors were always found in the inner nuclear layer, usually filling the area behind the lens. One case showed a mixed pattern of growth as tumor was also found between the photoreceptor outer segments and retinal pigment epithelium (data not shown).
Figure 4.
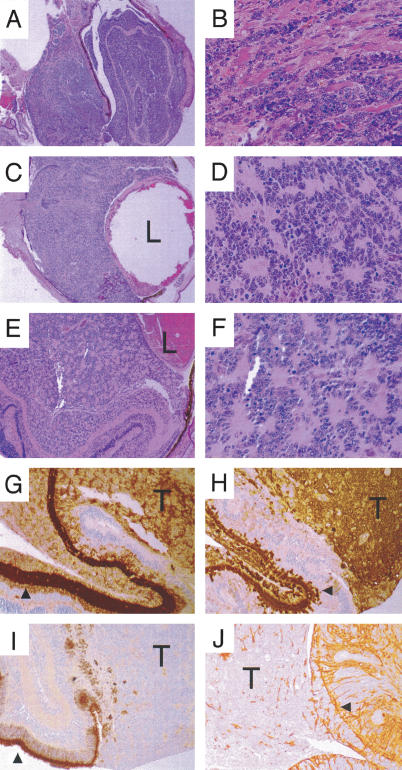
Retinoblastomas in p130-/-; NesCre1 Rb mosaics. (A-F) H&E staining of three retinoblastomas. (A,B) Invasive retinoblastoma at 40× (A) and 400× (B) showing tumor invasion into muscle. (C,D) Retinoblastoma at 40× (C) with rosette-like structures visible at 400× (D). (E,F) Retinoblastoma (100×) with rosette-like structures visible at 400× (F). (G) Immunohistochemistry of retinoblastoma from E with amacrine marker syntaxin showing positive staining throughout tumor (T; 100×). Arrowhead shows area of normal staining of the amacrine cell bodies and processes in the retina inner nuclear layer. (H) Calretinin immunohistochemistry labeling a subset of amacrine cells in the normal area of retina (arrowhead), with strong staining in tumor (T). (I) IRBP immunohistochemistry labeling photoreceptor outer segments (arrowhead) but not staining the tumor (T). (J) GFAP immunohistochemistry labeling Muller glia, with a pattern indicative of gliosis in the mutant retina (arrowhead) with some cells in the area of the tumor staining positively. The lens is indicated (L).
Immunostaining with different retinal cell markers showed positive staining for the amacrine marker syntaxin (Fig. 4G). This is in agreement with results found in chimeras with mutation of p107 and Rb in which tumors were also syntaxin-positive (Robanus-Maandag et al. 1998). To further explore the possibility that these tumors were of the amacrine lineage, we immunostained them for calretinin, a marker of AII amacrine cells. As shown in Figure 4, the mouse retinoblastomas were intensely calretinin-positive (Fig. 4H). The tumors did not stain for interstitial retinoid-binding protein (IRBP), expressed in photoreceptor outer segments (Fig. 4I), the bipolar cell marker protein kinase C-α (PKC-α), or the retinal ganglion cell marker Brn3b (data not shown). We also performed immunostaining for glial fibrillary acidic protein (GFAP), which is up-regulated in Muller glia exhibiting gliosis, and found some GFAP-positive cells, both in adjacent, normal retina and, infrequently, in the tumor (Fig. 4J). This staining pattern is suggestive of reactive gliosis, as the vast majority of cells in the area of the tumors were GFAP-negative. Our data and the results of Robanus-Maandag et al. (1998) indicate that mutation of either p107 or p130 on a Rb-deficient background can lead to retinoblastomas of similar histology and, likely, histogenesis.
Apoptosis after retinal-specific Rb deletion
In order to study the specific effects of Rb deletion in the major retinal cell types, including amacrine cells, we crossed the Rblox/lox mice to α-Cre transgenic mice. In this strain Cre expression is controlled by elements of the Pax6 promoter, allowing for retinal-specific gene deletion in retinal progenitors in distal retina as early as E10.5 (Marquardt et al. 2001). Complete gene deletion has been reported in the distal retina, but not in proximal retina (Marquardt et al. 2001). This approach allowed us to characterize the specific developmental defects in retinal cell types that might have been less apparent or more variable in the Rb mosaics.
As shown in Figure 5, retinas from 3-week-old α-Cre Rblox/lox animals exhibited widespread photoreceptor loss. To determine whether apoptosis contributed to the photoreceptor phenotype, we performed immunostaining for active caspase3 at various times during development. Whereas few apoptotic cells were apparent in controls (Fig. 5E), extensive apoptosis was evident throughout the Rb-deficient distal retina at postnatal day 4 (P4; Fig. 5F). At P12, the photoreceptor-containing outer nuclear layer was visible in control animals; cells with photoreceptor-like morphology were present, and no significant apoptosis in the outer nuclear layer was detected (Fig. 5G). However, in the α-Cre Rblox/lox distal retina, pyknotic nuclei (not shown) and caspase3 immunopositive cells were observed in the outer nuclear layer (Fig. 5H). This suggests that photoreceptors are born, but die at some point in differentiation in the absence of Rb. Apoptosis was also found in the inner part of the Rb mutant retina at P4 and P12 (Fig. 5F,H).
Given the observed cell cycle defects in NesCre1(p) Rblox/lox retinas at E18.5 (Figs. 1, 2), we examined cell-cycle activity in postnatal embryos. DNA synthesis in the normal murine retina has been reported to be completed by P11 (Young 1985), and we did not observe BrdU-positive cells in control retinas at P12 (Fig. 5I). In contrast, in Rb mutants very high levels of BrdU-positive cells were present at this stage, indicative of inappropriate S-phase entry (Fig. 5J). We noted that the inappropriate BrdU incorporation and apoptosis were not restricted to the photoreceptor-containing outer nuclear layer, but were found throughout the retina (Fig. 5J). Widespread apoptosis was also observed in the αCre Rb mutant retina at P4 and P12.
Bipolar loss and inner nuclear layer defects with Rb deletion
In addition to the reduced outer nuclear layer (ONL), the inner nuclear layer (INL) of α-Cre Rb mutants was clearly affected although to a lesser extent than the ONL (Fig. 5A,B). Along with the diminished INL, the corresponding inner plexiform layer of axons was also reduced. We used immunohistochemical analyses with cell type-specific markers to further characterize effects of Rb deletion on development of specific cell types at 3 wk of age, when retinal histogenesis is complete. Labeling with PKC-α, a bipolar cell marker (Fig. 5C,D), revealed that Rb deletion led to near complete loss of bipolar neurons (Fig. 5D). Loss of bipolar cells occurred prior to P12, as PKC-α-positive cells were also absent at this time (data not shown).
In contrast to the stark depletion of photoreceptors and bipolar cells with Rb gene mutation, some INL cell types survived the absence of Rb. In the 3-week-old αCre Rb mutants, we observed aberrant, very large cells in the upper part of the INL that were never seen in controls (Fig. 6A,B). Calbindin immunostaining was consistent with these cells being in the horizontal cell lineage (Fig. 6C,D).
Figure 6.
Immunohistochemical characterization of different retinal cell types with Rb deletion. (A,B) H&E staining on inner nuclear layer (1000×) of distal retina from controls (A) and Rbloxlox α-Cre mutants (B) at 3 wk of age. Note the decreased INL thickness in the mutant INL and the presence of cells with abnormally large nuclei (arrows) in mutant. (C,D) Calbindin immunostaining of distal retina from controls (C) and Rbloxlox α-Cre mutants (D) labeling horizontal cells strongly in the upper INL as well as some lower amacrine cells. (E,F) Calretinin immunostaining labeling a subset of amacrine cells in controls (E) and Rbloxlox α-Cre mutants (F). (G,H) Syntaxin immunostaining labeling amacrine cells and processes in controls (G) and Rbloxlox α-Cre mutants (H). (I,J) GFAP immunostaining of distal retina in controls (I) and Rbloxlox α-Cre mutants (J). Note the presence of GFAP-positive Muller glia vertical processes only in the Rbloxlox α-Cre mutants (J). In controls, GFAP signal is restricted to astrocytes in the inner limiting membrane. (K,L) Brn3b immunostaining labeling retinal ganglion cells in the GCL in controls (K, arrows) and very rarely in Rbloxlox α-Cre mutants (L, arrow). Images for C-K taken at 400× magnification. (g) Ganglion cell layer; (i) inner nuclear layer; (o) outer nuclear layer.
In control mice, calbindin also labeled a subset of amacrine cells in the INL and displaced amacrine cells and ganglion cells in the GCL (Fig. 6C). The postnatal αCre Rb mutants had calbindin-positive amacrine cells as well, but there was clear disorganization and loss of laminar structure in the inner plexiform layer (Fig. 6D). Calretinin immunostaining of AII amacrine cells also indicated a loss of laminar structure in the inner plexiform layer in αCre Rb mutants (Fig. 6E,F), and syntaxin staining revealed a slight decrease in the thickness of the amacrine layer compared to controls (Fig. 6G,H). Given the presence of amacrine cell markers in murine retinoblastomas (see Fig. 4), the survival of many Rb-deficient amacrine cells may be highly significant.
GFAP is up-regulated in Muller glia in response to retinal injury, and most mouse models of photoreceptor degeneration also show GFAP up-regulation. Staining with GFAP showed that Muller glia were also present and were in an active state indicative of gliosis (Fig. 6I,J) in the αCre Rb mutants at 3 wk of age. Finally, consistent with the observed apoptosis in the Rb-deficient retinal ganglion cell layer at E18.5, we found that αCre Rb mutants had only rare cells positive for Brn3b, a marker of retinal ganglion cells (Fig. 6K,L).
In summary, Rb deletion in the retina leads to profound effects on retinal ganglion cells, photoreceptors, and bipolar cells. Horizontal cells, Muller glia, and amacrine cells can survive in the absence of Rb function, although disorganization in the inner nuclear layer is apparent and horizontal cells show cytologic abnormalities. We conclude that Rb is a critical regulator of cell-cycle exit in the retina and that the consequences of failure to exit the cell cycle are cell type-specific, with apoptosis a frequent endpoint for some cells. The survival of some Rb-deficient amacrine cells may help explain the development of retinoblastomas with amacrine cell features in mice mutant for Rb and p130 (Fig. 4G,H).
Discussion
In this report we describe a critical role for the retinoblastoma gene in normal retinal development. Rb deletion in this tissue leads to failure in cell-cycle exit and p53-independent apoptosis in multiple cell types. Importantly, Rb mutation combined with p107 and with p130 mutation caused retinal dysplasia or retinoblastomas with amacrine cell characteristics. Whereas most major cell types were affected by Rb mutation, amacrine cells were able to develop and survive.
We and others have previously described apoptosis in response to Rb deletion during development of the CNS (Clarke et al. 1992; Jacks et al. 1992; Lee et al. 1992; Wu et al. 2003). However, apoptosis in the Rb germ-line mutant CNS is secondary to hypoxia caused by abnormal placental development (MacPherson et al. 2003; Wu et al. 2003). In contrast, we have demonstrated that retinal cell apoptosis in the absence of Rb is p53-independent and a direct consequence of Rb deletion. Previous studies involving expression of human papillomavirus E7 (which binds and inactivates all pRB family members) in the photoreceptor compartment also showed links between loss of Rb family function and apoptosis (Howes et al. 1994). Interestingly, retinal E7 expression on a p53-mutant background led to retinal tumors in the photo-receptor layer (Howes et al. 1994), and photoreceptor expression of SV40 T antigen (which inhibits the pRB family as well as p53) also caused photoreceptor layer tumors (al-Ubaidi et al. 1992). However, we have shown that combined mutation of Rb and p53 does not lead to retinoblastoma development, a result which is consistent with the fact that p53 has not been found to be mutated in human retinoblastomas (Gallie et al. 1999).
Although mutation of p53 did not strongly cooperate with Rb mutation in NesCre1 Rb mosaics, we did observe striking cooperativity between Rb and p130 in retinal tumor development. All five p130-/- NesCre1 Rb mosaic animals examined developed retinoblastomas, which had remarkable histological similarities to their human counterparts. Bilateral tumors were present in four of five of these animals. Beyond the similarities in tumor histology, the mouse tumors were also notable due to the presence of Homer-Wright rosettes, high apoptotic and mitotic levels, and tumor invasiveness. However, the mouse retinoblastomas did not contain Flexner-Wintersteiner rosettes, which have been found in some human retinoblastomas and have evidence of photoreceptor differentiation. All of the tumors in our model appeared to have inner nuclear layer characteristics, although one tumor also had outer layer growth between the photoreceptor outer segments and pigment epithelium. Of note, Bellan et al. (2002) reported that lack of p130 expression was strongly associated with poor differentiation in human retinoblastomas. Thus, our model may recapitulate this subset of human retinoblastomas. A thorough mutational analysis of p130 in human retinoblastomas has not been performed. However, it is interesting to note that the p130 genomic region (at 16q12) is frequently lost in human retinoblastomas (Mairal et al. 2000; Chen et al. 2001; Herzog et al. 2001; Lillington et al. 2003).
These data complement those of Robanus-Maandag et al. (1998), who showed that 5/7 chimeras with Rb/p107-/- cells developed retinoblastomas. We could not generate p107-/-;NesCre1 Rb mosaic adult mice due to early lethality. However, it is very likely that retinoblastomas would have developed had these animals survived, given the extensive retinal dysplasia in embryonic retinas lacking Rb and p107. In addition, Chen et al. (2004) found that retinal-specific conditional mutants lacking Rb and p107 develop retinoblastomas at high frequency. The characteristics of the p130-/- NesCre1 Rb tumors reported here and those from Rb/p107 mutant retinas (Robanus-Maandag et al. 1998; Chen et al. 2004) are similar, and these studies point to an amacrine cell (or precursor) as a cell of origin in retinoblastoma. Because the necessary animals can be generated by breeding, our retinoblastoma model using maternal expression of the NesCre1 transgene on a p130-/- background is more tractable than chimera-based models and should facilitate further analysis of the genetics and cell biology of retinoblastoma development.
A simple explanation for the apparent functional compensation between Rb family members in the developing retina is that in response to Rb loss, p107 and/or p130 is up-regulated to provide additional cell-cycle inhibitory function. In fact, in Rb-deficient embryo fibroblasts, muscle progenitors, and other cell types, p107 levels are clearly elevated (Schneider et al. 1994; Hurford et al. 1997). In whole tissue extracts from Rb-deficient retinas, however, we found that the absolute levels of p107 were not increased. Instead, there was a subtle shift in the phosphorylation state of p107 upon Rb deletion to more of the active, hypophosphorylated form. Cyclin D1 is expressed at extremely high levels in the developing retina and the hypoplastic retinas in mice lacking cyclin D1 point to a critical role for cyclin D1 in retinal cell proliferation (Fantl et al. 1995; Sicinski et al. 1995). Interestingly, we observed that the expression of cyclin D1 was reduced in Rb-/- retinas, which could account for p107 hypophosphorylation. Both the p107 phosphorylation shift and decrease in cyclin D1 levels were magnified with mutation of both Rb and p130. We postulate that cyclin D1 is down-regulated in order to induce the activity of other Rb family members. This mechanism could suppress tumorigenic effects of Rb loss alone and provide a selective force for mutation of p107 and p130. This is the first demonstration of the negative control of cyclin D1 expression by Rb in vivo, and extends observations made by others in Rb-deficient cells in vitro (Muller et al. 1994; Takebayashi et al. 2003). An important question is the nature of the Rb activity for which p107 might compensate that is essential for suppression of retinoblastoma. We showed that Rb is important for cell-cycle exit in the retina with Rb loss leading to ectopic S-phase entry and extensive S-phase entry when normal cell division had stopped. Interestingly, in a study of the effects of retinal-specific deletion of both Rb and p107, there was an exacerbation of cell-cycle exit defects compared to Rb mutation alone, although Rb/p107-/- cells did eventually exit the cell cycle (Chen et al. 2004). It is possible that in the murine retina lacking Rb alone, active hypophosphorylated p107 may allow the retinoblastoma precursor cell to exit the cell cycle before other events critical for retinoblastoma development occur.
A striking phenotype apparent in Rb mosaics and in α-Cre conditional Rb mutants was a dramatic reduction in the photoreceptor cell layer. Rb-deficient photoreceptors were born but underwent apoptosis while differentiating. This was surprising given a report by Vooijs et al. (2002), who used the photoreceptor-specific IRBP-promoter to drive Cre-mediated Rb deletion in the retina and did not report phenotypic effects. In that model, Cre was expressed in only a fraction of photoreceptors, and thus may have led to only a partial decrease in the photoreceptor layer. Alternatively, the specific timing of deletion could be critical. In our studies, Cre was expressed in early retinal progenitors by E10.5 (Marquardt et al. 2001), whereas IRBP-driven CRE expression was first detected at E14.5. (Vooijs et al. 2002). Zhang et al. (2004) recently reported that Rb is critical for proliferation and for rod photoreceptor development in the mouse. Those authors used an in vitro retinal explant system to study Rb retinal function, and they also deleted Rb in the retina using retinal-specific Cre expression. However, Cre-mediated Rb deletion occurred in a “patchy” fashion, and only defects in proliferation and in rod photoreceptor development were reported (Zhang et al. 2004). Also, those authors did not find increased apoptosis in their system, which contrasts with the results of our study, and with those of the Bremner lab, who have also generated retinal-specific Rb-mutant mice (Chen et al. 2004). Here we were able to achieve more complete Rb deletion, in vivo, allowing the discovery of much broader cell type-specific effects of Rb deletion in the retina and a critical role for Rb in suppression of retinal apoptosis.
We characterized the effects of Rb deletion on the major cell types of the mouse retina. In addition to loss of photoreceptors, we found high levels of apoptosis in the retinal ganglion cell layer at a time when differentiation is occurring. Strikingly, we also found that bipolar cells were rarely present in Rb mutant adult retinas. This phenotype correlated with the high levels of apoptosis in Rb mutant retinas at various times in development. However, in contrast to the photoreceptor compartment, where apoptotic cells were clearly evident at P12, we have not determined the time at which the bipolar cells are lost. It remains possible that Rb deletion could affect the birth of these cells or cause apoptosis very early on in bipolar cell differentiation. Our data show that Rb deletion causes a failure in cell-cycle exit and cell death, either as an indirect consequence of these cell-cycle defects or more direct functions of Rb in regulation of apoptosis.
In the midst of high levels of cell death, some Rb-/- retinal cells survive. For example, horizontal cells can develop, but these often have abnormally large nuclei. This phenotype is reminiscent of Purkinje cells from Rb-/-:+/+ chimeras and NesCre1 Rb mosaics (Williams et al. 1994b; D. MacPherson and T. Jacks, unpubl.) and Rb-/- skeletal muscle (Zacksenhaus et al. 1996; de Bruin et al. 2003; MacPherson et al. 2003). In Rb-/- skeletal muscle, ectopic S-phase entry occurs in differentiating cells, and loss of Rb has been linked to endoreduplication cycles and polyploidy (Zacksenhaus et al. 1996). We also found Muller glia to be present and in an activated state associated with up-regulation of GFAP. Interestingly, inactivating mutation in p27 (an upstream regulator of pRB) led to constitutive Muller glia activation with GFAP up-regulation (Dyer and Cepko 2000). However, retinal defects involving photoreceptor degeneration usually cause Muller glia activation with GFAP up-regulation, and our experiments have not addressed whether the gliosis-like phenotype in the Rb-deficient retina is a primary defect or secondary to the photoreceptor loss. We were particularly interested in the fate of the amacrine cells due to the positive staining of the p130-/- NesCre1 Rb mosaic retinoblastomas for the amacrine cell markers syntaxin and calretinin. Most of the cells remaining in the αCre Rb mutant inner nuclear layer appeared to be amacrine cells, based on syntaxin staining. There appeared to be some decrease in the amount of these cells in Rb mutants compared to controls, but clearly many amacrine cells can survive deletion of Rb.
The observation of high levels of apoptosis in the developing Rb-/- retina is difficult to reconcile with the tumor-suppressive function of Rb in this tissue. This observation leads to the possibility that apoptosis would have to be suppressed during tumor initiation or progression, as has been suggested in other settings (Howes et al. 1994; Symonds et al. 1994). Indeed, there is evidence that retinoblastomas do undergo consistent, unknown genetic alterations in addition to RB deletion that have been suggested to be linked to apoptosis suppression (Mairal et al. 2000; Chen et al. 2001; Herzog et al. 2001; Lillington et al. 2003). However, our data indicate that mutations in apoptotic pathways may not be necessary for retinoblastoma development. Whereas many cells in the retina, including photoreceptors, bipolar cells, and retinal ganglion cells respond to Rb loss by undergoing apoptosis, amacrine cells and some other cell types can tolerate Rb mutation. Thus, the specific tumorigenic effects of RB mutation in human retinas and Rb/p107 or Rb/p130 in the mouse may be linked to the fate of different cells harboring these mutations. The careful study of this process should finally reveal the origins of retinoblastoma and the exact contribution of loss of Rb family function to the development of this tumor.
Materials and methods
Mice
NesCre1 transgenic mice were obtained from Andreas Trumpp (Swiss Institute for Experimental Cancer Research, Lausanne, Switzerland), and generation of the Rb/NesCre1 mutants has been described (MacPherson et al. 2003). All embryo studies were performed using paternal inheritance of Cre, to achieve complete Rb deletion in the retina. NesCre1 Rb mosaics are animals in which the NesCre1 transgene was maternally inherited. p107, p130, p53 germ-line mutant mice have been described (Jacks et al. 1994; Cobrinik et al. 1996; Lee et al. 1996). αCre transgenic mice express Cre from elements of the pax6 promoter, as described (Marquardt et al. 2001). Lox-STOP-lox LacZ reporter mice Gt(ROSA)26Sortm1Sor (Soriano 1999) were obtained from Jackson Laboratories.
Immunohistochemistry, BrdU, and TUNEL assays
Eyes were fixed in Bouins solution or 10% neutral buffered formalin (3.7% formaldehyde in PBS) for 24 h before processing into paraffin. Four-micron paraffin sections were cut and processed from xylene through a graded ethanol series to PBS. Unmasking was performed using microwave heating in citrate buffer (0.01 M sodium citrate at pH 6). Endogenous peroxidases were blocked with 1% H202, and immunohistochemistry was performed with an overnight incubation in the following antibodies: active caspase3 (Cell Signaling, 1/200), phospho-histone H3 (Upstate, 1/200), calbindin D28 (Chemicon, 1/500), syntaxin (Sigma, 1/3000), calretinin (Chemicon, 1/1500), IRBP (polyclonal anti-monkey, gift from Dr. Rachel Caspi, Laboratory of Immunology, National Institutes of Health, Bethesda, MD; 1/3000), PKCα (BD Transduction Laboratories, 1/200), GFAP (DAKO, 1/600), Brn3b (Santa Cruz, 1/100). Five-percent normal horse serum was used in blocking for all mouse primary antibodies, and 5% normal goat serum was used in blocking for all rabbit primary antibodies. Biotin-conjugated secondary antibodies (Vector Laboratories) were used at a dilution of 1/200 in blocking solution. After secondary antibody binding, detection was performed via a biotin-peroxidase complex (Vectastain ABC, Vector Laboratories) with diaminobenzidine substrate (Vector Laboratories). TUNEL and BrdU assays were performed as described (MacPherson et al. 2003). For BrdU analysis, an intraperitoneal injection of pups, or for embryo studies, pregnant mothers, was done with 100 μg/g body weight, and eyes were collected/fixed 1 h later.
β-Galactosidase histochemistry
Whole embryos or adult retinas were dissected and fixed in 4% paraformaldehyde (PFA) for 1 h at 4°C, rinsed, and then assayed for β-galactosidase activity. Tissue was incubated in X-gal staining solution [1 mg/mL 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal), 5 mmole/L K3Fe(CN)3, 5 mmole/L K4Fe(CN)6, 2 mmole/L MgCl2, 0.015% sodium deoxycholate, 0.03% IGEPAL CA-630 in PBS] overnight at 37°C, washed, and postfixed in 10% NBF (3.7% formaldehyde in PBS) at 4°C. Stained tissue was transferred through an ethanol series to xylene and then paraffin-embedded; 4-μM sections were cut.
Western and Northern blotting
Retinas were microdissected from E13.5 or E18.5 embryos under a Zeiss microscope and immediately frozen on dry ice. For Western blots on E13.5 embryos, four retinas were pooled following embryo genotyping. Protein was isolated by extraction in 100 μL lysis buffer (100 mM Tris at pH 8, 100 mM NaCl, 1% Nonidet P-40, with Complete protease inhibitor tablets [Roche Molecular Biochemicals], 1 mM sodium vanadate, 1 mM sodium fluoride). For phosphatase treatment, sodium fluoride and sodium vanadate were excluded, and 10 mM MgCl2 and 1 mM dithiothreitol were added to the lysis buffer. Lysates were incubated for 15 min with calf-intestine phosphatase and then with λ-phosphatase at 30°C. Western blots were performed as described (MacPherson et al. 2003). Antibodies to the following antigens were used: pRB (BD Biosciences), p107 (C-18 Santa Cruz), p130 (C-20 Santa Cruz), cyclin D1 (Santa Cruz), actin (I-20 Santa Cruz). For Northern blots, pooled E18.5 retinas were lysed in TRIZOL (Invitrogen) using a 30-gauge syringe, and RNA was isolated following the manufacturer's instructions. Next, 130ng total RNA was loaded, and Northern blotting was performed using standard protocols. cDNAs for cyclin D1 or ARPP P0 were used to generate probes.
Acknowledgments
We thank David Kirsch and Carla Kim for critical reading of the manuscript. We thank Rod Bremner for communicating data prior to publication. We thank Denise Crowley for excellent assistance with histology. We are grateful to Andreas Trumpp for providing the NesCre1 transgenic mice, as well as Peter Gruss and Ruth Ashery-Padan for providing the α-Cre transgenic mice. T.J. is an Investigator of the Howard Hughes Medical Institute, and this work was supported in part by a grant from the National Cancer Institute.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Supplemental material is available at http://www.genesdev.org.
Article published online ahead of print. Article and publication date are at http://www.genesdev.org/cgi/doi/10.1101/gad.1203304.
References
- al-Ubaidi M.R., Font, R.L., Quiambao, A.B., Keener, M.J., Liou, G.I., Overbeek, P.A., and Baehr, W. 1992. Bilateral retinal and brain tumors in transgenic mice expressing simian virus 40 large T antigen under control of the human interphotoreceptor retinoid-binding protein promoter. J. Cell Biol. 119: 1681-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beijersbergen R.L., Carlee, L., Kerkhoven, R.M., and Bernards, R. 1995. Regulation of the retinoblastoma protein-related p107 by G1 cyclin complexes. Genes & Dev. 9: 1340-1353. [DOI] [PubMed] [Google Scholar]
- Bellan C., De Falco, G., Tosi, G.M., Lazzi, S., Ferrari, F., Morbini, G., Bartolomei, S., Toti, P., Mangiavacchi, P., Cevenini, G., et al. 2002. Missing expression of pRb2/p130 in human retinoblastomas is associated with reduced apoptosis and lesser differentiation. Invest. Ophthalmol. Vis. Sci. 43: 3602-3608. [PubMed] [Google Scholar]
- Bogenmann E., Lochrie, M.A., and Simon, M.I. 1988. Cone cell-specific genes expressed in retinoblastoma. Science 240: 76-78. [DOI] [PubMed] [Google Scholar]
- Cepko C.L., Austin, C.P., Yang, X., Alexiades, M., and Ezzeddine, D. 1996. Cell fate determination in the vertebrate retina. Proc. Natl. Acad. Sci. 93: 589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau B.N. and Wang, J.Y. 2003. Coordinated regulation of life and death by RB. Nat. Rev. Cancer 3: 130-138. [DOI] [PubMed] [Google Scholar]
- Chen D., Gallie, B.L., and Squire, J.A. 2001. Minimal regions of chromosomal imbalance in retinoblastoma detected by comparative genomic hybridization. Cancer Genet. Cytogenet. 129: 57-63. [DOI] [PubMed] [Google Scholar]
- Chen D., Livne-bar, I., Vanderluit, J.L., Slack, R.S., Agochiya, M., and Bremner, R. 2004. Cell-specific effects of RB or RB/p107 loss on retinal development implicate an intrinsically death resistant cell-of-origin in retinoblastoma. Cancer Cell (in press). [DOI] [PubMed]
- Clarke A.R., Maandag, E.R., van Roon, M., van der Lugt, N.M., van der Valk, M., Hooper, M.L., Berns, A., and te Riele, H. 1992. Requirement for a functional Rb-1 gene in murine development. Nature 359: 328-330. [DOI] [PubMed] [Google Scholar]
- Classon M. and Harlow, E. 2002. The retinoblastoma tumour suppressor in development and cancer. Nat. Rev. Cancer 2: 910-917. [DOI] [PubMed] [Google Scholar]
- Cobrinik D., Lee, M.H., Hannon, G., Mulligan, G., Bronson, R.T., Dyson, N., Harlow, E., Beach, D., Weinberg, R.A., and Jacks, T. 1996. Shared role of the pRB-related p130 and p107 proteins in limb development. Genes & Dev. 10: 1633-1644. [DOI] [PubMed] [Google Scholar]
- de Bruin A., Wu, L., Saavedra, H.I., Wilson, P., Yang, Y., Rosol, T.J., Weinstein, M., Robinson, M.L., and Leone, G. 2003. Rb function in extraembryonic lineages suppresses apoptosis in the CNS of Rb-deficient mice. Proc. Natl. Acad. Sci. 100: 6546-6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCiommo D., Gallie, B.L., and Bremner, R. 2000. Retinoblastoma: The disease, gene and protein provide critical leads to understand cancer. Semin. Cancer Biol. 10: 255-269. [DOI] [PubMed] [Google Scholar]
- Dyer M.A. and Cepko, C.L. 2000. Control of Muller glial cell proliferation and activation following retinal injury. Nat. Neurosci. 3: 873-880. [DOI] [PubMed] [Google Scholar]
- Eng C., Li, F.P., Abramson, D.H., Ellsworth, R.M., Wong, F.L., Goldman, M.B., Seddon, J., Tarbell, N., and Boice Jr., J.D. 1993. Mortality from second tumors among long-term survivors of retinoblastoma. J. Natl. Cancer Inst. 85: 1121-1128. [DOI] [PubMed] [Google Scholar]
- Fan G., Beard, C., Chen, R.Z., Csankovszki, G., Sun, Y., Siniaia, M., Biniszkiewicz, D., Bates, B., Lee, P.P., Kuhn, R., et al. 2001. DNA hypomethylation perturbs the function and survival of CNS neurons in postnatal animals. J. Neurosci. 21: 788-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantl V., Stamp, G., Andrews, A., Rosewell, I., and Dickson, C. 1995. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes & Dev. 9: 2364-2372. [DOI] [PubMed] [Google Scholar]
- Ferguson K.L., Vanderluit, J.L., Hebert, J.M., McIntosh, W.C., Tibbo, E., MacLaurin, J.G., Park, D.S., Wallace, V.A., Vooijs, M., McConnell, S.K., et al. 2002. Telencephalon-specific Rb knockouts reveal enhanced neurogenesis, survival and abnormal cortical development. EMBO J. 21: 3337-3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend S.H., Bernards, R., Rogelj, S., Weinberg, R.A., Rapaport, J.M., Albert, D.M., and Dryja, T.P. 1986. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature 323: 643-646. [DOI] [PubMed] [Google Scholar]
- Fung Y.K., Murphree, A.L., T'Ang, A., Qian, J., Hinrichs, S.H., and Benedict, W.F. 1987. Structural evidence for the authenticity of the human retinoblastoma gene. Science 236: 1657-1661. [DOI] [PubMed] [Google Scholar]
- Gallie B.L., Campbell, C., Devlin, H., Duckett, A., and Squire, J.A. 1999. Developmental basis of retinal-specific induction of cancer by RB mutation. Cancer Res. 59: 1731s-1735s. [PubMed] [Google Scholar]
- Herzog S., Lohmann, D.R., Buiting, K., Schuler, A., Horsthemke, B., Rehder, H., and Rieder, H. 2001. Marked differences in unilateral isolated retinoblastomas from young and older children studied by comparative genomic hybridization. Hum. Genet. 108: 98-104. [DOI] [PubMed] [Google Scholar]
- Horowitz J.M., Park, S.H., Bogenmann, E., Cheng, J.C., Yandell, D.W., Kaye, F.J., Minna, J.D., Dryja, T.P., and Weinberg, R.A. 1990. Frequent inactivation of the retinoblastoma anti-oncogene is restricted to a subset of human tumor cells. Proc. Natl. Acad. Sci. 87: 2775-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes K.A., Ransom, N., Papermaster, D.S., Lasudry, J.G., Albert, D.M., and Windle, J.J. 1994. Apoptosis or retinoblastoma: Alternative fates of photoreceptors expressing the HPV-16 E7 gene in the presence or absence of p53. Genes & Dev. 8: 1300-1310. [DOI] [PubMed] [Google Scholar]
- Hurford R.K., Cobrinik, D., Lee, M.H., and Dyson, N. 1997. pRB and p107/p130 are required for the regulated expression of different sets of E2F responsive genes. Genes & Dev. 11: 1447-1463. [DOI] [PubMed] [Google Scholar]
- Jacks T., Fazeli, A., Schmitt, E.M., Bronson, R.T., Goodell, M.A., and Weinberg, R.A. 1992. Effects of an Rb mutation in the mouse. Nature 359: 295-300. [DOI] [PubMed] [Google Scholar]
- Jacks T., Remington, L., Williams, B.O., Schmitt, E.M., Halachmi, S., Bronson, R.T., and Weinberg, R.A. 1994. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 4: 1-7. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Zacksenhaus, E., Gallie, B.L., and Phillips, R.A. 1997. The retinoblastoma gene family is differentially expressed during embryogenesis. Oncogene 14: 1789-1797. [DOI] [PubMed] [Google Scholar]
- Kyritsis A.P., Tsokos, M., Triche, T.J., and Chader, G.J. 1984. Retinoblastoma—Origin from a primitive neuroectodermal cell? Nature 307: 471-473. [DOI] [PubMed] [Google Scholar]
- Lee W.H., Bookstein, R., Hong, F., Young, L.J., Shew, J.Y., and Lee, E.Y. 1987. Human retinoblastoma susceptibility gene: Cloning, identification, and sequence. Science 235: 1394-1399. [DOI] [PubMed] [Google Scholar]
- Lee E.Y., Chang, C.Y., Hu, N., Wang, Y.C., Lai, C.C., Herrup, K., Lee, W.H., and Bradley, A. 1992. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature 359: 288-294. [DOI] [PubMed] [Google Scholar]
- Lee M.H., Williams, B.O., Mulligan, G., Mukai, S., Bronson, R.T., Dyson, N., Harlow, E., and Jacks, T. 1996. Targeted disruption of p107: Functional overlap between p107 and Rb. Genes & Dev. 10: 1621-1632. [DOI] [PubMed] [Google Scholar]
- Lillington D.M., Kingston, J.E., Coen, P.G., Price, E., Hungerford, J., Domizio, P., Young, B.D., and Onadim, Z. 2003. Comparative genomic hybridization of 49 primary retinoblastoma tumors identifies chromosomal regions associated with histopathology, progression, and patient outcome. Genes Chromosomes Cancer 36: 121-128. [DOI] [PubMed] [Google Scholar]
- Lipinski M.M. and Jacks, T. 1999. The retinoblastoma gene family in differentiation and development. Oncogene 18: 7873-7882. [DOI] [PubMed] [Google Scholar]
- Maandag E.C., van der Valk, M., Vlaar, M., Feltkamp, C., O'Brien, J., van Roon, M., van der Lugt, N., Berns, A., and te Riele, H. 1994. Developmental rescue of an embryonic-lethal mutation in the retinoblastoma gene in chimeric mice. EMBO J. 13: 4260-4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod K.F., Hu, Y., and Jacks, T. 1996. Loss of Rb activates both p53-dependent and independent cell death pathways in the developing mouse nervous system. EMBO J. 15: 6178-6188. [PMC free article] [PubMed] [Google Scholar]
- MacPherson D., Sage, J., Crowley, D., Trumpp, A., Bronson, R.T., and Jacks, T. 2003. Conditional mutation of Rb causes cell cycle defects without apoptosis in the central nervous system. Mol. Cell Biol. 23: 1044-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mairal A., Pinglier, E., Gilbert, E., Peter, M., Validire, P., Desjardins, L., Doz, F., Aurias, A., and Couturier, J. 2000. Detection of chromosome imbalances in retinoblastoma by parallel karyotype and CGH analyses. Genes Chromosomes Cancer 28: 370-379. [PubMed] [Google Scholar]
- Marquardt T. and Gruss, P. 2002. Generating neuronal diversity in the retina: One for nearly all. Trends Neurosci. 25: 32-38. [DOI] [PubMed] [Google Scholar]
- Marquardt T., Ashery-Padan, R., Andrejewski, N., Scardigli, R., Guillemot, F., and Gruss, P. 2001. Pax6 is required for the multipotent state of retinal progenitor cells. Cell 105: 43-55. [DOI] [PubMed] [Google Scholar]
- Muller H., Lukas, J., Schneider, A., Warthoe, P., Bartek, J., Eilers, M., and Strauss, M. 1994. Cyclin D1 expression is regulated by the retinoblastoma protein. Proc. Natl. Acad. Sci. 91: 2945-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robanus-Maandag E., Dekker, M., van der Valk, M., Carrozza, M.L., Jeanny, J.C., Dannenberg, J.H., Berns, A., and te Riele, H. 1998. p107 is a suppressor of retinoblastoma development in pRb-deficient mice. Genes & Dev. 12: 1599-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim A., Wiknjosastro, G.H., Danukusumo, D., Barnas, B., and Zalud, I. 1998. Fetal retinoblastoma. J. Ultrasound Med. 17: 717-720. [DOI] [PubMed] [Google Scholar]
- Schneider J.W., Gu, W., Zhu, L., Mahdavi, V., and Nadal-Ginard, B. 1994. Reversal of terminal differentiation mediated by p107 in Rb-/- muscle cells. Science 264: 1467-1471. [DOI] [PubMed] [Google Scholar]
- Sicinski P., Donaher, J.L., Parker, S.B., Li, T., Fazeli, A., Gardner, H., Haslam, S.Z., Bronson, R.T., Elledge, S.J., and Weinberg, R.A. 1995. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82: 621-630. [DOI] [PubMed] [Google Scholar]
- Soriano P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21: 70-71. [DOI] [PubMed] [Google Scholar]
- Symonds H., Krall, L., Remington, L., Saenz-Robles, M., Lowe, S., Jacks, T., and Van Dyke, T. 1994. p53-dependent apoptosis suppresses tumor growth and progression in vivo. Cell 78: 703-711. [DOI] [PubMed] [Google Scholar]
- Tajima Y., Munakata, S., Ishida, Y., Nakajima, T., Sugano, I., Nagao, K., Minoda, K., and Kondo, Y. 1994. Photoreceptor differentiation of retinoblastoma: An electron microscopic study of 29 retinoblastomas. Pathol. Int. 44: 837-843. [DOI] [PubMed] [Google Scholar]
- Takebayashi T., Higashi, H., Sudo, H., Ozawa, H., Suzuki, E., Shirado, O., Katoh, H., and Hatakeyama, M. 2003. NF-κB-dependent induction of cyclin D1 by retinoblastoma protein (pRB) family proteins and tumor-derived pRB mutants. J. Biol. Chem. 278: 14897-14905. [DOI] [PubMed] [Google Scholar]
- Trimarchi J.M. and Lees, J.A. 2002. Sibling rivalry in the E2F family. Nat. Rev. Mol. Cell Biol. 3: 11-20. [DOI] [PubMed] [Google Scholar]
- Turner D.L. and Cepko, C.L. 1987. A common progenitor for neurons and glia persists in rat retina late in development. Nature 328: 131-136. [DOI] [PubMed] [Google Scholar]
- Turner D.L., Snyder, E.Y., and Cepko, C.L. 1990. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron 4: 833-845. [DOI] [PubMed] [Google Scholar]
- Vooijs M., te Riele, H., van der Valk, M., and Berns, A. 2002. Tumor formation in mice with somatic inactivation of the retinoblastoma gene in interphotoreceptor retinol binding protein-expressing cells. Oncogene 21: 4635-4645. [DOI] [PubMed] [Google Scholar]
- Vrabec T., Arbizo, V., Adamus, G., McDowell, J.H., Hargrave, P.A., and Donoso, L.A. 1989. Rod cell-specific antigens in retinoblastoma. Arch. Ophthalmol. 107: 1061-1063. [DOI] [PubMed] [Google Scholar]
- Weinberg R.A. 1995. The retinoblastoma protein and cell cycle control. Cell 81: 323-330. [DOI] [PubMed] [Google Scholar]
- Williams B.O., Remington, L., Albert, D.M., Mukai, S., Bronson, R.T., and Jacks, T. 1994a. Cooperative tumorigenic effects of germline mutations in Rb and p53. Nat. Genet. 7: 480-484. [DOI] [PubMed] [Google Scholar]
- Williams B.O., Schmitt, E.M., Remington, L., Bronson, R.T., Albert, D.M., Weinberg, R.A., and Jacks, T. 1994b. Extensive contribution of Rb-deficient cells to adult chimeric mice with limited histopathological consequences. EMBO J. 13: 4251-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., de Bruin, A., Saavedra, H.I., Starovic, M., Trimboli, A., Yang, Y., Opavska, J., Wilson, P., Thompson, J.C., Ostrowski, M.C., et al. 2003. Extra-embryonic function of Rb is essential for embryonic development and viability. Nature 421: 942-947. [DOI] [PubMed] [Google Scholar]
- Young R.W. 1985. Cell proliferation during postnatal development of the retina in the mouse. Brain Res. 353: 229-239. [DOI] [PubMed] [Google Scholar]
- Zacksenhaus E., Jiang, Z., Chung, D., Marth, J.D., Phillips, R.A., and Gallie, B.L. 1996. pRb controls proliferation, differentiation, and death of skeletal muscle cells and other lineages during embryogenesis. Genes & Dev. 10: 3051-3064. [DOI] [PubMed] [Google Scholar]
- Zhang J., Gray, J., Wu, L., Leone, G., Rowan, S., Cepko, C.L., Zhu, X., Craft, C.M., and Dyer, M.A. 2004. Rb regulates proliferation and rod photoreceptor development in the mouse retina. Nat. Genet. 36: 351-360. [DOI] [PubMed] [Google Scholar]