Abstract
Background
Frontotemporal dementia (FTD) is a heterogeneous neurodegenerative disorder with a strong genetic component. The cerebellum has not traditionally been felt to be involved in FTD but recent research has suggested a potential role.
Methods
We investigated the volumetry of the cerebellum and its subregions in a cohort of 44 patients with genetic FTD (20 MAPT, 7 GRN, and 17 C9orf72 mutation carriers) compared with 18 cognitively normal controls. All groups were matched for age and gender. On volumetric T1-weighted magnetic resonance brain images we used an atlas propagation and label fusion strategy of the Diedrichsen cerebellar atlas to automatically extract subregions including the cerebellar lobules, the vermis and the deep nuclei.
Results
The global cerebellar volume was significantly smaller in C9orf72 carriers (mean (SD): 99989 (8939) mm3) compared with controls (108136 (7407) mm3). However, no significant differences were seen in the MAPT and GRN carriers compared with controls (104191 (6491) mm3 and 107883 (6205) mm3 respectively). Investigating the individual subregions, C9orf72 carriers had a significantly lower volume than controls in lobule VIIa-Crus I (15% smaller, p < 0.0005), whilst MAPT mutation carriers had a significantly lower vermal volume (10% smaller, p = 0.001) than controls. All cerebellar subregion volumes were preserved in GRN carriers compared with controls.
Conclusion
There appears to be a differential pattern of cerebellar atrophy in the major genetic forms of FTD, being relatively spared in GRN, localized to the lobule VIIa-Crus I in the superior-posterior region of the cerebellum in C9orf72, the area connected via the thalamus to the prefrontal cortex and involved in cognitive function, and localized to the vermis in MAPT, the ‘limbic cerebellum’ involved in emotional processing.
Keywords: Cerebellum, Genetic frontotemporal dementia, C9orf72
Highlights
-
•
Different pattern of cerebellar atrophy in the major genetic forms of FTD
-
•
Involvement of the lobule VIIa-Crus I in C9orf72 carriers, related to cognition
-
•
MAPT carriers show vermal involvement, linked to emotional processing
-
•
GRN carriers show relative sparing of the cerebellum
1. Introduction
Frontotemporal dementia (FTD) is a clinically, pathologically and genetically heterogeneous neurodegenerative disorder, commonly presenting with progressive impairment in behaviour (behavioural variant FTD, bvFTD) or language (primary progressive aphasia, PPA). Around a third of patients with FTD have an autosomal dominant mutation in one of three genes: microtubule-associated protein tau (MAPT), progranulin (GRN) and chromosome 9 open reading frame 72 (C9orf72) (Rohrer and Warren, 2011). Neuroimaging and pathological studies of FTD have emphasized the key roles of the frontal, temporal, insular and cingulate cortices as well as subcortical structures such as the striatum and thalamus, but minimal attention has been paid to the potential role of the cerebellum.
Although its function has traditionally been felt to be related solely to the co-ordination of movement, research over the past twenty years has highlighted a role for the cerebellum in cognitive and emotional processing (Schmahmann, 1991, D'Angelo and Casali, 2013, Middleton and Strick, 2000, Strick et al., 2009, Makris et al., 2003). It is extensively connected with different brain regions, including key areas involved in FTD, e.g. via the thalamus to the prefrontal cortex (Behrens et al., 2003, Palesi et al., 2015), and to the limbic system via a direct cerebello-limbic pathway (Arrigo et al., 2014).
Interest in the cerebellum in FTD has arisen from the association of C9orf72 mutations with cerebellar pathology at post-mortem and a number of voxel-based morphometry MRI studies have now found involvement of the cerebellum in this group (Whitwell et al., 2012, Irwin et al., 2013, Mahoney et al., 2012, Rohrer et al., 2015). However detailed region of interest studies have not been performed, nor direct comparison across the different major genetic forms of FTD. Therefore, the aim of this study was to investigate the volume of the cerebellum and its subregions in a cohort of genetic FTD patients, and to determine whether specific cerebellar regions are associated with genetic mutations in MAPT, GRN and C9orf72 genes.
2. Methods
We reviewed the UCL Dementia Research Centre FTD database to identify all patients who were symptomatic carriers of a mutation in the MAPT, GRN or C9orf72 genes and who had also undergone a volumetric T1-weighted MRI. 44 patients were identified: 20 MAPT (19 with bvFTD and one with a corticobasal syndrome), 7 GRN (3 bvFTD and 4 PPA) and 17 C9orf72 (13 bvFTD, 2 FTD with motor neurone disease and 2 PPA). No significant differences were seen in age at scan (p = 0.071, Kruskal–Wallis test) or gender (p = 0.301 Chisquare test) between the groups: 57.6 (7.1) years for MAPT (75% male), 63.0 (7.0) years for GRN (43% male) and 61.4 (6.7) years for C9orf72 (65% male). Disease duration at time of scan was significantly different between the groups (p = 0.026, Kruskal–Wallis test): 7.0 (4.3) years for MAPT, 2.7 (1.9) for GRN and 6.0 (4.1) years for C9orf72. However, each genetic FTD cause is known to vary in its disease progression and so we examined whole brain volume (corrected for total intracranial volume, TIV) as a proxy of overall disease stage. No significant differences were seen between the groups (p = 0.610, Kruskal–Wallis test): 1108.8 (388.7) cm3 for MAPT, 1118.9 (411.7) cm3 for GRN and 1098.1 (661) cm3 for C9orf72, suggesting that the groups were approximately matched for disease severity. Eighteen cognitively normal subjects, with a similar age and gender to the carriers, were identified as controls: 56.4 (14.3) years (50% male). The study was approved by the local ethics committee and written informed consent was obtained from all participants.
Raw MR images were pre-processed to correct for magnetic field bias (inhomogeneity) using a non-parametric non-uniform intensity normalization (N3) algorithm (Sled et al., 1998, Boyes et al., 2008). We then used an atlas propagation and label fusion strategy of the Diedrichsen cerebellar atlas to automatically extract subregions of the cerebellum: the cerebellar lobules (I-IV, V, VI, VIIa-Crus I, VIIa-Crus II, VIIb, VIIIa, VIIIb, IX and X), the vermis and the deep nuclei (dentate, interposed and fastigial) (Cardoso et al., 2015, Diedrichsen et al., 2009, Diedrichsen et al., 2011). No significant asymmetry was noted in the volumes and so right and left-sided results were combined for each subregion. All volumes were corrected for TIV, which was calculated using the Statistical Parametric Mapping (SPM) 12 software, version 6470 (www.fil.ion.ucl.ac.uk/spm), running under Matlab R2014b (Math Works, Natick, MA, USA) (Malone et al., 2015). Statistical analyses were performed in SPSS software (SPSS Inc., Chicago, IL, USA) version 22.0, with differences in volumes of the cerebellar lobules, vermis and deep nuclei between all groups tested using the Mann–Whitney U test.
3. Results
C9orf72 mutation carriers showed the lowest global cerebellar volume (mean (SD): 99989 (8939) mm3), significantly smaller than controls (108136 (7407) mm3, p = 0.006 on Mann–Whitney U test), and the GRN group (107883 (6205) mm3, p = 0.028), but not from the MAPT group (104191 (6491) mm3; p = 0.080). No significant differences were seen in the GRN or MAPT groups in comparison with each other or the control group (p = 0.196, MAPT versus controls; p = 0.836, GRN versus controls; p = 0.370, GRN versus MAPT).
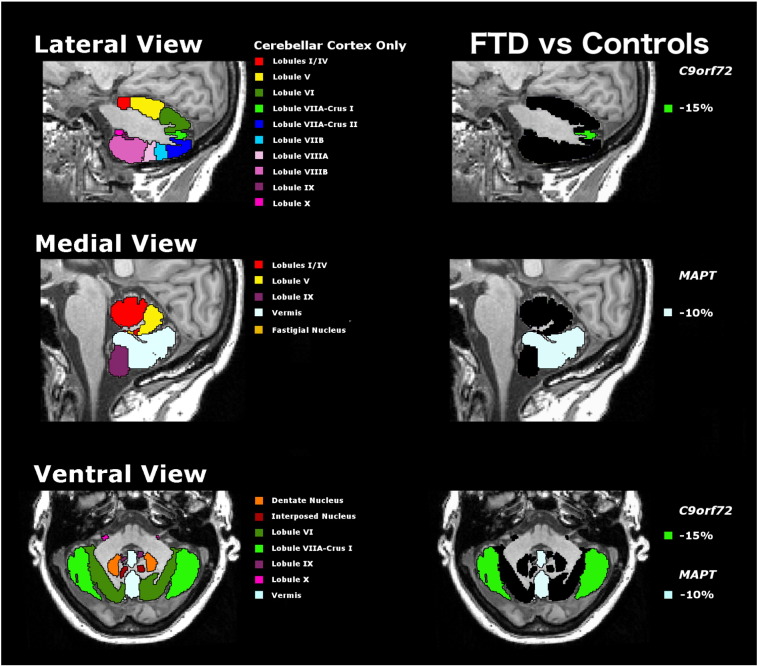
For the 14 individual subregions of the cerebellum, a Bonferroni correction for multiple comparisons was made so that only a threshold of p < 0.003 was considered significant. The C9orf72 group showed a significantly lower volume compared with controls of the lobule VIIa-Crus I only (p < 0.0005, 15.2% smaller than controls), whilst the MAPT group showed a significantly lower volume of the vermis only (p = 0.001, 9.5% smaller than controls) (Table 1 and Fig. 1). No other subregion reached statistical significance but there was a trend for lower volumes of the interposed nuclei in both the C9orf72 and MAPT groups compared with controls (p = 0.004 and p = 0.007, respectively). GRN carriers showed preserved cerebellar volumetry with no significant differences from controls.
Table 1.
Volumetry of cerebellar subregions in 18 healthy non-carrier controls and 44 genetic FTD patients. Values denote mean and standard deviation (SD) volumes in mm3 or n (%). p-values denote significance on Mann–Whitney U test. Bold represents a significant difference between groups after correcting for multiple comparisons.
| Controls (18) |
C9orf72 (17) |
MAPT (20) |
GRN (7) |
C9orf72 vs Controls |
MAPT vs Controls |
GRN vs Controls |
C9orf72 |
MAPT |
GRN |
GRN vs C9orf72 |
GRN vs MAPT |
C9orf72 vs MAPT |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Structure | Mean | SD | Mean | SD | Mean | SD | Mean | SD | p-Value | p-Value | p-Value | % difference vs controls | % difference vs controls | % difference vs controls | p-Value | p-Value | p-Value |
| Lobule I–IV | 6236 | 670 | 6363 | 861 | 6250 | 433 | 7053 | 674 | 0.660 | 0.534 | 0.012 | − 2.0 | − 0.2 | − 13.1 | 0.099 | 0.013 | 0.775 |
| Lobule V | 8911 | 897 | 8623 | 905 | 8922 | 742 | 9371 | 737 | 0.503 | 0.553 | 0.125 | 3.2 | − 0.1 | − 5.2 | 0.034 | 0.145 | 0.311 |
| Lobule VI | 15639 | 1452 | 14587 | 2000 | 15277 | 1390 | 16590 | 1930 | 0.067 | 0.534 | 0.244 | 6.7 | 2.3 | − 6.1 | 0.034 | 0.104 | 0.133 |
| Lobule VIIa-Crus I | 21091 | 2467 | 17885 | 1984 | 20360 | 3303 | 19298 | 2237 | < 0.0005 | 0.675 | 0.125 | 15.2 | 3.5 | 8.5 | 0.099 | 0.498 | 0.013 |
| Lobule VIIa-Crus II | 17610 | 2311 | 15807 | 2863 | 16015 | 1822 | 17666 | 752 | 0.067 | 0.033 | 0.534 | 10.2 | 9.1 | − 0.3 | 0.147 | 0.036 | 0.845 |
| Lobule VIIb | 7565 | 802 | 7330 | 976 | 7488 | 781 | 7460 | 611 | 0.483 | 0.515 | 0.534 | 3.1 | 1.0 | 1.4 | 0.951 | 0.850 | 0.729 |
| Lobule VIIIa | 7799 | 704 | 7724 | 927 | 7957 | 558 | 8033 | 756 | 0.909 | 0.361 | 0.495 | 1.0 | − 2.0 | − 3.0 | 0.534 | 0.766 | 0.517 |
| Lobule VIIIb | 7451 | 900 | 6926 | 894 | 7318 | 1077 | 7233 | 834 | 0.245 | 0.806 | 0.790 | 7.0 | 1.8 | 2.9 | 0.288 | 0.685 | 0.270 |
| Lobule IX | 6200 | 828 | 5563 | 799 | 5520 | 709 | 5659 | 864 | 0.013 | 0.010 | 0.158 | 10.3 | 11.0 | 8.7 | 0.318 | 0.533 | 0.988 |
| Lobule X | 860 | 88 | 850 | 108 | 909 | 124 | 882 | 146 | 0.883 | 0.228 | 1.000 | 1.1 | − 5.7 | − 2.6 | 0.757 | 0.607 | 0.149 |
| Vermis | 5034 | 375 | 4624 | 584 | 4554 | 412 | 4862 | 408 | 0.025 | 0.001 | 0.297 | 8.1 | 9.5 | 3.4 | 0.260 | 0.145 | 0.940 |
| Dentate nuclei | 3291 | 426 | 3303 | 406 | 3214 | 422 | 3325 | 524 | 1.000 | 0.361 | 0.836 | − 0.4 | 2.3 | − 1.0 | 0.951 | 0.766 | 0.341 |
| Interposed nuclei | 392 | 44 | 351 | 31 | 357 | 31 | 394 | 54 | 0.004 | 0.007 | 0.836 | 10.4 | 8.9 | − 0.4 | 0.099 | 0.104 | 0.821 |
| Fastigial nuclei | 57 | 10 | 51 | 6 | 51 | 6 | 56 | 10 | 0.062 | 0.033 | 0.836 | 10.5 | 11.1 | 0.9 | 0.147 | 0.104 | 0.988 |
Fig. 1.
Segmentation of the cerebellar lobules and deep nuclei (left side) mapped on a T1-weighted 3T MR image of a control subject. On the right panel significant volumetric differences in FTD are mapped on the same MR image.
4. Discussion
We found a differential pattern of regional cerebellar involvement in the different genetic forms of FTD: lobule VIIA-Crus I in C9orf72, the vermis in MAPT, and sparing in the GRN group. Lobule VIIa-Crus I has been associated with cognitive processing including the direction of complex goal-directed behaviours, through its connections via the ventrolateral and ventroanterior thalamus to the prefrontal cortex (Makris et al., 2003, Palesi et al., 2015, D'Angelo and Casali, 2013). Although the vermis is involved in the integration of sensory input with motor commands (Bogovic et al., 2013, Schmahmann, 2000, Makris et al., 2003), it is also considered the ‘limbic cerebellum’, involved in the modulation of emotions and social behaviours, based on its connections with the limbic brain structures (Schmahmann, 2000, Stoodley and Schmahmman, 2009, Arrigo et al., 2014, Makris et al., 2003).
The involvement of the cerebellum, and particularly the superior-posterior area, in C9orf72 mutation carriers is consistent with previous studies (Whitwell et al., 2012, Irwin et al., 2013, Mahoney et al., 2012), but here is localized to a specific lobule (VIIa–Crus I) which is known to be linked to the thalamus, a key area of atrophy in C9orf72 carriers, and then on to the prefrontal cortex (Behrens et al., 2003, Palesi et al., 2015). Degeneration of a cerebello-thalamic-cortical network has been proposed to underpin disturbances of body schema and related neuropsychiatric symptoms in this group (Downey et al., 2014), and is an early (presymptomatic) feature of the disease (Rohrer et al., 2015).
Identification of vermal involvement in MAPT carriers in this region of interest study is unsurprising given what is known about the pattern of atrophy in this group, where limbic structures are predominantly involved, particularly the amygdala, hippocampus and hypothalamus, which are linked to the cerebellum via the cerebello-limbic pathway (Rohrer et al., 2010, Bocchetta et al., 2015). Patients with MAPT mutations commonly present with impaired social conduct and behaviour, and it is likely that degeneration across the limbic network leads directly to these symptoms.
There is a clear trend to involvement of the interposed nuclei in both C9orf72 and MAPT groups. As part of the deep cerebellar nuclei, they receive intrinsic inputs from the cerebellar cortex to be sent to the other cortical regions via the ventroanterior and ventrolateral thalamic nuclei (Granziera et al., 2009, Makris et al., 2003), and are likely to be part of the cerebello-thalamic-cortical network in C9orf72 carriers and the cerebello-limbic network in MAPT carriers.
In summary, we found that mutations in MAPT and C9orf72 have a differential impact on the cerebellum with GRN carriers having no significant impact. The regions involved are part of different brain networks that are intrinsically linked to the development of the symptoms seen in each of these genetic disorders. It will be important to investigate how early these regions may become affected using data from presymptomatic FTD cohorts such as GENFI (Rohrer et al., 2015). Furthermore, future studies on functional and structural connections of the cerebellum will be helpful in clarifying the role of these networks in genetic FTD.
Disclosures
JDR is an MRC Clinician Scientist (MR/M008525/1) and has received funding from the NIHR Rare Diseases Translational Research Collaboration (BRC149/NS/MH). JDW is supported by a Wellcome Trust Senior Clinical Fellowship (091673/Z/10/Z). SO is funded by the Engineering and Physical Sciences Research Council (EP/H046410/1, EP/J020990/1, EP/K005278), the Medical Research Council (MR/J01107X/1), the EU-FP7 project VPH-DARE@IT (FP7- ICT-2011-9-601055), and the National Institute for Health Research University College London Hospitals Biomedical Research Centre (NIHR BRC UCLH/UCL High Impact Initiative BW.mn.BRC10269). All other authors have nothing to disclose.
Acknowledgments
This work was funded by the Medical Research Council, UK and Alzheimer's Research UK. The authors acknowledge the support of the NIHR Queen Square Dementia Biomedical Research Unit, Leonard Wolfson Experimental Neurology Centre, and the University College London Hospitals NHS Trust Biomedical Research Centre. The Dementia Research Centre is supported by Alzheimer's Research UK, Brain Research Trust, and The Wolfson Foundation.
References
- Arrigo A., Mormina E., Anastasi G.P., Gaeta M., Calamuneri A., Quartarone A., De Salvo S., Bruschetta D., Rizzo G., Trimarchi F., Milardi D. Constrained spherical deconvolution analysis of the limbic network in human, with emphasis on a direct cerebello-limbic pathway. Front. Hum. Neurosci. 2014;8:987. doi: 10.3389/fnhum.2014.00987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens T.E., Johansen-Berg H., Woolrich M.W., Smith S.M., Wheeler-Kingshott C.A., Boulby P.A., Barker G.J., Sillery E.L., Sheehan K., Ciccarelli O., Thompson A.J., Brady J.M., Matthews P.M. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat. Neurosci. 2003;6(7):750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Bocchetta M., Gordon E., Manning E., Barnes J., Cash D.M., Espak M., Thomas D.L., Modat M., Rossor M.N., Warren J.D., Ourselin S., Frisoni G.B., Rohrer J.D. Detailed volumetric analysis of the hypothalamus in behavioral variant frontotemporal dementia. J. Neurol. 2015 doi: 10.1007/s00415-015-7885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogovic J.A., Jedynak B., Rigg R., Du A., Landman B.A., Prince J.L., Ying S.H. Approaching expert results using a hierarchical cerebellum parcellation protocol for multiple inexpert human raters. NeuroImage. 2013;64:616–629. doi: 10.1016/j.neuroimage.2012.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes R.G., Gunter J.L., Frost C., Janke A.L., Yeatman T., Hill D.L., Bernstein M.A., Thompson P.M., Weiner M.W., Schuff N., Alexander G.E., Killiany R.J., DeCarli C., Jack C.R., Fox N.C., ADNI Study Intensity non-uniformity correction using N3 on 3-T scanners with multichannel phased array coils. NeuroImage. 2008;39(4):1752–1762. doi: 10.1016/j.neuroimage.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso M.J., Modat M., Wolz R., Melbourne A., Cash D., Rueckert D., Ourselin S. Geodesic information flows: spatially-variant graphs and their application to segmentation and fusion. IEEE TMI. 2015 doi: 10.1109/TMI.2015.2418298. [DOI] [PubMed] [Google Scholar]
- D'Angelo E., Casali S. Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Front. Neural Circuits. 2013;6:116. doi: 10.3389/fncir.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J., Balsters J.H., Flavell J., Cussans E., Ramnani N. A probabilistic MR atlas of the human cerebellum. NeuroImage. 2009;46(1):39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J., Maderwald S., Küper M., Thürling M., Rabe K., Gizewski E.R., Ladd M.E., Timmann D. Imaging the deep cerebellar nuclei: a probabilistic atlas and normalization procedure. NeuroImage. 2011;54(3):1786–1794. doi: 10.1016/j.neuroimage.2010.10.035. [DOI] [PubMed] [Google Scholar]
- Downey L.E., Fletcher P.D., Golden H.L., Mahoney C.J., Agustus J.L., Schott J.M., Rohrer J.D., Beck J., Mead S., Rossor M.N., Crutch S.J., Warren J.D. Altered body schema processing in frontotemporal dementia with C9ORF72 mutations. J. Neurol. Neurosurg. Psychiatry. 2014;85(9):1016–1023. doi: 10.1136/jnnp-2013-306995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granziera C., Schmahmann J.D., Hadjikhani N., Meyer H., Meuli R., Wedeen V., Krueger G. Diffusion spectrum imaging shows the structural basis of functional cerebellar circuits in the human cerebellum in vivo. PLoS One. 2009;4(4):e5101. doi: 10.1371/journal.pone.0005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin D.J., McMillan C.T., Brettschneider J., Libon D.J., Powers J., Rascovsky K., Toledo J.B., Boller A., Bekisz J., Chandrasekaran K., Wood E.M., Shaw L.M., Woo J.H., Cook P.A., Wolk D.A., Arnold S.E., Van Deerlin V.M., McCluskey L.F., Elman L., Lee V.M., Trojanowski J.Q., Grossman M. Cognitive decline and reduced survival in C9orf72 expansion frontotemporal degeneration and amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry. 2013;84(2):163–169. doi: 10.1136/jnnp-2012-303507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney C.J., Beck J., Rohrer J.D., Lashley T., Mok K., Shakespeare T., Yeatman T., Warrington E.K., Schott J.M., Fox N.C., Rossor M.N., Hardy J., Collinge J., Revesz T., Mead S., Warren J.D. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain. 2012;135(Pt 3):736–750. doi: 10.1093/brain/awr361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N., Hodge S.M., Haselgrove C., Kennedy D.N., Dale A., Fischl B., Rosen B.R., Harris G., Caviness V.S., Schmahmann J.D. Human cerebellum: surface-assisted cortical parcellation and volumetry with magnetic resonance imaging. J. Cogn. Neurosci. 2003;15(4):584–599. doi: 10.1162/089892903321662967. [DOI] [PubMed] [Google Scholar]
- Malone I.B., Leung K.K., Clegg S., Barnes J., Whitwell J.L., Ashburner J., Fox N.C., Ridgway G.R. Accurate automatic estimation of total intracranial volume: a nuisance variable with less nuisance. NeuroImage. 2015;104:366–372. doi: 10.1016/j.neuroimage.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton F.A., Strick P.L. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res. Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Palesi F., Tournier J.D., Calamante F., Muhlert N., Castellazzi G., Chard D., D'Angelo E., Wheeler-Kingshott C.A. Contralateral cerebello-thalamo-cortical pathways with prominent involvement of associative areas in humans in vivo. Brain Struct. Funct. 2015;220(6):3369–3384. doi: 10.1007/s00429-014-0861-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J.D., Warren J.D. Phenotypic signatures of genetic frontotemporal dementia. Curr. Opin. Neurol. 2011;24(6):542–549. doi: 10.1097/WCO.0b013e32834cd442. [DOI] [PubMed] [Google Scholar]
- Rohrer J.D., Ridgway G.R., Modat M., Ourselin S., Mead S., Fox N.C., Rossor M.N., Warren J.D. Distinct profiles of brain atrophy in frontotemporal lobar degeneration caused by progranulin and tau mutations. NeuroImage. 2010;53(3):1070–1076. doi: 10.1016/j.neuroimage.2009.12.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J.D., Nicholas J.M., Cash D.M., van Swieten J., Dopper E., Jiskoot L., van Minkelen R., Rombouts S.A., Cardoso M.J., Clegg S., Espak M., Mead S., Thomas D.L., De Vita E., Masellis M., Black S.E., Freedman M., Keren R., MacIntosh B.J., Rogaeva E., Tang-Wai D., Tartaglia M.C., Laforce R., Jr., Tagliavini F., Tiraboschi P., Redaelli V., Prioni S., Grisoli M., Borroni B., Padovani A., Galimberti D., Scarpini E., Arighi A., Fumagalli G., Rowe J.B., Coyle-Gilchrist I., Graff C., Fallström M., Jelic V., Ståhlbom A.K., Andersson C., Thonberg H., Lilius L., Frisoni G.B., Pievani M., Bocchetta M., Benussi L., Ghidoni R., Finger E., Sorbi S., Nacmias B., Lombardi G., Polito C., Warren J.D., Ourselin S., Fox N.C., Rossor M.N. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the genetic frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. Lancet Neurol. 2015;14(3):253–262. doi: 10.1016/S1474-4422(14)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J.D. An emerging concept. The cerebellar contribution to higher function. Arch. Neurol. 1991;48(11):1178–1187. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D. The role of the cerebellum in affect and psychosis. J. Neurolinguistics. 2000;13:189–214. [Google Scholar]
- Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmman J.D. Functional topography in the human cerebellum: a meta-analysis if neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Strick P.L., Dum R.P., Fiez J.A. Cerebellum and nonmotor function. Annu. Rev. Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Whitwell J.L., Weigand S.D., Boeve B.F., Senjem M.L., Gunter J.L., DeJesus-Hernandez M., Rutherford N.J., Baker M., Knopman D.S., Wszolek Z.K., Parisi J.E., Dickson D.W., Petersen R.C., Rademakers R., Jack C.R., Jr., Josephs K.A. Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72, tau, progranulin and sporadics. Brain. 2012;135(Pt 3):794–806. doi: 10.1093/brain/aws001. [DOI] [PMC free article] [PubMed] [Google Scholar]