Abstract
Harbour seals (Phoca vitulina) are frequently infected with the lungworms Otostrongylus circumlitus and Parafilaroides gymnurus. The infection is often accompanied by secondary bacterial infections and can cause severe bronchopneumonia and even death in affected animals. Hitherto, the detection of lungworm infections was based on post mortem investigations from animals collected within stranding networks and a valid detection method for live free-ranging harbour seals was not available. Recently, an ELISA was developed for detecting lungworm antibodies in harbour seal serum, using major sperm protein (MSP) of the bovine lungworm, Dictyocaulus viviparus as recombinant diagnostic antigen. To determine lungworm seroprevalence in free-ranging harbour seals, serum was taken from four different seal age groups (n = 313) resulting in an overall prevalence of 17.9% (18.9% of males, 16.7% of females). 0.7% of harbour seals up to six weeks of age were seropositive, as were 89% of seals between six weeks and six months, 53.6% between six and 18 months and 24.2% of seals over 18 months of age. In the 18 months and over age group, seropositive animals showed statistically significant reductions in body weight (P = 0.003) and length (P < 0.001). Sera from lungworm infected harbour seals in rehabilitation (n = 6) revealed that duration of antibody persistence may be similar to that of lungworm infected cattle, but further studies are needed to confirm this. Phylogenetic analyses of MSP sequences of different marine and terrestrial mammal parasitic nematodes revealed that lungworm MSP of the genus Dictyocaulus (superfamily Trichostrongyloidea) is more closely related to metastrongylid marine mammal lungworms than to trichostrongylid nematodes of terrestrial hosts.
Keywords: Phoca vitulina, Lungworm infection, Otostrongylus circumlitus, Parafilaroides gymnurus, ELISA, Major sperm protein
Graphical abstract
Highlights
-
•
First study on lungworm seroprevalence in live free-ranging harbour seals.
-
•
Total seroprevalence was 17.9%, but age-dependent differences were observed.
-
•
Six weeks to six months old seals showed highest prevalences (89% positives).
-
•
Seropositive adult seals showed significantly reduced body weight and length.
-
•
Phylogenetic tree construction using MSP of marine and terrestrial mammal parasites.
1. Introduction
After the first seal epidemic occurred in 1988/89, caused by a phocine distemper virus, a network along the coasts of the German Federal State Schleswig–Holstein was established to monitor stranded marine mammals. During necropsy of stranded harbour porpoises and harbour seals findings frequently showed nematodes including lungworms Otostrongylus circumlitus (Crenosomatidae) and Parafilaroides gymnurus (Filaroididae) of harbour seals (Lehnert et al., 2005, Lehnert et al., 2007, Siebert et al., 2007). O. circumlitus was found in the bronchi, in the right heart chamber, the Vena pulmonalis and in the blood vessels of the liver (De Bruyn, 1933, Onderka, 1989, Claussen et al., 1991, Measures, 2001, Lehnert et al., 2007), while P. gymnurus mainly parasitised the alveoles and bronchioles (Stroud and Dailey, 1978; Claussen et al., 1991). Varying lungworm prevalence, up to 76%, was reported in harbour seals found in the German Wadden Sea (Claussen et al., 1991, Lehnert et al., 2007, Siebert et al., 2007). Infections were age-related, with most infections occurring in young animals between two and 18 months of age. Harbour seals may start acquiring lungworm infections after nursing for four weeks and after a post-weaning fast of 15–17 days (Muelbert and Bowen, 1993, Ross et al., 1994) when they start to consume prey species (Measures, 2001). Benthic fish were identified as potential intermediate hosts of lungworms (Dailey, 1970, Bergeron et al., 1997a, Lehnert et al., 2010); however, the complete life cycle is yet unknown.
Lungworms in harbour seals can cause severe pathological changes, like obstruction of bronchial tubes, and are often accompanied by bacterial infections leading to severe bronchopneumonia and death (Measures, 2001, Lehnert et al., 2007, Siebert et al., 2007, Rijks et al., 2008). Clinical symptoms include bronchospasm, anorexia, dehydration, and individual O. circumlitus specimens can be observed in sputum (Bergeron et al., 1997a, Measures, 2001). Lungworm infections are mainly reported from stranded harbour seals during post mortem examinations (Claussen et al., 1991, Siebert et al., 2007, Rijks et al., 2008), but those data can be biased by different influences such as age, diseases and anthropogenic activities (Claussen et al., 1991, Measures, 2001, Siebert et al., 2007). Diagnosis in living seals is difficult, as detecting lungworm larvae in faeces has limited sensitivity (Schnieder, 1992). Collecting harbour seal faeces is logistically challenging and assigning samples to individual free ranging seals is not feasible. Due to the difficulties in diagnosing lungworm infections in living seals, prevalence data in the free-ranging harbour seal populations is missing. Therefore, an existing ELISA for immunodiagnosis of the bovine lungworm Dictyocaulus viviparus (Schnieder, 1992, von Holtum et al., 2008) was adapted to harbour and grey seals with a resulting sensitivity of 98% and a specificity of 100% (Ulrich et al., 2015). The ELISA represents a reliable method for diagnosing lungworm antibodies in serum samples of free-ranging harbour seals. Recombinant major sperm protein (MSP), a protein family occurring in nematode sperm only (Klass and Hirsh, 1981, Ward et al., 1988) serves as diagnostic antigen.
Information about the molecular structure of MSP from nematodes infecting harbour seals and harbour porpoises is missing. Previous phylogenetic analyses within the Metastrongyloidea have been performed on the base of large-subunit and small-subunit ribosomal (r)RNA (Carreno and Nadler, 2003), the ITS-2 region of rDNA (Lehnert et al., 2010) and the 18S and 28S rRNA (Chilton et al., 2006). Those analyses confirmed the close relationship of marine mammal lungworms within their superfamily Metastrongyloidea, an evolutionary old group that was derived from the terrestrial ancestors of seals and porpoises (Anderson, 1984, Carreno and Nadler, 2003).
The aim of this study was to assess lungworm seroprevalence in free-ranging harbour seals in different age groups. Furthermore, consecutive serum samples of harbour seals in rehabilitation were analysed to obtain first information on the persistence of serum anti-lungworm-MSP antibodies. Additionally, MSP genes from different nematodes infecting harbour porpoises and harbour seals were identified and sequenced to explore phylogenetic relationships between marine and terrestrial parasitic nematodes.
2. Material and methods
2.1. ELISA
2.1.1. Age determination of harbour seals
The approximate age of sampled harbour seals was determined and sorted in age groups, considering sampling date, body-length and body-weight. In young seals, navel and canine development was additionally considered. Age group (AG) 1 included harbour seals from birth to six weeks of age, AG 2 harbour seals from six weeks to six months, AG 3 from six to 18 months and AG 4 above 18 months of age.
2.1.2. Sera of free-ranging harbour seals
All experimental procedures involving harbour seals were approved by the Ministry of Energy, Agriculture, the Environment and Rural Areas of the federal state Schleswig Holstein, Germany [permit number: V312-72241.121-19 (70-6/07)], the Danish Nature Agency (SNS-3446-00054 and SN 2001-34461/SN-0005) and the Animal Welfare Division (Ministry of Justice, Denmark, 2005/561-976).
Serum was taken from a total of 313 free-ranging harbour seals. 141 serum samples were taken in June, one sample in May and two samples in July. As 95% of harbour seals are born in June, and nursing takes four weeks, pups sampled in May and June were considered as not weaned (Ross et al., 1994, Abt, 2002). The two animals sampled in July had an unknown weaning status and harbour seals captured in September 2014 were designated as weaned because sampling was conducted when the harbour seals had already finished nursing.
Because of their approximate age, AG 3 and AG 4 animals were considered as weaned regardless of the sampling date. Detailed information on sampled harbour seals is given in Table 1.
Table 1.
Lungworm seroprevalence in harbour seals.
| Harbour seal age group (AG) | Origin/coordinates | No. of samples (male/female) | Seroprevalence % (proportion) |
|---|---|---|---|
| AG 1 free-ranging (not weaneda) | North Sea coast of the federal state Schleswig–Holstein, Germany/ 54°48′55.5″N 8°39′10.0″E |
144 (74 m/70 f) | 1 (m)/0.7% (100% m) |
| AG 2 free-ranging (weaned) | Anholt island in the Kattegat, Denmark/ 56°42′40.9″N 11°31′06.5″E |
9 (5 m/4 f) | 8 (4 m, 4 f)/88.9% (50.0% m, 50.0% f) |
| AG 3 free-ranging (weaned) | Lorenzensplate, sandbank off the North Sea coast of the federal state Schleswig–Holstein, Germany/54°25′00.3″N 8°29′35.7″E | 28 (18 m/10 f) | 15 (10 m, 5 f)/53.7% (66.6% m/33.3% f) |
| AG 4 free-ranging (weaned) | Lorenzensplate, sandbank off the North Sea coast of the federal state Schleswig–Holstein, Germany/54°25′00.3″N 8°29′35.7″E | 132 (72 m/60 f) | 32 (17 m, 15 f)/24.2% (53.1% m, 46.9% f) |
| AG 3 in rehabilitation (weaned) | North Sea coast of The Netherlands/ 53°25′32.2″N 6°27′20.0″E |
6 (5 m/1 f) | 6 (5 m, 1 f)/100% (83.3% m, 16.7% f) |
Except two individuals with an unknown weaning status.
Blood was taken with a 1.20 × 100 mm needle (SUPRA, Ehrhardt Medizinprodukte, Geislingen, Germany) from the extradural intervertebral sinus 5 cm cranial to the pelvis (Dierauf and Gulland, 2001), or from the tarsal sinus of the hind flippers (Sanchez Contreras, 2014) and filled in tubes containing serum separation gel. Serum was obtained by centrifugation at 3000x g for 15 min and stored at −20 °C until use.
2.1.3. Sera of harbour seals in rehabilitation
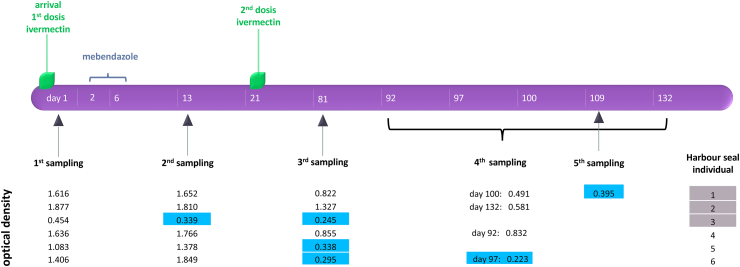
Consecutive serum samples were taken for routine diagnostic examinations from six harbour seals at the Seal Rehabilitation and Research Center (SRRC), Pieterburen, The Netherlands (Table 1). The animals were found between January and June 2015 and were suspected or diagnosed to have lungworm infections. O. circumlitus specimens were found in sputum in three of six individuals, and all six harbour seals showed symptoms like dyspnoe, coughing and a poor body condition. Samples were taken on three to five different occasions during a period of about 12–19 weeks (Fig. 1). Blood was sampled and processed as described above.
Fig. 1.
Anthelmintic treatment pattern, blood sampling time points and corresponding OD values of harbour seals in rehabilitation. Harbour seals (n = 6) were treated twice with ivermectin at arrival (day 1) at the Seal Rehabilitation and Research Centre, Pieterburen, The Netherlands, and 21 days later and once with mebendazole between day 2 and 6. OD values in grey boxes show lungworm-ELISA positive serum samples, those highlighted in blue lungworm-ELISA negative samples. Harbour seal individuals highlighted in dark grey coughed up lungworms one day after arrival. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.1.4. Statistical analyses
Statistical significance of differences between body weight and body length and age distribution within the lungworm infected and negative animals was tested using SigmaStat (version 3.11, Systat Software GmbH, Erkrath, Germany).
2.1.5. ELISA
All sera were tested with the harbour/grey seal adapted recombinant MSP-ELISA as previously described (Ulrich et al., 2015). All samples were analysed in duplicates and the optical density (OD) arithmetic mean of the duplicates was corrected for the blank value. Serum samples were assigned as positive or negative considering the evaluated cut-off value of 0.422 OD (Ulrich et al., 2015).
2.2. Molecular characterisation of MSP in parasitic nematodes of harbour seals and harbour porpoises
2.2.1. Parasite material
Adult nematodes were collected during necropsies of harbour seals and harbour porpoises and their species identified by stereomicroscopic examination (45x magnification; Olympus® SZ 61, Hamburg, Germany). Nematodes of harbour seals included lungworms (O. circumlitus, Crenosomatidae: Metastrongyloidea and P. gymnurus, Filaroididae: Metastrongyloidea), heartworms (Acanthocheilonema spirocauda, Onchocercidae: Filarioidea), intestinal nematodes (Contracaecum osculatum and Pseudoterranova decipiens, Anisakidae: Ascaridoidea) and lungworms of harbour porpoises (Pseudalius inflexus, Torynurus convolutus, Halocercus invaginatus and Stenurus minor, Pseudaliidae: Metastrongyloidea). Nematodes were washed with a 0.9% sodium chloride solution and stored at −80 °C until use.
2.2.2. Characterisation of genomic MSP sequences
Genomic DNA was isolated from female and male individuals using NucleoSpin® Tissue Kit (Macherey–Nagel, Dueren, Germany) following the manufacturer's instructions. Degenerated primers spanning the MSP gene from the start to the stop codon were designed based on nine known MSP sequences [D. viviparus (GenBank accession no. EF012201), Dictyocaulus arnfieldi (KR267306), Dictyocaulus eckerti (KR267310), Oesophagostomum dentatum (AJ627870), Ancylostoma ceylanicum (CB176474), Haemonchus contortus (BM138909), Nippostrongylus brasiliensis (BU493394), Teladorsagia circumcincta (CB037984) and Trichostrongylus vitrinus (AJ616498)]. Selected primer sequences were MSP deg for: 5′-ATGGCHWCAGTTCCHCCHGGHGAYATC-3′ and MSP deg rev: 5′-TCADGGRTTGTAYTCRATVGG-3′. PCR reaction setup was as follows: 16 μl deionized H2O, 2.5 μl 10 × buffer, 0.5 μl dNTP (10 mM each), 1.25 μl forward and reverse primer (10 μM each) and 0.5 μl Perfect Taq Polymerase (5 U/μl, 5 PRIME GmbH, Hilden, Germany), respectively were added to 3 μl genomic DNA as template. PCR cycling (40 cycles) was performed using the following temperature profile: Initial denaturing at 95 °C for 4 min, denaturing at 95 °C for 30 s, annealing primers at 55 °C for 1 min, extending primers at 72 °C for 1 min, and final extension at 72 °C for 10 min. Gel electrophoresis [1% agarose gels stained with Gel Red® (Biotium, Inc., Hayward, Canada)] was performed and visible bands were cut out of the gel, ligated into pCR® 4-TOPO® vector followed by transformation of Escherichia coli One Shot® TOP 10 cells (TOPO TA Cloning® Kit for Sequencing; Invitrogen, Karlsruhe, Germany). Plasmid inserts obtained by using the DNA NucleoSpin® Plasmid Kit (Macherey–Nagel, Dueren, Germany) were sequenced at the SEQLAB Sequence Laboratories (Göttingen, Germany). Sequences of the degenerated primers were removed from the received MSP sequences before submission to GenBank (accession nos. KR267315-KR267323) and phylogenetic analyses.
2.2.3. Generation of the complete MSP mRNA transcript sequence of O. circumlitus
To obtain the full-length MSP mRNA transcript, gene-specific 3′ and 5′ RACE primers were designed based on the obtained O. circumlitus genomic MSP sequence with the PrimerSelect program (DNASTAR, version 5.06; GATC Biotech, Konstanz, Germany). Designed 3′ RACE primer sequence was 5′-ATGCGATACGTTTGAGTATGGTCGTGAGGACACCA-3′, the 5′ RACE primer sequence was 5′-GTGTTGCACCACTCAACAGTGATACGGTCG-3’. RACE experiments were performed using the SMART™ RACE cDNA Amplification Kit (Clontech, Heidelberg, Germany) following the manufacturer's recommendations. The full length transcript was generated by overlapping sequences using Clone Manager 9 (Professional Edition, Scientific and Educational Software, Morrisville, North Carolina, USA) and submitted to GenBank (accession no. KR267324).
2.2.4. Phylogenetic analyses
For phylogenetic analyses, intron sequences were removed from the genomic MSP sequences to compare coding sections only. MSP nucleotide and deduced amino acid sequences of harbour seal and harbour porpoise nematodes were compared to those of nematodes affecting terrestrial animals. The sequences were aligned with the Clustal W method and phylogenetic analyses were performed by bootstrap tests of phylogeny (1000 replicates) using the maximum likelihood method of the software package MEGA 6.0 (Tamura et al., 2013), respectively. The best fit model was determined comparing maximum likelihood fits of 24 different nucleotide substitution models including General Time Reversible (GTR), Hasegawa-Kishino-Yano (HKY), Tamura-Nei (TN93), Tamura 3-parameter (T92), Kimura 2-parameter (K2), Jukes-Cantor (JC) and maximum likelihood fits of 48 different amino acid substitution models including General Time Reversible (GTR), Jones-Taylor-Thornton (JTT), General Reverse Transcriptase (rtREV), General Reversible Chloroplast (cpREV), General Reversible Mitochondrial (mtREV24). Models were determined in consideration of the corrected Akaike Information Criterion (AICc), the Bayesian Information Criterion (BIC), the Maximum Likelihood value (InL) and the number of parameters (including branch lengths) by using the software MEGA 6.0 (Nei and Kumar, 2000, Tamura et al., 2013).
3. Results
3.1. ELISA
3.1.1. Sera of free-ranging harbour seals
In all four age groups of habour seals the lungworm seroprevalence was 17.9% (56/313 individuals). Separated according to sex, 18.9% male (32/169) and 16.7% female (24/144) harbour seals were seropositive.
In AG 1 only one serum sample of the male harbour seal pups sampled in mid-July (unknown weaning status) showed a positive ELISA result with 0.582 OD (0.7%, Table 1). The arithmetic mean OD value of all 144 analysed samples of AG 1 was 0.056 OD (SD 0.075). Within the AG 2 to 4, a lungworm seroprevalence of 32.5% was determined with 55 lungworm-positives out of 169 total samples (Table 1). The arithmetic mean of the positive samples was 0.912 OD [standard deviation (SD) = 0.51], whereas the arithmetic mean of the negative samples was 0.219 OD (SD = 0.09). In AG 2, 88.9% of harbour seals were seropositive, 53.6% in AG3 and 24.2% in AG 4 which was significantly less prevalent (P=<0.0001) than in AG 2 and AG 3. A significant decrease in body weight (P = 0.003) and body length (P=<0.001) was determined in lungworm-positive animals of AG 4 (Table 2). There was no significant difference between male and female harbour seal lungworm infection rates. Detailed results on arithmetic means of the positive and negative OD values and SD of the different age groups are given in Table 2.
Table 2.
Differences between body weight and body length within age groups.
| Lungworm seropositives | Lungworm seronegatives | P-value | |
|---|---|---|---|
| AG 2 | |||
| No. of seals | 8 | 1 | |
| Mean OD | 1.264 (SD 0.72) | 0.325 | |
| Mean body weight [kg] | 22.6 (SD 2.6) | 24 | n.d.a |
| Mean body length [cm] | 112.4 (SD 7.6) | 116 | n.d.a |
| AG 3 | |||
| No. of seals | 15 | 13 | |
| Mean OD | 1.019 (SD 0.516) | 0.275 (SD 0.089) | |
| Mean body weight [kg] | 35.8 (SD 5.6) | 40 (SD 5.2) | 0.051 |
| Mean body length [cm] | 134.3 (SD 11.3) | 136.2 (SD 11.8) | 0.67 |
| AG 4 | |||
| No. of seals | 32 | 100 | |
| Mean OD | 0.774 (SD 0.399) | 0.211 (SD 0.087) | |
| Mean body weight [kg] | 56.2 (SD 15.5) | 67.5 (SD 16.2) | 0.003b |
| Mean body length [cm] | 151.8 (SD 12.7) | 160.9 (SD12.1) | <0.001b |
Not determined because of low sample size.
Statistically significant (P ≤ 0.05).
3.1.2. Sera of harbour seals in rehabilitation
Serum samples of all six investigated individuals (AG 3) showed positive OD values on arrival at the Seal Rehabilitation and Research Centre, Pieterburen, The Netherlands (arithmetic mean: 1.345 OD; SD 0.512). Three of six animals coughed up lungworms on arrival (individuals 1–3, Fig. 1). One harbour seal showed a negative OD (0.339 OD) seven days after the first antiparasitic treatment, two more animals turned antibody-negative 60 days after, and a further individual 88 days after the second antiparasitic treatment. Two of six individuals still showed positive ODs at the final examination before release 71 and 112 days after finishing the antiparasitic treatment scheme (Fig. 1).
3.2. Molecular characterisation of MSP in harbour seal and harbour porpoise parasitic nematodes
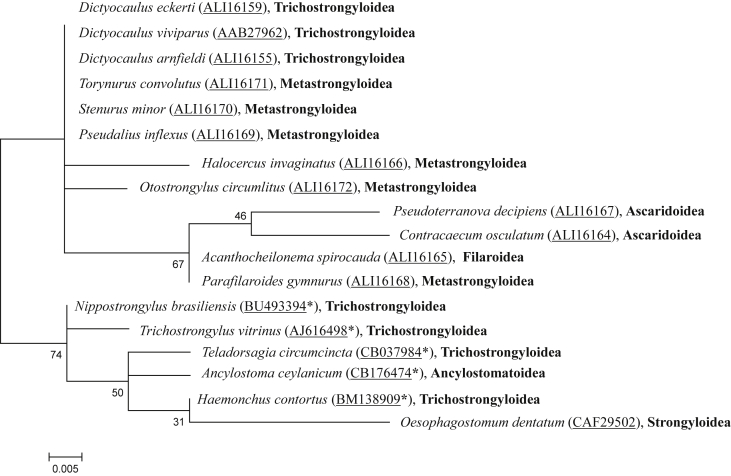
Genomic MSP sequences of the nine investigated marine mammal nematode species contained intron sequences between 59 and 83 base pairs (bp) in length. The full-length MSP mRNA of O. circumlitus included 415 bp without poly(A)+ tail. The 5′ untranslated region (UTR) consisted of 14 bp, the 3’ UTR of 17 bp. The full coding sequence comprised 381 bp encoding 126 amino acids as well as D. viviparus. Sequence comparison showed that O. circumlitus and D. viviparus differed in one amino acid at position 34, at which isoleucine of D. viviparus was substituted by methionine in O. circumlitus. P. gymnurus differed in three amino acids from D. viviparus, most substitutions (six amino acids) were found for P. decipiens (Anisakidae, Ascaridoidea). Alignments are provided in Fig. 2, Fig. 3. Phylogenetic trees including 18 MSP sequences of marine and terrestrial mammal parasitic nematodes on nucleotide and amino acid level are given in Fig. 4, Fig. 5, respectively. The Kimura 2- Parameter using a discrete Gamma distribution with five rate categories was defined as the best fit nucleotide substitution model with 35 parameters, a BIC of 3519,661638, a AICc of 3290,173685 and an InL of −1609,846293. The best fit amino acid substitution model was determined to be Jones-Taylor-Thornton using a discrete Gamma distribution with five rate categories with 34 parameters, a BIC of 1137,728758, a AICc of 951,1837439 and an InL of −440,9376663.
Fig. 2.
Alignment of MSP nucleotide sequences of marine and terrestrial mammal parasitic nematodes using the Clustal W method.
Fig. 3.
Alignment of MSP amino acid sequences of marine and terrestrial mammal parasitic nematodes using the Clustal W method.
Fig. 4.
Phylogenetic tree from maximum likelihood analysis of MSP nucleotide sequences of marine and terrestrial mammal parasitic nematodes including GenBank accession numbers. The percentage of replicate trees in which the associated species clustered together in the bootstrap test (1000 replicates) is shown next to the branches.
Fig. 5.
Phylogenetic tree from maximum likelihood analysis of MSP amino acid sequences of marine and terrestrial mammal parasitic nematodes including GenBank accession numbers. The percentage of replicate trees in which the associated species clustered together in the bootstrap test (1000 replicates) is shown next to the branches. *amino acid sequence translated from EST sequences.
On nucleotide level, D. viviparus, D. arnfieldi and D. eckerti, belonging to the subfamily Dictyocaulidae (Trichostrongylidea), showed a genetic similarity of 94% and 82% (Fig. 4) and were 100% identical on amino acid level (Fig. 5). On amino acid level, MSP of marine mammal parasites grouped in the same cluster as the Dictyocaulidae (Trichostrongyloidea). The other included species of the superfamily Trichostrongyloidea (N. brasiliensis, T. vitrinus, T. circumcincta, H. contortus), together with the two further terrestrial mammal parasites Oesophagostomum dentatum (Strongyloidea) and A. ceylanicum (Ancylostomatoidea), formed a separate cluster from the Dictyocaulidae and marine mammal parasites (Fig. 5).
4. Discussion
In the present study, prevalence of lungworm infection in live free-ranging harbour seal populations was investigated for the first time. Assuming lungworm larvae transmission to be dependent on preying upon the intermediate hosts, unweaned seal pups (AG 1) were unlikely to be infected by O. circumlitus and P. gymnurus as they did not consume fish or other potential intermediate hosts (Dailey, 1970, Bergeron et al., 1997a, Lehnert et al., 2010). Supporting that hypothesis, lungworm seroprevalence in AG 1 was 0.7%. The one seropositive individual of AG 1 was sampled in mid-July, about six weeks after majority of harbour seals give birth to their offspring (Bonner, 1972; Siebert et al., 2012). After nursing and the post-weaning fast, which takes approximately six weeks in total (Muelbert and Bowen, 1993, Ross et al., 1994), the lungworm-positive specimen may have been already preying and developing adult parasites. Even though a lungworm-infection in mid-July seems early, it may be explained by early births during the breeding period (Measures, 2001). Additionally, the measured OD value of the seropositive seal pup is 0.582 OD, which is relatively close to the cut-off value of 0.422 OD and distinctively away from the mean positive OD values of AG 2 (1.264 OD) and AG 3 (1.019 OD). This indicates that the lungworm positive seal pup was in early patency and had just started to develop anti-MSP antibodies, which supports the aforementioned aspects on infection time pattern.
In AGs 2 to 4, 32.5% of the free-ranging harbour seal population in the German Wadden Sea and Danish Kattegat was seropositive for lungworm infections. No correlation between sex distribution and lungworm infection was found. The majority of seropositive animals belonged to AG 2 (88.9%) and AG 3 (53.6%), covering 7 weeks–18 months of life, confirming age-related lungworm infection reported previously (Onderka, 1989, Claussen et al., 1991, Lehnert et al., 2007, Siebert et al., 2007). AG 3 represents a transition between young seals (AG 2) being infected often by lungworms and adult seals (AG 4) showing a seroprevalence of only 24.2%.
In contrast to harbour seals, lungworm infections in harbour porpoises seem to accumulate with age and could possibly have infections throughout their life-time (Measures, 2001, Lehnert et al., 2005). This may be due to harbour porpoises being weaned eight to ten months later than harbour seals (Lockyer, 2003). They therefore get infected at a later point when they start ingesting prey species (Lockyer, 2003). However, transmission of marine lungworm larvae is not completely understood. Besides food-borne infection, transplacental transmission in cetaceans has been hypothesised (Dailey et al., 1991) and transmammary infection cannot be excluded (Conlogue et al., 1985). Another explanation for parasite accumulation in harbour porpoises is the possible lack of protective immunity to lungworm infections in this marine mammal species.
Because adult harbour seals were observed to be less infected than young seals, a development of a protective immunity has been assumed (Claussen et al., 1991, Bergeron et al., 1997b). Such immunity has been described in cattle against the bovine lungworm D. viviparus (Jarrett et al., 1958, Jarrett et al., 1959, Enigk and Hildebrandt, 1969, McKeand et al., 1995), lasting for a period of approximately six to twelve months (Michel et al., 1965). Whether seropositive animals of AG 4 are due to a reinfection event after loss of immunity or due to remaining antibodies against a lungworm infection in previous age groups remains unknown. To gain first information on the persistence of anti-lungworm antibodies in harbour seals, six individuals were monitored serologically during rehabilitation. Animals were treated with anthelmintics after arrival to the rehabilitation center, but this may not have an impact on antibody persistence. For cattle it has been shown that anthelmintic treatment in advanced patency did not influence lungworm antibody titers (Fiedor et al., 2009). All six harbour seals revealed positive OD values on arrival. The longest seropositivity determined was for an individual that was still positive at release 132 days after arrival. Another animal turned seronegative between day 100 (fourth sampling) and day 109 (fifth sampling), and another still, was seropositive at its release on day 92. The second half of monitored harbour seals showed a shorter period of antibody persistence. Two animals turned seronegative between day 13 (second sampling) and day 81 (third sampling) after arrival, and one individual was already seronegative 13 days after arrival. As the latter had a very low positive OD value on arrival, it may be concluded that this individual was already in the post-patency phase. However, as the time point of infection and the duration of patency in harbour seals is unknown (Measures, 2001), it can only be assumed that those harbour seals with longer lasting antibody titers got infected at a later point compared to those whose antibodies decreased earlier. Studies on serum persistence of lungworm-antibodies in cattle revealed positive OD values until day 41–203 post infection (pi) with an overall mean detection period until day 126–143 pi (Cornelissen et al., 1997, Fiedor et al., 2009). Antibody persistence in harbour seals may be similar, but further studies with a larger sample size or experimental infection studies are needed to confirm this presumption.
Harbour seals that had never been affected by lungworms or those who had overcome a lungworm infection relatively early, are assumed to be able to dive and prey appropriately to their needs as opposed to infected seals. As a consequence, lungworm infected seals are not able to grow as fast as their healthy conspecifics due to respiratory difficulties and shorter diving and feeding times (Onderka, 1989, Measures, 2001). This became obvious in AG 4, in which lungworm seropositive harbour seals showed statistically significantly reduced body weights and lengths compared to the sero-negative individuals. Lungworm infected harbour seals in AG 3 showed a trend towards reduced body weight (P = 0.051), whereas body length was unaffected. Similarly, a negative correlation between infection with O. circumlitus and sternal blubber thickness has been reported for ringed seals (Bergeron et al., 1997b).
Lungworms, as well as all other nematodes, possess the sperm protein MSP, which enables sperm motility (Miller et al., 2001, Smith, 2014). The molecular characteristics of this protein has been described in several terrestrial host species, including the bovine lungworm D. viviparus (Strube et al., 2009), whose MSP serves as diagnostic antigen in the cattle as well as harbour seal lungworm ELISA (von Holtum et al., 2008, Fiedor et al., 2009, Ulrich et al., 2015). However, no molecular information on MSP from nematodes infecting harbour seals and harbour porpoises was available. As MSP is essential for nematode reproduction, and thus for conservation of the species, conservation of the protein was expected (Klass and Hirsh, 1981, Miller et al., 2001, Smith, 2014). The phylogenetic analyses revealed that MSP sequences of the trichostrongylid Dictyocaulidae are more closely related to MSP of the metastrongylid lungworms of marine mammals than to trichostrongylid intestinal nematodes of terrestrial hosts, which formed a separate cluster with strongylid and ancylostomatid representatives. This might indicate that dictyocaulid lungworms may belong to the superfamily Metastrongyloidea rather than to the Trichostrongyloidea. Phylogenetic analysis of mitochondrial encoded proteins showed that the Dictyocaulidae grouped with the exclusion of the terrestrial metastrongylid lungworms of the genus Metastrongylus and Angiostrongylus as well as trichostrongylid (H. contortus), strongylid (O. dentatum) and ancylostomatid (Ancylostoma caninum) nematodes (Gasser et al., 2012). As the present study was based on one gene, further analyses are necessary to verify or falsify the current taxonomical allocation of the Dictyocaulidae to the superfamily Trichostrongyloidea.
5. Conclusions
In all age groups of live free-ranging harbour seals an age-related lungworm seroprevalence of 17.5% was found. Young seals between six weeks and six months were most often infected with a seroprevalence of 88.9% and seals of six to 18 months had a seroprevalence of 56.5%. Analyses on the persistence of lungworm anti-MSP antibodies in harbour seals showed that a protective immunity may be assumed and antibody persistence patterns may be similar to that of cattle. The MSP-ELISA proved to be a valuable method to screen live seals for lungworm infections, providing important data for health surveillance, management and conservation of free-ranging harbour seal populations. Moreover, the molecular MSP similarity within the superfamily Metastrongyloidea provides potential for future development of an ELISA for lungworm detection in harbour porpoises.
Conflict of interest
None of the authors of this paper has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
Acknowledgements
The authors would like thank all helpers at seal catches, the national park rangers, seal hunters, Danish colleagues and other helpers for the collection of harbour seals and Jörg Driver for blood sampling. Parts of the project were funded by the Agency for Coastal Defence National Park and Marine Conservation (ACNM-SH) (4121.5-201-522F) as well as The Ministry of Energy, Agriculture, the Environment and Rural Affairs of the federal state Schleswig–Holstein, Germany (V 5012-0635.70-6).
References
- Abt K.F. Forschungs-u. Technologiezentrum Westküste, Universität Kiel; 2002. Phänologie und Populationsdynamik des Seehundes (Phoca vitulina) im Wattenmeer: Grundlagen zur Messung von Statusparametern. (doctoral thesis) [Google Scholar]
- Anderson R.C. The origins of zooparasitic nematodes. Can. J. Zool. 1984;62:317–328. [Google Scholar]
- Bergeron E., Huot J., Measures L.N. Experimental transmission of Otostrongylus circumlitus (Railliet, 1899) (Metastrongyloidea: Crenosomatidae), a lungworm of seals in eastern arctic Canada. Can. J. Zool. 1997;75:1364–1371. [Google Scholar]
- Bergeron E., Measures L.N., Huot J. Lungworm (Otostrongylus circumlitus) infections in ringed seals (Phoca hispida) from eastern Arctic Canada. Can. J. Fish. Aquat. Sci. 1997;54:2443–2448. [Google Scholar]
- Bonner N. The grey seal and common seal in European waters. Oceanogr. Mar. Biol. Ann. Rev. 1972;10:461–507. [Google Scholar]
- Carreno R.A., Nadler S.A. Phylogenetic analysis of the Metastrongyloidea (Nematoda: Strongylida) inferred from ribosomal RNA gene sequences. J. Parasitol. 2003;89:965–973. doi: 10.1645/GE-76R. [DOI] [PubMed] [Google Scholar]
- Chilton N.B., Huby-Chilton F., Gasser R.B., Beveridge I. The evolutionary origins of nematodes within the order Strongylida are related to predilection sites within hosts. Mol. Phylogenet. Evol. 2006;40:118–128. doi: 10.1016/j.ympev.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Claussen D., Strauss V., Ising S., Jäger M., Schnieder T., Stoye M. The helminth fauna from the common seal (Phoca vitulina vitulina, Linné, 1758) of the Wadden Sea in Lower Saxony. J. Vet. Med. Ser. B. 1991;38:649–656. doi: 10.1111/j.1439-0450.1991.tb00924.x. [DOI] [PubMed] [Google Scholar]
- Conlogue G.J., Ogden J.A., Foreyt W.J. Parasites of the Dall's porpoise (Phocoenoides dalli True) J. Wildl. Dis. 1985;21:160–166. doi: 10.7589/0090-3558-21.2.160. [DOI] [PubMed] [Google Scholar]
- Cornelissen J.B.W.J., Borgsteede F.H.M., Van Milligen F.J. Evaluation of an ELISA for the routine diagnosis of Dictyocaulus viviparus infections in cattle. Vet. Parasitol. 1997;70:153–164. doi: 10.1016/s0304-4017(96)01141-7. [DOI] [PubMed] [Google Scholar]
- Dailey M.D. The transmission of Parafilaroides decorus (Nematoda: Metastrongyloidea) in the California sea lion (Zalophus californianus) Proc. Helminthol. Soc. Wash. 1970;37:215–222. [Google Scholar]
- Dailey M., Walsh M., Odell D., Campbell T. Evidence of prenatal infection in the bottlenose dolphin (Tursiops truncates) with the lungworm Halocercus lagenorhynchi (Nematoda: Pseudaliidae) J. Wildl. Dis. 1991;27:164–165. doi: 10.7589/0090-3558-27.1.164. [DOI] [PubMed] [Google Scholar]
- Dierauf L., Gulland F.M.D. second ed. CRC press; Boca Raton: 2001. CRC Handbook of Marine Mammal Medicine: Health, Disease, and Rehabilitation. [Google Scholar]
- De Bruyn W. Beiträge zur Kenntnis von Strongylus circumlitus, Railliet, aus den Lungen des Seehundes: die neue Gattung Otostrongylus. Zool. Anz. 1933;103:142–153. [Google Scholar]
- Enigk K., Hildebrandt J. Zur Empfänglichkeit der Wiederkäuer für Dictyocaulus viviparus und Dictyocaulus filaria (Strongyloidea, Nematoda) Zentralbl. Veterinarmed. Reihe B. 1969;16:67–76. [PubMed] [Google Scholar]
- Fiedor C., Strube C., Forbes A., Buschbaum S., Klewer A.M., von Samson-Himmelstjerna G., Schnieder T. Evaluation of a milk ELISA for the serodiagnosis of Dictyocaulus viviparus in dairy cows. Vet. Parasitol. 2009;166:255–261. doi: 10.1016/j.vetpar.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Gasser R.B., Jabbar A., Mohandas N., Höglund J., Hall R.S., Littlewood D.T., Jex A.R. Assessment of the genetic relationship between Dictyocaulus species from Bos taurus and Cervus elaphus using complete mitochondrial genomic datasets. Parasit. Vectors. 2012;5:241. doi: 10.1186/1756-3305-5-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett W.F.H., Jennings F.W., McIntyre W.I.M., Mulligan W., Urquhart G.M. Irradiated helminth larvae in vaccination. J. R. Soc. Med. 1958;51:743. doi: 10.1177/003591575805100912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett W.F.H., Jennings F.W., McIntyre W.I.M., Mulligan W.T., Beatrice A.C., Urquhart G.M. Dictyocaulus viviparus: immunological studies on infection: the immunity resulting from experimental infection. Immunology. 1959;2:252. [PMC free article] [PubMed] [Google Scholar]
- Klass M.R., Hirsh D. Sperm isolation and biochemical analysis of the major sperm protein from Caenorhabditis elegans. Dev. Biol. 1981;84:299–312. doi: 10.1016/0012-1606(81)90398-5. [DOI] [PubMed] [Google Scholar]
- Lehnert K., Raga J.A., Siebert U. Macroparasites in stranded and by caught harbour porpoises from German and Norwegian waters. Dis. Aquat. Org. 2005;64:265–269. doi: 10.3354/dao064265. [DOI] [PubMed] [Google Scholar]
- Lehnert K., Raga J.A., Siebert U. Parasites in harbour seals (Phoca vitulina) from the German Wadden Sea between two phocine distember virus epidemics. Helgol. Mar. Res. 2007;61:239–245. [Google Scholar]
- Lehnert K., von Samson-Himmelstjerna G., Schaudien D., Bleidorn C., Wohlsein P., Siebert U. Transmission of lungworms of harbour porpoises and harbour seals: molecular tools determine potential vertebrate intermediate hosts. Int. J. Parasitol. 2010;40:845–853. doi: 10.1016/j.ijpara.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Lockyer C. Harbour porpoises (Phocoena phocoena) in the North Atlantic: biological parameters. NAMMCO Sci. Publ. 2003;5:71–89. [Google Scholar]
- McKeand J.B., Knox D.P., Duncan J.L., Kennedy M.W. Protective immunisation of guinea pigs against Dictyocaulus viviparus using excretory/secretory products of adult parasites. Int. J. Parasitol. 1995;25:95–104. doi: 10.1016/0020-7519(94)e0066-v. [DOI] [PubMed] [Google Scholar]
- Measures L.N. Lungworms of marine mammals. In: Samuel W.M., Pybus M.J., Kocan A.A., editors. Parasitic Diseases of Wild Mammals. second ed. Iowa Sate University Press; Ames: 2001. pp. 279–300. [Google Scholar]
- Michel J.F., Mackenzie A., Bracewell C.D., Cornwell R.L., Eliot J., Herbert C.N., Holman H.H., Sinclair I.J.B. Duration of the acquired resistance of calves to infections with Dictyocaulus viviparus. Res. Vet. Sci. 1965;6:344–395. [PubMed] [Google Scholar]
- Miller M.A., Nguyen V.Q., Lee M.-H., Kosinski M., Schedl T., Caprioli R.M., Greenstein D. A sperm cytoskeletal protein that signals oocyte meiotic maturation and ovulation. Science. 2001;291:2144–2147. doi: 10.1126/science.1057586. [DOI] [PubMed] [Google Scholar]
- Muelbert M.M.C., Bowen W.D. Duration of lactation and postweaning changes in mass and body composition of harbour seal, Phoca vitulina, pups. Can. J. Zool. 1993;71:1405–1414. [Google Scholar]
- Nei M., Kumar S. Oxford University Press; New York: 2000. Molecular Evolution and Phylogenetics. [Google Scholar]
- Onderka D.K. Prevalence and pathology of nematode infections in the lungs of ringed seals (Phoca hispida) of the western arctic of Canada. J. Wildl. Dis. 1989;25:218–224. doi: 10.7589/0090-3558-25.2.218. [DOI] [PubMed] [Google Scholar]
- Rijks J.M., Hoffman J.I., Kuiken T., Osterhaus A.D.M.E., Amos W. Heterozygosity and lungworm burden in harbour seals (Phoca vitulina) Hered. Edinb. 2008;100:587–593. doi: 10.1038/hdy.2008.18. [DOI] [PubMed] [Google Scholar]
- Ross P.S., de Swart R.L., Visser I.K.G., Vedder L.J., Murk W., Bowen W.D., Osterhaus A.D.M.E. Relative immunocompetence of the newborn harbour seal, Phoca vitulina. Vet. Immunol. Immunopathol. 1994;42:331–348. doi: 10.1016/0165-2427(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Sanchez Contreras G.J. 42nd European Association for Aquatic Mammals (EAAM) Symposium. Spain. 2014. Drawing seal blood from the tarsal sinus. [Google Scholar]
- Schnieder T. Use of a recombinant Dictyocaulus viviparus antigen in an enzyme-linked immunosorbent assay for immunodiagnosis of bovine dictyocaulosis. Parasitol. Res. 1992;78:298–302. doi: 10.1007/BF00937087. [DOI] [PubMed] [Google Scholar]
- Siebert U., Wohlsein P., Lehnert K., Baumgärtner W. Pathological findings in harbour seals (Phoca vitulina): 1996-2005. J. Comp. Pathol. 2007;137:47–58. doi: 10.1016/j.jcpa.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Siebert U., Müller S., Gilles A., Sundermeyer J., Narberhaus U.I. Species profiles marine mammals. In: Narberhaus I., Krause J., Bernitt U., editors. Threatened Biodiversity in the German North and Baltic Seas – Sensitivities towards Human Activities and the Effects of Climate Change. vol. 116. 2012. pp. 487–542. (Naturschutz und Biologische Vielfalt). [Google Scholar]
- Smith H.E. The C. elegans Research Community. WormBook; 2014. Nematode sperm motility. [Google Scholar]
- Stroud R.K., Dailey M.D. Parasites and associated pathology observed in pinnipeds stranded along the Oregon coast. J. Wildl. Dis. 1978;14:292–298. doi: 10.7589/0090-3558-14.3.292. [DOI] [PubMed] [Google Scholar]
- Strube C., Sandra B., Schnieder T. Molecular characterization and real-time PCR transcriptional analysis of Dictyocaulus viviparus major sperm proteins. Parasitol. Res. 2009;104:543–551. doi: 10.1007/s00436-008-1228-5. [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich S.A., Lehnert K., Siebert U., Strube C. A recombinant antigen-based enzyme-linked immunosorbent assay (ELISA) for lungworm detection in seals. Parasit. Vectors. 2015;8:443. doi: 10.1186/s13071-015-1054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Holtum C., Strube C., Schnieder T., von Samson-Himmelstjerna G. Development and evaluation of a recombinant antigen-based ELISA for serodiagnosis of cattle lungworm. Vet. Parasitol. 2008;151:218–226. doi: 10.1016/j.vetpar.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Ward S., Burke D.J., Sulston J.E., Coulson A.R., Albertson D.G., Ammons D., Klass M., Hogan E. Genomic organization of major sperm protein genes and pseudogenes in the nematode Caenorhabditis elegans. J. Mol. Biol. 1988;1:1–13. doi: 10.1016/0022-2836(88)90374-9. [DOI] [PubMed] [Google Scholar]