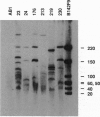
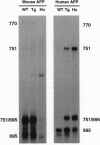
Abstract
One hallmark of Alzheimer disease is the formation in the brain of amyloid plaques containing a small peptide derived from the beta-amyloid precursor protein (APP). The APP gene exhibits a complex pattern of expression in peripheral tissues and in the brain. The entire human APP gene was introduced into embryonic stem (ES) cells by co-lipofection of a 650-kb yeast artificial chromosome (YAC). Three ES lines containing an essentially intact YAC were isolated, and expression of human APP mRNAs at levels comparable to those of endogenous mouse APP transcripts was obtained. A transgenic mouse line was established by germ-line transmission of the APP YAC. RNase protection analysis of human APP mRNAs demonstrated appropriate splicing of the primary APP transcript in ES cells and in the brain of a transgenic animal. These mice may be useful for elucidating the function of the various APP isoforms in vivo.
Full text
PDF
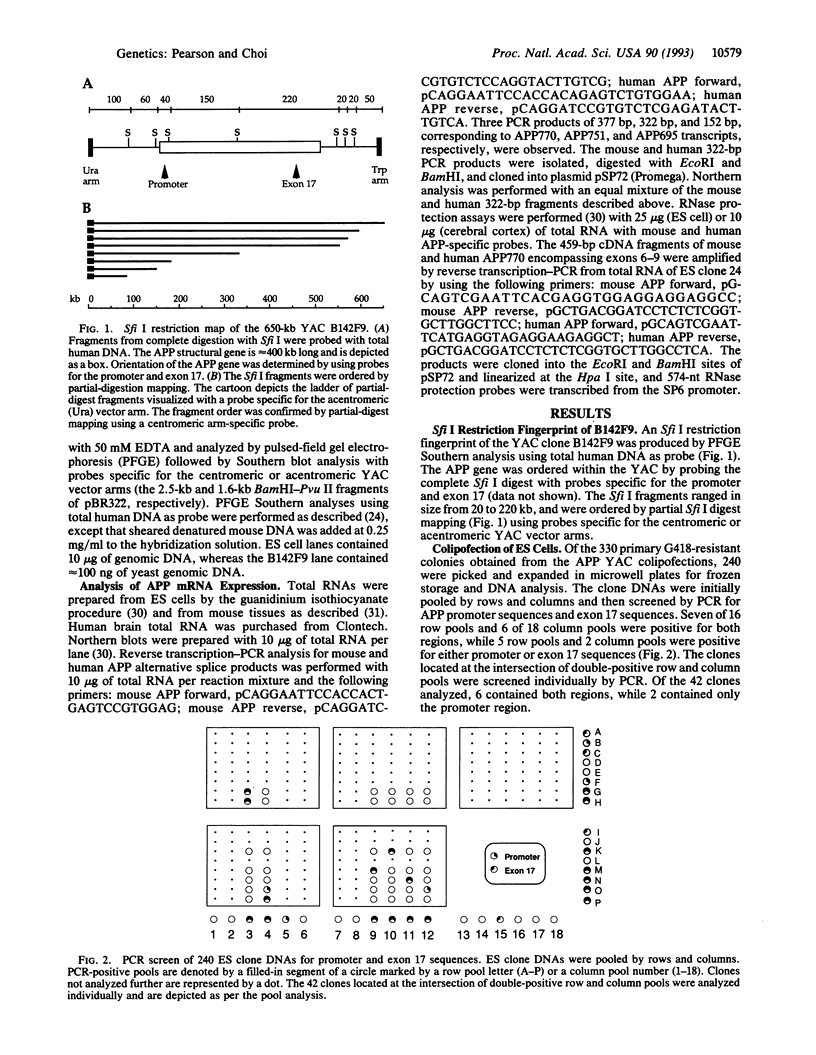
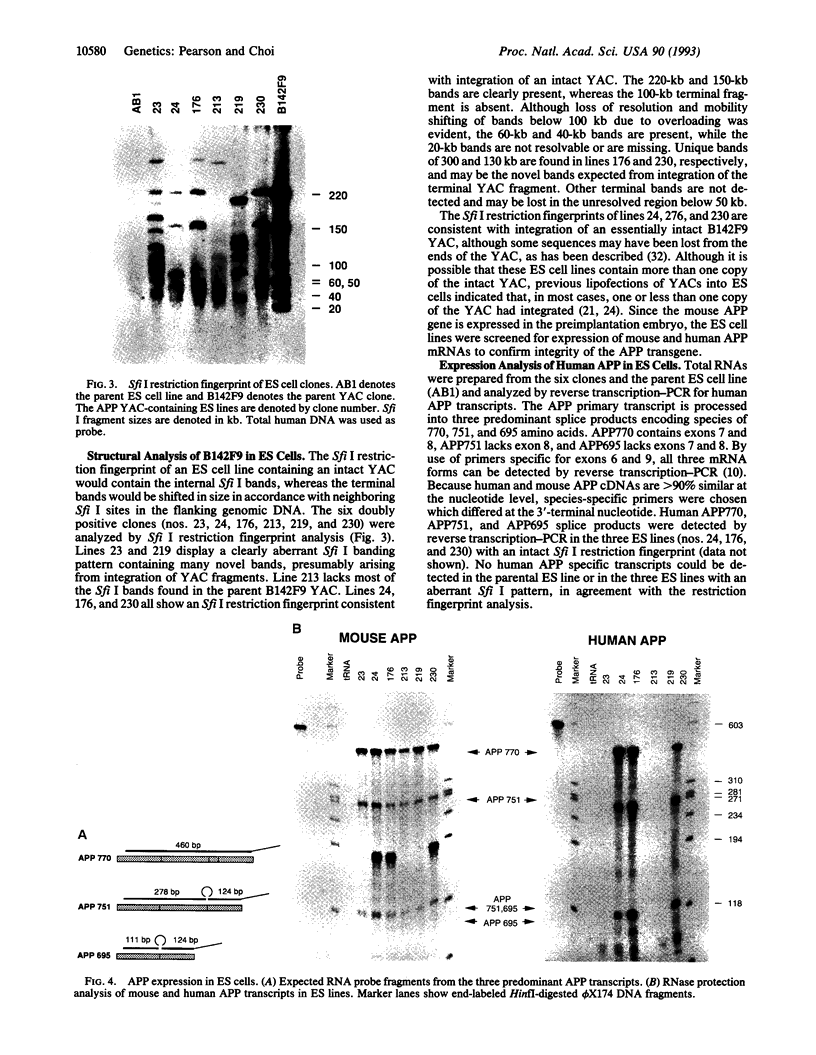

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendotti C., Forloni G. L., Morgan R. A., O'Hara B. F., Oster-Granite M. L., Reeves R. H., Gearhart J. D., Coyle J. T. Neuroanatomical localization and quantification of amyloid precursor protein mRNA by in situ hybridization in the brains of normal, aneuploid, and lesioned mice. Proc Natl Acad Sci U S A. 1988 May;85(10):3628–3632. doi: 10.1073/pnas.85.10.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. L., Allen J. M., Behringer R. R., Gelinas R. E., Palmiter R. D. Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci U S A. 1988 Feb;85(3):836–840. doi: 10.1073/pnas.85.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi T. K., Hollenbach P. W., Pearson B. E., Ueda R. M., Weddell G. N., Kurahara C. G., Woodhouse C. S., Kay R. M., Loring J. F. Transgenic mice containing a human heavy chain immunoglobulin gene fragment cloned in a yeast artificial chromosome. Nat Genet. 1993 Jun;4(2):117–123. doi: 10.1038/ng0693-117. [DOI] [PubMed] [Google Scholar]
- Choi T., Huang M., Gorman C., Jaenisch R. A generic intron increases gene expression in transgenic mice. Mol Cell Biol. 1991 Jun;11(6):3070–3074. doi: 10.1128/mcb.11.6.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidani L., Rooke K., Chartier-Harlin M. C., Hughes D., Tanzi R., Mullan M., Roques P., Rossor M., Hardy J., Goate A. Screening for mutations in the open reading frame and promoter of the beta-amyloid precursor protein gene in familial Alzheimer's disease: identification of a further family with APP717 Val-->Ile. Hum Mol Genet. 1992 Jun;1(3):165–168. doi: 10.1093/hmg/1.3.165. [DOI] [PubMed] [Google Scholar]
- Fisher S., Gearhart J. D., Oster-Granite M. L. Expression of the amyloid precursor protein gene in mouse oocytes and embryos. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1779–1782. doi: 10.1073/pnas.88.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaccone G., Tagliavini F., Linoli G., Bouras C., Frigerio L., Frangione B., Bugiani O. Down patients: extracellular preamyloid deposits precede neuritic degeneration and senile plaques. Neurosci Lett. 1989 Feb 13;97(1-2):232–238. doi: 10.1016/0304-3940(89)90169-9. [DOI] [PubMed] [Google Scholar]
- Goate A., Chartier-Harlin M. C., Mullan M., Brown J., Crawford F., Fidani L., Giuffra L., Haynes A., Irving N., James L. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991 Feb 21;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Golde T. E., Estus S., Usiak M., Younkin L. H., Younkin S. G. Expression of beta amyloid protein precursor mRNAs: recognition of a novel alternatively spliced form and quantitation in Alzheimer's disease using PCR. Neuron. 1990 Feb;4(2):253–267. doi: 10.1016/0896-6273(90)90100-t. [DOI] [PubMed] [Google Scholar]
- Huxley C., Hagino Y., Schlessinger D., Olson M. V. The human HPRT gene on a yeast artificial chromosome is functional when transferred to mouse cells by cell fusion. Genomics. 1991 Apr;9(4):742–750. doi: 10.1016/0888-7543(91)90369-p. [DOI] [PubMed] [Google Scholar]
- Jacobsen J. S., Blume A. J., Vitek M. P. Quantitative measurement of alternatively spliced amyloid precursor protein mRNA expression in Alzheimer's disease and normal brain by S1 nuclease protection analysis. Neurobiol Aging. 1991 Sep-Oct;12(5):585–592. doi: 10.1016/0197-4580(91)90090-7. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Transgenic animals. Science. 1988 Jun 10;240(4858):1468–1474. doi: 10.1126/science.3287623. [DOI] [PubMed] [Google Scholar]
- Jakobovits A., Moore A. L., Green L. L., Vergara G. J., Maynard-Currie C. E., Austin H. A., Klapholz S. Germ-line transmission and expression of a human-derived yeast artificial chromosome. Nature. 1993 Mar 18;362(6417):255–258. doi: 10.1038/362255a0. [DOI] [PubMed] [Google Scholar]
- Johnson S. A., McNeill T., Cordell B., Finch C. E. Relation of neuronal APP-751/APP-695 mRNA ratio and neuritic plaque density in Alzheimer's disease. Science. 1990 May 18;248(4957):854–857. doi: 10.1126/science.2111579. [DOI] [PubMed] [Google Scholar]
- Kitaguchi N., Takahashi Y., Tokushima Y., Shiojiri S., Ito H. Novel precursor of Alzheimer's disease amyloid protein shows protease inhibitory activity. Nature. 1988 Feb 11;331(6156):530–532. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- König G., Mönning U., Czech C., Prior R., Banati R., Schreiter-Gasser U., Bauer J., Masters C. L., Beyreuther K. Identification and differential expression of a novel alternative splice isoform of the beta A4 amyloid precursor protein (APP) mRNA in leukocytes and brain microglial cells. J Biol Chem. 1992 May 25;267(15):10804–10809. [PubMed] [Google Scholar]
- Lamb B. T., Sisodia S. S., Lawler A. M., Slunt H. H., Kitt C. A., Kearns W. G., Pearson P. L., Price D. L., Gearhart J. D. Introduction and expression of the 400 kilobase amyloid precursor protein gene in transgenic mice [corrected]. Nat Genet. 1993 Sep;5(1):22–30. doi: 10.1038/ng0993-22. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990 Sep 21;62(6):1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- Mullan M., Crawford F., Axelman K., Houlden H., Lilius L., Winblad B., Lannfelt L. A pathogenic mutation for probable Alzheimer's disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet. 1992 Aug;1(5):345–347. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- Neve R. L., Rogers J., Higgins G. A. The Alzheimer amyloid precursor-related transcript lacking the beta/A4 sequence is specifically increased in Alzheimer's disease brain. Neuron. 1990 Sep;5(3):329–338. doi: 10.1016/0896-6273(90)90169-g. [DOI] [PubMed] [Google Scholar]
- Olson M. I., Shaw C. M. Presenile dementia and Alzheimer's disease in mongolism. Brain. 1969 Mar;92(1):147–156. doi: 10.1093/brain/92.1.147. [DOI] [PubMed] [Google Scholar]
- Palmert M. R., Golde T. E., Cohen M. L., Kovacs D. M., Tanzi R. E., Gusella J. F., Usiak M. F., Younkin L. H., Younkin S. G. Amyloid protein precursor messenger RNAs: differential expression in Alzheimer's disease. Science. 1988 Aug 26;241(4869):1080–1084. doi: 10.1126/science.2457949. [DOI] [PubMed] [Google Scholar]
- Quon D., Wang Y., Catalano R., Scardina J. M., Murakami K., Cordell B. Formation of beta-amyloid protein deposits in brains of transgenic mice. Nature. 1991 Jul 18;352(6332):239–241. doi: 10.1038/352239a0. [DOI] [PubMed] [Google Scholar]
- Ramírez-Solis R., Rivera-Pérez J., Wallace J. D., Wims M., Zheng H., Bradley A. Genomic DNA microextraction: a method to screen numerous samples. Anal Biochem. 1992 Mar;201(2):331–335. doi: 10.1016/0003-2697(92)90347-a. [DOI] [PubMed] [Google Scholar]
- Richards S. J., Waters J. J., Beyreuther K., Masters C. L., Wischik C. M., Sparkman D. R., White C. L., 3rd, Abraham C. R., Dunnett S. B. Transplants of mouse trisomy 16 hippocampus provide a model of Alzheimer's disease neuropathology. EMBO J. 1991 Feb;10(2):297–303. doi: 10.1002/j.1460-2075.1991.tb07950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumble B., Retallack R., Hilbich C., Simms G., Multhaup G., Martins R., Hockey A., Montgomery P., Beyreuther K., Masters C. L. Amyloid A4 protein and its precursor in Down's syndrome and Alzheimer's disease. N Engl J Med. 1989 Jun 1;320(22):1446–1452. doi: 10.1056/NEJM198906013202203. [DOI] [PubMed] [Google Scholar]
- Sandhu F. A., Salim M., Zain S. B. Expression of the human beta-amyloid protein of Alzheimer's disease specifically in the brains of transgenic mice. J Biol Chem. 1991 Nov 15;266(32):21331–21334. [PubMed] [Google Scholar]
- Schedl A., Montoliu L., Kelsey G., Schütz G. A yeast artificial chromosome covering the tyrosinase gene confers copy number-dependent expression in transgenic mice. Nature. 1993 Mar 18;362(6417):258–261. doi: 10.1038/362258a0. [DOI] [PubMed] [Google Scholar]
- Strauss W. M., Dausman J., Beard C., Johnson C., Lawrence J. B., Jaenisch R. Germ line transmission of a yeast artificial chromosome spanning the murine alpha 1(I) collagen locus. Science. 1993 Mar 26;259(5103):1904–1907. doi: 10.1126/science.8096090. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., McClatchey A. I., Lamperti E. D., Villa-Komaroff L., Gusella J. F., Neve R. L. Protease inhibitor domain encoded by an amyloid protein precursor mRNA associated with Alzheimer's disease. Nature. 1988 Feb 11;331(6156):528–530. doi: 10.1038/331528a0. [DOI] [PubMed] [Google Scholar]
- Wirak D. O., Bayney R., Ramabhadran T. V., Fracasso R. P., Hart J. T., Hauer P. E., Hsiau P., Pekar S. K., Scangos G. A., Trapp B. D. Deposits of amyloid beta protein in the central nervous system of transgenic mice. Science. 1991 Jul 19;253(5017):323–325. doi: 10.1126/science.1857970. [DOI] [PubMed] [Google Scholar]
- Yamada T., Sasaki H., Dohura K., Goto I., Sakaki Y. Structure and expression of the alternatively-spliced forms of mRNA for the mouse homolog of Alzheimer's disease amyloid beta protein precursor. Biochem Biophys Res Commun. 1989 Feb 15;158(3):906–912. doi: 10.1016/0006-291x(89)92808-8. [DOI] [PubMed] [Google Scholar]
- Yamaguchi F., Richards S. J., Beyreuther K., Salbaum M., Carlson G. A., Dunnett S. B. Transgenic mice for the amyloid precursor protein 695 isoform have impaired spatial memory. Neuroreport. 1991 Dec;2(12):781–784. doi: 10.1097/00001756-199112000-00013. [DOI] [PubMed] [Google Scholar]
- Yoshikai S., Sasaki H., Doh-ura K., Furuya H., Sakaki Y. Genomic organization of the human amyloid beta-protein precursor gene. Gene. 1990 Mar 15;87(2):257–263. doi: 10.1016/0378-1119(90)90310-n. [DOI] [PubMed] [Google Scholar]
- de Sauvage F., Octave J. N. A novel mRNA of the A4 amyloid precursor gene coding for a possibly secreted protein. Science. 1989 Aug 11;245(4918):651–653. doi: 10.1126/science.2569763. [DOI] [PubMed] [Google Scholar]