Abstract
As part of a presurgical investigation for a resection of a tumor located in the left temporal brain region, we evaluated pre- and postsurgical language lateralization in a right-handed boy with refractory epilepsy. In this study, we compared functional near infrared spectroscopy (fNIRS) results obtained while the participant performed expressive and receptive language tasks with those obtained using functional magnetic resonance imaging (fMRI). This case study illustrates the potential for NIRS to contribute favorably to the localization of language functions in children with epilepsy and cognitive or behavioral problems and its potential advantages over fMRI in presurgical assessment. Moreover, it suggests that fNIRS is sensitive in localizing an atypical language network or potential brain reorganization related to epilepsy in young patients.
Keywords: Refractory epilepsy, fNIRS, fMRI, EEG, Language localization
1. Introduction
Individuals with refractory epilepsy are investigated for surgical candidacy using noninvasive techniques such as functional magnetic resonance imaging (fMRI) and functional near infrared spectroscopy (fNIRS) for presurgical assessment of language lateralization. However, the clinical value of these techniques is still unclear, especially in the pediatric population. This case study describes the repeated use of pre- and postsurgical fMRI and simultaneous functional near infrared spectroscopy and electroencephalography (fNIRS–EEG) for language localization in a boy with refractory epilepsy who underwent multiple resective surgeries for a left temporal lobe tumor. A comparison of the value of both neuroimaging techniques in this patient is provided.
2. Case report
The patient was born at term and had no family history of epilepsy. He presented with normal language and motor development and early right-handed dominance. Seizure onset occurred at 5 years with drug-resistant focal dyscognitive seizures occurring three to four times a day. His seizures involved a loss of consciousness for 20 to 45 s accompanied by automatisms. His MRI revealed changes compatible with a neuroglial tumor located in the left temporal lobe including Wernicke's area. At 6 years old, the patient underwent a presurgical assessment followed by a first resection of the tumor. Two months postsurgery, daily refractory seizures reoccurred. A second presurgical assessment was performed and led to a second resection of an enlarged tumor at age 10. Initial pre- and postsurgical cognitive assessments revealed normal intellectual functioning with attention and executive deficits as well as a moderate to severe receptive and expressive dysphasia. More specifically, language assessment revealed a poor lexical access and difficulties of phonological awareness and for the understanding of complex instructions. Overall, the cognitive profile remained stable despite repeat excisions.
3. Methods
At 6 and 10 years old, the patient underwent presurgical assessments for language lateralization and localization including fNIRS–EEG and fMRI while he performed expressive and receptive language tasks. At 11 years old, a postneurosurgical fMRI was performed for language investigation.
The expressive language task consisted of a categorical verbal fluency task (e.g., name as many animals as possible). The receptive language was assessed using a passive story listening task. Both tasks were done alternatively with rest periods.
Combined with fNIRS (Imagent, ISS; 11 detectors, 46 sources (690/830 nm)), EEG (19 electrodes) was acquired to identify and exclude epileptic discharges from fNIRS data. Simultaneous EEG was not possible during fMRI (Simens, Germany; 1.5 T) because of technical constraints.
Significant hemodynamic changes in regions of interest (ROIs: Broca's area or Brodmann's areas 44 and 45 and Wernicke's areas or Brodmann's areas 22, 39, and 40, and right homologous regions) were identified by computing a two-tailed paired t-test (fNIRS) and T statistic (fMRI) of a general linear model analysis on hemodynamic responses. For language lateralization, significant (p < 0.05) hemodynamic changes in the left (LH) and right (RH) hemisphere ROIs associated with each language task, compared with rest periods, were used to calculate a lateralization index (LI). The LI was calculated on mean around the peak (5 s) of activations measured in the ROIs (Broca's area for expressive language; Wernicke's area for receptive language). The LI was calculated using the following formula: ((LH − RH) / (LH + RH)). The LI values range between − 1 and 1, with a value between − 1 and − 0.26, indicating right hemisphere dominance, and between 0.26 and 1, indicating left language lateralization, and with a value close to zero (− 0.25 to 0.25) revealing a bilateral distribution [1].
4. Results
At 6 years old, the first presurgical fNIRS showed left hemisphere dominance for expressive language (LI = 0.5), with a significant activation in Broca's area (Fig. 1). The receptive language was not assessed because of time limitation. To validate fNIRS results, fMRI had to be repeated twice because of patient noncompliance. The fMRI showed left hemisphere dominance for receptive language (LI = 0.3) but inconclusive results for expressive language.
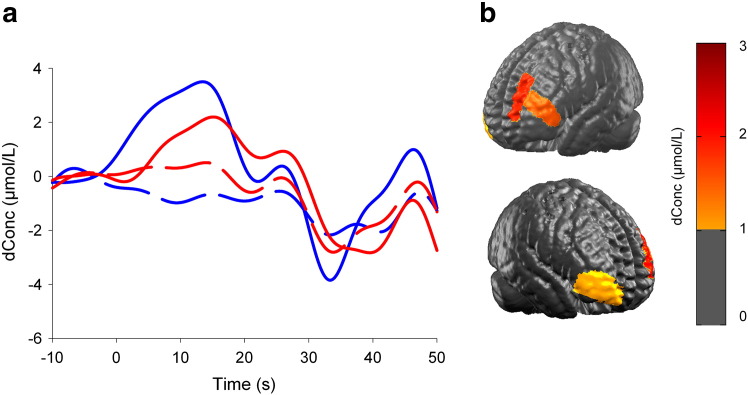
Fig. 1.
fNIRS results at 6 years old during the verbal fluency task (expressive language task). (a) The graph illustrates a cerebral activation characterized by a large increase in HbO concentration (solid lines) and a small decrease in HbR (dotted lines) that is larger in the left hemisphere (blue lines) compared with that in the right hemisphere (red lines), suggesting a left hemispheric language dominance. Hemoglobin data (molar units) represent an average of all channels covering the regions of interest during baseline (− 5–0 s), category naming (0–30 s), and resting period (30–50 s). (b) HbO concentration changes on the maximal cerebral activation during the verbal fluency task at 12 s after task onset. Results suggest larger activation in Broca's area compared with that in its right counterpart.
At 10 years old, a second presurgical fNIRS showed right hemisphere dominance for both expressive and receptive language (LI = − 0.5 and − 0.3, respectively, Fig. 2). Conversely, fMRI showed left hemisphere lateralization for receptive language (LI = 0.3) and inconclusive results for expressive language. A second surgical resection was performed in the left temporal area. A postsurgical fMRI performed at 11 years old, while the patient was seizure-free, showed right hemisphere dominance for receptive language (LI = − 0.3), confirming the second presurgical fNIRS findings.
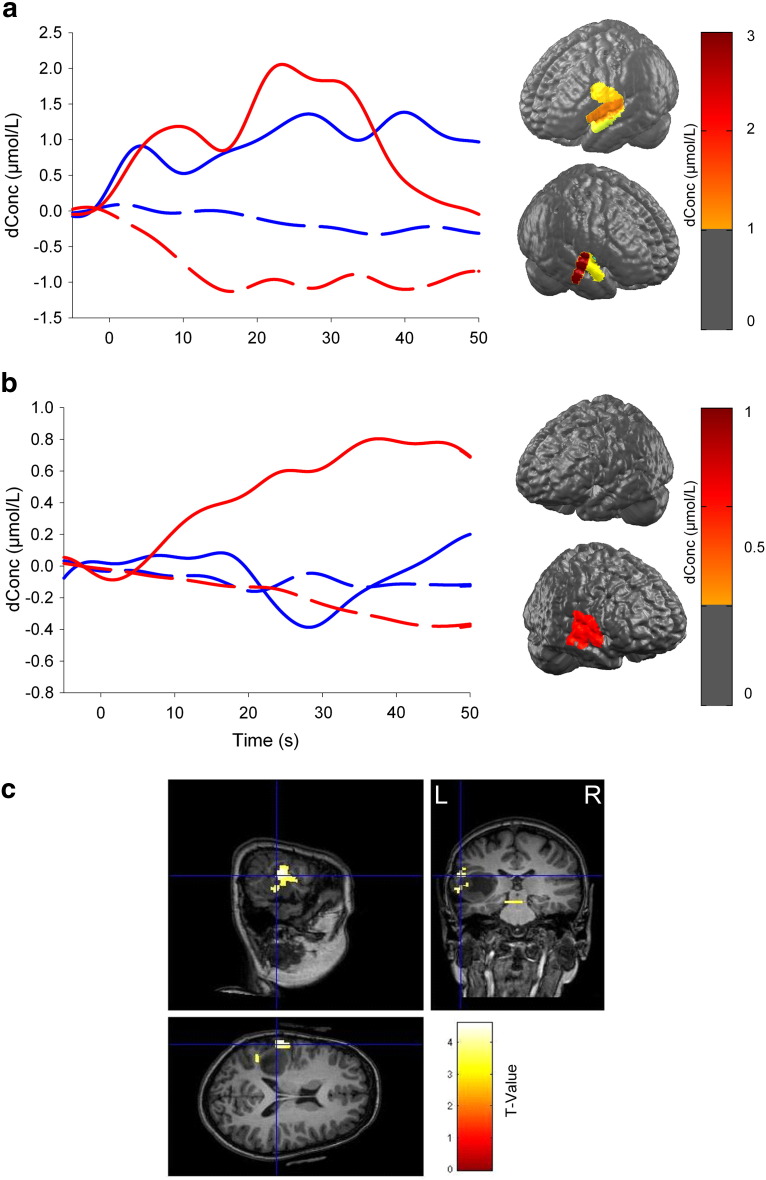
Fig. 2.
Presurgical fNIRS and fMRI results at age 10.
fNIRS results of the verbal fluency (a, expressive language task) and the story listening (b, receptive language task) paradigms. The curves represent HbO (solid lines) and HbR (dotted lines) concentration changes (molar units) over the left (blue lines) and the right (red lines) hemispheres. Maximum HbO activations at 10 s for both languages are shown on the brain 3D reconstructions. Both tasks (a and b) are associated with right-greater-than-left Wernicke's area activation, suggesting a functional cerebral reorganization following the first tumor excision performed at 6 years old. (c) The fMRI results during the expressive language task revealed left hemisphere lateralization in the area of the lesion, Wernicke's area.
5. Conclusion
This case study illustrates the potential for fNIRS to contribute favorably to the localization of language functions in children with epilepsy and cognitive or behavioral problems [1]. To verify whether the child correctly understands the instructions and performs the task properly during recordings, it is essential that expressive language tasks are performed aloud, therefore generating muscle and articulatory movement artifacts, which are less tolerated by fMRI. Conversely, fNIRS has no major restrictions on movements, making it suitable for investigations in young children with behavioral disorders [2]. As in this 6-year-old boy, it is not always possible to obtain conclusive results using fMRI because of movement constraint and the intimidating environment. Consequently, multiple scans may be required and are associated with significant costs.
As for receptive language tasks, the intimidating noise associated with the scanner makes it more difficult for listeners to entirely hear the auditory stimuli, subsequently disengaging the participants from performing the task, especially in a pediatric population. In contrast, fNIRS has no major ambient noise, which allows participants to clearly hear stimuli, thus providing better language localization. Another advantage of fNIRS over fMRI is that an experimenter is always present during the acquisition to ensure that the child adequately performs the tasks; this approach provides a more child-friendly recording session.
At 10 years old, contradictory results were obtained with fNIRS and fMRI. Since EEG was not simultaneously acquired with fMRI data, it could not be excluded that left hemisphere activation found during the language task could be related to epileptic discharges occurring during the acquisition and not to a specific language response. The EEG acquired simultaneously with fNIRS allowed us to exclude epileptic discharges from the analyses, providing more reliable language localization. Moreover, these results were supported by stable language functions found after the second left temporal resection and by the postsurgical fMRI showing right hemisphere language dominance while the patient was seizure-free. Therefore, the use of simultaneous EEG during fNIRS or fMRI data acquisition provides important information, especially in patients with temporal lobe epilepsy, considering that epileptic activity may interfere with language localization.
In the present case, the first presurgical fNIRS revealed a typical left hemisphere language lateralization whereas the one performed prior to the second resection showed atypical right language representation and brain reorganization, which is probably reflecting a compensatory mechanism supported by brain plasticity [3]. It has been reported that the damage associated with epilepsy stimulates the brain either to activate preexisting connections to the dormant functional language areas or to recruit new areas in the right hemisphere to compensate for the altered left hemisphere language centers [4]. For instance, an fMRI study investigating language patterns in 34 children with epilepsy aged from 7 to 18 years performing a verbal fluency task revealed a reorganization of the language network that was significantly influenced by both age of onset and duration of epilepsy [5]. In fact, early onset and a longer duration of the epilepsy are factors that increase the probability of cerebral language reorganization. In this young patient, we found evidence for interhemispheric language reorganization after his first resection using fNIRS, thus confirming that fNIRS is a sensitive tool in the localization of language network reorganization related to epilepsy.
Overall, this case report highlights the clinical value of fNIRS in the presurgical assessment of young patients with epilepsy and its potential advantages over fMRI.
To conclude, fNIRS is a promising technique, which may serve to determine language localization or to investigate atypical language representations in young patients with epilepsy.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Gallagher A., Thériault M., Maclin E., Low K., Gratton G., Fabiani M. Language mapping using near-infrared spectroscopy (NIRS) in young epileptic patients. Epileptic Disord. 2007;9:241–255. doi: 10.1684/epd.2007.0118. [DOI] [PubMed] [Google Scholar]
- 2.Paquette N., Lassonde M., Vannasing P., Tremblay J., Gonzalez-Frankenberger B. Developmental patterns of expressive language hemispheric lateralization in children, adolescents and adults using functional near-infrared spectroscopy. Neuropsychologia. 2015;68:117–125. doi: 10.1016/j.neuropsychologia.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Yuan W., Szaflarski J.P., Schmithorst V.J., Schapiro M., Byars A.W., Strawsburg R.H. fMRI shows atypical language lateralization in pediatric epilepsy patients. Epilepsia. 2006;47(3):593–600. doi: 10.1111/j.1528-1167.2006.00474.x. (PubMed PMID: 16529628; PubMed Central PMCID: PMC1402337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mbwana J., Berl M.M., Ritzl E.K., Rosenberger L., Mayo J., Weinstein S. Limitations to plasticity of language network reorganization in localization related epilepsy. Brain. 2009;132(Pt 2):347–356. doi: 10.1093/brain/awn329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadac J., Brozova K., Tintera J., Krsek P. Language lateralization in children with pre- and postnatal epileptogenic lesions of the left hemisphere: an fMRI study. Epileptic Disord. 2007;9(Suppl. 14):s19–s27. doi: 10.1684/epd.2007.0149. [DOI] [PubMed] [Google Scholar]