Abstract
Purpose
Drug resistance has been recognized to be a major obstacle to the chemotherapy for osteosarcoma. And the potential importance of hypoxia as a target to reverse drug resistance in osteosarcoma has been indicated, though the mechanism underlining such role is not clarified. The present study aims to investigate the role of hypoxia in the drug resistance in osteosarcoma cells via activating AMP-activated protein kinase (AMPK) signaling.
Experimental design
We investigated the promotion of the resistance to doxorubicin of osteosarcoma MG-63 and U2-os cells in vitro, and then determined the role of hypoxia-inducible factor-1 (HIF-1)α and HIF-1β, the activation and regulatory role of AMPK in the osteosarcoma U2-os cells which were treated with doxorubicin under hypoxia.
Results
It was demonstrated that hypoxia significantly reduced the sensitivity of MG-63 and U2-os cells to doxorubicin, indicating an inhibited viability reduction and a reduced apoptosis promotion. And such reduced sensitivity was not associated with HIF-1α, though it was promoted by hypoxia in U2-os cells. Interestingly, the AMPK signaling was significantly promoted by hypoxia in the doxorubicin-treated U2-os cells, with a marked upregulation of phosphorylated AMPK (Thr 172) and phosphorylated acetyl-CoA carboxylase (ACC) (Ser 79), which were sensitive to the AMPK activator, AICAR and the AMPK inhibitor, Compound C. Moreover, the promoted AMPK activity by AICAR or the downregulated AMPK activity by Compound C significantly reduced or promoted the sensitivity of U2-os cells to doxorubicin.
Conclusion
The present study confirmed the AMPK signaling activation in the doxorubicin-treated osteosarcoma cells, in response to hypoxia, and the chemical upregulation or downregulation of AMPK signaling reduced or increased the chemo-sensitivity of osteosarcoma U2-os cells in vitro. Our study implies that AMPK inhibition might be a effective strategy to sensitize osteocarcoma cells to chemotherapy.
Keywords: Hypoxia, Osteosarcoma cells, Doxorubicin, Resistance, AMPK signaling
Graphical abstract

Highlights
-
•
AMPK signaling is activated by hypoxia in the doxorubicin-treated osteosarcoma cells.
-
•
Chemical upregulation of AMPK desensitizes osteosarcoma U2-os cells to doxorubicin.
-
•
AMPK inhibition sensitizes osteosarcoma U2-os cells to doxorubicin.
1. Introduction
Osteosarcoma is the most frequent primary bone malignancy, mainly attacks adolescents [1]. The combined chemotherapy with intensive dose has greatly improved the overall survival for osteosarcoma patients to over 70% [2]. However, the prognosis remains poor for those with metastasis, or for those who relapse, and survival rates only reach 20–30% [3], [4]. Doxorubicin, methotrexate and Cisplatin are commonly used as anticancer drugs in osteosarcoma [5] for the last 20 years, and there has been no improvement in the survival of those osteosarcoma patients, who acquisite the drug-resistant phenotype. Thus, it is urgent to recognize the drug-resistance mechanism of osteosarcoma and to provide novel therapeutic options for this disease.
Hypoxia-induced drug resistance has been confirmed for a variety of anti-tumor agents in various types of tumors [8], [6], [7], and even in osteosarcoma [9]. Such hypoxia markers as hypoxia-inducible factor-1 (HIF-1), vascular endothelial growth factor (VEGF) and carbonic anhydrase IX (CA IX) are detectable in osteosarcomas [10], and correlate with poor progress of osteosarcoma patients, suggesting the important role of hypoxia in the survival of osteosarcoma cells [11], [12], and implying the potential importance of hypoxia as a target to antagonize drug resistance in osteosarcoma. However, it is indicated that the drug resistance in osteosarcoma is independent on the upregulated HIF-1α, suggesting other hypoxia-related signaling may be more relevant in the drug resistance to osteosarcoma.
Multiple other signaling pathways are deregulated in hypoxia and may exert regulatory roles in the hypoxia-induced drug resistance. Wild-type p53 is confirmed to be inactivated in some tumor cells by hypoxia [14], [13]. c-jun, activator protein-1 (AP-1), Phosphoinositol-3-kinase (PI3K) pathway and nuclear factor kappa-B (NF-κB) have also been indicated to involve in the hypoxia-induced drug resistance, mainly by inhibiting the drug-induced apoptosis [16], [15], [17]. And the target inhibiting of these signaling pathways sensitizes cells to cytotoxic agents under the condition of hypoxia, implying these markers as possible targets to counteract the hypoxia-induced drug resistance.
AMP-activated protein kinase (AMPK) is the most important sensor of cellular energy [19], [18], and is also activated by hypoxia as a compensatory response to the reduced mitochondrial respiration [20]. The heterotrimeric AMPK composes of two regulatory subunits and one catalytic subunit, which is activated the increased AMP/ATP ratio [21]. Then the activity was promoted of upstream kinases and phosphatases that control AMPK phosphorylation and dephosphorylation at the Thr-172 [22], [23], particularly by the tumor suppressor LKB1, which phosphorylates the catalytic subunit of AMPK in an AMP-dependent manner [24], [25]. AMPK is also activated by the Ca2 /calmodulin-dependent protein kinase kinase (CaMKK) [27], [26], independently of cellular AMP levels. In addition, the activation of AMPK can also be induced in response to oxidant stress [28], [29] with or without hypoxia. However, the role of the AMPK activation in hypoxia in osteosarcoma is still unclarified.
In the current study, we investigated the resistance of osteosarcoma cells to the widely utilized in clinic cytotoxic drug, doxorubicin under hypoxia, and then examined the association of such resistance with the activation of AMPK signaling. On the other side, we investigated the influence by the chemical inhibition of AMPK signaling on the hypoxia-induced resistance to doxorubicin. The present study indicated the key regulatory role of AMPK in the hypoxia-induced resistance to doxorubicin in osteosarcoma cells, suggesting a possible target of AMPK against the drug-resistance of osteosarcoma cells.
2. Materials and methods
2.1. Cell culture and treatment
Human osteosarcoma cell line MG-63 and U2-os were purchased from American Type Culture Collection (ATCC) (Rockville, MD, USA). MG-63 cells were cultured in Eagle's minimal essential medium (EMEM), supplemented with 2 mM Glutamine, 1% Non Essential Amino Acids (NEAA) and 10% Fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA), and were routinely incubated at 37 °C with 5% CO2. U2-os cells were grown in RPMI-1640 medium (Ameresco, Framingham, MA, USA) supplemented with 10% FBS (Invitrogen, Carlsbad, CA, USA). For hypoxia culture, MG-63 or U2-os cells were incubated in a hypoxia incubator infused with a gas mixture of 5% CO2, 2% oxygen and nitrogen, whereas cells were incubated in an ordinary incubator with 5% CO2. For the doxorubicin treatment, MG-63 or U2-os cells with more than 85% confluence were updated with medium containing 2% FBS and the docorubicin with various concentrations. To induce or inhibit the AMPK activity, AICAR and Compound C (Sigma-Aldrich, St. Louis, MO, USA) were added to the medium with a concentration of 1 mM and 20 μM respectively.
2.2. MTT assay and apoptosis assay
The viability of MG-63 or U2-os cells which were seeded in 96-well plates with more than 85% confluence post various treatments were measured with MTT assay according to the standard protocol. The absorbance was measured at 570 nm with a reference wavelength of 750 nm using a spectrophotometer.
Apoptosis of U2-os cells was examined with an Annexin V-FITC apoptosis detection kit (Sigma-Aldrich, USA). Briefly, 6×105 U2-os cells were stained with Annexin V-FITC and propidium iodide and then were detected by a FACScan flow cytometer (Bio-Rad, Hercules, CA, USA). The results were presented as a percentage of apoptotic cells from total cells.
2.3. Western blot analysis
U2-os cells, post treatment, were harvested and were homogenized in an ice-cold Cell lysis buffer (Bio-Rad, Hercules, CA, USA). Cellular lysates was centrifugated with 12,000×g for 30 min at 4 °C, and the supernatant was collected. Protein samples were separated with 8–12% SDS-PAGE gel and were transferred to a nitrocellulose membrane (Millipore, Bedford, MA, USA). The membrane was blocked for non-specific binding targets with 5% Skimmed milk powder overnight at 4 °C, and were incubated with the rabbit polyclone antibody (against caspase 3, Poly (ADP-ribose) polymerase (PARP), HIF-1α, HIF-1β, AMPKα with or without phoshorylated Thr 172, ACC with or without phoshorylated Ser 79, or β-actin) overnight at 4 °C, and then were incubated with HRP-linked secondary anti-rabbit antibody (New England Biolab, Ipswich, UK) for 1 h at room temperature. The specific binding band was scanned and quantified according to the band density by Image J software.
2.4. HIF-1α knockdown via RNA interference
The HIF-1α specific siRNA (siRNA- HIF-1α) (25 or 50 nM) or the scrambler oligonucleotides as control (siRNA-Con) (25 or 50 nM) were purchased from Thermo Fisher (Waltham, MA, USA), and were transfected into U2-os cells with Opti-MEM containing Lipofectamine RNAiMax (Invitrogen, Carlsbad, CA, USA). 6 h post transfection, cells were updated with fresh RPMI-1640 medium, which was supplemented with 2% FBS, and were subject to other treatment or were assayed for the knockdown efficiency post another inoculation of 24 h.
2.5. DCFH-DA assay ELISA for AMPK activity
Reactive oxygen species (ROS) was assessed using 2,7-dichlorofluorescin diacetate (DCFH-DA). U2-os cells were added with BSS containing DCFH-DA (5 µM), and fluorescence signaling was collected at 535 nm using excitation at 484 nm. Cellular fluorescence density from individual cell well was collected and was averaged to provide an overall assessment for each group.
The AMPK activity was examined with AMPK Kinase Assay Kit AMPK Kinase Assay Kit (CY-1182; CycLex, Nagano, Japan) according to the manufacturer’s manual. U2-os cells post various treatments were directly lysed with ice-cold lysis buffer; then the cellular lysates were serially diluted in Kinase buffer and supplemented with phosohorylation substrate, and the amount of phosphorylated substrate specifically bonded to an anti-phospho-mouse IgG, which then assayed with anti mouse Ig-G conjugated with horseradish peroxidase and its substrate, by absorbance 450 nm.
2.6. Statistical evaluations
Quantitative results are presented as mean±SE. For the analysis between two groups, the Student’s t test was performed. A p value less than 0.05 was considered significant.
3. Results
3.1. Hypoxia reduces the sensitivity of osteosarcoma cells to doxorubicin in vitro
We firstly examined the viability of doxorubicin-treated osteosarcoma MG-63 cells under normoxia or hypoxia. It was shown in Fig. 1A, that the doxorubicin with 4, 8 or 16 μM significantly reduced the viability of MG-63 cells under normoxia (p<0.01 or p<0.001). Interestingly, the cellular viability reduction in the MG-63 cells under hypoxia was significantly less than in the cells under normoxia (p<0.05 respectively for 4, 8 or 16 μM). Then we reevaluated such regulation by hypoxia in U2-os osteosarcoma cells. Fig. 1B also indicated that the doxorubicin treatment with 5, 10, 20 or 40 μM significantly reduced the cellular viability (p<0.05, p<0.01 or p<0.001). And the hypoxia pre-treatment could significantly ameliorate such viability reduction in U2-os cells (p<0.05 for 5 μM, p<0.01 respectively for 10, 20 or 40 μM). Moreover, to reconfirm the influence by hypoxia on the doxorubicin sensitivity of osteosarcoma cells, we determined the apoptosis induction in U2-os cells treated with doxorubicin under normoxia or hypoxia. It was indicated that less apoptotic U2-os cells were induced by the doxorubicin with 10 or 20 μM in the hypoxia group (Fig. 1C; p<0.05 respectively).
Fig. 1.
Hypoxia reduces the sensitivity of osteosarcoma cells to doxorubicin in vitro. A: Viability of MG-63 cells, post a 24 h pre-treatment incubation in normoxia or hypoxia, and subject to the treatment with doxorubicin of 0, 2, 4, 8, or 16 μM for 2 h. MTT assay was performed post another 48 h inoculation; B: Viability of U2-os cells, post a 24 h pre-treatment incubation in normoxia or hypoxia, and subject to the treatment with doxorubicin of 0, 5, 10, 20, or 40 μM for 2 h. MTT assay was performed post another 24 h inoculation; C: Apoptosis induction in U2-os cells post a 24 h pre-treatment incubation in normoxia or hypoxia, and after an exposure to a 2 h treatment with doxorubicin (5, 10 or 20 μM); D: Western blot analysis of cleaved caspase 3 (c casp 3) and lyzed PARP (L PARP) in U2-os cells post the hypoxia and the treatment with 20 μM doxorubicin. E and F: Relative c casp 3 or L PARP level in U2-os cells post the hypoxia and the treatment with 20 μM doxorubicin. Experiments were repeated independently in triplicate. *p<0.05, **p<0.01, ***p<0.001, ns: no significance.
In addition, we analyzed the activation of caspase 3, which is the executive marker of apoptosis [30], in the doxorubicin-treated U2-os cells under normoxia or hypoxia. As shown in Fig. 1D and E, the cleaved caspase 3 (activated form) was markedly lower in the U2-os cells under hypoxia (p<0.01 respectively). And the substrate of caspase 3, Poly ADP ribose polymerase (PARP) was also markedly less lyzed in the U2-os cells under hypoxia (Fig. 1F; p<0.05 respectively for 10, 20 or 40 μM). Therefore, hypoxia reduces the sensitivity of osteosarcoma cells to doxorubicin in either MG-63 or U2-os osteosarcoma cells.
3.2. Hypoxia-induced HIF-1α exerts no influence on the sensitivity of osteosarcoma cells to doxorubicin
HIF-1α is the best characterized marker for hypoxia and is sensitively accumulated under hypoxia [32], [31]. To investigate a possible regulatory role of HIF-1α on the sensitivity of osteosarcoma cells to doxorubicin, we then quantified the level of HIF-1α and the constitutive HIF-1β in the doxorubicin-treated U2-os cells with or without hypoxia. The western blotting results demonstrated that the HIF-1α level was dramatically promoted by hypoxia in the doxorubicin-treated U2-os cells (Figs. 2A and 1B; p<0.01 or p<0.001) at 6, 12 or 24 h post inoculation. However, there was no marked difference in the HIF-1β level between the hypoxia and normoxia groups (Fig. 2C). Then the siRNA targeting HIF-1α was used to knockdown the expression of HIF-1α and re-evaluated the influence of hypoxia on the drug resistance. Fig. 2D and E confirmed the significant blockage of the HIF-1α induction by hypoxia in U2-os cells (p<0.01 respectively). However, cleaved caspase 3 and lyzed PARP were not significantly downregulated by the HIF-1α blockage (Fig. 2F); and the cellular viability (Fig. 2G) and the apoptosis (Fig. 2H) induction were also not regulated by the HIF-1α blockage. Thus, we deduced that the reduced drug sensitivity of U2-os cells under hypoxia was not associated with HIF-1α.
Fig. 2.
Hypoxia-induced chemo-resistance is HIF-1-independent in U2-os cells. A–C: Western blot analysis of HIF-1α and HIF-1β in U2-os cells with the pre-incubation in normoxia or hypoxia for 6, 12 or 24 h and the doxorubicin treatment with 20 μM for 2 h, the level of HIF-1α or HIF-1β was relative to β-actin; D–F: Western blot analysis of HIF-1α, cleaved caspase 3 and lyzed PARP, in U2-os cells post the transfection with 20 or 50 nM siRNA- HIF-1α or control siRNA, with β-actin as internal control; G and H: Viability reduction and apoptosis induction of U2-os cells post the transfection with 20 or 50 nM siRNA- HIF-1α or control siRNA, via MTT assay and FACScan flow assay. Each result was averaged for triple independent experiments. **p<0.01, ***p<0.001, ns: no significance.
3.3. AMPK signaling was promoted by hypoxia in osteosarcoma cells in the presence of doxorubicin
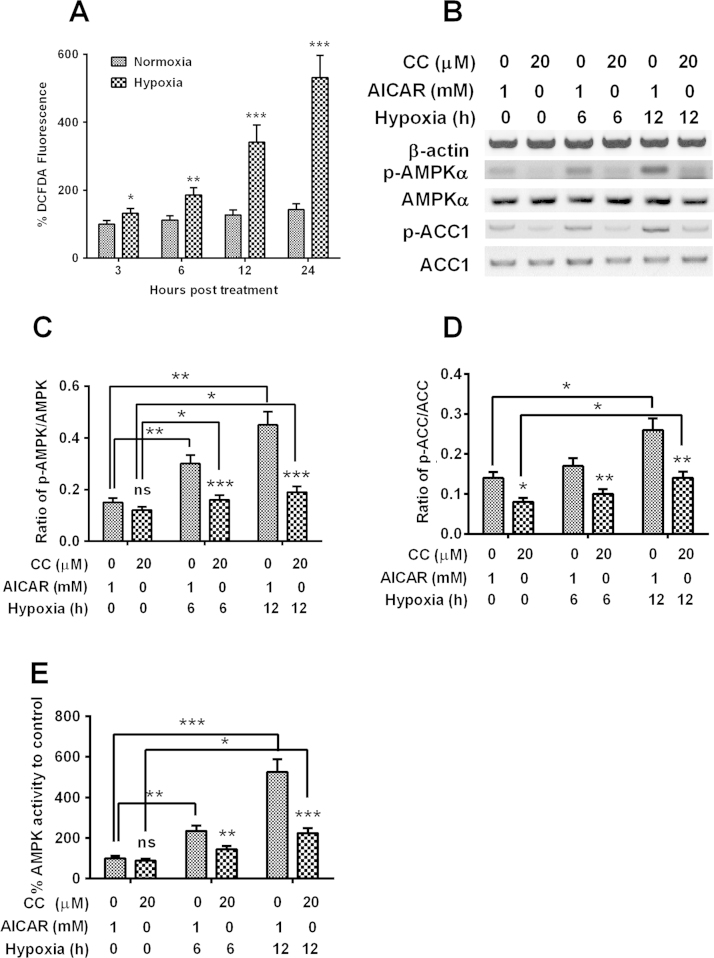
It has been conceived that the oxygen deprivation under hypoxia upregulates the production of reactive oxygen species (ROS), which then triggers the AMPK activation, but independent of an increase in AMP/ATP ratio [34], [33]. We then determined the activation of AMPK signaling under hypoxia in U2-os cells and examined the role of AMPK activation in drug sensitivity of U2-os osteosarcoma cells. It was indicated that the U2-os cells under hypoxia produced markedly increased ROS (Fig. 3A; p<0.05, p<0.01 or p<0.001) via the DCFH-DA fluorescence assay. And the western blot analysis demonstrated that the levels of phosphorylated AMPK and phosphorylated ACC, both of which are the active forms of AMPK signaling, were significantly promoted in the U2-os cells under hypoxia for 6 or 12 h (Fig. 3B–D; whichever in the presence of AMPK activator, AICAR, or AMPK inhibitor, Compound C; p<0.05 or p<0.01). Moreover, the levels of phosphorylated AMPK and phosphorylated ACC was markedly up-regulated by the AMPK activator, AICAR, or downregulated by AMPK inhibitor, Compound C (p<0.01 or p<0.001); and the AMPK activity assay also indicated the significant promotion of AMPK signaling by hypoxia, and the marked up- or down-regulation of AMPK signaling by AICAR or Compound C (Fig. 3E; p<0.05, p<0.01 or p<0.001).
Fig. 3.
Hypoxia induces AMPK signaling in U2-os cells, which are sensitive to the AMPK activator and inhibitor. A: Reactive oxygen species (ROS) level in the U2-os cells post the inoculation under hypoxia or normoxia for 3, 6, 12 or 24 h and the doxorubicin treatment with 20 μM for 2 hs; B–D: Western blotting of AMPK with or without Thr 172 phosphorylation, of ACC with or without Ser 79 phosphorylation in the U2-os cells successively post the inoculation under hypoxia and the treatment with 20 μM Compound C or 1 mM AICAR for 24 h and the doxorubicin treatment with 20 μM for 2 h; E: Relativel AMPK activity in the combined treatment with hypoxia and 1 mM AICAR (or 20 μM Compound C) for 24 h and the doxorubicin treatment with 20 μM for 2 h. Experiments were repeated in triplicate. *p<0.05, **p<0.01, ***p<0.001, ns: no significance.
3.4. Chemical manipulation of AMPK signaling regulates the sensitivity of osteosarcoma cells to doxorubicin
Then we examined the influence of AMPK activation on the U2-os cell sensitivity to doxorubicin. Fig. 4A indicated that the promotion by AICAR and the reduction by Compound C of the viability of U2-os cells were not significant. However, it was significant under hypoxia of the cellular viability promotion by AICAR and the reduction by Compound C (Fig. 4B; p<0.05 respectively). And the apoptosis level was also significantly regulated by both chemical agents (reduced by AICAR, whereas promoted by Compound C; Fig. 4C; p<0.05 or p<0.01) in the U2-os cells under hypoxia. In addition, levels of cleaved caspase 3 and the lyzed PARP were also reduced by the treatment with AICAR, whereas were upregulated by Compound C (Fig. 4D–F; p<0.05 or p<0.01). Thus, we confirmed that AMPK signaling was promoted by the hypoxia-mediated resistance of U2-os cells to doxorubicin.
Fig. 4.
Manipulation of AMPK activity with the AMPK activator or inhibitor exerts influence on the sensitivity of U2-os cells to doxorubicin. A and B: Influence of the treatment with 1 mM AICAR or 20 μM Compound C on the viability of U2-os cells under normoxia (A) or hypoxia (B) for 24 or 48 h; C and D: Apoptosis induction (C) and western blot analysis of cleaved caspase 3 and lyzed PARP (D) in the U2-os cells post the treatment with 1 mM AICAR or 20 μM Compound C under hypoxia for 24 or 48 h and the doxorubicin treatment with 20 μM for 2 h; E and F: Relative levels of cleaved caspase 3 (E) or lyzed PARP (F) to β-actin in the hypoxia-treated U2-os cells post the post the treatment with 1 mM AICAR or 20 μM Compound C. Results were averaged for triple independent experiments. *p<0.05, **p<0.01, ns: no significance.
4. Discussion
The role of hypoxia in the drug sensitivity has not been universally concluded, varying according to the type of tumor and the drug used [35], [36]. It was observed to be resistant in rhabdomyosarcoma, Ewing’s sarcoma and neuroblastoma [36], [37]. Evidence exists of the importance of hypoxia in osteosarcoma [39], [38]. In particular, the patients with a moderate or strong expression of HIF-1α showed significantly shorter overall survival (OS) and disease-free survival (DFS), compared with HIF-1α negative/weak expression [40]. And recently, a significant hypoxia-induced drug resistance in osteosarcoma cells has been confirmed in intro [9], highlighting the potential importance of hypoxia in the drug resistance in osteosarcoma. However, though the HIF-1α was also found to be upregulated by hypoxia, it was irrelevant to such drug resistance.
In the present study, we firstly confirmed the resistance of osteosarcoma U2OS cells to doxorubicin under hypoxia via inhibiting the drug-induced apoptosis. The hypoxia pre-treatment could significantly ameliorate the viability reduction in U2-os cells which were treated with doxorubicin with 5, 10, 20 or 40 μM. And less apoptotic U2-os cells were induced, and less cleaved caspase (activated form) and less lyzed PARP were promoted by the doxorubicin treatment in the hypoxia group. Therefore, hypoxia reduces the sensitivity of osteosarcoma cells to doxorubicin in either MG-63 or U2-os osteosarcoma cells. Then we investigated the role of HIF-1α, which is the best characterized marker for hypoxia [32], [31], in the hypoxia-mediated resistance of U2-os cells to doxorubicin. Interestingly, HIF-1α was significantly promoted by hypoxia in U2-os cells which were treated with doxorubicin, whereas exerted no influence on the sensitivity of U2-os cells to doxorubicin.
Reactive oxygen species (ROS) has been conceived to be promoted under hypoxia and to trigger the AMPK activation, independent of an increase in AMP/ATP ratio [34], [33]. In the present study, we also confirmed the activation of AMPK by hypoxia in U2-os cells. Markedly increased ROS was indicated in the U2-os cells under hypoxia. And significantly upregulated levels of phosphorylated AMPK and phosphorylated ACC, both of which are the active forms of AMPK signaling, were also promoted in the U2-os cells under hypoxia. And the levels of phosphorylated AMPK and phosphorylated ACC were significantly up-regulated by the AMPK activator, AICAR, or downregulated by AMPK inhibitor, Compound C in the doxorubicin-treated U2-os cells. Moreover, AMPK signaling has been recognized to involve in the hypoxia-mediated drug resistance in several types of cancers, such as non–small cell lung cancers to cisplatin and doxorubicin [41], prostate cancers [42]. However, there was a controversy about the activation of AMPK signaling in the drug resistance. AMPK is activated in the prostate cancer cells [42], while is inactivated in the lung cancer cells [41], during hypoxia leading to drug resistance, implying a cancer type-dependence. And the present study also confirmed the activation of AMPK signaling during the hypoxia-induced drug resistance in osteosarcoma cells. The chemical manipulation of AMPK signaling regulates the sensitivity of osteosarcoma cells to doxorubicin. The promotion by AICAR and the reduction by Compound C to the AMPK signaling significantly ameliorated or deteriorated the viability reduction or apoptosis induction of U2-os cells under hypoxia. In addition, the level of cleaved caspase 3 and the lyzed PARP were also reduced by the treatment with AICAR, whereas were upregulated by Compound C. Thus, the AMPK signaling mediated the hypoxia-induced resistance of U2-os cells to doxorubicin.
Autophagy is a dynamic self-degradation process for cellular components by cellular lysosome under a stringent regulation [43], [44], and has also been recognized during ischemic heart disease [45]. Autophagy normally maintains at a low level in heart, and is sharply promoted as a response to such environmental stress conditions as ATP depletion, excessive ROS and mitochondrial dysfunction [46], [47]. Moreover, autophagy has been identified to mediate the hypoxia-induced resistance by regulating the angiogenesis in malignancies [48]. The present study has recognized the involvement of autophagy in the hypoxia-induced resistance in osteosarcoma cells. However, it is not clear about the orchestrated pathways about AMPK signaling and autophagy promotion during the hypoxia-induced resistance. The reduced adenosine-triphosphate (ATP) has been recognized to activate autophay [49]. The increased AMP/ATP ratio activates AMP-activated protein kinase (AMPK) [50], which successively induces autophagy via inhibiting mammalian target of rapamycin (mTOR) [46]. The promoted reactive oxygen species (ROS) has also been recognized to induce autophagy in cardiocytes [51], in choriocarcinoma cells [52], and in breast cancer cells [53]. Therefore, the role of autophagy in the AMPK-mediated chemoresistance of osteocarcoma cells needs to be further clarified.
5. Conclusion
The present study confirmed the hypoxia induced resistance of osteocarcoma cells to doxorubicin, and such chemo-resistance was HIF-1α-independent. Moreover, we recognized the involvement to AMPK signaling in such hypoxia-induced chemoresistance in U2-os osteocarcoma cells, chemical manipulation of AMPK activity exerted an influence of such chemoresistance in U2-os osteocarcoma cells. Our study implies that AMPK inhibition might be a effective strategy to sensitize osteocarcoma cells to chemotherapy.
Conflict of interest
Authors declare no conflict of interests regarding the current study.
References
- 1.Stiller C.A. International patterns of cancer incidence in adolescents. Cancer Treat. Rev. 2007;33:631–645. doi: 10.1016/j.ctrv.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Gatta G., Corazziari I., Magnani C., Peris-Bonet R., Roazzi P., Stiller C. Childhood cancer survival in Europe. Ann. Oncol. 2003;14(Suppl. 5):v119–v127. doi: 10.1093/annonc/mdg755. [DOI] [PubMed] [Google Scholar]
- 3.Meyers P.A., Heller G., Healey J.H., Huvos A., Applewhite A., Sun M. Osteogenic sarcoma with clinically detectable metastasis at initial presentation. J. Clin. Oncol. 1993;11:449–453. doi: 10.1200/JCO.1993.11.3.449. [DOI] [PubMed] [Google Scholar]
- 4.Harting M.T., Blakely M.L., Jaffe N., Cox C.J., Hayes-Jordan A., Benjamin R.S. Long-term survival after aggressive resection of pulmonary metastases among children and adolescents with osteosarcoma. J. Pediatr. Surg. 2006;41:194–199. doi: 10.1016/j.jpedsurg.2005.10.089. [DOI] [PubMed] [Google Scholar]
- 5.Wittig J.C., Bickels J., Priebat D., Jelinek J., Kellar-Graney K., Shmookler B. Osteosarcoma: a multidisciplinary approach to diagnosis and treatment. Am. Family Phys. 2002;65:1123–1132. [PubMed] [Google Scholar]
- 6.Brown L.M., Cowen R.L., Debray C., Eustace A., Erler J.T., Sheppard F.C. Reversing hypoxic cell chemoresistance in vitro using genetic and small molecule approaches targeting hypoxia inducible factor-1. Mol. Pharmacol. 2006;69:411–418. doi: 10.1124/mol.105.015743. [DOI] [PubMed] [Google Scholar]
- 7.Song X., Liu X., Chi W., Liu Y., Wei L., Wang X. Hypoxia-induced resistance to cisplatin and doxorubicin in non-small cell lung cancer is inhibited by silencing of HIF-1alpha gene. Cancer Chemother. Pharmacol. 2006;58:776–784. doi: 10.1007/s00280-006-0224-7. [DOI] [PubMed] [Google Scholar]
- 8.Erler J.T., Cawthorne C.J., Williams K.J., Koritzinsky M., Wouters B.G., Wilson C. Hypoxia-mediated down-regulation of Bid and Bax in tumors occurs via hypoxia-inducible factor 1-dependent and -independent mechanisms and contributes to drug resistance. Mol. Cell. Biol. 2004;24:2875–2889. doi: 10.1128/MCB.24.7.2875-2889.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamski J., Price A., Dive C., Makin G. Hypoxia-induced cytotoxic drug resistance in osteosarcoma is independent of HIF-1Alpha. PLoS One. 2013;8:e65304. doi: 10.1371/journal.pone.0065304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizobuchi H., Garcia-Castellano J.M., Philip S., Healey J.H., Gorlick R. Hypoxia markers in human osteosarcoma: an exploratory study. Clin. Orthop. Relat. Res. 2008;466:2052–2059. doi: 10.1007/s11999-008-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Q.C., Zeng B.F., Dong Y., Shi Z.M., Jiang Z.M., Huang J. Overexpression of hypoxia-inducible factor-1alpha in human osteosarcoma: correlation with clinicopathological parameters and survival outcome. Jpn. J. Clin. Oncol. 2007;37:127–134. doi: 10.1093/jjco/hyl137. [DOI] [PubMed] [Google Scholar]
- 12.Chen W.L., Feng H.J., Li H.G. Expression and significance of hypoxemia-inducible factor-1alpha in osteosarcoma of the jaws. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008;106:254–257. doi: 10.1016/j.tripleo.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 13.Cosse J.P., Ronvaux M., Ninane N., Raes M.J., Michiels C. Hypoxia-induced decrease in p53 protein level and increase in c-jun DNA binding activity results in cancer cell resistance to etoposide. Neoplasia. 2009;11:976–986. doi: 10.1593/neo.09632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Achison M., Hupp T.R. Hypoxia attenuates the p53 response to cellular damage. Oncogene. 2003;22:3431–3440. doi: 10.1038/sj.onc.1206434. [DOI] [PubMed] [Google Scholar]
- 15.Cosse J.P., Ronvaux M., Ninane N., Raes M.J., Michiels C. Hypoxia-induced decrease in p53 protein level and increase in c-jun DNA binding activity results in cancer cell resistance to etoposide. Neoplasia. 2009;11:976–986. doi: 10.1593/neo.09632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boller Y.C., Brandes L.M., Russell R.L., Lin Z.P., Patierno S.R., Kennedy K.A. Prostaglandin A1 inhibits stress-induced NF-kappaB activation and reverses resistance to topoisomerase II inhibitors. Oncol. Res. 2000;12:383–395. doi: 10.3727/096504001108747846. [DOI] [PubMed] [Google Scholar]
- 17.Rohwer N., Dame C., Haugstetter A., Wiedenmann B., Detjen K., Schmitt C.A. Hypoxia-inducible factor 1alpha determines gastric cancer chemosensitivity via modulation of p53 and NF-kappaB. PLoS One. 2010;5:e12038. doi: 10.1371/journal.pone.0012038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardie D.G., Ross F.A., Hawley S.A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shackelford D.B., Shaw R.J. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat. Rev. Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greer E.L., Dowlatshahi D., Banko M.R., Villen J., Hoang K., Blanchard D. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr. Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker L.B., Vanden H.T., Shao Z.H., Li C.Q., Schumacker P.T. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am. J. Physiol. 1999;277:H2240–H2246. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]
- 22.Davies S.P., Helps N.R., Cohen P.T., Hardie D.G. 5’-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- 23.Hawley S.A., Selbert M.A., Goldstein E.G., Edelman A.M., Carling D., Hardie D.G. 5’-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J. Biol. Chem. 1995;270:27186–27191. doi: 10.1074/jbc.270.45.27186. [DOI] [PubMed] [Google Scholar]
- 24.Woods A., Johnstone S.R., Dickerson K., Leiper F.C., Fryer L.G., Neumann D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 25.Hawley S.A., Boudeau J., Reid J.L., Mustard K.J., Udd L., Makela T.P. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woods A., Dickerson K., Heath R., Hong S.P., Momcilovic M., Johnstone S.R. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Hawley S.A., Davison M., Woods A., Davies S.P., Beri R.K., Carling D. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J. Biol. Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 28.Hwang J.T., Lee M., Jung S.N., Lee H.J., Kang I., Kim S.S. AMP-activated protein kinase activity is required for vanadate-induced hypoxia-inducible factor 1alpha expression in DU145 cells. Carcinogenesis. 2004;25:2497–2507. doi: 10.1093/carcin/bgh253. [DOI] [PubMed] [Google Scholar]
- 29.Toyoda T., Hayashi T., Miyamoto L., Yonemitsu S., Nakano M., Tanaka S. Possible involvement of the alpha1 isoform of 5′AMP-activated protein kinase in oxidative stress-stimulated glucose transport in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2004;287:E166–E173. doi: 10.1152/ajpendo.00487.2003. [DOI] [PubMed] [Google Scholar]
- 30.Porter A.G., Janicke R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 31.Kaelin W.J., Ratcliffe P.J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Semenza G.L. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007:m8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- 33.Mungai P.T., Waypa G.B., Jairaman A., Prakriya M., Dokic D., Ball M.K. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol. Cell. Biol. 2011;31:3531–3545. doi: 10.1128/MCB.05124-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emerling B.M., Weinberg F., Snyder C., Burgess Z., Mutlu G.M., Viollet B. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic. Biol. Med. 2009;46:1386–1391. doi: 10.1016/j.freeradbiomed.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teicher B.A., Lazo J.S., Sartorelli A.C. Classification of antineoplastic agents by their selective toxicities toward oxygenated and hypoxic tumor cells. Cancer Res. 1981;41:73–81. [PubMed] [Google Scholar]
- 36.Hussein D., Estlin E.J., Dive C., Makin G.W. Chronic hypoxia promotes hypoxia-inducible factor-1alpha-dependent resistance to etoposide and vincristine in neuroblastoma cells. Mol. Cancer Ther. 2006;5:2241–2250. doi: 10.1158/1535-7163.MCT-06-0145. [DOI] [PubMed] [Google Scholar]
- 37.Kilic M., Kasperczyk H., Fulda S., Debatin K.M. Role of hypoxia inducible factor-1 alpha in modulation of apoptosis resistance. Oncogene. 2007;26:2027–2038. doi: 10.1038/sj.onc.1210008. [DOI] [PubMed] [Google Scholar]
- 38.Yamada N., Yamanegi K., Ohyama H., Hata M., Nakasho K., Futani H. Hypoxia downregulates the expression of cell surface MICA without increasing soluble MICA in osteosarcoma cells in a HIF-1alpha-dependent manner. Int. J. Oncol. 2012;41:2005–2012. doi: 10.3892/ijo.2012.1630. [DOI] [PubMed] [Google Scholar]
- 39.Mizobuchi H., Garcia-Castellano J.M., Philip S., Healey J.H., Gorlick R. Hypoxia markers in human osteosarcoma: an exploratory study. Clin. Orthop. Relat. Res. 2008;466:2052–2059. doi: 10.1007/s11999-008-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Q.C., Zeng B.F., Dong Y., Shi Z.M., Jiang Z.M., Huang J. Overexpression of hypoxia-inducible factor-1alpha in human osteosarcoma: correlation with clinicopathological parameters and survival outcome. Jpn. J. Clin. Oncol. 2007;37:127–134. doi: 10.1093/jjco/hyl137. [DOI] [PubMed] [Google Scholar]
- 41.Shin D.H., Choi Y.J., Park J.W. SIRT1 and AMPK mediate hypoxia-induced resistance of non-small cell lung cancers to cisplatin and doxorubicin. Cancer Res. 2014;74:298–308. doi: 10.1158/0008-5472.CAN-13-2620. [DOI] [PubMed] [Google Scholar]
- 42.Chhipa R.R., Wu Y., Ip C. AMPK-mediated autophagy is a survival mechanism in androgen-dependent prostate cancer cells subjected to androgen deprivation and hypoxia. Cell. Signal. 2011;23:1466–1472. doi: 10.1016/j.cellsig.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shintani T., Klionsky D.J. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Todde V., Veenhuis M., van der Klei I.J. Autophagy: principles and significance in health and disease. Biochim. Biophys. Acta. 2009;1792:3–13. doi: 10.1016/j.bbadis.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 45.Hamacher-Brady A., Brady N.R., Gottlieb R.A. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J. Biol. Chem. 2006;281:29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 46.Matsui Y., Takagi H., Qu X., Abdellatif M., Sakoda H., Asano T. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ. Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 47.Gustafsson A.B., Gottlieb R.A. Autophagy in ischemic heart disease. Circ. Res. 2009;104:150–158. doi: 10.1161/CIRCRESAHA.108.187427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu Y.L., Jahangiri A., De Lay M., Aghi M.K. Hypoxia-induced tumor cell autophagy mediates resistance to anti-angiogenic therapy. Autophagy. 2012;8:979–981. doi: 10.4161/auto.20232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arad M., Seidman C.E., Seidman J.G. AMP-activated protein kinase in the heart: role during health and disease. Circ. Res. 2007;100:474–488. doi: 10.1161/01.RES.0000258446.23525.37. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y., McMillan-Ward E., Kong J., Israels S.J., Gibson S.B. Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. J. Cell Sci. 2007;120:4155–4166. doi: 10.1242/jcs.011163. [DOI] [PubMed] [Google Scholar]
- 51.Shen Y., Yang J., Zhao J., Xiao C., Xu C., Xiang Y. The switch from ER stress-induced apoptosis to autophagy via ROS-mediated JNK/p62 signals: a survival mechanism in methotrexate-resistant choriocarcinoma cells. Exp. Cell Res. 2015 doi: 10.1016/j.yexcr.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 52.Jiang K., Wang W., Jin X., Wang Z., Ji Z., Meng G. Silibinin, a natural flavonoid, induces autophagy via ROS-dependent mitochondrial dysfunction and loss of ATP involving BNIP3 in human MCF7 breast cancer cells. Oncol. Rep. 2015 doi: 10.3892/or.2015.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsui Y., Takagi H., Qu X., Abdellatif M., Sakoda H., Asano T. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ. Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]