Abstract
Purpose
Osteosarcoma is an aggressive malignant neoplasm, and conflicting findings have been reported on the survival and function recovery in osteosarcoma patients experiencing limb salvage or amputation. In the present study, we compared clinical outcomes regarding limb salvage surgery vs. amputation for osteosarcoma patients by a meta-analysis.
Method
Literature search was conducted in CNKI, Medline, Embase, the Cochrane Database, and Web of Sciences, and the quality of included studies was evaluated based on Newcastle-Ottawa scale quality assessment. Odds ratio and 95% confidence interval of the local recurrence, 5-year overall survival, and metastasis occurrence were calculated.
Results
17 articles were included according to selection criteria. There were 1343 patients in total derived from these studies. Our result showed that there was no significant difference between limb salvage surgery and amputation with respect to local recurrence, and patients with limb salvage surgery had a higher 5-year overall survival, and a lower metastasis occurrence.
Conclusions
The present study provided more comprehensive evidences to support limb salvage surgery as an optimal treatment of osteosarcoma patients.
Abbreviations: LSS, limb salvage surgery
Keywords: Meta-analysis, Limb-salvage treatment, Amputation, Osteosarcoma
1. Introduction
Osteosarcoma is an aggressive bone neoplasm arising from primitive transformed cells of mesenchymal origin. It was such a fatal disease that “months to metastasis” rather than actual survival time, was used to measure the outcomes of treatment in studies of early stage. In the 1950s, there was no optional therapy that could significantly increase the survival rate, with a 5-year survival rate of 22% [1]. However, with the aid of effective chemotherapeutic drugs the survival rate of osteosarcoma has been significantly improved since the late 1970s [2], [3]. Recently, the gold standard of osteosarcoma chemotherapy have been based on around 5 drugs; high-dose methotrexate (HDMTX) with leucovorin rescue, doxorubicin (adriamycin), cisplatin, ifosfamide, and etoposide [4]. Combinations of these drugs, mostly in the form of neoadjuvant as well as adjuvant MAP, are the current management for osteosarcoma [5], and various chemotherapy protocols are still under investigation. The experience with radiotherapy is limited, as osteosarcoma is long considered resistant to applicable doses of radiation. However, recent data suggest that the combined approach of irradiation with chemotherapy may be useful in patients who have microscopic residual tumor foci following intralesional resection [6].
With the advent of effective neoadjuvant chemotherapy in the 1970s, limb salvage surgery (LSS) has been taken as a potential treatment for osteosarcoma [7], [8]. Usually, LSS has functional and physiological advantages over traditional amputative procedures when combined with neoadjuvant or adjuvant chemotherapy [9]. It is now generally accepted that LSS is indicative for localized osteosarcoma, while surgical amputation is adopted for high malignancy osteosarcoma. However, there are still some surgeons holding the view that immediate and aggressive removal of the tumor will prevent the progression of fracture-induced disease, and consequently amputation is considered to be a better option for osteosarcoma patients with pathologic fracture [10], [11], [12], [13].
Conflicting findings have been reported on the survival and function recovery between treatments of LSS and amputation in patients with osteosarcoma. Toward this end, a meta-analysis of published clinical trials was performed to compare the clinical efficacy of LSS and amputation treatments in terms of local recurrence, 5-year overall survival rate, and metastatic occurrence. Several studies have attempted similar meta-analysis [14]; however, the included studies were much smaller, and their scopes were restricted to specific therapies compared with this meta-analysis. Through more extensive osteosarcoma literature, this meta-analysis tries to give a comprehensive conclusion on the outcomes in osteosarcoma patients receiving LSS and amputation. Such information will help us determine the most appropriate osteosarcoma-treating method.
2. Material and methods
2.1. Literature search
A comprehensive and complete search of Medline, Embase, Cochrane Database, Web of Sciences, and CNKI was performed from June 2014 to July 2014, using the search terms: “osteosarcoma”, “limb salvage” and “amputation”. There was no language or other restrictions. All articles with raw descriptive data were included, including original research, clinical trials, case reports, databases, letters, and reviews.
2.2. Included studies
Articles were included if they were (1) comparative study between LSS and amputation groups, (2) patients with osteosarcoma in their lower limb, (3) sufficient data was provide in terms of local recurrence, 5-year overall survival rate, or occurrence of metastasis. Exclusion criteria were as follows: (1) studies only reported data related to LSS or amputation groups without a comparison, (2) general case series with less than 20 total patients, (3) letters, case reports, editorials or reviews.
2.3. Data extraction
Outcome data were collected from the articles by two authors of our study. The authors used a structured sheet, and then gathered all the data into a database. Study characteristics included year of publication, number of patient with LSS and amputation, study period, gender, Enneking stage, response to chemotherapy, follow-up, etc. Any disagreement was resolved by continuing discussions until a consensus was reached.
2.4. Study quality
With the Newcastle–Ottawa scale (NOS) quality assessment as recommended by the Cochrane Observational Studies Method Working Group, the quality of included articles was evaluated by two independent reviewers. This scale has a maximum nine points concerning quality of selection, comparability, exposure, and outcome of study participants. Because of the variable quality of the observational studies, we took the criteria of 5 or more NOS scores as studies with good quality.
2.5. Statistical analysis
The outcome of measurement used in our study was local recurrence, 5-year overall survival rate, and occurrence of metastasis, which were all dichotomous data. We used the software of the Cochrane Collaboration (Review Manager 5.2) to calculate OR and 95% CI for all outcomes. Statistical heterogeneity among the included studies was assessed by the Chi squared and I2 tests. Statistically significant heterogeneity was defined as an I2 value>0.5. A random effects model was selected for heterogeneous data; otherwise, a fixed effect model was selected.
Funnel plots were used to test the possibility of publication bias, which exhibited the intervention effect from the individual study against the respective standard error. A symmetrical plot represents no bias, and any asymmetry of the plot suggests the existence of publication bias.
3. Results
3.1. Literature information
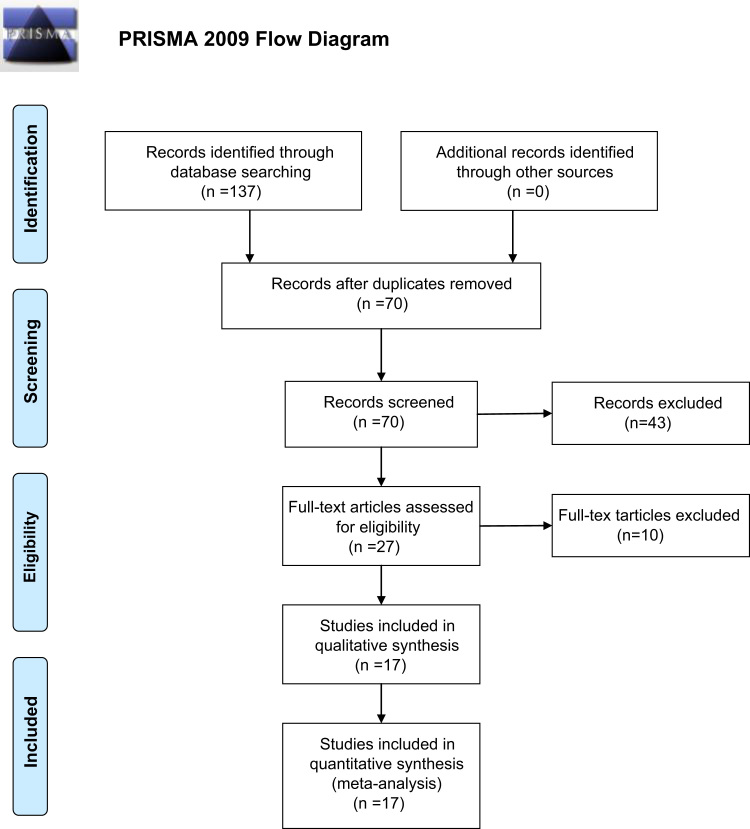
In the preliminary literature search, 137 potentially relevant articles were identified. However, according to the inclusion criteria, only 17 articles [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31] were selected (Fig. 1; Table S1). All of the 17 research articles were retrospective studies. The publication dates ranged from 1996 to 2012. 1343 patients with osteosarcoma were comprised totally, of whom 617 patients received LSS and 726 received amputation. The results of quality assessment by NOS are shown in Table 1, and the detail information of patients in each articles were listed in Table 2. Among 5 of the studies [20], [22], [25], [30], [31] patients with Enneking Stage-IIA, and Stage-IIB osteosarcoma were both included. For other 5 studies [15], [16], [26], [28], [29], patients with Enneking Stage-IIB osteosarcoma were included. Histologic response to preoperative chemotherapy is reported to be a independent prognostic factor of osteosarcoma [32]. Among the included studies, 7 studies assessed the histologic response to preoperative chemotherapy [16], [17], [18], [21], [23], [25], [28].
Fig. 1.
Flow chart of studies included and excluded.
Table 1.
Quality assessment for the 17 included articles based on Newcastle–Ottawa quality assessment scale.
| Selection |
Comparability |
Exposure |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Refs. | Inclusion criteria | Sample size >50 | Endpoint | Anatomical location | Enneking stage | Chemotherapy | Local recurrence | 5-year overall survival | Metastatic | NOS |
| Abudu et al. [12] | * | – | * | * | * | * | * | * | * | 8* |
| Bacci et al. [13] | * | – | * | * | * | * | * | – | – | 6* |
| Bacci et al.b[14] | * | * | * | – | – | * | * | * | * | 7* |
| Bramer et al. [15] | * | * | * | * | – | * | * | – | – | 6* |
| Ferguson et al. [16] | * | – | * | * | – | * | * | – | – | 5* |
| Guo et al. [17] | * | – | * | * | – | * | * | – | * | 6* |
| Hegyi et al. [18] | * | * | * | – | – | * | * | * | * | 7* |
| Jiang et al. [19] | * | * | * | * | * | * | * | – | – | 8* |
| Kim et al. [20] | * | – | * | * | – | * | * | – | – | 5* |
| Ma et al. [21] | * | * | * | * | – | * | – | * | – | 6* |
| Mavrogenis et al. [22] | * | – | * | * | – | * | * | – | * | 6* |
| Niu et al. [23] | * | – | * | * | * | * | * | * | * | 8* |
| Robert et al. [24] | * | * | * | * | – | – | * | * | – | 6* |
| Scully et al. [25] | * | * | * | * | * | * | * | * | – | 8* |
| Xu et al. [26] | * | * | * | * | * | * | * | * | – | 8* |
| Zhang et al. [27] | * | – | * | * | * | * | * | – | * | 7* |
| Zhao et al. [28] | * | * | * | * | * | * | * | – | – | 7* |
Table 2.
Characteristic of the 17 included studies.
| Ref. | Abudu et al. [12] | Bacci et al. [13] | Bacci et al.b[14] | Bramer et al. [15] | Ferguson et al. [16] | Guo et al. [17] | Hegyi et al. [18] | Jiang et al. [19] | Kim et al. [20] |
|---|---|---|---|---|---|---|---|---|---|
| Country | England | Italy | Italy | UK | Canada | China | Hungary | China | Korea |
| Patient Number | 40 | 46 | 560 | 56 | 31 | 21 | 122 | 64 | 37 |
| LSS, | 27 | 35 | 95 | 44 | 19 | 13 | 92 | 32 | 3 |
| Amputation | 13 | 11 | 465 | 12 | 12 | 8 | 30 | 32 | 4 |
| Study period | 1975–1994 | 1983–1999 | 1983–1995 | 1983–2003 | 1989–2006 | 1998–2008 | 1988–2006 | 2001–2011 | – |
| Male | 25 | 24 | 320 | 36 | 14 | 14 | 65 | 46 | 26 |
| Female | 14 | 22 | 240 | 20 | 17 | 7 | 57 | 18 | 11 |
| Median age(range), years | 18 (2–46) | 11 (3–20) | – | 16 (4–57) | 30 (11–8) | 14.5 (9–17) | 13.8±3.6a | 18.4±6.3 (12–30) | – |
| Enneking stage | Stage-IIB | Stage-IIB | – | – | – | Stage-IIA, Stage-IIB | – | Stage-IIA, Stage-IIB | – |
| Follow-up(range), months | 55 (8–175) | 132 (36–240) | 22.6 (3–96) | 117 (7–252) | – | 38 (28–62) | – | 8–42 | 43 (10–228) |
| Poor chemotherapy | – | 12 (26%) | 194 (35%) | 43 (78%) | – | – | 67 (55%) | – | 23 (62%) |
| Local recurrence | |||||||||
| LSS | 5 | 1 | 6 | 6 | 2 | 2 | – | 2 | 4 |
| Amputation | 0 | 1 | 20 | 2 | 0 | – | – | 2 | 0 |
| 5-year Survival | |||||||||
| LSS | 17 | – | 60 | – | – | 9 | 58 | 25 | – |
| Amputation | 6 | – | 230 | – | – | 3 | 23 | 19 | – |
| Metastatic occurrence | |||||||||
| LSS | 12 | – | – | – | – | 1 | – | – | – |
| Amputation | 9 | – | – | – | – | 4 | – | – | – |
| Ref. | Ma et al. [21] | Mavrogenis et al. [22] | Niu et al. [23] | Robert et al. [24] | Scully et al. [25] | Xu et al. [26] | Zhang et al. [27] | Zhao et al. [28] |
| Country | China | Italy | China | USA | USA | China | China | China |
| Patient Number | 51 | 42 | 22 | 57 | 52 | 58 | 31 | 53 |
| LSS, | 32 | 23 | 12 | 33 | 30 | 43 | 17 | 37 |
| Amputation | 19 | 19 | 10 | 24 | 22 | 15 | 14 | 16 |
| Study period | 1991–1999 | 1985–2010 | 1992–2001 | 2007–2008 | 1977–1996 | 1992–2002 | – | 1996–2007 |
| Male | 35 | 23 | 15 | 20 | 28 | 30 | 26 | – |
| Female | 16 | 19 | 7 | 37 | 24 | 28 | 5 | – |
| Median Age(range), years | 22.3 (10–47)a | 26 (7–78)a | 18 (3–36) | 33.8 (16.1–52) | 23±17.4a | 20.26 (12–55) | 17a | 19.5 (5–45)a |
| Enneking stage | – | Stage-IIA, Stage-IIB | Stage-IIB | – | Stage-IIB | Stage-IIB | Stage-IIA, Stage-IIB | Stage-IIA, Stage-IIB |
| Follow-up(range), months | 72 (60–144) | 60 (8–288) | 54.7 (8–146) | 223.2 (39.6–338.4) | 54 (6–152.4) | 129.6 (72–192) | 43.2 | – |
| Poor chemotherapy | – | 7 (17%) | – | – | 29 (64%) | – | – | – |
| Local recurrence | ||||||||
| LSS | 2 | 5 | 2 | – | 7 | 7 | 5 | 3 |
| Amputation | – | 2 | 1 | – | 4 | 1 | 12 ( | 1 |
| 5-year Survival | ||||||||
| LSS | 12 | – | 8 | – | 19 | 19 | 6 | 19 |
| Amputation | 7 | – | 4 | – | 12 | 8) | 1 | |
| Metastatic Occurrence | ||||||||
| LSS | – | 1 | 3 | – | – | – | ||
| Amputation | – | 3 | 6 | – | – | – |
3.2. Study heterogeneity and publication bias
The P-value for study heterogeneity was 0.26, 0.29, 0.80 for three outcomes including local recurrence, 5-year overall survival rate, and metastatic occurrence. As a result of the true difference in terms of treatment effect, clinical characteristics, etc., the variability (I2) across all studies was 18%, 16%, and 0% for three outcomes respectively. All the results above indicated that there was no heterogeneity among the included studies ( Table 3, Table 4, Table 5). Moreover, the funnel plots for all outcome measurements were symmetrical (Fig. 2A–C). All the spots representing individual studies fell evenly within the top of the inverted funnel, indicating that there was no publication bias.
Table 3.
Statistic summary of forest plot of comparison: local recurrence of LSS vs. amputation for the treatment of osteosarcoma.
| Study | LSS |
Amputation |
Weight (%) | Odds Ratio | ||
|---|---|---|---|---|---|---|
| Events | Total | Events | Total | M–H, Fixed, 95% CI | ||
| Abudu et al. | 5 | 27 | 0 | 13 | 1.5 | 6.60 [0.34, 128.99] |
| Bacci et al. | 1 | 35 | 1 | 11 | 4.2 | 0.29 [0.02, 5.14] |
| Bacci et al.b | 6 | 95 | 20 | 465 | 17.9 | 1.50 [0.59, 3.84] |
| Bramer et al. | 6 | 44 | 2 | 12 | 7.6 | 0.78 [0.14, 4.52] |
| Ferguson et al. | 2 | 19 | 0 | 12 | 1.5 | 3.57 [0.16, 81.03] |
| Guo et al. | 2 | 13 | 5 | 8 | 14.8 | 0.11 [0.01, 0.87] |
| Jiang et al. | 2 | 32 | 2 | 32 | 5.3 | 1.00 [0.13, 7.57] |
| Kim et al. | 4 | 33 | 0 | 4 | 2.1 | 1.37 [0.06, 30.04] |
| Mavrogenis et al. | 5 | 23 | 2 | 19 | 4.8 | 2.36 [0.40, 13.84] |
| Niu et al. | 2 | 12 | 1 | 10 | 0.0 | 1.80 [0.14, 23.37] |
| Scully et al. | 7 | 30 | 4 | 22 | 10.0 | 1.37 [0.35, 5.41] |
| Xu et al. | 7 | 43 | 1 | 15 | 3.5 | 2.72 [0.31, 24.19] |
| Zhang et al. | 5 | 17 | 12 | 18 | 23.2 | 0.21 [0.05, 0.87] |
| Zhao et al. | 3 | 37 | 1 | 16 | 3.6 | 1.32 [0.13, 13.79] |
| Total (95%) CI | 57 | 480 | 50 | 647 | 100.0 | 1.03 [0.65, 1.64] |
Heterogeneity: χ2=14.47, df=12 (P=0.26); I2=18%.
Test for over effect: Z=0.14 (P=0.89).
Table 4.
Statistic summary of forest plot of comparison: 5-year overall survival of LSS vs. amputation for the treatment of osteosarcoma.
| Study | LSS |
Amputation |
Weight (%) | Odds Ratio | ||
|---|---|---|---|---|---|---|
| Events | Total | Events | Total | M–H, Fixed, 95% CI | ||
| Abudu et al. | 17 | 27 | 6 | 13 | 4.0 | 1.98 [0.52, 7.58] |
| Bacci et al.b | 60 | 95 | 230 | 465 | 38.5 | 1.75 [1.11, 2.76] |
| Guo et al. | 9 | 13 | 3 | 8 | 1.5 | 3.75 [0.59, 23.94] |
| Hegyi et al. | 58 | 92 | 23 | 30 | 17.2 | 0.52 [0.20, 1.34] |
| Jiang et al. | 25 | 32 | 19 | 32 | 5.6 | 2.44 [0.82, 7.31] |
| Ma et al. | 12 | 32 | 7 | 19 | 7.4 | 1.03 [0.32, 3.33] |
| Niu et al. | 8 | 12 | 4 | 10 | 1.9 | 3.00 [0.52, 17.16] |
| Scully et al. | 19 | 30 | 12 | 22 | 6.8 | 1.44 [0.47, 4.41] |
| Xu et al. | 19 | 43 | 8 | 15 | 8.9 | 0.69 [0.21, 2.25] |
| Zhang et al. | 6 | 17 | 1 | 14 | 1.0 | 7.09 [0.74, 68.24] |
| Zhao et al. | 19 | 37 | 8 | 16 | 7.3 | 1.06 [0.33, 3.41] |
| Total (95%) CI | 252 | 430 | 321 | 644 | 100.0 | 1.47 [ 1.10, 1.97] |
Heterogeneity: χ2=11.94, df=10 (P=0.29); I2=16%.
Test for overall effect: Z=2.61 (P=0.009).
Table 5.
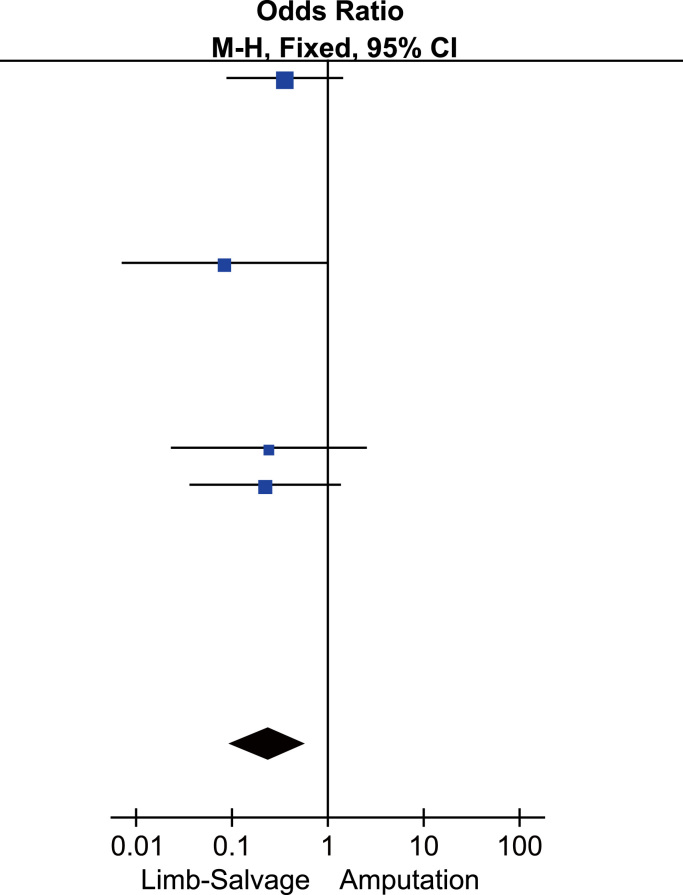
Statistic summary of forest plot of comparison: metastatic occurrence in patients receiving LSS vs. amputation for the treatment of osteosarcoma.
| Study | LSS |
Amputation |
Weight (%) | Odds ratio | ||
|---|---|---|---|---|---|---|
| Events | Total | Events | Total | M–H, Fixed, 95% CI | ||
| Abudu et al. | 12 | 27 | 9 | 13 | 34.8 | 0.36 [0.09, 1.44] |
| Guo et al. | 1 | 13 | 4 | 8 | 23.6 | 0.08 [0.01, 0.98] |
| Mavrogenis et al. | 1 | 23 | 3 | 19 | 16.2 | 0.24 [0.02, 2.55] |
| Niu et al. | 3 | 12 | 6 | 10 | 25.3 | 0.22 [0.04, 1.37] |
| Total (95%) CI | 17 | 75 | 22 | 50 | 100.0 | 0.24 [0.10, 0.60] |
Heterogeneity: χ2=1.02, df=3 (P=0.80); I2=0%.
Test for overall effect: Z=3.05 (P=0.002).
Fig. 2.
Funnel plot of comparison. A: local recurrence of LSS vs. amputation; B: 5-year overall survival of LSS vs. amputation; C: metastatic occurrence of LSS vs. amputation.
3.3. Meta-analysis of local recurrence
1127 cases from 14 studies were selected for meta-analysis of local recurrence. The overall incidence of local recurrence in LSS and amputation group was 11.88% (57 of 480) and 7.73% (50 of 647), respectively. The results showed that there was no significant differences between LSS and amputation group (OR: 1.03 with 95% CI ranging from 0.65 to 3.30; Z=0.14, P=0.89) (Table 3; Fig. 3).
Fig. 3.
Forest plot of comparison, local recurrence of LSS vs. amputation for the treatment of osteosarcoma.
3.4. Meta-analysis of 5-year overall survival
Totally, 1074 cases from 10 studies were analyzed for 5-year overall survival. The 5-year overall survival rate in LSS and amputation group was 58.60% (252 of 430) and 49.84% (321 of 644), respectively. In patients treated with LSS, the 5-year overall survival rate was significantly higner than those treated with amputation (OR: 1.47 with 95% CI ranging from 1.10 to 1.97; Z=2.61, P<0.05) (Table 4; Fig. 4).
Fig. 4.
Forest plot of comparison, 5-year overall survival of LSS vs. amputation for the treatment of osteosarcoma.
3.5. Meta-analysis of metastasis occurrence
125 cases from 4 studies were analyzed for metastatic analysis. The overall incidence of metastasis occurrence in LSS and amputation group was 22.67% (17 of 75) and 44% (22 of 50), respectively. Patients treated with LSS had a significantly lower metastasis occurrence compared those with amputation (OR: 0.24 with 95% CI ranging from 0.10 to 0.60; Z=3.05, P<0.05) (Table 5; Fig. 5).
Fig. 5.
Forest plot of comparison, metastatic occurrence of LSS vs. amputation for the treatment of osteosarcoma.
4. Discussion
With the improved efficacy of chemotherapy, the number of patients with osteosarcoma who received LSS instead of amputation has significantly increased recent years [33], [34], [35], [36], [37]. Moreover, LSS benefits not only malignant primary osteosarcoma patients, but also high-grade, localized osteosarcoma patients. However, there are substantial studies showing that the survival rate and local recurrence between LSS and amputation for osteosarcoma have been conflicting [25], [38]. In this study, it was concluded that patients treated with LSS had a similar local recurrence and a lower metastasis occurrence compared with those treated with amputation, which was identical with that of Yin [14] but with more expansive literature included in our study. In addition, we found that 5-year overall survival rate of patients treated with LSS was higher than those treated with amputation. Therefore, our results provide more comprehensive evidence to support LSS for the treatment of osteosarcoma patients.
In the meta-analysis of local recurrence of LSS vs. amputation for the treatment of osteosarcoma, there was no significant difference in the two surgery methods (OR: 1.03 with 95% CI ranging from 0.65 to 3.30; Z=0.14, P=0.89) (Table 3; Fig. 3). In five of 17 articles, the local recurrence rate in patients undergoing LSS was dramatically higher than those receiving amputation [15], [19], [23], [25], [29]. The sample sizes of these five studies were relatively small. Differently from these studies, other included studies revealed similar local recurrence rates between the two surgery methods. Moreover, in a study of Bacci et al. [17] with more than 500 samples investigated, local recurrence rates were found to be similar between LSS and amputation, which offered solid evidence to evaluate the local recurrence of LSS for the treatment of osteosarcoma.
In this meta-analysis, the overall survival at 5 years was slightly better in those treated by LSS than those who had amputation(OR: 1.47 with 95% CI ranging from 1.10 to 1.97; Z=2.61, P<0.05)for treating osteosarcoma patients. Among the included studies, only two studies of Xu et al., Hegyi et al. [21], [29] found that the amputation resulted in better 5-year survival. Abudu et al. [15] found that amputation didn’t come with a prolonged overall survival, though it provide better eradication of local tumor than LSS. However, in another article which was not included in the meta-analysis [39], it was indicated that LSS did not affect the survival rate. Even through our analysis results were somewhat inconsistent with previous research, we still concluded that LSS had a similar 5-year overall survival rate to that of amputation.
The metastatic occurrence rate for patients treated with LSS was significantly lower than those treated with amputation (OR: 0.24 with 95% CI ranging from 0.10 to 0.60; Z=3.05, P<0.05) (Table 5; Fig. 5), which was identical with the results of Yin et al. [14]. However, only 4 of 17 studies reported the metastasis, including 125 patients. Abudu [15] found that the treatment of LSS or amputation influenced the development of metastases to some degree, 44% in patients with LSS and 69% in patients with amputation; Niu [26] reported that metastasis happened in 25% patients treated with LSS compared with 60% in patients treated with amputation; in the study of Mavrogenis et al. [22], one of 23 patients receiving LSS developed metastasis while 3 of 19 patients receiving amputation did; in another study, one of 13 patients undergoing LSS had metastatic occurrence and 4 of 8 patients undergoing amputation had metastatic occurrence [17]. Our analysis of metastatic occurrence was based on only four articles. There are some important prognostic factors of osteosarcoma, such as radical resectability of the tumor, extent of disease at diagnosis, initial tumor volume, and response to neoadjuvant chemotherapy[40], which makes the comparison of the two surgery methods complicate. Thus, additional high-quality, randomized controlled studies are needed to confirm the conclusions.
5. Conclusions
In conclusion, our meta-analysis highlighted that LSS can be safely used in localized osteosarcoma patients with lower metastatic occurrence and better survival, which won’t increase the risk for local recurrence. And our meta-analysis supported the conclusions proposed by Yin et al. and provided more comprehensive evidence to support application of LSS for the treatment of osteosarcoma patients.
6. Limitation
In our study, all the articles were retrospective designed, and most of the included studies had small sample sizes that were subjected to systematic and random bias. The small sample size here was more likely to be the main reason for the failure in detecting heterogeneity in articles if it did exist, as the test power of heterogeneity was low in this situation. Moreover, the number in some studies of events for the outcome measurement was very low. Finally, the details of Enneking Stage and response to preoperative chemotherapy were missing for some studies. Therefore, even our study represented more comprehensive evidence compared with previous studies, the conclusions should be confirmed by high-quality, randomized-controlled, large-sample studies.
Acknowledgments
This research was supported in part by the China Postdoctoral Science Foundation (Nos. 2013M542478 and 2015T81139), a Grant (no. 2014FB059) from the Joint Special Funds for the Department of Science and Technology of Yunnan Province-Kunming Medical University, a Grant (no. D-201242) from the specialty fund of High-level Talents Medical Personnel Training of Yunnan Province. A Grant (no. 2015HC026) from the Yunnan Provincial Innovative Team of Bone and Soft Tissue Tumor. A Grant (no. 2014HB034) from the Young and Middle-aged Academic and Technical Leaders of Yunnan Province, Grant (no. 2014NS013/2014NS014/2014NS016) from the Health Medical Units with Research Institutions Construction Project of Yunnan Province, and Doctor Scientific Research Startup funds of the Third Affiliated Hospital of Kunming Medical University (No. BSJJ201406).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jbo.2016.01.001.
Appendix A. Supplementary material
Supplementary material
References
- 1.Coventry M.B., Dahlin D.C. Osteogenic sarcoma: a critical analysis of 430 cases. J. Bone Jt. Surg. 1957;39:741–758. [PubMed] [Google Scholar]
- 2.Sampo M.M., Tarkkanen M., Kivioja A.H., Taskinen M.H., Sankila R. Osteosarcoma in Finland from1971 through 1990: a nationwide study of epidemiology and outcome. Acta Orthop. 2008;79:861–866. doi: 10.1080/17453670810016966. [DOI] [PubMed] [Google Scholar]
- 3.Wilkins R.M., Cullen J.W., Odom L., Jamroz B.A., Cullen P.M. Superior survival in treatment of primary nonmetastaticpediatric osteosarcoma of the extremity. Ann. Surg. Oncol. 2003;10:498–507. doi: 10.1245/aso.2003.03.061. [DOI] [PubMed] [Google Scholar]
- 4.Ta H.T., Dass C.R., Choong P.F., Dunstan D.E. Osteosarcoma treatment: state of the art. Cancer Metastasis Rev. 2009;28:247–263. doi: 10.1007/s10555-009-9186-7. [DOI] [PubMed] [Google Scholar]
- 5.Luetke A., Meyers P.A., Lewis I., Juergens H. Osteosarcoma treatment-where do we stand? A state of the art review. Cancer Treat. Rev. 2014;40(4):523–532. doi: 10.1016/j.ctrv.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Ozaki T., Flege S., Kevric M. Osteosarcoma of the pelvis: experience of the Cooperative Osteosarcoma Study Group (COSS) J. Clin. Oncol. 2003;21:334–341. doi: 10.1200/JCO.2003.01.142. [DOI] [PubMed] [Google Scholar]
- 7.Messerschmitt P.J., Garcia R.M., Abdul-Karim F.W., Greenfield E.M., Getty P.J. Osteosarcoma. J. Am. Acad. Orthop. Surg. 2009;17:515–527. doi: 10.5435/00124635-200908000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Carty C.P., Dickinson I.C., Watts M.C., Crawford R.W., Steadman P. Impairment and disability following limb salvage procedures for bone sarcoma. Knee. 2007;16:405–408. doi: 10.1016/j.knee.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Ferrari S., Palmerini E. Adjuvant and neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr. Opin. Oncol. 2007;19:341–346. doi: 10.1097/CCO.0b013e328122d73f. [DOI] [PubMed] [Google Scholar]
- 10.Ebeid W., Amin S., Abdelmegid A. Limb salvage management of pathologic fractures of primary malignant bone tumors. Cancer Control. 2005;12:57–61. doi: 10.1177/107327480501200107. [DOI] [PubMed] [Google Scholar]
- 11.Natarajan M.V., Govardhan R.H., Williams S., Raja G.T.S. Limb salvage surgery for pathological fractures in osteosarcoma. Int. Orthop. 2000;24:170–172. doi: 10.1007/s002640000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui Q., Li D.F., Liu C., Guo J., Liu S.B., Liu Y.S. Two case-reports of the limb salvage treatment of osteosarcoma consolidated with obvious pathological fractures. Pathol. Oncol. Res. 2011;17:973–979. doi: 10.1007/s12253-010-9347-6. [DOI] [PubMed] [Google Scholar]
- 13.Godley K., Watts A.C., Robb J.E. Pathological femoral fracture caused by primary bone tumour: a population-based study. Scott. Med. J. 2011;56:5–9. doi: 10.1258/smj.2010.010006. [DOI] [PubMed] [Google Scholar]
- 14.Yin K., Liao Q.D., Zhong D., Ding J., Niu B. Meta-analysis of limb salvage versus amputation for treating high-grade and localized osteosarcoma in patients with pathological fracture. Exp. Ther. Med. 2012;4:889–894. doi: 10.3892/etm.2012.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abudu A., Sferopoulos N.K., Tillman R.M., Carter S.R., Grimer R.J. The surgical treatment and outcome of pathological fractures in localised osteosarcoma. J. Bone Jt. Surg. Br. 1996;78:694–698. [PubMed] [Google Scholar]
- 16.Bacci G., Ferrari S., Longhi A., Donati D., Manfrini M., Giacomini S. Nonmetastatic osteosarcoma of the extremity with pathologic fracture at presentation: local and systemic control by amputation or limb salvage after preoperative chemotherapy. Acta Orthop. Scand. 2003;74:449–454. doi: 10.1080/00016470310017776. [DOI] [PubMed] [Google Scholar]
- 17.Bacci G., Ferrari S., Lari S., Mercuri M., Donati D., Longhi A. Osteosarcoma of the limb: amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J. Bone Jt. Surg. Br. 2002;2002(84):88–92. doi: 10.1302/0301-620x.84b1.12211. [DOI] [PubMed] [Google Scholar]
- 18.Bramer J.A., Abudu A.A., Grimer R.J., Carter S.R., Tillman R.M. Do pathological fractures influence survival and local recurrence rate in bony sarcomas? Eur. J. Cancer. 2007;43:1944–1951. doi: 10.1016/j.ejca.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson P.C., McLaughlin C.E., Griffin A.M., Bell R.S., Deheshi B.M., Wunder J.S. Clinical and functional outcomes of patients with a pathologic fracture in high-grade osteosarcoma. J. Surg. Oncol. 2010;102:120–124. doi: 10.1002/jso.21542. [DOI] [PubMed] [Google Scholar]
- 20.Guo X.J., Zhou D.K., Zhu Z.J. Therapeutic analysis of combined treatment and amputation therapy for osteosarcoma in children and adolescent patients. J. Appl. Clin. Pediatr. 2011;26:1804–1805. 2011. In Chinese. [Google Scholar]
- 21.Hegyi M., Semei A.F., Jakab Z., Antal I., Kiss J., Miklos S. Good prognosis of localized osteosarcoma in young patients treated with limb-salvage surgery and chemotherapy. Pediatr. Blood Cancer. 2011;57:415–422. doi: 10.1002/pbc.23172. [DOI] [PubMed] [Google Scholar]
- 22.Jiang H., Chi X.F., Yang D. Effect of limb salvage surgery on 32 cases of osteosarcoma. J. Jilin Med. 2012;33:1832. In Chinese. [Google Scholar]
- 23.Kim M.S., Lee S.Y., Lee T.R., Cho W.H., Song W.S., Cho S.H. Prognostic effect of pathologic fracture in localized osteosarcoma: a cohort/case controlled study at a single institute. J. Surg. Oncol. 2009;100:233–239. doi: 10.1002/jso.21265. [DOI] [PubMed] [Google Scholar]
- 24.Ma C., Sun X.C. Effects of operation combined with chemotherapy for osteogenic sarcoma. J. Southeast Univ. 2004;23:406–408. [Google Scholar]
- 25.Mavrongenis A.F., Abati C.N., Romagnoli C., Ruggieri P. Similar survival but better function for patients after limb salvage versus amputation for distal tibia osteosarcoma. Clin. Orthop. Relat. Res. 2012;470:1735–1748. doi: 10.1007/s11999-011-2238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niu X.H., Ding Y. The surgical treatment and outcome of nonmetastatic osteosarcoma of the extremity with pathologic fractures. Zhonghua Wai Ke Za Zhi. 2008;46:1730–1733. In Chinese. [PubMed] [Google Scholar]
- 27.Robert R.S., Ottaviani G., Huh W.W., Palla S., Jaffe N. Psychosocial and functional outcomes in long-term survivors of osteosarcoma: a comparison of limb-salvage surgery and amputation. Pediatr. Blood Cancer. 2010;54:990–999. doi: 10.1002/pbc.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scully S.P., Ghert M.A., Zurakowski D., Thompson R.C., Gebhardt M.C. Pathologic fracture in osteosarcoma: prognostic importance and treatment implications. J. Bone Jt. Surg. Am. 2002;84:49–57. [PubMed] [Google Scholar]
- 29.Xu B., Cai Z.D., Chen Z.R., Yao Z.J., Zhang G.J. A preliminary evaluation of limb salvage surgery for osteosarcoma around knee joint. PLoS One. 2012;7:e33492. doi: 10.1371/journal.pone.0033492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H.Y., Luo X.Z., Wang Z.Y. Limb salvage and amputation in osteosarcoma: report of 31 cases. Chin. J. Surg. 1997;35:556–557. [PubMed] [Google Scholar]
- 31.Zhao Y.J. The limb-salvage treatment of limb osteosarcoma a clinical outcome study study at a single institute. J. Surg. Oncol. 2008;100:233–239. [Google Scholar]
- 32.Bramer J.A., van Linge J.H., Grimer R.J. Prognostic factors in localized extremity osteosarcoma: a systematic review. Eur. J. Surg. Oncol. 2009;35:1030–1036. doi: 10.1016/j.ejso.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Bacci G., Ferrari S., Mercuri M., Longhi A., Capanna R., Tienghi A. Neoadjuvant chemotherapy for extremity osteosarcoma: preliminary results of the Rizzoli's 4th study. Acta Oncol. 1998;37:41–48. doi: 10.1080/028418698423168. [DOI] [PubMed] [Google Scholar]
- 34.Delepine N., Delepine G., Alkallaf S. Local relapses following limb sparing salvage surgery for osteosarcoma: prognostic factors and influence of chemotherapy. ASCO Proc. 1996;15:526. [Google Scholar]
- 35.Saeter G., Wiebe T., Wiklund T., Monge O., Wahlqvist Y., Engstrom K. Chemotherapy in osteosarcoma: the Scandinavian sarcoma group experience. Acta Orthop. Scand. Suppl. 1999;285:74–82. [PubMed] [Google Scholar]
- 36.Tsuchiya H., Tomita K. Prognosis of osteosarcoma treated by limbsalvage surgery: the ten-year intergroup study in Japan. Jpn. J. Clin. Oncol. 1992;22:347–353. [PubMed] [Google Scholar]
- 37.Winkler K., Bieling P., Bielack S. Local control and survival from the co-operative osteosarcoma study group studies of the German Society of Pediatric Oncology and the Vienna Bone Tumour Registry. Clin. Orthop. 1991;270:79–86. [PubMed] [Google Scholar]
- 38.Allison D.C., Carney S.C., Ahlmann E.R., Hendifar A., Chawla S., Fedenko A. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma. 2011;2012:1–10. doi: 10.1155/2012/704872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeifang F., Sabo D., Ewerbeck V. Vol. 71. 2000. Pathological fracture in primary malignant bone tumors; pp. 1121–1125. (Chirurg). 2000. In German. [DOI] [PubMed] [Google Scholar]
- 40.Davis A.M., Bell R.S., Goodwin P.J. Prognostic factors in osteosarcoma: a critical review. J. Clin. Oncol. 1994;21:423–431. doi: 10.1200/JCO.1994.12.2.423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material