Abstract
Bone metastasis occurs in the majority of late-stage tumors with poor prognosis. It is mainly classified as osteoblastic metastasis and osteolytic metastasis. The pathogenesis of osteolytic metastasis is a “vicious cycle” between tumor cells and bone cells (primarily the osteoclasts), which is mediated by secretory factors. The P62 adapter protein is a versatile multitasker between tumor cells and bone cells. The overexpression of P62 has been detected among a variety of tumors, playing positive roles in both tumorigenesis and metastasis. Moreover, P62 is an important modulator of the osteoclastogenesis pathway. Therefore, the ability of P62 to modulate tumors and osteoclasts suggests that it may be a feasible oncotarget for bone metastasis, especially for osteolytic metastasis. Recent research has shown that a P62 DNA vaccine triggered effective anti-tumor, anti-metastatic and anti-osteoporotic activities. Growing lines of evidence point to P62 as an emerging oncotarget for osteolytic metastasis. In this review, we outline the different roles of P62 in tumor cells and osteoclasts, focusing on the P62-related signaling pathway in key steps of osteolytic metastasis, including tumorigenesis, metastasis and osteoclastogenesis. Finally, we discuss the newest observations on P62 as an oncotarget for osteolytic metastasis treatment.
Keywords: P62/SQSTM1, Bone metastasis, Cancer, Osteoclasts, Autophagy
1. Introduction
Up to 90% of patients with multiple myeloma, and 60–75% patients with prostate cancer and breast cancer develop bone metastasis at the later stages of their diseases [1]. Metastatic lesions in bone are significantly associated with bone pain, hypercalcemia or hypocalcemia, pathological fracture and spinal cord compression, which mostly correlated to low 5-years survival-rate less than 30% [2]. Relative to osteoblastic metastasis, osteolytic metastasis is more difficult to treat, making the need to study its pathogenesis. Recent advances in the understanding of osteolytic metastasis revealed that it is associated with characteristic modulations of the bone microenvironment and crosstalk between the tumor cells and bone cells (primarily the osteoclasts). Tumor cells condition the “metastatic niche” through the secretion of soluble factors that stimulate bone resorption by the osteoclasts. Osteoclastic bone resorption results in the release and activation of growth factors in the bone microenvironment that further stimulate tumor growth, leading to a “vicious cycle” [3]. It is involved with numerous signaling factors, but the crucial molecules are still uncertain [1].
Since its initial discovery as an atypical protein kinase C (PKC)-interacting protein, P62 (also known as sequestosome-1, SQSTM-1 or A170) has emerged as a crucial molecule in the regulation of cell growth, survival and proliferation [4]. The human P62 protein has 440 amino acid residues and contains different types of protein–protein interaction domains. The multi-functional domains of the P62 adapter protein are consistent with its role as a versatile multitasker in signal transduction in tumors [5], (Fig. 1). P62 accumulation promotes tumorigenesis [6]. It is found to be either not expressed or found at low levels of expression in normal tissues, but is over-expressed among various types of tumors and, for the most part, is correlated with tumor migration, invasion or metastasis [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]. Thus, the P62 gene is generally acknowledged to be an oncogene. In addition, many studies on Paget's disease of bone (PDB), which is a skeletal disorder characterized by osteolytic lesions and overactive osteoclasts, have identified P62 as an important modulator of the osteoclastogenesis pathway [25], [26], [27]. The dysregulated expression of the P62 protein promotes osteoclastogenesis, bone resorption and osteolytic lesions. Therefore, P62 has long been thought of as a promising molecular target in PDB and other bone metabolic diseases [28].
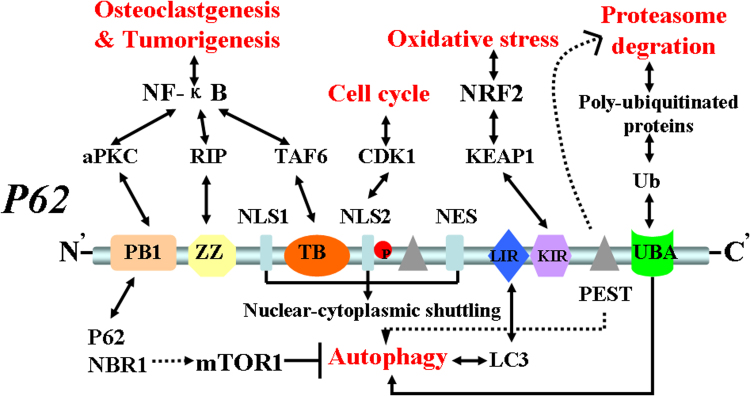
Fig. 1.
P62 structure, interaction domains and function. P62 has six main functional domains: PB1, ZZ, TB, LIR, KIR and UBA. The PB1 domain binds PKC. The ZZ zinc-finger domain binds receptor interacting protein (RIP).The TRAF6 binding (TB) domain binds TRAF6. Three domains link p62 to NF-κB activation, which is relevant in RANK-induced osteoclastogenesis, as well as in Ras-induced tumorigenesis. PB1 domain also self- and hetero- oligomerizes with NBR1, which might restrain autophagy through mTOR activation. The LIR domain interacts with autophagosome protein LC3, serving to control p62 levels in autophagy. The KIR domain binds Keap1, which might be important for the regulation of Nrf2 and the control of ROS levels in oxidative stress. The UBA domain regulates the interaction of p62 with poly-ubiquitylated proteins targeted for degradation by the proteasome or autophagy. In addition, p62 has two PEST sequences that are targets for post-translation modifications and degradation. P62 also contains two NLS sequences and a NES sequence which allow p62 shuttling into and out of nucleus. NLS2 domain phosphorylated by CDK1 that regulates cell cycle.
Many in vitro and in vivo studies employing knockdown have shown that P62 can inhibit tumor formation, proliferation and/or progression [29], [30], [31]. Recent research on intramuscularly or intravenously administered P62 DNA vaccines showed that they induced anti-P62 antibodies and exhibited strong anti-tumor and anti-metastatic activities in transplantable mouse tumors [32] and canine spontaneous mammary neoplasm models [33]. The latest research reported the unexpected finding that intramuscular delivery of P62 DNA vaccines exerts a powerful anti-osteoporotic activity in a mouse model of inflammatory bone loss [34]. These studies promoted P62 as an oncotarget for bone metastasis, especially for osteolytic metastasis.
In this review, we outline the different role of P62 in tumor cell and osteoclast, focusing on the P62-related signal pathway in key steps of osteolytic metastasis, including tumorigenesis, metastasis and osteoclastogensis. Finally, we discuss the newest observations about P62 as an oncotarget for osteolytic metastasis treatment.
2. The role of P62 in tumors
2.1. P62 accumulation promotes tumorigenesis
P62 is over-expressed among almost all differently primary tumors, including prostate [7], [8], [9], breast [10], [11], [12], [13], lung [14], kidney [15], head and neck [16], esophageal [17], gastric [18], liver [19], colon [20], and ovarian [21] tumors, and myelomas [22], melanomas [23], and glioblastomas [24]. This implies that P62 may play an extensive role in tumorigenesis. Using immunohistochemical staining and an enzyme-linked immunosorbent assay, P62 over-expression was shown to be localized to the tumor cell cytoplasm and nucleus [12], [22], [24], which suggested that P62 may shuttle in transcription and translation signaling of tumor cells.
First, P62 promotes tumorigenesis through the autophagy pathway. Autophagy is a homeostatic process that occurs in all eukaryotic cells and involves the sequestration of cytoplasmic components (proteins and organelles) in double-membraned autophagosomes [35]. P62 has been shown to be both an autophagy substrate and an autophagy adapter protein that acts as a link between ubiquitination and autophagy. Defects in autophagy promote a failure of protein and organelle quality control in cells, which leads to P62 accumulation, resulting in perturbation of gene expression, increased genome damage and tumorigenesis [6]. Meanwhile, autophagy can be categorized as either nonselective or selective. Nonselective autophagy randomly engulfs a portion of the cytoplasm into autophagosomes and then delivers them to lysosomes for degradation. Selective autophagy, however, specifically recognizes and degrades a particular cargo, either a protein complex, an organelle, or an invading microbe [36]. It is likely that the failure to properly remove the damaged cargo (i.e., the aggregation-prone proteins) by selective autophagy (aggrephagy) contributes to tumors [37]. Recent studies have implied that P62 plays a receptor role in aggrephagy. The phosphorylation of P62 at Ser403 regulates the selective autophagic clearance of the ubiquitinated aggregated proteins [38], [39]. The mechanism of function in the aggrephagy of P62 can be described as a cargo–ligand–receptor–adapter model (Fig. 2). In short, as a receptor protein in the aggrephagy pathway, P62 plays a positive role in tumorigenesis.
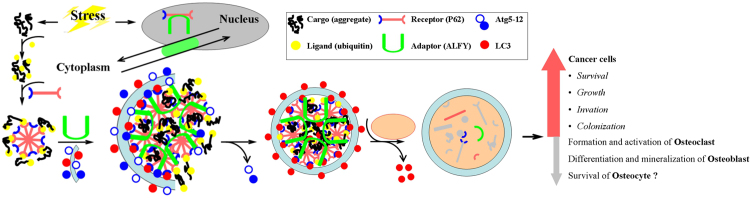
Fig. 2.
Receptor P62 in aggrephagy model. The ligand (ubiquitin) is the recognition component on the cargo (aggregate-prone proteins) that binds a receptor (P62). The interaction between the receptor and adapter is vital for cargo recruitment to the phapophore assembly site, where an autophagosome forms. Adapter autophagy-linked FYVE protein (ALFY) interacts both with the receptor P62 and with components of the core Atg5–Atg12 machinery to facilitate formation of the autophagosomal membrane around the cargo to be degraded.
Secondly, P62 is required for Ras-induced tumorigenesis. The Ras proto-oncogene has been found to be mutated in at least 25% of human tumors and is a potent activator of NF-κB, which is important for cell survival and tumor transformation [40]. P62 is an important NF-κB mediator of Ras-induced tumorigenesis [41], [42]. In a model of Ras-induced lung adenocarcinoma, in vitro and in vivo, P62 was induced by Ras to trigger IκB kinase (IKK) through the polyubiquitination of tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6). Then, activation of NF-κB increased inflammation and tumorigenesis. Deficiency of P62 also accounted for enhanced cell death and reduced the tumorigenicity of Ras [41]. Another recent study found that P62 plays an important role in the development of pancreatic ductal adenocarcinomas through a feedforward loop, whereby Ras activates NF-κB; NF-κB then transcriptionally induces P62 [42]. Therefore, P62 is a positive mediator in Ras-induced tumorigenesis by activation of NF-κB.
Finally, P62 is a new mediator of oxidative stress signaling in tumorigenesis. Oxidative stress is common in tumor microenvironment. Oxidative stress produces additional reactive oxygen species (ROS), which increase tumorigenesis through the induction of genome instability [43]. As an emerging regulator at the interface of oxidative stress signaling and tumorigenesis, P62 was shown to interact with Kelch-like ECH-associated protein 1 (KEAP1) at the transcription factor NF-E2-related factor 2 (NRF2) binding site, thereby competing with KEAP1 binding and thus promoting NRF2 release from KEAP1 [44]. Finally NRF2 activation induced many antioxidant gene expressions, which promoted cell survival and tumorigenesis [45]. Additionally, animal models with genetically-impaired autophagy initiation also reveal P62 accumulation, prolonged NRF2 activation, which leaded to tumor cell survival and tumorigenesis [46].
2.2. P62 plays different roles in tumor metastasis under different autophagy conditions
Recently, we also reviewed complex correlation between autophagy and cancer metastasis [47]. Autophagy may serve as a double-edged sword depending on the contextual demands placed on tumor cells throughout the metastatic process [48]. Latest research implied autophagy may play a role in cancer metastasis via promoting metastatic colonization [49]. P53 status may determine the role of autophagy in tumor development [50]. When p53 is functional, autophagy is required for tumor progression, but that autophagy might function as part of the tumor-suppression machinery when the function of p53 is lost [51].
P62 is over-expressed in primary tumor tissues, but there is little research on P62 expression in metastatic tumor tissues. The results appear to be contradictory. Kim evaluated autophagy activity in pulmonary metastasis of colorectal tumors and compared recurrence and non-recurrence groups. The results showed enhanced autophagy activity and decreased P62 expression in the recurrence group [52]. Another study showed there was no difference in P62 expression according to histologic subtypes in metastatic tumor tissues of unknown primary tumors [53]. However, in a mouse colorectal lung metastatic model, P62 levels were increased because autophagy activity was inhibited [54]. Generally speaking, in established tumors, autophagic responses constitute a means to cope with intracellular and environmental stress, thus favoring tumor metastasis [47], [55]. As a substrate protein of autophagy, overactive autophagy leads to P62 degradation. Therefore, it seems that P62 expression is negatively correlated with tumor metastasis.
However, in autophagy deficient cells, P62 plays different roles in tumor progression or metastasis. Qiang demonstrated that, in autophagy deficiency cells, P62 accumulation promotes cell proliferation and migration through P62-dependent stabilization of the oncogenic transcription factor Twist1, and in a mice model, P62 up-regulation promoted tumor growth and metastasis [56]. Another study showed that interrupting P62 with TRB3 causes P62 accumulation, which produces potent anti-tumor efficacies against tumor growth and metastasis [57]. Interestingly, recent research demonstrated that P62 and autophagy synergize to promote tumor growth and metastasis. In autophagy-deficient established murine tumors, increased P62 potentiated NF-κB signaling and the elaboration of pro-tumorigenic inflammatory cytokines to promote tumor growth and metastasis [58]. Therefore, “doubling down” on the P62 and autophagy pathway reveals an unexpected synergism [59]. It could be more effective than targeting either pathway alone to suppress tumor growth and metastasis. We then speculated P62 might play a dual role in tumor metastasis: under active autophagy status, as an autophagy receptor and substrate, P62 degradation promotes tumor metastasis, but under deficient autophagy status, P62 accumulation potentiates NF-κB signaling to promote tumor metastasis.
3. The role of P62 in osteoclastogenesis
3.1. P62 plays a positive role in osteoclastogenesis through the NF-κB activation pathway
Bone is continuously renewed through the dynamic balance between bone resorption and bone formation. Osteoclasts play a crucial role in both physiological and pathological bone resorption, and the interactions of tumor cells with osteoclasts disturb this balance and lead to osteolytic metastasis. The receptor activator of the nuclear factor-κB ligand (RANKL) is the key cytokine that induces osteoclastogenesis. After secretion by osteoblast, RANKL binds to its membrane-bound RANK receptor and results in signaling cascades that ultimately activate transcription factors, particularly NF-κB, which play a crucial role in the formation, survival, and bone resorption activity of the osteoclasts [60], [61]. The effects of NF-κB activation are to increase osteoclastogenesis, decrease osteoblast maturation and function, and increase chondrocyte hypertrophy [62]. RANKL–RANK-NF-κB activation is readily detectable in human disease samples, including inflammatory arthritis, osteoporosis, osteoarthritis, PDB, multiple myeloma and osteolytic bone metastasis [63], [64]. Many clinical trails had proved monoclonal antibody targeting RANKL (Denosumab) was more effective at delaying skeletal-related events than zoledronic acid in solid tumors and multiple myeloma [65], [66], [67].
P62 is an adapter protein that modulates protein–protein interactions using its different types of protein–protein interaction domains. Three of the domains; i.e., PB1, ZZ and TB, interact with the RANKL–RANK-NF-κB pathway. The PB1 domain of P62 binds PKCζ through a PB1–PB1 interaction, the ZZ zinc-finger domain binds receptor interacting protein (RIP), and the TRAF6 binding (TB) domain binds TRAF6. These three domains are relevant in the canonical and noncanonical NF-κB activation pathways of osteoclastogenesis (Fig. 3).
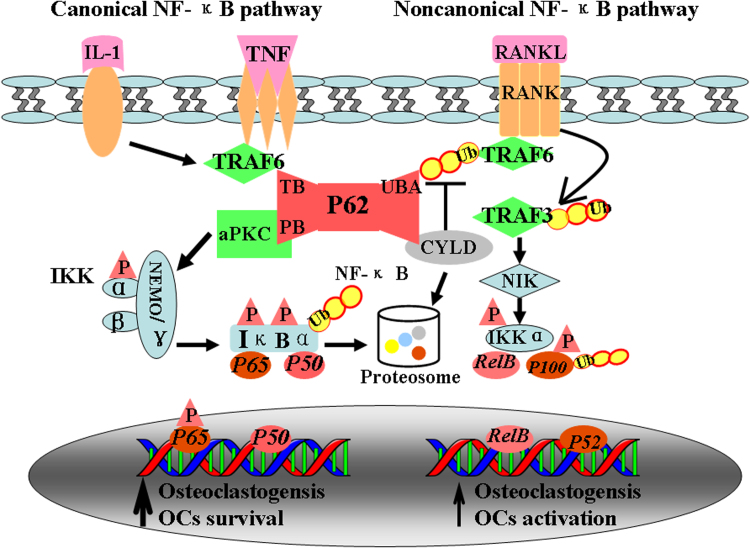
Fig. 3.
Osteoclastogenesis induced by adapter P62 in NF-κB activation pathway. RANKL activates both canonical and noncanonical pathways of NF-κB in osteoclastic like cells. In the canonical pathway, RANKL and IL-1 binding to RANK leads quickly TRAF-6 binding to TB domain of P62. Deubiquitinating enzyme CYLD targets TRAF6 via its interaction with the UBA domain of P62. P62 activated aPKC, which induce activation of IKK and IκBα. IκBα consequently undergoes rapid degradation by proteasome, resulting in release of p65 and p50 and their translocation to nuclei where they prevent apoptosis of osteoclast precursors, thus allowing them to continue differentiating. In the noncanonical pathway, RANKL binding to RANK interacts with TRAF3, activation of the NF-kB inducing kinase (NIK) and IKKa, lead to the phosphorylation of p100 and the processing of p100–p52.
P62 gene mutation was considered to be the main cause of Paget's disease of bone (PDB) [25], [26], [27], [28], which is a skeletal disorder characterized by osteolytic lesions and overactive osteoclasts. To date, 28 distinct P62 mutations have been reported in PDB patients and the most commonly identified mutation leads to a proline to leucine substitution at residue 392 (P392L) [28], [68]. Dysregulated expression of P62 protein promotes osteoclastogenesis, bone resorption and osteolytic lesions through the NF-κB activation pathway, leading to focal areas of aberrant and excessive bone turnover of PDB patients [69]. In vivo, six- to-eight-week-old P62 knockout mice showed complex signs caused by inactivation of the osteoclasts, as well as inhibition of IKK activation and NF-κB nuclear translocation [70]. Therefore, it is certain that P62 plays a positive role in osteoclastogenesis through the NF-κB activation pathway.
3.2. P62 may play an essential role in RANKL-induced autophagy and osteoclastogenesis
Autophagy plays a changing role in osteoclastogenesis. Recently, the roles of autophagy in the function of cartilage cells and bone cells (primarily the osteoclasts, osteoblasts, and osteocytes) were reviewed exhaustively [71], [72]. Autophagy has dual roles, regulating the osteoclast precursor cell pool and subsequent osteoclast formation and activation. On the one hand, the activation of autophagy promotes osteoclast formation and function. The deletion of ATG7 and ATG5 reduced the formation and resorptive capacity of osteoclasts in conditional knockout mice [73], [74]. On the other hand, the induction of autophagy may negatively regulate both osteoclast formation and activity. Treatment with rapamycin (which induces autophagy by inhibiting mTOR) has been reported to reduce the number of osteoclasts in young rats [75] and to reduce osteoclast formation and bone resorption in experimental mouse models of arthritis [76]. Therefore, as a substrate of autophagy, P62 expression was changed according to autophagy activity [77]. A recent study investigated the correlation of P62-autophagy–osteoclastgenesis. Using RT-PCR and Western-blot immunofluorescence analyses, the data showed that the expression of P62 was significantly altered during RANKL-induced osteoclast differentiation. Importantly, the knockdown of P62 obviously attenuated RANKL-induced expression of autophagy- and osteoclastogenesis-related genes. These indicated that P62 may play essential roles in RANKL-induced autophagy and osteoclastogenesis [78].
3.3. P62 plays a novel role between oxidative stress and osteoclastogenesis
In oxidative stress, reactive oxygen species (ROS) are crucial in bone homeostasis through the stimulation of osteoclasts, the inhibition of osteoblasts, and the induction of osteocyte apoptosis [79]. RANKL induces the production of long-lived ROS that are crucial for the late stages of osteoclastogenesis and regulation of bone resorption [80]. As previously mentioned, P62 was a novel mediator in KEAP1/NRF2 signaling of oxidative stress [44]. A recent study showed that the S349T mutation of P62 linked KEAP1/NRF2 signaling to PDB. The S349T mutant P62 showed reducing ability to activate NRF2 signaling in differentiating osteoclast-like cells [81]. Therefore, NRF2 deficiency promoted RANKL-induced osteoclast differentiation [82]. This may be a new mechanism for the role of P62 in PDB [68]. On the other hand, the correlation between oxidative stress and autophagy has long been known [83]. As a key role in oxidative stress and autophagy crosstalk, the phosphorylation of P62 activates the NRF2-KEAP1 pathway during selective autophagy [84]. Interestingly, the P62 NRF2-autophagy regulation appears to rely on dual feedback loops. First, a positive feedback loop is the regulation of P62 expression by NRF2, which in turn regulates NRF2 stabilization and degradation [85]. Therefore, P62 regulates its own transcription by controlling NRF2 activation. Second, starvation has been reported to induce ROS formation, which in turn triggers autophagy [86]. It creates a negative feedback loop whereby, under the conditions of starvation, ROS formation stimulates autophagy, which will result in the degradation of P62 and the attenuation of NRF2 activation [45]. In short, P62-NRF2-autophagy feedback loops may play a novel role in oxidative stress and osteoclastogenesis.
4. P62 as an oncotarget for treatment
4.1. Knockdown of P62 for tumor and bone metastasis
As previously mentioned, the multi-functional domains of the P62 adapter protein determine its role as a versatile multitasker in signal transduction [5]. P62 plays different roles in tumorigenesis, osteoclastogenesis, autophagy and oxidative stress. In theory, knockdown of P62 expression would lead intricate cellular reactions. However, the existing research showed knockdown of P62 expression was an attractive target for tumor and bone disease. The earlier studies found that some medicines, such as chloroquine [87] and calpain inhibitor [88], induced anti-tumor activity through P62 clearance. Later studies showed RNAi silencing of P62 significantly inhibited tumor growth both in vitro and in xenograft tumors model [29], [30], [31]. A puzzling aspect of the anti-tumor mechanism is interaction between P62 and autophagy. Down-regulation of P62 showed anti-tumor activity, but it also activated autophagy, which promoted tumor growth conversely. Nihira studied P62 inhibition on the regulation of autophagy and cell survival in P62-positive carcinoma cells. The results showed P62-silencing dramatically suppressed cell proliferation and activated autophagy. However, this autophagy is deficient, which was manifested as formation of mis-regulated autophagosomes with multilayer membranes and an autophagic cell death [29]. Islam showed similar results in a K-rasLA1 mice model. Using an osmotically active polysorbitol-mediated transporter (PSMT) to downregulate P62 by an RNAi strategy, autophagy was over-activated and remarkably reduced the size and number of tumors by suppressing proliferative cell nuclear antigen, CD 31, and VEGF levels [30]. In short, knockdown of P62 was a promising strategy for tumor targeted treatment.
The ability of P62 to modulate tumors and osteoclasts suggests that it may be a feasible oncotarget for bone metastasis, especially for osteolytic metastasis. First, P62 is required for the cell survival of apoptosis-resistant bone metastatic prostate tumor (PCa) cells. In bone metastatic lines that escape the bone marrow stromal cell paracrine factors (BMSC-PF), induced apoptosis can upregulate cytoprotective autophagy. In these PCa cell lines, the BMSC-PF signaling upregulated the P62 mRNA and protein by IL-1β [89], the siRNA knockdown of P62 was cytotoxic and the immunostaining showed a peri-nuclear clustering of the autolysosomes. Therefore, P62 could be exploited to target and kill these apoptosis-resistant PCa cells in the bone [9]. Secondly, P62 is also an attractive therapeutic target for multiple myeloma (MM) that is characterized by osteolytic destruction. Knocking-down P62 in patient-derived stromal cells significantly decreased the PKCζ, VCAM-1, and IL-6 levels as well as decreasing the stromal cell support of MM cell growth. Similarly, marrow stromal cells from P62-/- mice produced much lower levels of IL-6, TNF-α, RANKL and supported MM cell growth and osteoclast formation to a much lower extent than in normal cells [22]. The latest study reveals that P62 aggregation is a faithful marker of defective proteostasis, defining a novel prognostic and therapeutic framework for MM [90]. An exciting recent study showed that osteolytic metastasis is hampered by impinging on the interplay among autophagy, anoikis and ossifications. Osteolytic metastasis was largely prevented because of an autophagy failure marked by P62 without Beclin-1 [91]. However, an accurate molecular mechanism is still unclear.
4.2. P62 vaccine for tumor and bone disease treatment
The abundant studies on P62, tumors and PDB raises the possibility of a P62 vaccine for tumor and bone disease treatment. In 2013, Franco first performed a preclinical study of a novel DNA vaccine encoding P62 [32]. Surprisingly, the P62 vaccine showed broad-spectrum anti-tumor and anti-metastatic activity. Intramuscularly or intravenously administered P62 DNA vaccine induced anti-P62 antibodies and exhibited strong antitumor activity in four models of allogeneic mouse tumors; i.e., B16 melanoma, Lewis lung carcinoma, S37 sarcoma, and Ca755 breast carcinoma. A P62 DNA vaccine also dramatically decreased the number or sizes (volumes) of lung or lymphatic metastases. Then, Vladimir analyzed the feasibility a P62 DNA vaccine as a tumor immunotherapy target [92]. P62 protein fits the following antigen criteria: (i) immunogenic; (ii) essential for tumors cells (to avoid its loss through immune-editing), but dispensable for normal tissues to reduce the risk of toxicity, and (iii) overexpressed in tumors compared to the normal tissues. They subsequently demonstrated that P62 DNA plasmids, when administered in a preoperative setting, decreased and/or stabilized growth of advanced lesions in canine mammary tumors. These studies proved the P62 vaccine not only had good antitumor and anti-metastatic activity but also little toxicity [32], [33]. Recently, this team reported an unexpected finding that intramuscular delivery of a P62 vaccine exerts a powerful anti-osteoporotic activity in a mouse model of inflammatory bone loss by combining bone-sparing and osteosynthetic effects. Notably, the suppression of osteoporosis by a P62 vaccine was associated with sharp down-regulation of master inflammatory cytokines, and up-regulation of endogenous P62 protein by bone-marrow stromal cells. However, the study failed to detect the osteoclast activity when the P62 vaccine was administered in vivo [34]. On the whole, the data provide a solid rational to apply a P62 DNA vaccine as a safe, new therapeutic treatment for tumor and bone loss diseases. Therefore, in an osteolytic metastasis model, which was characterized by tumor and bone loss disease, we could hypothesis that a P62 vaccine may exert a combined effect in osteolytic metastasis treatment.
5. Concluding remarks
P62 is a cellular “Swiss army knife,” with its diverse cellular functions arising from its unique functional motifs and protein–protein interaction properties. P62 may play different roles in different type cells or different states of cells. For example, in tumor cells, P62 accumulation results in perturbation of gene expression, increased genome damage and tumorigenesis [6]. However, in tumor stromal cells, P62 up-regulation is an anti-inflammatory tumor suppressor that acts through the modulation of metabolism [93]. At the same time, its multifunctional nature may contribute to its complex role in osteolytic metastasis. For example, the up-regulation of P62 can promote osteoclastogenesis and differentiation by activating RANKL–RANK-NF-κB signaling, thereby accelerating bone resorption. This implies that P62 may promote osteolytic metastasis. However, as a receptor protein of autophagy, P62 accumulated because of autophagy failure, which hampered osteolytic metastasis. This implies that P62 may be, contradictorily, a reducer of osteolytic metastasis. In another example, as previously noted, the P62-NRF2-autophagy signal feedback loop includes two contradictory types, also playing an important role in osteoclastogenesis, and appears to be similar to the P62-mTOR-autophagy feedback in fat metabolism [94]. As a whole, these apparently contradictory roles showed that P62 is a multi-domain protein that may play different roles in different types of cells and in different microenvironments. Nevertheless, the ability of P62 to modulate tumors and osteoclasts suggests that it may be a feasible oncotarget for bone metastasis, especially for osteolytic metastasis. P62 may be an attractive target for therapeutic intervention if we are able to selectively modulate the interactions of P62 with specific signaling molecules, perhaps by using different interaction modules inherent in its structure.
Molecule targeted therapy is an important developing direction for tumor treatments, and the key to its success is the identification of crucial molecular targets. The mechanism of osteolytic metastasis involves different types of cells and intricate molecular signaling. P62 is at the interface of both tumor cells and osteoclasts, at the juncture of different signaling pathways that are correlated with tumorigenesis, metastasis and bone turnover. Therefore, selective regulation of P62 expression may be a feasible strategy for bone metastasis, especially for osteolytic metastasis. To date, several animal studies using the P62 vaccine showed broad-spectrum anti-tumor, anti-metastatic and anti-osteoporotic activity. A number of human clinical trials of DNA vaccines have been performed or are ongoing for tumors and showed encouraging results [95]. Further studies should be designed to detect P62 expression in osteolytic metastasis tissues. Meanwhile, evaluating the curative effects of down-regulating P62 expression in osteolytic metastasis animal models may provide an experimental basis for new targeted treatments.
Competing interests
The authors declare that they have no competing interests.
Authors contributions
Study concept and design: Jian Dong, Jing Zhang. Figure Preparing: Zuozhang Yang, Jing Zhang. Manuscript preparation: Jing Zhang. Manuscript review: Jian Dong. All authors have read and approval the final manuscript.
Acknowledgments
This research was supported in part by Grants (no. 81302343/H1624) from the National Natural Science Foundation of China, a Grant (no. 2014FB0 592014FB067) from the Joint Special Funds for the Department of Science and Technology of Yunnan Province – Kunming Medical University.
References
- 1.Roodman G.D. Genes associate with abnormal bone cell activity in bone metastasis. Cancer Metastasis Rev. 2012;31:569–578. doi: 10.1007/s10555-012-9372-x. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R., Ma J., Zou Z., Jemal A. CA Cancer J. Clin. Cancer Stat. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Ell B., Kang Y. SnapShot: bone metastasis. Cell. 2012;151:690. doi: 10.1016/j.cell.2012.10.005. 690. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez P., De Carcer G., Sandoval, Moscat J., Diaz-Meco M.T. Localization of atypical protein kinase C isoforms into lysosome-targeted endosomes through interaction withp62. Mol. Cell. Biol. 1998;18:3069–3080. doi: 10.1128/mcb.18.5.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moscat J., Diaz-Meco M.T. p62: a versatile multitasker takes on cancer. Trends Biochem. Sci. 2012;37:230–236. doi: 10.1016/j.tibs.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathew R., Karp C.M., Beaudoin B., Vuong N., Chen G., Chen H.Y. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giatromanolaki A., Sivridis E., Mendrinos S., Koutsopoulos A.V., Koukourakis M.I. Autophagy proteins in prostate cancer: relation with anaerobic metabolism and Gleason score. Urol. Oncol. 2014;32(39):e11–e18. doi: 10.1016/j.urolonc.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Kitamura H., Torigoe T., Asanuma H., Hisasue S.I., Suzuki K., Tsukamoto T. Cytosolic overexpression of p62 sequestosome 1 in neoplastic prostate tissue. Histopathology. 2006;48:157–161. doi: 10.1111/j.1365-2559.2005.02313.x. [DOI] [PubMed] [Google Scholar]
- 9.Chang M.A., Morgado M., Warren C.R., Hinton C.V., Farach-Carson M.C., Delk N.A. P62/SQSTM1 is required for cell survival of apoptosis-resistant bone metastatic prostate cancer cell lines. Prostate. 2014;74:149–163. doi: 10.1002/pros.22737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo R.Z., Yuan Z.Y., Li M., Xi S.Y., Fu J., He J. Accumulation of p62 is associated with poor prognosis in patients with triple-negative breast cancer. Onco Targets Ther. 2013;19:883–888. doi: 10.2147/OTT.S46222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rolland P., Madjd Z., Durrant L., Ellis I.O., Layfield R., Spendlove I. The ubiquitin-binding protein p62 is expressed in breast cancers showing features of aggressive disease. Endocr. Relat. Cancer. 2007;14:73–80. doi: 10.1677/erc.1.01312. [DOI] [PubMed] [Google Scholar]
- 12.Choi J., Jung W., Koo J.S. Expression of autophagy-related markers beclin-1, light chain 3A, light chain 3B and p62 according to the molecular subtype of breast cancer. Histopathology. 2013;62:275–286. doi: 10.1111/his.12002. [DOI] [PubMed] [Google Scholar]
- 13.Thompson H.G., Harris J.W., Wold B.J., Lin F., Brody J.P. P62 overexpression in breast tumors and regulation by prostate-derived Ets factor in breast cancer cells. Oncogene. 2003;22:2322–2333. doi: 10.1038/sj.onc.1206325. [DOI] [PubMed] [Google Scholar]
- 14.Inoue D., Suzuki T., Mitsuishi Y., Miki Y., Suzuki S., Sugawara S. Accumulation of p62/SQSTM1 is associated with poor prognosis in patients with lung adenocarcinoma. Cancer Sci. 2012;103:760–766. doi: 10.1111/j.1349-7006.2012.02216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L., Shen C., Nakamura E., Ando K., Signoretti S., Beroukhim R. SQSTM1 is a pathogenic target of 5q copy number gains in kidney cancer. Cancer Cell. 2013;24:738–750. doi: 10.1016/j.ccr.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuo W.L., Sharifi M.N., Lingen M.W., Ahmed O., Liu J., Nagilla M. P62/SQSTM1 accumulation in squamous cell carcinoma of head and neck predicts sensitivity to phosphatidylinositol 3-kinase pathway inhibitors. PLoS One. 2014;9:e90171. doi: 10.1371/journal.pone.0090171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou S.L., Yue W.B., Fan Z.M., Du F., Liu B.C., Li B. Autoantibody detection to tumor-associated antigens of P53, IMP1, P16, cyclin B1, P62, C-myc, Survivn, and Koc for the screening of high-risk subjects and early detection of esophageal squamous cell carcinoma. Dis. Esophagu. 2014;27:790–797. doi: 10.1111/dote.12145. [DOI] [PubMed] [Google Scholar]
- 18.Zhou S.L., Ku J.W., Fan Z.M., Yue W.B., Du F., Zhou Y.F. Detection of autoantibodies to a panel of tumor-associated antigens for the diagnosis values of gastric cardia adenocarcinoma. Dis. Esophagus. 2015;28:371–379. doi: 10.1111/dote.12206. [DOI] [PubMed] [Google Scholar]
- 19.Qian H.L., Chen S.H., Peng X.X. Significance of a novel fetal RNA-binding protein p62 expression in hepatocellular carcinoma. Zhonghua Bing Li Xue Za Zhi. 2003;32:329–332. [PubMed] [Google Scholar]
- 20.Park J. Myung, Huang S., Wu T.T., Foster N.R., Sinicrope F.A. Prognostic impact of Beclin 1, p62/sequestosome 1 and LC3 protein expression in colon carcinomas from patients receiving 5-fluorouracil as adjuvant chemotherapy. Cancer Biol. Ther. 2013;14:100–107. doi: 10.4161/cbt.22954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu H., Su J., Xu Y., Kang J., Li H., Zhang L. P62/SQSTM1 involved in cisplatin resistance in human ovarian cancer cells by clearing ubiquitinated proteins. Eur. J. Cancer. 2011;47:1585–1594. doi: 10.1016/j.ejca.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Hiruma Y., Honjo T., Jelinek D.F., Windle J.J., Shin J., Roodman G.D. Increased signaling through p62 in the marrow microenvironment increases myeloma cell growth and osteoclast formation. Blood. 2009;113:4894–4902. doi: 10.1182/blood-2008-08-173948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis R.A., Horswell S., Ness T., Lumsdon J., Tooze S.A., Kirkham N. Prognostic impact of p62 expression in cutaneous malignant melanoma. J. Investig. Dermatol. 2014;134:1476–1478. doi: 10.1038/jid.2013.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galavotti S., Bartesaghi S., Faccenda D., Shaked-Rabi M., Sanzone S., McEvoy A. The autophagy-associated factors DRAM1 and p62 regulate cell migration and invasion in glioblastoma stem cells. Oncogene. 2013;32:699–712. doi: 10.1038/onc.2012.111. [DOI] [PubMed] [Google Scholar]
- 25.Laurin N., Brown J.P., Morissette J., Raymond V. Recurrent mutation of the gene encoding sequestosome 1 (SQSTM1/p62) in Paget disease of bone. Am. J. Hum. Genet. 2002;70:1582–1588. doi: 10.1086/340731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundaram K., Shanmugarajan S., Rao D.S., Reddy S.V. Mutant p62P392L stimulation of osteoclast differentiation in Paget's disease of bone. Endocrinology. 2011;152:4180–4189. doi: 10.1210/en.2011-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Najat D., Garner T., Hagen T., Shaw B., Sheppard P.W., Falchetti A. Characterization of a non-UBA domain missense mutation of sequestosome 1 (SQSTM1) in Paget's disease of bone. J. Bone Miner. Res. 2009;24:632–642. doi: 10.1359/jbmr.081204. [DOI] [PubMed] [Google Scholar]
- 28.Singer F.R. Paget's disease of bone-genetic and environmental factors. Nat. Rev. Endocrinol. 2015;11:662–671. doi: 10.1038/nrendo.2015.138. [DOI] [PubMed] [Google Scholar]
- 29.Nihira K., Miki Y., Ono K., Suzuki T., Sasano H. An inhibition of p62/SQSTM1 caused autophagic cell death of several human carcinoma cells. Cancer Sci. 2014;105:568–575. doi: 10.1111/cas.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Islam M.A., Shin J.Y., Yun C.H., Cho C.S., Seo H.W., Chae C. The effect of RNAi silencing of p62 using an osmotic polysorbitol transporter on autophagy and tumorigenesis in lungs of K-rasLA1 mice. Biomaterials. 2014;35:1584–1596. doi: 10.1016/j.biomaterials.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Ren F., Shu G., Liu G., Liu D., Zhou J., Yuan L. Knockdown of p62/sequestosome 1 attenuates autophagy and inhibits colorectal cancer cell growth. Mol. Cell. Biochem. 2014;385:95–102. doi: 10.1007/s11010-013-1818-0. [DOI] [PubMed] [Google Scholar]
- 32.Venanzi F., Shifrin V., Sherman M., Gabai V., Kiselev O., Komissarov A. Broad-spectrum anti-tumor and anti-metastatic DNA vaccine based on p62-encoding vector. Oncotarget. 2013;4:1829–1835. doi: 10.18632/oncotarget.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabai V., Venanzi F.M., Bagashova E., Rud O., Mariotti F., Vullo C. Pilot study of p62 DNA vaccine in dogs with mammary tumors. Oncotarget. 2014;5:12803–12810. doi: 10.18632/oncotarget.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabbieti M.G., Agas D., Capitani M., Marchetti L., Concetti A., Vullo C. Plasmid DNA-coding p62 as a bone effective anti-inflammatory/anabolic agent. Oncotarget. 2015;6:3590–3599. doi: 10.18632/oncotarget.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi A.M., Ryter S.W., Levine B. Autophagy in human health and disease. N. Engl. J. Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 36.Jin M., Liu X., Klionsky D.J. SnapShot: selective autophagy. Cell. 2013;152(368–368):e2. doi: 10.1016/j.cell.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green D.R., Levine B. To be or not to be? How selective autophagy and cell death govern cell fate. Cell. 2014;157:65–75. doi: 10.1016/j.cell.2014.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsumoto G., Wada K., Okuno M., Kurosawa M., Nukina N. Serine 403 phosphorylation of p62/SQSTM1 regulates selective autophagic clearance of ubiquitinated proteins. Mol. Cell. 2011;44:279–289. doi: 10.1016/j.molcel.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 39.Rogov V., Dötsch V., Johansen T., Kirkin V. Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Mol. Cell. 2014;53:167–178. doi: 10.1016/j.molcel.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Mayo M.W., Wang C.Y., Cogswell P.C., Rogers-Graham K.S., Lowe S.W., Der C.J. Requirement of NF-kappaB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- 41.Duran A., Linares J.F., Galvez A.S., Wikenheiser K., Flores J.M., Diaz-Meco M.T. The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis. Cancer Cell. 2008;13:343–354. doi: 10.1016/j.ccr.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Ling J., Kang Y., Zhao R., Xia Q., Lee D.F., Chang Z. KrasG12d-induced IKK2/β/NF-κB activation by IL-1α and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:105–120. doi: 10.1016/j.ccr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cerdá C., Sánchez C., Climent B., Vázquez A., Iradi A., El Amrani F. Oxidative stress and DNA damage in obesity-related tumorigenesis. Adv. Exp. Med. Biol. 2014;824:5–17. doi: 10.1007/978-3-319-07320-0_2. [DOI] [PubMed] [Google Scholar]
- 44.Komatsu M., Kurokawa H., Waguri S., Taguchi K., Kobayashi A., Ichimura Y. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 45.Nezis I.P., Stenmark H. P62 at the interface of autophagy, oxidative stress signaling, and cancer. Antioxid. Redox Signal. 2012;17:786–793. doi: 10.1089/ars.2011.4394. [DOI] [PubMed] [Google Scholar]
- 46.Jiang T., Harder B., de la Vega M. Rojo, Wong P.K., Chapman E., Zhang D.D. p62 links autophagy and Nrf2 signaling. Free Radic. Biol. Med. 2015;88:199–204. doi: 10.1016/j.freeradbiomed.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J., Yang Z., Xie L., Xu L., Xu D., Liu X. Statins, autophagy and cancer metastasis. Int. J. Biochem. Cell Biol. 2013;45:745–752. doi: 10.1016/j.biocel.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Kenific C.M., Thorburn A., Debnath J. Autophagy and metastasis: another double-edged sword. Curr. Opin. Cell Biol. 2010;22:241–245. doi: 10.1016/j.ceb.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng Y.F., Shi Y.H., Shen Y.H., Ding Z.B., Ke A.W., Zhou J. Promoting colonization in metastatic HCC cells by modulation of autophagy. PLoS One. 2013;8:e74407. doi: 10.1371/journal.pone.0074407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenfeldt M.T., O’Prey J., Morton J.P., Nixon C., MacKay G., Mrowinska A. P53 status determines the role of autophagy in pancreatic tumour development. Nature. 2013;504:296–300. doi: 10.1038/nature12865. [DOI] [PubMed] [Google Scholar]
- 51.McCarthy N. Autophagy: directed development. Nat. Rev. Cancer. 2014;14:74–75. doi: 10.1038/nrc3673. [DOI] [PubMed] [Google Scholar]
- 52.Kim Y.S., Shin J.H., Bae M.K., Lee C.Y., Kim D.J., Chung K.Y. Autophagy activity in pulmonary metastatic tumor tissues from colorectal cancer: a pilot study. Yonsei Med. J. 2014;55:1484–1488. doi: 10.3349/ymj.2014.55.6.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim J.Y., Kim do H., Jung W.H., Koo J.S. Autophagy and redox status in carcinoma of an unknown primary. Tumori. 2014;100:118e–129e. doi: 10.1700/1636.17924. [DOI] [PubMed] [Google Scholar]
- 54.Jang J.H., Baerts L., Waumans Y., De Meester I., Yamada Y., Limani P. Suppression of lung metastases by the CD26/DPP4 inhibitor Vildagliptin in mice. Clin. Exp. Metastasis. 2015;32:677–687. doi: 10.1007/s10585-015-9736-z. [DOI] [PubMed] [Google Scholar]
- 55.Galluzzi L., Pietrocola F., Bravo-San Pedro J.M., Amaravadi R.K., Baehrecke E.H., Cecconi F. Autophagy in malignant transformation and cancer progression. EMBO J. 2015;34:856–880. doi: 10.15252/embj.201490784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiang L., Zhao B., Ming M., Wang N., He T.C., Hwang S. Regulation of cell proliferation and migration by p62 through stabilization of Twist1. Proc. Natl. Acad. Sci. USA. 2014;111:9241–9246. doi: 10.1073/pnas.1322913111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Hua F., Li K., Yu J.J., Lv X.X., Yan J., Zhang X.W. TRB3 links insulin/IGF to tumour promotion by interacting with p62 and impeding autophagic/proteasomal degradations. Nat. Commun. 2015;13:7951. doi: 10.1038/ncomms8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei H., Wang C., Croce C.M., Guan J.L. P62/SQSTM1 synergizes with autophagy for tumor growth in vivo. Genes Dev. 2014;28:1204–1216. doi: 10.1101/gad.237354.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leidal A.M., Debnath J. ‘Doubling down’ on the autophagy pathway to suppress tumor growth. Genes Dev. 2014;28:1137–1139. doi: 10.1101/gad.244681.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakashima T., Hayashi M., Takayanagi H. New insights into osteoclastogenic signaling mechanisms. Trends Endocrinol. Metab. 2012;23:582–590. doi: 10.1016/j.tem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 61.Boyce B.F. Advances in osteoclast biology reveal potential new drug targets and new roles for osteoclasts. J. Bone Miner. Res. 2013;28:711–722. doi: 10.1002/jbmr.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Novack D.V. Role of NF-κB in the skeleton. Cell Res. 2011;21:169–182. doi: 10.1038/cr.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Demchenko Y.N., Kuehl W.M. A critical role for the NF-kB pathway in multiple myeloma. Oncotarget. 2010;1:59–68. doi: 10.18632/oncotarget.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu J., Wu H.F., Ang E.S., Yip K., Woloszyn M., Zheng M.H. NF-kappaB modulators in osteolytic bone diseases. Cytokine Growth Factor Rev. 2009;20:7–17. doi: 10.1016/j.cytogfr.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 65.Fizazi K., Carducci M., Smith M., Damião R., Brown J., Karsh L. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stopeck A.T., Lipton A., Body J.J., Steger G.G., Tonkin K., de Boer R.H. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J. Clin. Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 67.Henry D.H., Costa L., Goldwasser F., Hirsh V., Hungria V., Prausova J. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 68.Rea S.L., Walsh J.P., Layfield R., Ratajczak T., Xu J. New insights into the role of sequestosome 1/p62 mutant proteins in the pathogenesis of Paget's disease of bone. Endocr. Rev. 2013;34:501–524. doi: 10.1210/er.2012-1034. [DOI] [PubMed] [Google Scholar]
- 69.McManus S., Roux S. The adaptor protein p62/SQSTM1 in osteoclast signaling pathways. J. Mol. Signal. 2012;4:1–8. doi: 10.1186/1750-2187-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Durán A., Serrano M., Leitges M., Flores J.M., Picard S., Brown J.P. The atypical PKC-interacting protein p62 is an important mediator of RANK-activated osteoclastogenesis. Dev. Cell. 2004;6:303–309. doi: 10.1016/s1534-5807(03)00403-9. [DOI] [PubMed] [Google Scholar]
- 71.Lotz M.K., Caramés B. Autophagy and cartilage homeostasis mechanisms in joint health, aging and OA. Nat. Rev. Rheumatol. 2011;7:579–587. doi: 10.1038/nrrheum.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hocking L.J., Whitehouse C., Helfrich M.H. Autophagy: a new player in skeletal maintenance? J. Bone Miner. Res. 2012;27:1439–1447. doi: 10.1002/jbmr.1668. [DOI] [PubMed] [Google Scholar]
- 73.Mortensen M., Soilleux E.J., Djordjevic G., Tripp R., Lutteropp M., Sadighi-Akha E. The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J. Exp. Med. 2011;208:455–467. doi: 10.1084/jem.20101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DeSelm C.J., Miller B.C., Zou W., Beatty W.L., van Meel E., Takahata Y. Autophagy proteins regulate the secretory component of osteoclastic bone resorption. Dev. Cell. 2011;21:966–974. doi: 10.1016/j.devcel.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sanchez C.P., He Y.Z. Bone growth during rapamycin therapy in young rats. BMC Pediatr. 2007;22:954–961. doi: 10.1186/1471-2431-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cejka D., Hayer S., Niederreiter B., Sieghart W., Fuereder T., Zwerina J. Mammalian target of rapamycin signaling is crucial for joint destruction in experimental arthritis and is activated in osteoclasts from patients with rheumatoid arthritis. Arthritis Rheum. 2010;62:2294–2302. doi: 10.1002/art.27504. [DOI] [PubMed] [Google Scholar]
- 77.Owen H.C., Vanhees I., Gunst J., Van Cromphaut S., Van den Berghe G. Critical illness-induced bone loss is related to deficient autophagy and histone hypomethylation. Intensive Care Med. Exp. 2015;3:52. doi: 10.1186/s40635-015-0052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li R.F., Chen G., Ren J.G., Zhang W., Wu Z.X., Liu B. The adaptor protein p62 is involved in RANKL-induced autophagy and osteoclastogenesis. J. Histochem. Cytochem. 2014;62:879–888. doi: 10.1369/0022155414551367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wauquier F., Leotoing L., Coxam V., Guicheux J., Wittrant Y. Oxidative stress in bone remodelling and disease. Trends Mol. Med. 2009;15:468–477. doi: 10.1016/j.molmed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 80.Rahim A.H., Setiawan B., Dewi F.R., Noor Z. Regulation by phloroglucinol of Nrf2/Maf-mediated expression of antioxidant enzymes and inhibition of osteoclastogenesis via the RANKL/RANK signaling pathway: in silico study. Acta Inf. Med. 2015;23:228–232. doi: 10.5455/aim.2015.23.228-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wright T., Rea S.L., Goode A., Bennett A.J., Ratajczak T., Long J.E. The S349T mutation of SQSTM1 links Keap1/Nrf2 signalling to Paget's disease of bone. Bone. 2013;52:699–706. doi: 10.1016/j.bone.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 82.Hyeon S., Lee H., Yang Y., Jeong W. Nrf2 deficiency induces oxidative stress and promotes RANKL-induced osteoclast differentiation. Free Radic. Biol. Med. 2013;65:789–799. doi: 10.1016/j.freeradbiomed.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 83.Kiffin R., Bandyopadhyay U., Cuervo A.M. Oxidative stress and autophagy. Antioxid. Redox Signal. 2006;8:152–162. doi: 10.1089/ars.2006.8.152. [DOI] [PubMed] [Google Scholar]
- 84.Ichimura Y., Waguri S., Sou Y.S., Kageyama S., Hasegawa J., Ishimura R. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol. Cell. 2013;51:618–631. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 85.Jain A., Lamark T., Sjøttem E., Larsen K.B., Awuh J.A., Øvervatn A., McMahon M., Hayes J.D., Johansen p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J. Biol. Chem. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song C., Song C., Tong F. Autophagy induction is a survival response against oxidative stress in bone marrow-derived mesenchymal stromal cells. Cytotherapy. 2014;6:1361–1370. doi: 10.1016/j.jcyt.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 87.Shen C., Wang W., Tao L., Liu B., Yang Z., Tao H. Chloroquine blocks the autophagic process in cisplatin-resistant osteosarcoma cells by regulating the expression of p62/SQSTM1. Int. J. Mol. Med. 2013;32:448–456. doi: 10.3892/ijmm.2013.1399. [DOI] [PubMed] [Google Scholar]
- 88.Colunga A., Bollino D., Schech A., Aurelian L. Calpain-dependent clearance of the autophagy protein p62/SQSTM1 is a contributor to ΔPK oncolytic activity in melanoma. Gene Ther. 2014;21:371–378. doi: 10.1038/gt.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chang M., Patel V., Gwede M., Morgado M., Tomasevich K., Fong E. IL-1β induces p62/SQSTM1 and represses androgen receptor expression in prostate cancer cells. J. Cell. Biochem. 2014;115:2188–2197. doi: 10.1002/jcb.24897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Milan E., Perini T., Resnati M., Orfanelli U., Oliva L., Raimondi A. A plastic SQSTM1/p62-dependent autophagic reserve maintains proteostasis and determines proteasome inhibitor susceptibility in multiple myeloma cells. Autophagy. 2015;11:1161–1178. doi: 10.1080/15548627.2015.1052928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maroni P., Bendinelli P., Matteucci E., Locatelli A., Nakamura T., Scita G. Osteolytic bone metastasis is hampered by impinging on the interplay among autophagy, anoikis and ossification. Cell Death Dis. 2014;16:5:e1005. doi: 10.1038/cddis.2013.465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92.Gabai V.L., Shifrin V.I. Feasibility analysis of p62 (SQSTM1)-encoding DNA vaccine as a novel cancer immunotherapy. Int. Rev. Immunol. 2014;33:375–382. doi: 10.3109/08830185.2014.954699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Valencia T., Kim J.Y., Abu-Baker S., Moscat-Pardos J., Ahn C.S., Reina-Campos M. Metabolic reprogramming of stromal fibroblasts through p62-mTORC1 signaling promotes inflammation and tumorigenesis. Cancer Cell. 2014;26:121–135. doi: 10.1016/j.ccr.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moscat J., Diaz-Meco M.T. Feedback on fat: p62-mTORC1-autophagy connections. Cell. 2011;147:724–727. doi: 10.1016/j.cell.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bloy N., Buqué A., Aranda F., Castoldi F., Eggermont A., Cremer I. Trial watch: Naked and vectored DNA-based anticancer vaccines. Oncoimmunology. 2015;4:e1026531. doi: 10.1080/2162402X.2015.1026531. [DOI] [PMC free article] [PubMed] [Google Scholar]