Abstract
Lung engineering is a potential alternative to transplantation for patients with end-stage pulmonary failure. Two challenges critical to the successful development of an engineered lung developed from a decellularized scaffold include (i) the suppression of resident infectious bioburden in the lung matrix, and (ii) the ability to sterilize decellularized tissues while preserving the essential biological and mechanical features intact. To date, the majority of lungs are sterilized using high concentrations of peracetic acid (PAA) resulting in extracellular matrix (ECM) depletion. These mechanically altered tissues have little to no storage potential. In this study, we report a sterilizing technique using supercritical carbon dioxide (ScCO2) that can achieve a sterility assurance level 10−6 in decellularized lung matrix. The effects of ScCO2 treatment on the histological, mechanical, and biochemical properties of the sterile decellularized lung were evaluated and compared with those of freshly decellularized lung matrix and with PAA-treated acellular lung. Exposure of the decellularized tissue to ScCO2 did not significantly alter tissue architecture, ECM content or organization (glycosaminoglycans, elastin, collagen, and laminin), observations of cell engraftment, or mechanical integrity of the tissue. Furthermore, these attributes of lung matrix did not change after 6 months in sterile buffer following sterilization with ScCO2, indicating that ScCO2 produces a matrix that is stable during storage. The current study's results indicate that ScCO2 can be used to sterilize acellular lung tissue while simultaneously preserving key biological components required for the function of the scaffold for regenerative medicine purposes.
Introduction
Tissue engineering and regenerative medicine technologies have made great strides in the past two decades, including the development of decellularized scaffolds for use in lung engineering.1–7 The creation of sterile, autologous cell-sourced bioengineered lungs would enable the construction of lungs that address patient-specific needs, decrease the morbidity associated with immunosuppression, and also address the worsening donor shortage of lungs for transplantation. Two remaining challenges critical to the clinical success of a donor-compatible organ transplant are (i) the ability to suppress the growth of resident infectious bacteria and other microorganisms, and (ii) the ability to produce a scaffold that is stable over long-term storage.8,9 The availability of sterile lung scaffolds with significant storage capacity would be a critical tool for one day constructing clinically viable, patient-specific lungs.
Unlike most organs, healthy lungs naturally have a surprisingly diverse population of resident bacterial, fungal, and viral organisms, including pathogens such as Haemophilus and Neisseria.10 The body's resident macrophages and immune mechanisms naturally keep these bioburden levels low during health in vivo; however, unless ex vivo microbial growth is controlled or stopped, donor lungs are overgrown by bacteria and matrix damage ensues due to bacterial collagenases and elastases.11 Even trace levels of these native bacteria in implantable lungs would be contraindicated in lung recipients who are on immunosuppressant therapy. Therefore, great care must be taken toward ensuring the sterility of implantable materials.12
The industry standard for surgically implanted medical devices to be deemed terminally sterile is a sterility assurance level of 10−6 (SAL6). SAL6 is defined as having a probability of one in a million that a given product is still contaminated after sterilization treatment when starting with an initial bioburden of 106 colony-forming units (CFUs).13 Three commonly utilized methods of achieving SAL6 sterility in soft tissues are γ-irradiation, ethylene oxide (ETO) sterilization, or perfusion with peracetic acid (PAA).14–16 Both γ-irradiation and ETO sterilization are known to produce important structural damage to tissues. Therefore, these methods are not recommended for use in soft tissue sterilization.14,17 PAA, a standard sterilizing agent, works through oxidative disinfection and is highly effective against bacteria, viruses, and spores.14,18,19 It has been cleared for some applications by the Food and Drug Administration,20 and recently has been used in the sterilization of lung tissue.21,22 However, reports on the impact of PAA on extracellular matrix (ECM) and growth factor retention in the acellular matrix are conflicting. While some groups report mild architectural damage,8,23 others report the depletion of critical growth factors, elution of soluble ECM protein, and reduced mechanical integrity of decellularized matrix.8,17,18 It is likely that these discrepancies are due to differences in PAA concentrations (0.1–0.3%) and tissue exposure times (20–160 min). In addition to the potential for ECM damage, PAA treatment alone may be insufficient for matrix preservation in soft tissues such as the lung.8,14 For example, decellularized lungs treated with PAA are reported to have severely compromised tissue architecture relative to freshly decellularized lungs after 6 months of storage.8
Supercritical carbon dioxide (ScCO2) has recently been developed as a means to achieve SAL6 sterility in medical devices, implantables, and allograft tissues.24–26 ScCO2 utilizes extremely low levels of PAA (0.005–0.05%), and is shown to achieve SAL6 with bacterial endospores.26 ScCO2 achieves sterilization through enhanced mass transfer of CO2 during the supercritical phase and disruption of the outer membrane of bacterial, viral, or fungal bioburden.26 Furthermore, given that ScCO2 has a diffusion capacity that allows it to penetrate matrix fibers, this process can sterilize at low temperatures (35–39°C) and remove unwanted compounds such as blood or potentially residual DNA. Furthermore, ScCO2 does not leave toxic residuals rendering it ideal as a means to sterilize delicate ECM.27 Previous reports indicate that soft tissues that undergo ScCO2 sterilization retained their mechanical and molecular characteristics.25 To date, the use of ScCO2 has not yet been investigated as a means to sterilize or enable long-term storage capacity of decellularized lung scaffolds. In the present study, we examined the effects of ScCO2 sterilization on freshly decellularized rat lungs to a standard application of PAA sterilization. We then assessed the mechanical and biological composition of scaffolds, and examined the cell seeding capacity of two different cell types on ScCO2-treated matrix.
Materials and Methods
Organ harvest and decellularization
All animal experimental work was performed with approval from the Yale University Institutional Animal Care and Use Committee. All animal care complied with the Guide for the Care and Use of Laboratory Animals. Briefly, 3-month-old Sprague Dawley rats were pretreated intravenously with 500 U/kg heparin (Sigma-Aldrich) and euthanized.28 Immediately after euthanasia, the abdomen was entered through a transverse incision just below the costal margin. The diaphragm was punctured, and the rib cage was cut to reveal the lungs. The lungs were then perfused through the right ventricle with phosphate-buffered saline (PBS) containing 50 U/mL heparin and 1 mg/mL sodium nitroprusside (Fluka). The heart, lungs, and trachea were dissected free from the surrounding muscles and connective tissue and removed en bloc. The thymus was removed, and care was taken not to disrupt the esophagus to minimize tissue damage and contamination during dissection. Tracheal and pulmonary artery cannulae were inserted and sutured into place to provide access for perfusion and decellularization.
The lungs were decellularized as described previously.2 Briefly, the lungs were perfused with 0.0035% Triton X-100 in PBS, followed by Benzonase buffer and Benzonase nuclease. After the Benzonase treatment, a series of solutions with increasing concentrations of sodium deoxycholate was applied through the vasculature. The lungs were then rinsed with 0.5% Triton X-100 and ethylenediaminetetraaceticacid (EDTA) in PBS. Finally, an additional Benzonase step was introduced at the end of the previously published method to remove residual DNA. The reagent volumes were scaled to tissue wet weight (average rat lung wet weight ∼2.5 g). For all blood and debris clearance and decellularization steps, the tissues were either perfused using a gravity feed at 22 mm Hg pressure through the pulmonary artery or gently flushed manually through the trachea. No antibiotics were used during the decellularization process or before sterilization treatments.
Sterilization procedures
Lung sterilization was completed at NovaSterilis in a Nova2200 sterilizer. Decellularized lungs were stored in Tyvek pouches, suspended in 100 mL PBS, and sealed before ScCO2 sterilization. To prevent tissue collapse, the airways of the decellularized lungs were manually filled with 5–7 mL PBS and allowed a preconditioning time. To establish what amounts of sterilization and preconditioning time were required for SAL6 sterilization, the Nova2200 was run for a varied preconditioning time (0.5–2 h), and varied supercritical exposure time (2–4 h). The chamber was filled at ∼1 psi/s, and the system reached supercritical in ∼4–7 min. During the sterilization procedure, the Nova2200 was maintained at ∼1440 psi and 35°C, and stirrer speed at ∼650 rpm. Following the 2-h exposure to ScCO2, slow depressurization lasting 30–45 min was performed to maintain tissue temperature at or above 25°C. Slow depressurization prevents freezing of the lung tissue and fluid or expansion of gas in the lung resulting in tissue destruction. For each sterilization run, the sterilizer was loaded with 2 mL of NovaKillTIM Gen2 (Novasterilis) additive and samples before start of conditioning. Although PAA is a component in the NovaKill Gen2 (13.5–18.5% PAA and 4.5–6% hydrogen peroxide), the PAA level in the lung effluent measured at an average of 0.018% (∼10× less than typical PAA concentration). For comparison, PAA lung tissues were sterilized as established previously.8 Briefly, 30 mL of 0.1% PAA in 4% ethanol solution was perfused through the trachea and the pulmonary artery of the decellularized lung. The lung was then submerged in the solution for 2 h and rinsed with PBS for 15 min.8 All samples were stored in PBS at 4°C without antibiotics before examination.
Validating SAL6 sterility
To develop a supercritical protocol that would produce a terminally sterile tissue, SAL6 sterility was assessed in accordance with industrial sterilization validation guidelines.28 Briefly, biological indicator (BI) strips per sterilization condition were inoculated with 106 Bacillus atrophaeus spores (Mesa Labs) and placed under the variety of sterilization conditions discussed above. BIs were either suspended in PBS directly or wrapped in lung proxy materials consisting of layers of semipermeable gauze (found to mimic rat lung intratracheal inoculation results) and suspended in PBS and tested at various run conditions. Samples were filter recovered, incubated on a tryptic soy agar plate (not supplemented with antibiotics) at 37°C for 2 days and examined for colony formation. Recovered BI strips were inoculated into tryptic soy broth, incubated at 37°C for 14 days, and examined daily for bacterial growth.
Testing sterility in tissues
After each sterilization procedure was performed, using decellularized lungs (ScCO2 or PAA), a subset of crushed tissue samples and effluent (PBS from suspension and from inside airways) were streaked on agar plates without selective inhibitor/antibiotics, as described previously.29 The plates were incubated at 37°C for 7 days, after which time the number of bacterial colonies on each plate was recorded. Native lung and freshly decellularized samples were used as positive controls and formed bacterial colonies on the agar plates.
Histology and immunostaining
Several portions of each lung (n = 3–5 areas, sampled randomly) were isolated, fixed with 10% formalin for 4–6 h at room temperature, stored overnight in 70% ethanol, embedded in paraffin, and sectioned at 5 μm. Tissue slides were stained for Hematoxylin and Eosin, Masson's Trichrome staining (Trichrome) for detecting collagen, Alcian Blue (AB) at pH 2.5 for detecting glycosaminoglycans (GAGs), and Verhoerff's Van Geison (EVG) for detecting elastin. Images were acquired using an Olympus BX/51 microscope and associated Olympus DB70 digital camera.
For immunofluorescence, antigen retrieval was performed in 1 mM EDTA, 10 mM Tris, and 0.05% Tween 20 buffer for 20 min at 75°C and allowed to cool to room temperature for 20 min. After blocking sections with PBS containing 10% fetal bovine serum (FBS) and 0.2% Triton X-100 for 45 min, primary antibodies were used against laminin (Abcam ab74164; two drops, prediluted to 1:100) for 2 h at room temperature. After washing slides with PBS, corresponding secondary antibodies (AlexaFluor 555) were used at 1:500 dilution for 45 min. The slides were visualized using a Leica DMI6000 B fluorescence microscope.
Tensile testing
Native, decellularized, PAA-treated, and ScCO2-treated lung samples were analyzed using an Instron 5848. Additionally, a subset of PAA-treated and ScCO2-treated samples were stored for 6 months in PBS and analyzed. Nominal 15 × 2 mm (length × width) strips were cut from tissue samples. Care was taken to analyze tissue from the distal region of the left rat lobe (so as not to include major airways and pleura that could dominate mechanical analysis). Tissue thickness of each sample was determined by a series of measurements at four different points using a digital micrometer (Mitutoyo). Specimens were glued to 1 mm sections of sand paper at each end of the tissue slices, and each end was affixed to grips. Tissues were then pretared to 0.01 N, cyclically preconditioned for three cycles to 15% strain, and pulled until failure at a strain rate of 1%/s. The axial force was measured with a 10 N load cell, and elongation assessed by cross-head displacement. Tissues were kept hydrated with PBS before and during the mechanical conditioning. Using tissue dimensions, engineering stress and strain were calculated from force and distance from the slope at the linear regions of the curve using the equations:
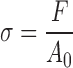 where, σ is engineering stress, F = force, and A0 = initial area
where, σ is engineering stress, F = force, and A0 = initial area
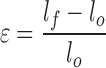 where ɛ = engineering strain, lf = final length, l0 = initial length.
where ɛ = engineering strain, lf = final length, l0 = initial length.
The Young's Modulus (E) for the tissue was determined by dividing the engineering stress σ, by the engineering strain ɛ, at low and high levels of deformation.
ECM analysis
Collagen was quantified with a colorimetric assay to detect hydroxyproline as previously reported.30 Lung samples were lyophilized and weighed, then incubated in papain (10 U/mL; 25 mg/mL) at 60°C overnight (Sigma). Papain-digested samples analyzed for hydroxyproline content and collagen content were calculated assuming a 1:12 w/w ratio of hydroxyproline to collagen. Sulfated GAGs (sGAGs) were quantified using the Blyscan GAG Assay Kit (Biocolor). Lung samples were lyophilized and weighed, then incubated in papain (25 mg/mL) at 60°C overnight (Sigma). Papain-digested samples (prepared as described above for the collagen assay) were assayed according to the manufacturer's instructions. Absorption was measured at a wavelength of 650 nm, and GAG content was quantified using a standard curve. Elastin was measured by determining the desmosine crosslinks as described previously.31 Briefly, desmosine crosslinks were measured by first lyophilizing the sample, hydrolyzing with 6 N HCl for 48 h, and reconstituting and filtering the tissue through a 0.45 μm filter. The desmosine levels were determined using a competitive enzyme-linked immunosorbent assay.
Cell culture
Human A549 cells (a type II epithelial-like cell line) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS (Hyclone) and 1% penicillin and 100 μg/mL streptomycin (Corning). Rat microvascular lung endothelial cells (RLMVECs; VEC Technologies) were cultured in MCDB-131C (VEC Technologies).
The biocompatibility of ScCO2-treated acellular lungs after 6 months of storage was evaluated in vitro by seeding either RLMVECs or A549 cells onto 1 cm2 decellularized tissue slices. Briefly, 300 μm thick acellular lung slices were seeded at a concentration of 500,000 cells/slice and cultured for 3 days as described previously.2 All cell-seeded slices were cultured in standard cell culture conditions at 37°C and 5% CO2.
Statistics
Data are presented as the mean with standard error bars representing the standard deviation. Data were analyzed by Student's t-test for significance and considered significantly different if p < 0.05. All sterilization samples were compared to freshly decellularized controls.
Results
Optimization of SAL6 sterility protocol using ScCO2
SAL6 sterilization using ScCO2 was evaluated across several processing times and levels of PAA-containing additive (Table 1). A minimum amount of 2 h of preconditioning time and 1.5 h of ScCO2 exposure were required for the inactivation of 106 Bacillus atrophaeus spores. To ensure confidence in SAL6 sterility, all subsequent ScCO2 treatments consisted of 2 h of preconditioning time and 2 h of ScCO2 exposure. ScCO2 alone without the addition of PAA was not sufficient to inactivate lung bioburden (data not shown).
Table 1.
Developing SAL6 Sterility
| NovaKillGen2 (mL) | Preconditioning time (h) | ScCO2 exposure (h) | Number of positive samples |
|---|---|---|---|
| 2 | — | 2 | 8/8 |
| 2 | 0.5 | 2 | 7/8 |
| 2 | 0.5 | 1.5 | 8/8 |
| 2 | 1 | 1.5 | 0/4 |
| 2 | 1 | 2 | 0/12 |
| 2 | 2 | 4 | 0/4 |
The NovaKill Gen2 additive amount was held constant, while length of conditioning and ScCO2 exposure varied. All samples inoculated with 106 Bacillus atrophaeus. A positive sample represents growth on an agar plate.
ScCO2, supercritical carbon dioxide.
Assessment of ScCO2 versus PAA sterility
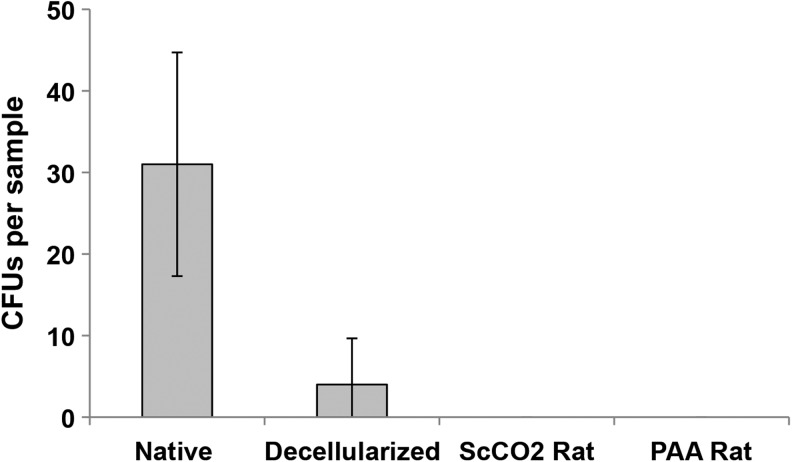
To compare the effectiveness of PAA and ScCO2 sterilization techniques, crushed tissue and effluent from native, freshly decellularized, and ScCO2- and PAA-sterilized lungs were streaked onto LB agar plates and examined for bacterial colonies (n ≥ 4 for all groups). ScCO2- and PAA-sterilized specimens were sampled 24 h after sterilization. Bacterial colonies were detected in both native and decellularized control tissues as early as 2 days after plating and were present in varying numbers after 7 days (Fig. 1). CFUs in freshly decellularized lungs (n = 5) ranged from 0 to 12 colonies, indicating variable presence of intrinsic bioburden postdecellularization. There were no detectible colonies in ScCO2-treated or PAA-treated decellularized rat lungs (n = 4 lungs per protocol) after 7 days (Fig. 1). After 6 months of storage, there was no presence of bacterial colonies in ScCO2- or PAA-treated lungs (data not shown). Therefore, both sterilization methods are effective in removing intrinsic bioburden in decellularized lungs.
FIG. 1.
Sterility assessment of decellularized lung matrix sterilized with peracetic acid (PAA) and supercritical carbon dioxide (ScCO2). Native, decellularized, ScCO2- and PAA-treated rat lung tissue (n ≥ 4) was crushed and streaked on agar plates without antibiotic. ScCO2-treated and PAA-treated tissues were tested 24 h poststerilization. The plates were incubated at 37°C for 7 days, after which the number of bacterial colony-forming units (CFUs) on each plate was recorded. Error bars represent ± standard deviation.
Histological evaluation of the lung ECM post ScCO2 and PAA sterilization
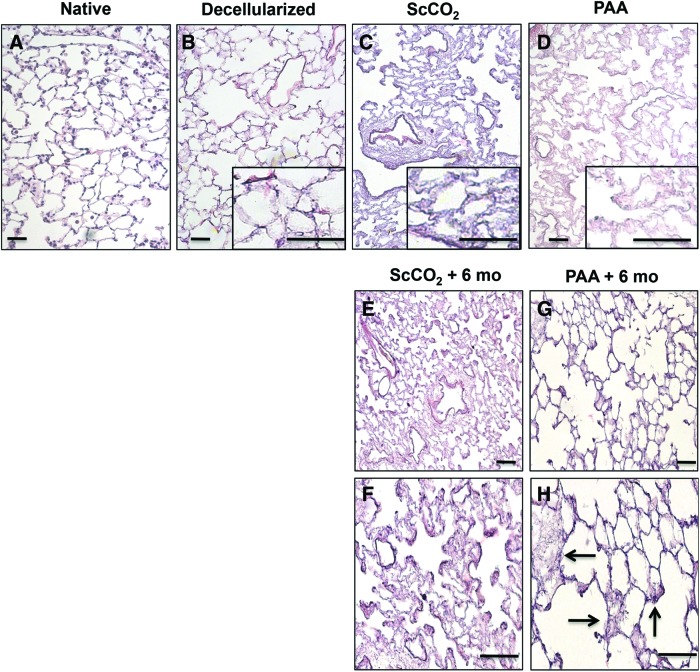
Histological comparison was done between native, decellularized, ScCO2-treated, and PAA-treated lungs (Fig. 2). The decellularized tissue retained microstructure similar to native tissue, with intact alveolar septae, vasculature, and airways (Fig. 2A, B).2 In terms of general architecture maintenance, tissues sterilized by either using PAA or ScCO2 did not demonstrate major morphological differences relative to freshly decellularized lungs, and the overall microarchitecture of the lung was preserved (Fig. 2B–D). After 6 months of storage, although the general architecture remained intact for all sterilized tissue (Fig. 2E–H), the PAA-treated lungs displayed several areas that appeared damaged (indicated by arrows) and the alveoli appeared distended (Fig. 2H). These data indicate that even after several months of storage, ScCO2-treated lungs retained structural integrity poststerilization.
FIG. 2.
Tissue characterization of decellularized lung matrix sterilized with PAA and ScCO2. Native (A), decellularized (B), PAA- (C) and ScCO2 (D)-treated rat lung tissue stained with Hematoxylin and Eosin (H&E) shows maintenance of tissue architecture in both ScCO2- and PAA-treated lung tissue immediately after sterilization. Insets (B–D) are at 400×. H&E images of ScCO2 (E, F) and PAA (G, H)-treated tissue 6 months after treatment shows compromised tissue in PAA-treated tissue (indicated by arrow) and not ScCO2-treated lung tissue after 6 months of storage. Scale bar = 50 μm applies to all panels. Color images available online at www.liebertpub.com/tec
Mechanical characterization of ScCO2- and PAA-treated lungs
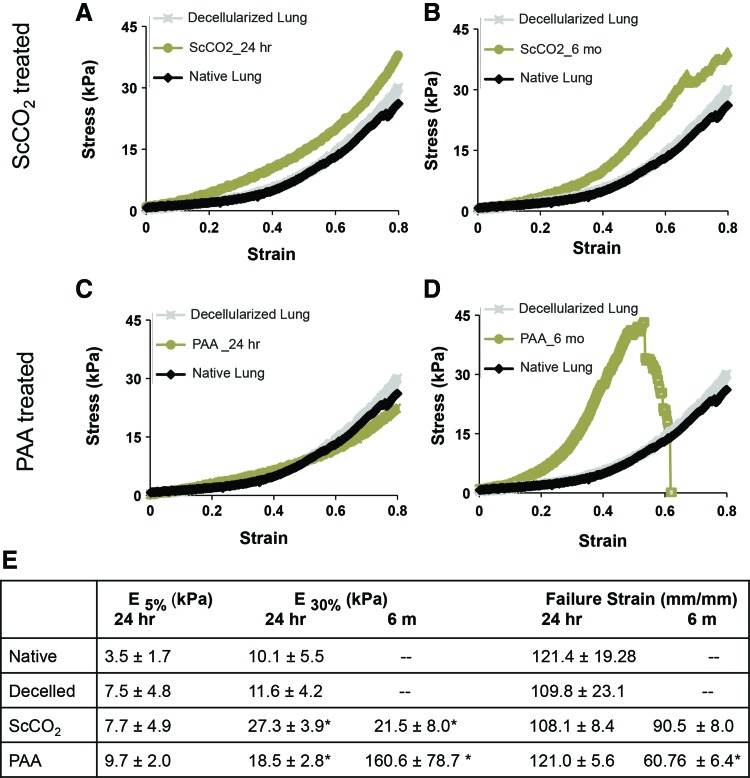
Results from mechanical testing of the native, decellularized, PAA-sterilized and ScCO2-treated tissues are shown in Figure 3 (n ≥ 5 for all groups). Figure 3A and C show stress–strain curves of ScCO2 and PAA 24 h poststerilization treatment, and Figure 3B and D show ScCO2 and PAA 6 months poststerilization treatment. At deformations within typical tidal volumes (i.e., 1–10% strain),32–34 all sterilized tissue closely resembled freshly decellularized lung and native lung (Fig. 3A–E). Specifically, at lower strain levels (i.e., 5% strain), all freshly sterilized tissue had Young's moduli that did not differ significantly from freshly decellularized lungs (Fig. 3E, p = 0.49, 0.96 respectively). However, under large deformations (30%), tissues sterilized with the ScCO2 (Fig. 3A–E) and PAA were significantly stiffer than freshly decellularized lungs (p = 0.047 and 0.046, respectively). In contrast to these differences in moduli, there were no statistical differences in freshly sterilized tissues relative to freshly decellularized tissues in terms of failure strain (24 h data, p = 0.07, 0.90, respectively Fig. 3E).
FIG. 3.
Mechanical properties of sterilized, decellularized lung tissue. Representative stress–strain curves of native, freshly decellularized and acellular rat lung tissue sterilized through (A, B) ScCO2 and (C, D) PAA, tested immediately after treatment and after 6 months of storage. Stress–strain curves of native and decellularized lung tissue (n ≥ 5 for all groups). (E) Biochemical and mechanical composition of tissues in native and decellularized conditions. Error bars show mean ± standard deviation, and “*” indicates significance from decellularized matrix at p ≤ 0.05. Color images available online at www.liebertpub.com/tec
When examining tissues that were sterilized and stored for 6 months in PBS, there was no significant change in stiffness between ScCO2 samples after 24 h of storage and 6 months of storage (27.3 vs. 21.5, p = 0.19, Fig. 3E). Tissues sterilized with PAA consistently failed under lower deformations (p = 0.01, Fig. 3E) than freshly decellularized tissues, whereas tissues sterilized with ScCO2 did not (p = 0.21, Fig. 3E). These data indicate that ScCO2 sterilization does not negatively impact the mechanical integrity of acellular lung tissue. Conversely, although PAA-treated samples appear unaltered mechanically 24 h after treatment, PAA-treated samples have a higher moduli and lower failure strain after 6 months of storage.
Quantitative biochemical analyses of sterilized lung matrix
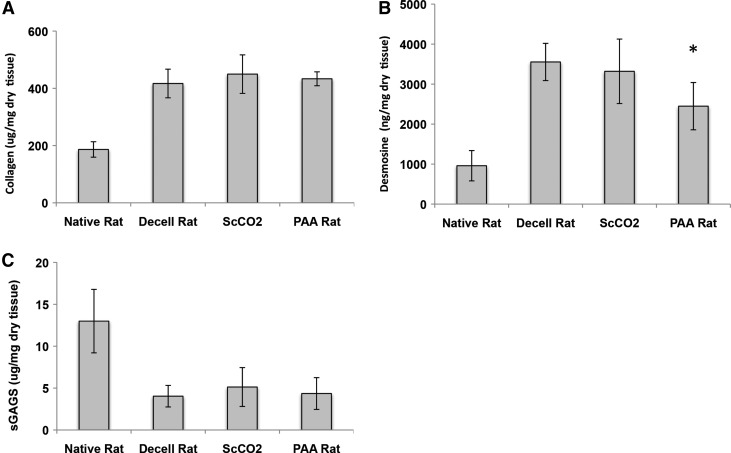
Collagen, elastin, and sGAG quantification of native, freshly decellularized, ScCO2-sterilized, and PAA-sterilized lungs are shown in Figure 4 (n ≥ 5). There is preservation of total collagen content after ScCO2 and PAA sterilization (Fig. 4A), as evidenced by a lack of statistical difference between acellular and ScCO2- or PAA-sterilized tissue by hydroxyproline assay (417, 449, and 433 μg/mg dry tissue, respectively; p = 0.19, 0.32). The amount of collagen in the decellularized samples is presented as a fraction of dry weight (both sterilized and non sterilized). It should be noted that the increase of collagen content relative to tissue mass is not a reflection of actual collagen production. Rather, this increase is an artificial concentration due to the removal of cellular material.
FIG. 4.
Quantifying impact of sterilization on extracellular matrix retention. Quantification of (A) collagen, (B) elastin, and (C) sulfated GAGs (sGAGs) in native (n ≥ 5), decellularized tissue (n = 15), PAA-treated and ScCO2-treated tissue immediately after processing. Data demonstrate retention of collagen, elastin, and sulfated GAGs in ScCO2-treated tissue and elastin loss in PAA-treated tissue. “*” Indicates significance from decellularized matrix at p ≤ 0.05. Error bars show mean ± standard deviation.
Desmosine concentrations indicate that ScCO2-treated lungs did not undergo any loss of elastin levels relative to native tissues (3553 vs. 3320 ng/mg dry weight; p = 0.52), whereas PAA-treated lungs did show a slight but significant level of elastin loss (15% loss, p = 0.048) (Fig. 4B). As seen previously, sGAG content was substantially depleted in decellularized lungs relative to native tissue.2 (Fig. 4C). The impact of sterilization (ScCO2 or PAA), however, had a negligible effect on the sGAG content of lung tissue (4.0, 5.1, and 4.3 μg/mg dry weight, respectively; p = 0.15, 0.52). These data indicate that ScCO2 sterilization does not adversely impact collagen, elastin, or sGAG content in acellular lungs. In addition, PAA sterilization does negatively impact elastin content in acellular lungs.
ECM retention in ScCO2-sterilized lungs
To visually examine ECM constructs in native, decellularized, and ScCO2-treated lungs, elastin fibers (EVG, Fig. 5A–C), collagen type I (Trichrome, Fig. 5D–F), GAGs (AB, Fig. 5G–I), and laminin (Fig. 5J–L) were imaged. ScCO2 samples were imaged 24 h after sterilization. As seen previously, the decellularized tissue retained the majority of elastin (black fibers, Fig. 5B, C), collagen (blue fibers, Fig. 5E, F), laminin (shown in red, Fig. 5K, L), and microstructure after decellularization. It should be noted that EVG stain (Fig. 5A–C) simultaneously stains cells and elastin, and that the difference in appearance between native and decellularized tissue is largely due to the decellularization process (Fig. 5A, B). These data indicate that ScCO2 sterilization does not adversely impact type I collagen, elastin, sGAG, or laminin architecture in acellular lungs.
FIG. 5.
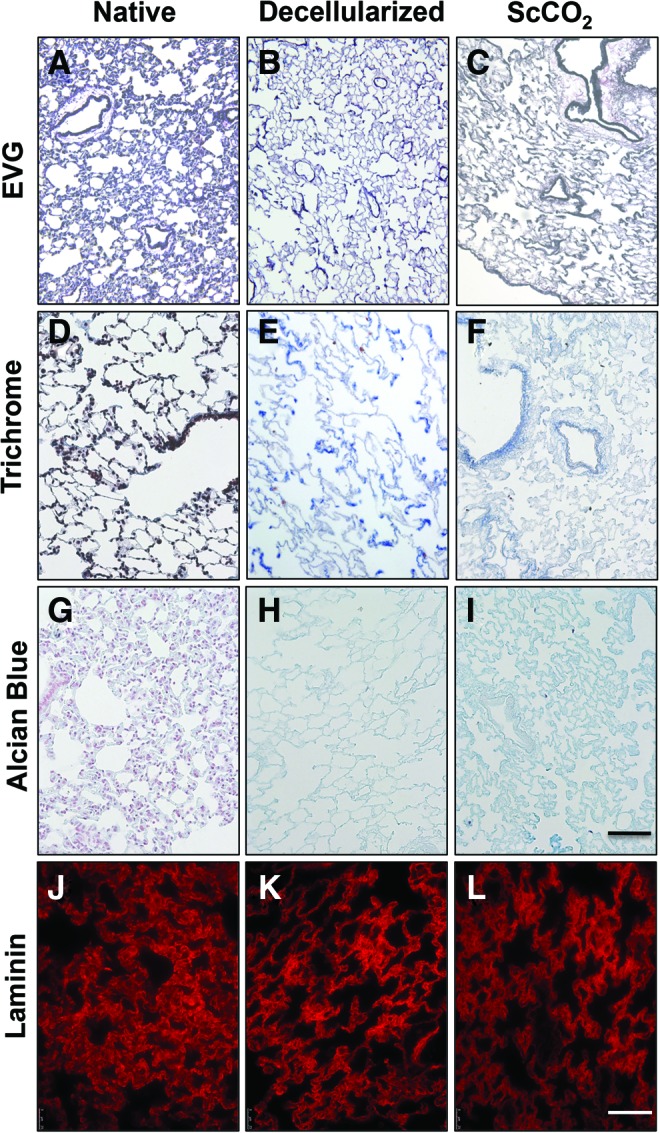
Characterization of sterilized acellular lung matrix. (A–C) Representative micrographs of native, decellularized, and ScCO2-treated lung tissue stained with Verhoeff's Van Gieson (EVG) to examine elastin, (D–F) Masson's Trichrome to examine tissue architecture and collagen preservation, (G–I) and Alcian Blue to observe GAG presence. The EVG, Trichrome, and Alcian Blue stain show maintenance of general tissue architecture, collagen type I and elastin fibers, and total GAGs content throughout the tissue after treatment with ScCO2. Representative fluorescence micrographs of (J) native, (K) decellularized, and (L) ScCO2-treated lung tissue show maintenance of laminin content throughout the tissue after treatment with ScCO2. Scale bar = 100 μm applies to all panels. Color images available online at www.liebertpub.com/tec
Recellularization and slice culture
To determine if the resulting sterilized acellular tissue is adequate for cell seeding after long-term storage, and also to investigate the in vitro potential of the rat scaffold for recellularization with relevant cell types (endothelial and epithelial cells), sections of lungs were reseeded with A549 (Fig. 6A) and RLMVEC cells (Fig. 6B). Acellular, ScCO2-sterilized lung tissues enabled homogeneous epithelial and endothelial cell engraftment. A549 cells attached to the matrices, elongated, lined the small and large airways, and remained viable for 3 days. RLMVECs also engrafted well, spread along the tissue, and remained viable for 3 days. Therefore, the resulting sterilized tissue is capable of providing a suitable scaffold for cell growth even after several months of storage.
FIG. 6.
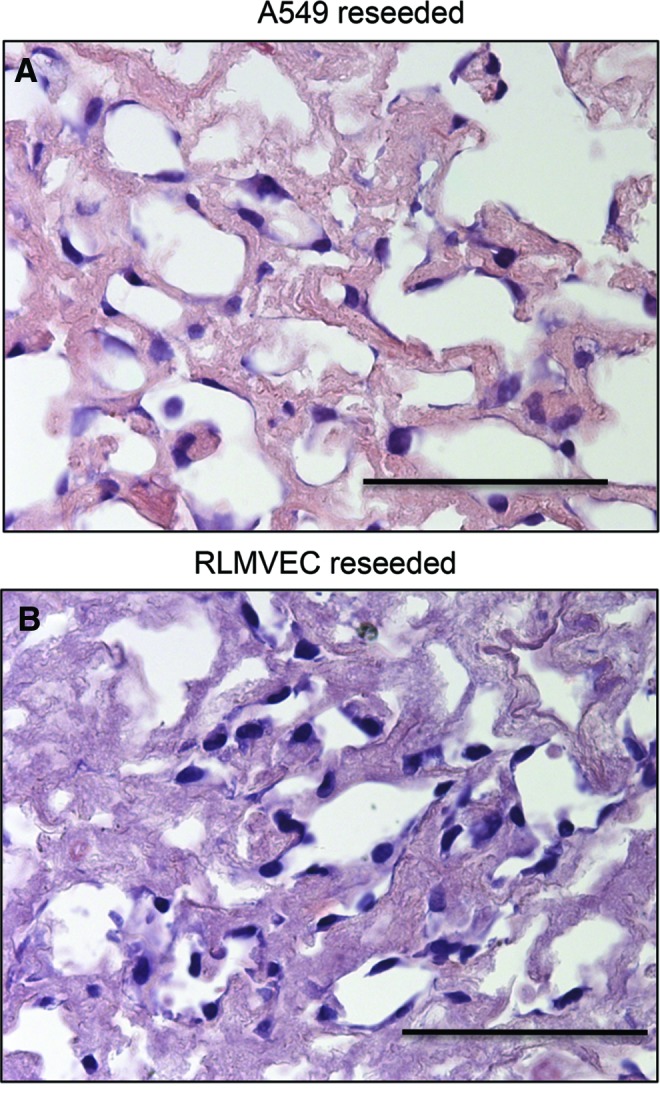
Recellularization of ScCO2-treated rat lung tissue slices. Representative H&E stained tissue slices seeded with (A) human epithelial (A549) and (B) rat endothelial (rat microvascular lung endothelial cells [RLMVECs]) cells. Cells were seeded at a concentration of 500,000 cells/slice and cultured for 3 days. Tissue was ScCO2 treated and stored in phosphate-buffered saline for 6 months before seeding. All cells homogenously engrafted in the tissue demonstrating the capacity for recellularization of sterilized acellular matrix. Scale bar = 50 μm. Color images available online at www.liebertpub.com/tec
Discussion
Producing a SAL6 sterile, biocompatible tissue scaffold for use in transplant therapies provides a very difficult set of challenges. The biomechanical and biological properties of the tissue can be adversely affected during terminal sterilization. For example, gamma radiation of acellular lung tissue causes significant structural damage and irreversible degradation of ECM.8 Our results indicate that sterilization of ScCO2 overcomes these limitations commonly associated with other sterilization methods while effectively eliminating bioburden. Specifically, ScCO2-sterilized tissue retains key ECM constituents (collagen, elastin, sGAGs) and mechanical characteristics (failure properties and stiffness) immediately after treatment, and retains these characteristics after 6 months of storage. Furthermore, this sterilized acellular tissue is hospitable to epithelial and endothelial engraftment even after 6-month storage of the tissue. To the best of our knowledge, this is the first report of a SAL6 method that preserves biological and mechanical properties in decellularized lung matrix.
To date, the majority of decellularization protocols for lung decellularization and lung engineering suppress resident bioburden using a combination of antibiotics.1,5,16,22,35–37 With recent improvements in decellularization that utilize gentler detergents and milder conditions to retain ECM components,2,4,38 antibiotics are increasingly ineffective in removing the pre-existing bioburden (Fig. 1). These antibiotics also have no effect on viral contaminants or multidrug-resistant bacterial growth, issues that become critical when advancing to human lung sources.12,14,39,40 Finally, if residual antibiotics remain in the scaffold postdecellularization, ultimate risk to patients who are allergic to these antibiotics becomes a potential issue.23 Therefore, developing an effective sterilization method independent of antibiotic use is an important advance for the field.
Currently, there are no published sterilization methods capable of attaining SAL6 sterility in acellular lungs without significant damage to the tissue. As with any implantable medical device utilized in the healthcare industry, tissue-engineered products must reach a sterility level of SAL6 for a claim of sterility to be made.41 To establish that a system has a sterility level of SAL6, the bacterial species used in sterility validations must exhibit resistance to inactivation. Spore-forming bacteria, such as Bacillus atrophaeus are commonly used for this purpose because of their resistance to traditional sterilization processes.42,43 Our results showed that traditional sterilization methods (i.e., 0.1% PAA treatment) of acellular lung did result in a complete reduction of bioburden; however, this treatment also resulted in a significant loss of elastin content and compromised tissue architecture (Figs. 2F and 4B). Although a minimal amount of PAA is required for ScCO2 sterilization to be effective, the concentration was only 10% of that traditionally used for PAA sterilization.
When examining ECM content and organization through biochemical quantification or histological staining, ScCO2 did not result in the significant removal of collagen, elastin, or sGAGs (Fig. 4). Furthermore, the tissue architecture closely resembled freshly decellularized tissue with respect to collagen, elastin, GAG, and laminin organization (Fig. 5). The sterilization procedure did produce a stiffer tissue, but this increase in stiffness was only significant in nonphysiological levels of stretch. Although the reason for the increase in stiffness is unclear, it is possibly due to crosslinking of matrix under high pressures during sterilization. It is possible that if this crosslinking exists, it could be providing protection from hydrolysis associated with long-term storage and contributing to the integrity of ScCO2-treated tissue over time.
We also investigated the sterilized tissues after 6 months in storage with PBS (without antibiotics) as a means to ensure the retention of these characteristics and examine the preservation of scaffolds. There were no significant differences between the mechanical properties of the freshly ScCO2-sterilized tissue and tissue that had been stored for 6 months in terms of stiffness or failure properties (Fig. 3A, B, and E). Interestingly, although PAA-treated tissues closely resembled decellularized tissues immediately after sterilization (Fig. 3C, E), after 6 months of storage the samples were brittle and could withstand significantly less stress before failing as compared to freshly PAA-treated tissue. The observed decrease in mechanical integrity of stored PAA-treated tissues is likely due, in part, to depletion of elastin and soluble matrix components and potentially due to dehydration associated with ethanol treatment.
Elastin loss is often associated with diseases such as emphysema.44,45 Furthermore, in addition to providing elastic recoil in the lung, elastin and elastin degradation products are able to influence cell function and promote cellular responses such as chemotaxis, proliferation, and cell adhesion.46,47 Elastin is also essential in lung development and could impair recellularization of the tissue.48,49 Finally, elastin is not a protein that is generally regenerated in the adult lung49 and, therefore, the depletion of elastin, unlike other ECM proteins, would likely be irreversible. Therefore, it is essential that the elastin content of the lung remain preserved during the sterilization process.
In this work, we demonstrated that ScCO2 did not cause any major structural and biological degradation of acellular lung matrix, and produced a sterile lung scaffold that can be stored long term. The stored, sterile tissues were also well suited for cell adhesion and survival. We showed that ScCO2 surpassed traditional PAA sterilization in the capacity for retention of key biological and mechanical features. Therefore, use of ScCO2 sterilization may be a powerful tool for whole organ decellularization technologies.
Conclusions
The use of ScCO2 presents a novel, user-friendly process to sterilize acellular tissue for use in tissue and organ engineering. The entire protocol can be accomplished in ∼3 h, and yields a sterile acellular matrix that retains critical structural, adhesive, and supportive proteins such as collagen, elastin, laminin, and polysaccharides such as sGAGs. Furthermore, after 6 months of storage, ScCO2-treated tissues maintain mechanical properties and cell seeding capacity. Taken together, these results suggest that the proposed sterilization protocol provides a time-efficient and reproducible method to create sterile scaffolds for use in tissue engineering.
Acknowledgments
The authors would like to thank Pavlina Baevova, Andrew Le, Sashka Dimitrievska Tuggle, and Katie Leiby for their technical assistance during experimentation. This work was supported by NIH U01 HL111016-01 (L.E.N.) and HL105314 (to R.P.M.).
Disclosure Statement
L.E.N. has a financial interest in Humacyte, Inc., a regenerative medicine company. Humacyte did not fund these studies, and Humacyte did not affect the design, interpretation, or reporting of any of the experiments herein. J.H. was formerly employed by and K.M.S.B. is currently employed by Novasterilis, Inc. (K.M.S.B. and J.H.). Novasterilis did not fund these studies, and Novasterilis did not affect the design, interpretation, or reporting of any of the experiments herein. No competing financial interests exist for any of the other authors (J.L.B., A.L, A.L.G., J.S., L.Z., T.J.B., R.P.M., and E.C.W.)
References
- 1.Petersen T.H., Calle E.A., Zhao L., Lee E.J., Gui L., Raredon M.B., Gavrilov K., Yi T., Zhuang Z.W., Breuer C., Herzog E., and Niklason L.E. Tissue-engineered lungs for in vivo implantation. Science 329, 538, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balestrini J.L., Gard A.L., Leiby K.L., Calle E., Sivarapatna A., Kunkemoeller B., Lin T., Dimitrievska S., and Niklason L.E. Production of decellularized porcine lung scaffolds for use in tissue engineering. Integr Biol 7, 1598, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price A.P., England K.A., Matson A.M., Blazar B.R., and Panoskaltsis-Mortari A. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A 16, 2581, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balestrini J.L., and Niklason L.E. Extracellular matrix as a driver for lung regeneration. Ann Biomed Eng 43, 568, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilpin S.E., and Ott H.C. Using nature's platform to engineer bio-artificial lungs. Ann Am Thorac Soc 12 Suppl 1, S45, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Booth A.J., Hadley R., Cornett A.M., Dreffs A.A., Matthes S.A., Tsui J.L., Weiss K., Horowitz J.C., Fiore V.F., Barker T.H., Moore B.B., Martinez F.J., Niklason L.E., and White E.S. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med 186, 866, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner D.E., Bonenfant N.R., Sokocevic D., DeSarno M.J., Borg Z.D., Parsons C.S., Brooks E.M., Platz J.J., Khalpey Z.I., Hoganson D.M., Deng B., Lam Y.W., Oldinski R.A., Ashikaga T., and Weiss D.J. Three-dimensional scaffolds of acellular human and porcine lungs for high throughput studies of lung disease and regeneration. Biomaterials 35, 2664, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonenfant N.R., Sokocevic D., Wagner D.E., Borg Z.D., Lathrop M.J., Lam Y.W., Deng B., Desarno M.J., Ashikaga T., Loi R., and Weiss D.J. The effects of storage and sterilization on de-cellularized and re-cellularized whole lung. Biomaterials 34, 3231, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badylak S.F., Weiss D.J., Caplan A., and Macchiarini P. Engineered whole organs and complex tissues. Lancet 379, 943, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Beck J.M., Young V.B., and Huffnagle G.B. The microbiome of the lung. Transl Res 160, 258, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yun J.H., Lee S.O., Jo K.W., Choi S.H., Lee J., Chae E.J., Do K.H., Choi D.K., Choi I.C., Hong S.B., Shim T.S., Kim H.R., Kim D.K., and Park S.I. Infections after lung transplantation: time of occurrence, sites, and microbiologic etiologies. Korean J Intern Med 30, 506, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torbeck L., Raccasi D., Guilfoyle D.E., Friedman R.L., and Hussong D. Burkholderia cepacia: this decision is overdue. PDA J Pharm Sci Technol 65, 535, 2011 [DOI] [PubMed] [Google Scholar]
- 13.von Woedtke T., and Kramer A. The limits of sterility assurance. GMS Krankenhaushygiene Interdisziplinar 3, Doc19, 2008 [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Q., Dawson R.A., Pegg D.E., Kearney J.N., and Macneil S. Use of peracetic acid to sterilize human donor skin for production of acellular dermal matrices for clinical use. Wound Repair Regen 12, 276, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Badylak S.F. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol 12, 367, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Crapo P.M., Gilbert T.W., and Badylak S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 32, 3233, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matuska A.M., and McFetridge P.S. The effect of terminal sterilization on structural and biophysical properties of a decellularized collagen-based scaffold; implications for stem cell adhesion. J Biomed Mater Res B Appl Biomater 103, 397, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodde J., Janis A., Ernst D., Zopf D., Sherman D., and Johnson C. Effects of sterilization on an extracellular matrix scaffold: part I. Composition and matrix architecture. J Mater Sci Mater Med 18, 537, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Marquis R.E., Rutherford G.C., Faraci M.M., and Shin S.Y. Sporicidal action of peracetic acid and protective effects of transition metal ions. J Ind Microbiol 15, 486, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Rutala W.A., Gergen M.F., and Weber D.J. Comparative evaluation of the sporicidal activity of new low-temperature sterilization technologies: ethylene oxide, 2 plasma sterilization systems, and liquid peracetic acid. Am J Infect Control 26, 393, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Wagner D.E., Bonenfant N.R., Parsons C.S., Sokocevic D., Brooks E.M., Borg Z.D., Lathrop M.J., Wallis J.D., Daly A.B., Lam Y.W., Deng B., Desarno M.J., Ashikaga T., Loi R., and Weiss D.J. Comparative decellularization and recellularization of normal versus emphysematous human lungs. Biomaterials 35, 3281, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilpin S.E., Guyette J.P., Gonzalez G., Ren X., Asara J.M., Mathisen D.J., Vacanti J.P., and Ott H.C. Perfusion decellularization of human and porcine lungs: bringing the matrix to clinical scale. J Heart Lung Transplant 33, 298, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Gilbert T.W., Sellaro T.L., and Badylak S.F. Decellularization of tissues and organs. Biomaterials 27, 3675, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Qiu Q.Q., Leamy P., Brittingham J., Pomerleau J., Kabaria N., and Connor J. Inactivation of bacterial spores and viruses in biological material using supercritical carbon dioxide with sterilant. J Biomed Mater Res B Appl Biomater 91, 572, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Wehmeyer J.L., Natesan S., and Christy R.J. Development of a sterile amniotic membrane tissue graft using supercritical carbon dioxide. Tissue Eng Part C Methods 21, 649, 2015 [DOI] [PubMed] [Google Scholar]
- 26.White A., Burns D., and Christensen T.W. Effective terminal sterilization using supercritical carbon dioxide. J Biotechnol 123, 504, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Nichols A., Burns D., and Christopher R. Studies on the sterilization of human bone and tendon musculoskeletal allograft tissue using supercritical Co2. J Orthop 6, e9, 2009 [Google Scholar]
- 28.Tsuchiya T., Balestrini J.L., Mendez J., Calle E.A., Zhao L., and Niklason L.E. Influence of pH on extracellular matrix preservation during lung decellularization. Tissue Eng Part C Methods 20, 1028, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiegand I., Hilpert K., and Hancock R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3, 163, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Woessner J.F., Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 93, 440, 1961 [DOI] [PubMed] [Google Scholar]
- 31.Swaminathan G., Gadepalli V.S., Stoilov I., Mecham R.P., Rao R.R., and Ramamurthi A. Pro-elastogenic effects of bone marrow mesenchymal stem cell-derived smooth muscle cells on cultured aneurysmal smooth muscle cells. J Tissue Eng Regen Med [Epub ahead of print]; DOI: 10.1002/term.1964, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Balestrini J.L., Skorinko J.K., Hera A., Gaudette G.R., and Billiar K.L. Applying controlled non-uniform deformation for in vitro studies of cell mechanobiology. Biomech Model Mechanobiol 9, 329, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Breen E.C. Mechanical strain increases type I collagen expression in pulmonary fibroblasts in vitro. J Appl Physiol 88, 203, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Sugihara T., Martin C.J., and Hildebrandt J. Length-tension properties of alveolar wall in man. J Appl Physiol 30, 874, 1971 [DOI] [PubMed] [Google Scholar]
- 35.Calle E.A., Petersen T.H., and Niklason L.E. Procedure for lung engineering. J Vis Exp pii: , 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Neill J.D., Anfang R., Anandappa A., Costa J., Javidfar J., Wobma H.M., Singh G., Freytes D.O., Bacchetta M.D., Sonett J.R., and Vunjak-Novakovic G. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann Thorac Surg 96, 1046, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sokocevic D., Bonenfant N.R., Wagner D.E., Borg Z.D., Lathrop M.J., Lam Y.W., Deng B., Desarno M.J., Ashikaga T., Loi R., Hoffman A.M., and Weiss D.J. The effect of age and emphysematous and fibrotic injury on the re-cellularization of de-cellularized lungs. Biomaterials 34, 3256, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Price A.P., Godin L.M., Domek A., Cotter T., D'Cunha J., Taylor D.A., and Panoskaltsis-Mortari A. Automated decellularization of intact, human-sized lungs for tissue engineering. Tissue Eng Part C Methods 21, 94, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biderman P., Bugaevsky Y., Ben-Zvi H., Bishara J., and Goldberg E. Multidrug-resistant Acinetobacter baumannii infections in lung transplant patients in the cardiothoracic intensive care unit. Clin Transplant 29, 756, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Gagermeier J.P., Rusinak J.D., Lurain N.S., Alex C.G., Dilling D.F., Wigfield C.H., and Love R.B. Subtherapeutic ganciclovir (GCV) levels and GCV-resistant cytomegalovirus in lung transplant recipients. Transpl Infect Dis 16, 941, 2014 [DOI] [PubMed] [Google Scholar]
- 41.ISO 14937: Sterilization of health care products. General requirements for characterization of a sterilizing agent and the development, validation and routine control of sterilization process for medical devices 2009, pp. 37
- 42.Pinzon-Arango P.A., Scholl G., Nagarajan R., Mello C.M., and Camesano T.A. Atomic force microscopy study of germination and killing of Bacillus atrophaeus spores. J Mol Recogn 22, 373, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Nicholson W.L., Munakata N., Horneck G., Melosh H.J., and Setlow P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev 64, 548, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pierce J.A., Hocott J.B., and Ebert R.V. The collagen and elastin content of the lung in emphysema. Ann Intern Med 55, 210, 1961 [DOI] [PubMed] [Google Scholar]
- 45.Suki B., andBates J.H. Extracellular matrix mechanics in lung parenchymal diseases. Respir Physiol Neurobiol 163, 33, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodgers U.R., and Weiss A.S. Cellular interactions with elastin. Pathol Biol (Paris) 53, 390, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Wagenseil J.E., and Mecham R.P. Vascular extracellular matrix and arterial mechanics. Physiol Rev 89, 957, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wendel D.P., Taylor D.G., Albertine K.H., Keating M.T., and Li D.Y. Impaired distal airway development in mice lacking elastin. Am J Respir Cell Mol Biol 23, 320, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Shifren A., and Mecham R.P. The stumbling block in lung repair of emphysema: elastic fiber assembly. Proc Am Thorac Soc 3, 428, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]