Abstract
Vitamin A deficiencies are common throughout the world and have a significant negative influence on immune protection against viral infections. Mouse models demonstrate that the production of IgA, a first line of defense against viruses at mucosal sites, is inhibited in the context of vitamin A deficiency. In vitro, the addition of vitamin A to activated B cells can enhance IgA expression, but downregulate IgE. Previous reports have demonstrated that vitamin A modifies cytokine patterns, and in so doing may influence antibody isotype expression by an indirect mechanism. However, we have now discovered hundreds of potential response elements among Sμ, Sɛ, and Sα switch sites within immunoglobulin heavy chain loci. These hotspots appear in both mouse and human loci and include targets for vitamin receptors and related proteins (e.g., estrogen receptors) in the nuclear receptor superfamily. Full response elements with direct repeats are relatively infrequent or absent in Sγ regions although half-sites are present. Based on these results, we pose a hypothesis that nuclear receptors have a direct effect on the immunoglobulin heavy chain class switch recombination event. We propose that vitamin A may alter S site accessibility to activation-induced deaminase and nonhomologous end-joining machinery, thereby influencing the isotype switch, antibody production, and protection against viral infections at mucosal sites.
Vitamin A is a critical nutrient for the protection of humans from infectious disease (9,30,34,37). Functions of vitamin A include upregulation of IgA among activated B cells (29), and downregulation of IgE (41). This vitamin is well known for its capacity to bind nuclear receptors and modulate gene expression. Examples of receptors are the retinoic acid receptor (RAR) and the retinoid X receptor (RXR), bound respectively by all-trans retinoic acid and 9-cis retinoic acid metabolites. RAR and RXR are members of the nuclear receptor superfamily, which is inclusive of retinoic acid, vitamin D, thyroid hormone, and steroid receptors. Typically, these receptor proteins have a central DNA-binding domain with which the protein is targeted to a particular DNA response element sequence. Receptors usually assemble as homodimers or heterodimers. The biological influences of vitamins and hormones are highly complex, because ligand and DNA-binding patterns of nuclear receptors are promiscuous (1,4–7,13,18). A common binding site for RAR-RXR heterodimers (retinoic acid response element [RARE]) is a direct repeat (DR) of two puG(G/T)TCA half-sites, but sequences, spacing, and half-site orientations are variable (15).
The upregulation of cytokines is one method by which vitamins influence antibody isotype expression patterns (21,29,32,35–37). For example, vitamin A can upregulate interleukin 6 (IL-6), a cytokine that supports maturation and stabilization of IgA-producing cells (29). However, we now hypothesize that vitamins may also have a direct influence on the B cell heavy chain class switch recombination (CSR) event. The CSR involves deletion of intervening sequences within the immunoglobulin heavy chain locus between a target S (e.g., Sα) and an upstream S (e.g., Sμ or Sγ2b) region during B cell maturation. S regions are each composed of a tandem array of repeating elements, and no two regions are alike (10). Coincident with the CSR is an increase of germline transcripts, initiated at promoters upstream of the targeted S and CH genes. Germline transcripts can form hybrids with DNA, visualized as R-loops (45). During the transcription process, RNA polymerase II molecules pause in S regions and proteins associated with paused polymerases can recruit activation-induced deaminase (AID) (2,24,27,39,44). Typically, AID drives the CSR process by converting cytosine to uracil in DNA (17,25). A potential next step is for uracil DNA glycosylase to excise uracil (26), rendering the abasic site vulnerable to cuts by apyrimidinic/apurinic endonucleases (33), followed by double-strand breaks and nonhomologous end joining (3).
To determine if potential response elements exist in the heavy chain locus, we searched for eight different RARE-like half-sites within published murine S regions, including direct and reverse complement sequences. As shown in Figure 1A, our search revealed an extraordinary number of juxtaposed half-sites in the Sα region. The most frequent distances between half-sites were 4 and 9 nucleotides (DR4 and DR9), the former most typical of heterodimeric, thyroid hormone, or liver X receptor (TR-RXR; LXR-RXR) binding motifs (38). Like Sα, Sμ, and Sɛ regions exhibited high-density response elements (Supplementary Figs. S1 and S2; Supplementary Data are available online at www.liebertpub.com/vim). One-half sites existed in Sγ regions, but these were rarely or never juxtaposed (Fig. 1B and Supplementary Figs. S3–S5).
FIG. 1.
Response element half-sites in the mouse Sα and Sγ2b regions. Response element half-sites are shown by color coding in the Sα (A) and Sγ2b (B) regions. Mouse and human immunoglobulin heavy chain S regions were accessed from the NCBI Nucleotide database (19,20,22,42). Access IDs included the following—Human IgA1: L19121.1, Human IgA2: AF030305.1, Human IgE: X56797.1, Human IgG1: U39737.1, Human IgG2: U39934.1, Human IgG3: U39935.1, Human IgG4: X56796.1, Human IgM: X54713.1, Mouse IgA: D11468.1, Mouse IgE: M57385.1, Mouse IgG1: M12389.2, Mouse IgG2a and IgG2b: D78344, Mouse IgG3: M12182.1, and Mouse IgM: AC073553.
In a separate analysis, we examined human S regions. As was the case for mouse S regions, the frequency of juxtaposed half-sites was very high in human Sα (Sα1 and Sα2), Sμ, and Sɛ with numerous DR4 and DR9 patterns, but juxtaposed half-sites in Sγ sequences were relatively rare or absent (Supplementary Figs. S6–S13).
Because the half-site TGAGCT (reverse complement AGCTCA) was especially frequent, a full scan for this sequence in mouse and human heavy chain loci was conducted. Figure 2 illustrates the high density of AGCTCA sites in Sμ, Sα, and Sɛ regions for both human and mouse loci, and shows that when half-sites were observed in Sγ regions, they were often oriented as the reverse complement. Results support a hypothesis that retinoid receptors directly bind Sμ, Sα, and Sɛ regions and thereby promote or inhibit Sμ-Sα and Sμ-Sɛ joins. The half-sites in Sγ regions are rarely adjacent to one another, atypical of consensus RARE motifs, but might nonetheless support weak binding of nuclear receptors or binding of related proteins.
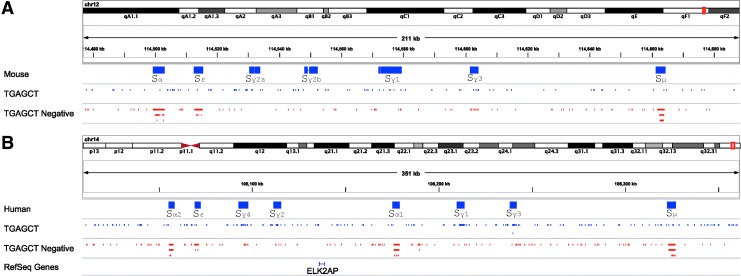
FIG. 2.
Clustered response elements in mouse and human heavy chain loci. Mouse and human immunoglobulin heavy chain switch regions were collected from NCBI Nucleotide database and aligned to mouse assembly mm9(NCBI37) or human assembly hg19(GRCh37) separately with BLAT (version 34 default parameters with maximum intron size 10 kb). We retained the best score alignment for each sequence. Alignment positions were loaded into Integrative Genomics Viewer (IGV, version 2.3). “TGAGCT” motif-matched tracks were created using the “find motif” tool in the IGV (28). Alignment positions are shown in (A) Mouse mm9 and (B) Human hg19. The “TGAGCT Negative” track shows TGAGCT motif matches on the anti-sense strand clustered in Sμ, Sɛ, and Sα, both in mouse and human loci. Parallel lines of blue/red tracks indicate highly concentrated motif hotspots.
To seek potential binding sites for other nuclear receptors, we asked if potential estrogen response elements (ERE) were frequent in S regions of heavy chain loci. RARE and ERE can be juxtaposed to mediate multihormonal responsiveness in gene regulation, and estrogen is known to influence AID and CSR (12,16,23). A canonical DNA motif for the estrogen receptor is a palindrome with half-sites GGTCA and TGACC separated by three nucleotides (11). To allow for sequence variability, we searched for G/A G/A T/C T/C G/A NNN TGA C/G C in S regions. In so doing, we found that high-density motifs appeared in Sμ and Sα regions of human and mouse loci, but not in Sγ regions. For example, there were more than 70 motifs in Sα regions for both human and mouse, yet not a single motif in Sγ2 regions in either species.
Altogether, data encourage investigation of potential interactions between nuclear receptors and response element hotspots in immunoglobulin heavy chain S region loci. We note that the response elements observed in this study were positioned in S regions rather than in the known promoter regions for sterile transcripts. We, therefore, propose that nuclear receptors may influence CSR by mechanisms other than transcription upregulation (31). Possibly, the tripartite binding of ligands (e.g., vitamins), nuclear receptors, and DNA, alter chromatin and the subsequent recruitment of enzymes (e.g., HDAC3, and AID) to enhance deamination and strand breaks in S regions. We note that a hotspot for AID binding is AGCT (8), a sequence within the half-site TGAGCT, supportive of our hypothesis. Should nuclear receptors bind S regions and recruit AID, they would not act in isolation. Previous research has shown that S regions are bound by diverse macromolecular complexes comprising a plethora of factors (e.g., RNA polymerase II, SPT5, RNA exosome, 14-3-3 adapters, AID, E-box-binding proteins, NFκB, and histone-modifying enzymes), each of which may influence isotype expression patterns by activated B cell populations (2,14,24,39,40,43,44).
In conclusion, our results reveal a large number of potential response elements for vitamins and hormones in immunoglobulin S loci. Future research may determine if and how nuclear receptors bind (or are prohibited from binding) these regions. Results should provide a better understanding of CSR mechanisms, isotype expression patterns, and strategies with which protection against infectious diseases may be improved.
Supplementary Material
Acknowledgments
The research was funded in part by NIH NIAID R01 AI088729, NCI P30 CA21765, the American Lebanese Syrian Associated Charities (ALSAC), and the Intramural Research Program of the NIH, National Institute on Aging.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bastien J, and Rochette-Egly C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene 2004;328:1–16 [DOI] [PubMed] [Google Scholar]
- 2.Basu U, Meng FL, Keim C, et al. The RNA exosome targets the AID cytidine deaminase to both strands of transcribed duplex DNA substrates. Cell 2011;144:353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boboila C, Alt FW, and Schwer B. Classical and alternative end-joining pathways for repair of lymphocyte-specific and general DNA double-strand breaks. Adv Immunol 2012;116:1–49 [DOI] [PubMed] [Google Scholar]
- 4.Boergesen M, Pedersen TA, Gross B, et al. Genome-wide profiling of liver X receptor, retinoid X receptor, and peroxisome proliferator-activated receptor alpha in mouse liver reveals extensive sharing of binding sites. Mol Cell Biol 2012;32:852–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bugge TH, Pohl J, Lonnoy O, et al. RXR alpha, a promiscuous partner of retinoic acid and thyroid hormone receptors. EMBO J 1992;11:1409–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans RM, and Mangelsdorf DJ. Nuclear receptors, RXR, and the big bang. Cell 2014;157:255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Villalba P, Jimenez-Lara AM, and Aranda A. Vitamin D interferes with transactivation of the growth hormone gene by thyroid hormone and retinoic acid. Mol Cell Biol 1996;16:318–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han L, Masani S, and Yu K. Overlapping activation-induced cytidine deaminase hotspot motifs in Ig class-switch recombination. Proc Natl Acad Sci U S A 2011;108:11584–11589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussey GD, and Klein M. A randomized, controlled trial of vitamin A in children with severe measles. N Engl J Med 1990;323:160–164 [DOI] [PubMed] [Google Scholar]
- 10.Iwasato T, Arakawa H, Shimizu A, et al. Biased distribution of recombination sites within S regions upon immunoglobulin class switch recombination induced by transforming growth factor beta and lipopolysaccharide. J Exp Med 1992;175:1539–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res 2001;29:2905–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee MO, Liu Y, and Zhang XK. A retinoic acid response element that overlaps an estrogen response element mediates multihormonal sensitivity in transcriptional activation of the lactoferrin gene. Mol Cell Biol 1995;15:4194–4207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leid M, Kastner P, and Chambon P. Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem Sci 1992;17:427–433 [DOI] [PubMed] [Google Scholar]
- 14.Ma L, Hu B, and Kenter AL. Ig S gamma-specific DNA binding protein SNAP is related to the helix-loop-helix transcription factor E47. Int Immunol 1997;9:1021–1029 [DOI] [PubMed] [Google Scholar]
- 15.Mader S, Leroy P, Chen JY, et al. Multiple parameters control the selectivity of nuclear receptors for their response elements. Selectivity and promiscuity in response element recognition by retinoic acid receptors and retinoid X receptors. J Biol Chem 1993;268:591–600 [PubMed] [Google Scholar]
- 16.Maul RW, and Gearhart PJ. Women, autoimmunity, and cancer: a dangerous liaison between estrogen and activation-induced deaminase? J Exp Med 2009;206:11–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maul RW, Saribasak H, Martomo SA, et al. Uracil residues dependent on the deaminase AID in immunoglobulin gene variable and switch regions. Nat Immunol 2011;12:70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mic FA, Molotkov A, Benbrook DM, et al. Retinoid activation of retinoic acid receptor but not retinoid X receptor is sufficient to rescue lethal defect in retinoic acid synthesis. Proc Natl Acad Sci U S A 2003;100:7135–7140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mills FC, Brooker JS, and Camerini-Otero RD. Sequences of human immunoglobulin switch regions: implications for recombination and transcription. Nucleic Acids Res 1990;18:7305–7316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mills FC, Mitchell MP, Harindranath N, et al. Human Ig S gamma regions and their participation in sequential switching to IgE. J Immunol 1995;155:3021–3036 [PubMed] [Google Scholar]
- 21.Mora JR, Iwata M, and Von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol 2008;8:685–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan-Hammarstrom Q, Zhao Y, and Hammarstrom L. Class switch recombination: a comparison between mouse and human. Adv Immunol 2007;93:1–61 [DOI] [PubMed] [Google Scholar]
- 23.Pauklin S, Sernandez IV, Bachmann G, et al. Estrogen directly activates AID transcription and function. J Exp Med 2009;206:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavri R, Gazumyan A, Jankovic M, et al. Activation-induced cytidine deaminase targets DNA at sites of RNA polymerase II stalling by interaction with Spt5. Cell 2010;143:122–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen-Mahrt SK, Harris RS, and Neuberger MS. AID mutates E. coli suggesting a DNA deamination mechanism for antibody diversification. Nature 2002;418:99–103 [DOI] [PubMed] [Google Scholar]
- 26.Rada C, Williams GT, Nilsen H, et al. Immunoglobulin isotype switching is inhibited and somatic hypermutation perturbed in UNG-deficient mice. Curr Biol 2002;12:1748–1755 [DOI] [PubMed] [Google Scholar]
- 27.Rajagopal D, Maul RW, Ghosh A, et al. Immunoglobulin switch mu sequence causes RNA polymerase II accumulation and reduces dA hypermutation. J Exp Med 2009;206:1237–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson JT, Thorvaldsdottir H, Winckler W, et al. Integrative genomics viewer. Nat Biotechnol 2011;29:24–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudraraju R, Jones BG, Surman SL, et al. Respiratory tract epithelial cells express retinaldehyde dehydrogenase ALDH1A and enhance IgA production by stimulated B cells in the presence of vitamin A. PLoS One 2014;9:e86554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudraraju R, Surman SL, Jones BG, et al. Reduced frequencies and heightened CD103 expression among virus-induced CD8(+) T cells in the respiratory tract airways of vitamin A-deficient mice. Clin Vaccine Immunol 2012;19:757–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo GY, Jang YS, Kim HA, et al. Retinoic acid, acting as a highly specific IgA isotype switch factor, cooperates with TGF-beta1 to enhance the overall IgA response. J Leukoc Biol 2013;94:325–335 [DOI] [PubMed] [Google Scholar]
- 32.Seo GY, Jang YS, Kim J, et al. Retinoic acid acts as a selective human IgA switch factor. Hum Immunol 2014;75:923–929 [DOI] [PubMed] [Google Scholar]
- 33.Stavnezer J, Linehan EK, Thompson MR, et al. Differential expression of APE1 and APE2 in germinal centers promotes error-prone repair and A:T mutations during somatic hypermutation. Proc Natl Acad Sci U S A 2014;111:9217–9222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stephensen CB, Blount SR, Schoeb TR, et al. Vitamin A deficiency impairs some aspects of the host response to influenza A virus infection in BALB/c mice. J Nutr 1993;123:823–833 [DOI] [PubMed] [Google Scholar]
- 35.Surman SL, Jones BG, Rudraraju R, et al. Intranasal administration of retinyl palmitate with a respiratory virus vaccine corrects impaired mucosal IgA response in the vitamin A-deficient host. Clin Vaccine Immunol 2014;21:598–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Surman SL, Jones BG, Sealy RE, et al. Oral retinyl palmitate or retinoic acid corrects mucosal IgA responses toward an intranasal influenza virus vaccine in vitamin A deficient mice. Vaccine 2014;32:2521–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Surman SL, Rudraraju R, Sealy R, et al. Vitamin A deficiency disrupts vaccine-induced antibody-forming cells and the balance of IgA/IgG isotypes in the upper and lower respiratory tract. Viral Immunol 2012;25:341–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Umesono K, Giguere V, Glass CK, et al. Retinoic acid and thyroid hormone induce gene expression through a common responsive element. Nature 1988;336:262–265 [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Wuerffel R, Feldman S, et al. S region sequence, RNA polymerase II, and histone modifications create chromatin accessibility during class switch recombination. J Exp Med 2009;206:1817–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Wuerffel R, and Kenter AL. NF-kappa B binds to the immunoglobulin S gamma 3 region in vivo during class switch recombination. Eur J Immunol 2006;36:3315–3323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Worm M, Krah JM, Manz RA, et al. Retinoic acid inhibits CD40 + interleukin-4-mediated IgE production in vitro. Blood 1998;92:1713–1720 [PubMed] [Google Scholar]
- 42.Wu X, and Stavnezer J. DNA polymerase beta is able to repair breaks in switch regions and plays an inhibitory role during immunoglobulin class switch recombination. J Exp Med 2007;204:1677–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu Z, Fulop Z, Wu G, et al. 14–3-3 adaptor proteins recruit AID to 5′-AGCT-3′-rich switch regions for class switch recombination. Nat Struct Mol Biol 2010;17:1124–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu Z, Zan H, Pone EJ, et al. Immunoglobulin class-switch DNA recombination: induction, targeting and beyond. Nat Rev Immunol 2012;12:517–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu K, Chedin F, Hsieh CL, et al. R-loops at immunoglobulin class switch regions in the chromosomes of stimulated B cells. Nat Immunol 2003;4:442–451 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.