Abstract
Genetically modified pigs have become available recently. In this study, we established the gnotobiotic pig model of human rotavirus (HRV) infection using cloned pigs with homozygous disruption in the gene encoding immunoglobulin heavy chain (HCKO), which totally impairs B-cell development. To clarify importance of B cells and cytotoxic T cells in rotavirus immunity, CD8 cells in a subset of the pigs were depleted by injecting antipig CD8 antibodies and the immune phenotypes of all pigs were examined. HCKO pigs, CD8 cell-depleted HCKO pigs, and wild-type (WT) pigs were vaccinated with an attenuated HRV vaccine and challenged with virulent HRV. Protection against HRV infection and diarrhea was assessed postchallenge and detailed T-cell subset responses were determined pre- and postchallenge. Significantly longer duration of virus shedding was seen in vaccinated HCKO pigs than in WT pigs, indicating the importance of B cells in vaccine-induced protective immunity. Vaccinated HCKO/CD8− pigs shed significantly higher number of infectious virus than WT pigs and non-CD8-depleted HCKO pigs, indicating the importance of CD8 T cells in controlling virus replication. Therefore, both B cells and CD8 T cells play an important role in the protection against rotavirus infection. HCKO and HCKO/CD8− pigs did not differ significantly in diarrhea and virus shedding postchallenge; increased CD4 and CD8− γδ T-cell responses probably compensated partially for the lack of CD8 T cells. This study demonstrated that HCKO pigs can serve as a valuable model for dissection of protective immune responses against viral infections and diseases.
Introduction
Genetically modified animal models (avian, rats, mice, pigs, etc.) are widely used in biomedical research (1,19,29,36). For virological research, genetically modified mice have been extensively used. For example, mice transferred with the genes coding for the measles receptor CD46 (30) and the poliomyelitis receptor (26) were used to study measles and poliovirus, respectively. Recently, humanized mice have been developed and are used in studies of a number of human-specific viruses such as hepatitis C, human immunodeficiency virus-1 (HIV-1), dengue, and Epstein-Barr virus (EBV) (1). BALB/c Rag-γc-deficient mice (humanized or not humanized) were found to support replication of a GII.4 strain of human norovirus and are being used as an infection model (35). In addition to transgenic mice, transgenic rabbits expressing the human CD4 gene (9) and transgenic rats expressing the HIV-1 provirus with a functional deletion of gag and pol (25) were generated for studying HIV-1 infection. However, the drawback is that many of these rodent-based models do not faithfully recapitulate human disease pathogenesis. Domestic pigs (Sus scrofa domesticus) share many anatomical, physiological, and immunological characteristics with humans and, therefore, are a superior model for preclinical testing of human vaccines and therapeutics. Genetically modified pigs have not been used in virological research previously, except that siRNA transgenic pigs were generated to knockdown porcine endogenous retrovirus expression for safe xenotransplantation (24) and more recently to investigate the infectivity of porcine reproductive and respiratory syndrome virus (17).
For rotavirus research, various gene knockout adult mice (i.e., Rag-2 mice devoid of both T and B cells, β2m mice that lack cytotoxic T-cell responses, JHD mice that lack B-cell responses, and IgA knockout mice that have no detectable IgA in the serum or in any secretions) have been extensively used in studying determinants of protective immunity against rotavirus infection (3,8,11,20,37). These studies have produced important observations regarding the roles of various components of humoral and cellular immunity (IgA antibody, CD4, or CD8 T cell) in resolution of primary infection or protection against chronic rotavirus infection. However, adult mice do not develop diarrhea after murine rotavirus infection. Also the pathogenesis of rotavirus infection in mice is very different from that in humans. Although neonatal mice are susceptible to rotavirus diarrhea for the first 14 days of life, the short susceptible time severely limits their use in rotavirus vaccine research (43). Moreover, different genetic backgrounds of mice lead to different, even conflicting, results (10,12). One study suggested that CD4 T cells are the only lymphocytes needed to protect mice against rotavirus shedding after immunization with rotavirus VP6 protein (20). Others have suggested that (i) neither CD4+, CD8+ T cells, nor antibodies were essential for protection against rotavirus primary infection in mice; (ii) B-cell responses were necessary for development of immunity against rotavirus reinfection; and (iii) the importance of each lymphocyte population as effectors of protection was found to be dependent on the immunogen (live, inactivated, or virus-like particles) and the route of immunization (oral or intranasal) (4,42).
In contrast to adult mice, the neonatal gnotobiotic (Gn) pig model of human rotavirus (HRV) infection and diarrhea more closely recapitulates rotavirus gastroenteritis in human infants and young children (27). The Gn pig is the only animal model susceptible to HRV diarrhea for up to 8 weeks of age and the model has been extensively used for investigating rotavirus vaccine-induced protective immunity against both infection and diseases (50). Our previous studies using Gn pigs found that the number of HRV-specific intestinal IgA antibody secreting cells and frequencies of intestinal interferon-gamma (IFN-γ)-producing T cells were significantly correlated with protection rates against rotavirus diarrhea, indicating the role of both B and T cells in the protection against rotavirus disease (51,52). However, these studies could not separate the humoral and cellular protective immunity from each other. Recently, Revivicor, Inc. has generated the total B-cell-deficient pigs. These genetically modified pigs have the gene encoding for the immunoglobulin HCKO using Revivicor's proprietary techniques. These pigs are impaired in their germinal center development and are completely incapable of producing antibodies, but theoretically maintain intact innate immune cell and adaptive T-cell responses. As such, these animals offer a unique model to elucidate the mechanisms of vaccine-induced protective immunity when challenged with virulent HRV (VirHRV). We characterized the development of cellular immune responses to an attenuated HRV (AttHRV) vaccine in the HCKO pigs compared with that in wild-type (WT) pigs at challenge and postchallenge. We also depleted CD8 cells in a subset of HCKO pigs by intravenously injecting antipig CD8 monoclonal antibodies before the time of challenge, examined the influence of the CD8-cell depletion on cellular immune responses, and assessed the contribution of CD8 T cells in protective immunity against rotavirus diarrhea conferred by the AttHRV vaccine.
Materials and Methods
Derivation and characterization of B-cell- and antibody-deficient HCKO pigs
All animal experimental procedures were conducted in accordance with protocols approved by Institutional Animal Care and Use Committees of Virginia Polytechnic Institute and State University. The uniquely modified HCKO pigs provided by Revivicor, Inc. (www.revivicor.com/) have the gene encoding for the Ig heavy chain knocked out to impair the variable (V), joining (J) and in some cases, diversity (D) gene segment rearrangement using Revivicor's proprietary techniques (U.S. patent application number 20080026457 and 20060130157). Briefly, the poly (A)-trap gene targeting technology was applied for disrupting the single functional heavy chain joining region (JH) in a porcine primary fetal fibroblast cell line, and the somatic cell nuclear transfer (SCNT) strategy was used to produce genetically modified piglets as described in previous studies (7). The JH− female pigs were outbred with WT male pigs of a similar large white breed to generate F1 offspring. The JH± F1 pigs (male and female) were subsequently crossbred to obtain the F2 generation (the ratio of JH−, JH±, and JH+ genotypes approximated the expected 1:2:1 Mendelian ratio).
F2 fetuses with confirmed JH− genotype from one sow were used to derive the JH− PPFF cells and produce reconstructed embryos, which were used to produce HCKO pigs through the SCNT technology. HCKO Gn pigs were delivered by hysterectomy and maintained in germ-free isolator units (22).
To characterize the immunophenotype of the B-cell-deficient HCKO (JH−) pigs, normal WT (n = 4 and 6 for postpartum days [PPDs] 0–7 and PPD 28, respectively) and cloned HCKO Gn pigs (n = 4 and 2 for PPDs 0–7 and PPD 28, respectively) were used to examine the presence of immature B cells and immunoglobulin-secreting cells (IgSCs) in various lymphoid tissues. Gn pigs were monoassociated with Lactobacillus acidophilus NCFM® (LA) strain to stimulate the development of the neonatal immune system (46) as was described previously (46). Mononuclear cells (MNCs) were isolated from ileum, duodenum, and mesenteric lymph nodes (same method), spleen and thymus (same method), and peripheral blood and bone marrow (same method) using the three different procedures as was previously described (46). MNCs were then subjected to cell surface staining and flow cytometry for detecting B cells (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/vim).
Viruses
The attenuated Wa strain (G1P1A) (8) HRV was used as the model monovalent AttHRV vaccine. The inoculum was derived from the 35th passage in African green monkey kidney cell (MA104) culture. Gn pigs were inoculated at a dose of 5 × 107 fluorescent focus-forming units (FFU) (46). AttHRV was also used as the detector antigen in enzyme-linked immunosorbent assay (ELISA) (18) and as the stimulating antigen in the intracellular IFN-γ staining assay as described previously (46).
The VirHRV Wa strain was passaged through Gn pigs and the pooled intestinal contents from the 27th passage were used to challenge Gn pigs at a dose of 105 FFU. The median infectious dose (ID50) and median diarrhea dose (DD50) of the VirHRV in Gn pigs were determined as ∼1 FFU (41).
CD8 T cell depletion
A subset of HCKO pigs was intravenously injected with a purified antipig CD8 monoclonal antibody (clone 76-2-11, Southern Biotech; 1.4 mg/kg of body weight in phosphate-buffered saline with pH 7.4) at 28 days of age to generate transiently CD8 T-cell-depleted pigs (designated as HCKO/CD8−). The concentration of antipig CD8 antibody was 1.0 mg/mL and the injected maximum volume of the antibody was up to 5 mL. The efficiency of CD8 T-cell depletion was monitored by flow cytometry.
Treatment groups and inoculation of WT and HCKO Gn pigs
Near-term WT and cloned HCKO Gn pigs were derived by hysterectomy and maintained in germ-free isolator units using the same procedure previously described (22). All pigs were confirmed sero-negative for antirotavirus antibodies and germ-free before AttHRV exposure. Gn pigs (both males and females) were randomly assigned to the following groups: (i) WT Gn pigs with AttHRV (n = 10, AttHRV/WT), (ii) HCKO Gn pigs with AttHRV (n = 12; 6 pigs for AttHRV/HCKO and 6 pigs for AttHRV/HCKO/CD8−), and (iii) control HCKO Gn pigs (n = 5, HCKO). Pigs in the AttHRV groups were orally inoculated with 5 × 107 FFU/dose of AttHRV in 5 mL of diluent (minimum essential medium; Invitrogen) at both 5 and 15 days of age (postinoculation days [PIDs] 0 and 10, respectively). Pigs not inoculated with AttHRV were given an equal volume of diluent as a control. At PID 28, subsets of pigs from AttHRV/WT (n = 5), AttHRV/HCKO (n = 3), AttHRV/HCKO/CD8− (n = 3), and HCKO (n = 3) groups were orally challenged with 105 FFU VirHRV as was previously described (46). Postchallenge, pigs were examined daily for clinical signs and fecal swabs were collected daily as was previously described (51).
For examining the efficacy of CD8-cell depletion, peripheral blood was collected from pigs in the AttHRV/HCKO/CD8− group at 31 days of age (3 days after the CD8 antibody injection and 2 days before the VirHRV challenge) and MNCs were isolated. MNCs were also isolated from ileum, spleen, and peripheral blood of pigs euthanized on PID 28 or postchallenge day (PCD) 7 as was previously described (46,51), to examine the efficacy of CD8-cell depletion and cellular immune responses. In addition, MNCs from ileum, spleen, and peripheral blood of AttHRV/HCKO pigs were subjected to the examination of rotavirus-specific IgM, IgA, and IgG antibody-secreting cell responses using the ELISPOT assay previously described (51).
Detection of rotavirus shedding and assessment of rotavirus caused diarrhea
Rectal swabs were collected for 7 days after VirHRV challenge to assess rotavirus diarrhea and shedding. Fecal consistency was scored as follows: 0, normal; 1, pasty; 2, semiliquid; and 3, liquid. Pigs with daily fecal scores of ≥2 were considered diarrheic. The mean cumulative score was calculated as the sum of daily fecal scores from PCDs 1 to 7 in each group divided by the number of pigs. Virus shedding was detected in processed rectal swab fluids by ELISA and infectious virus particles were measured by cell culture immunofluorescence (CCIF) assay as described previously (2). A sample was positive for rotavirus shedding if the mean absorbance of replicates was greater than the mean plus three times the standard deviations of the absorbance of the negative controls. Fecal samples from mock-infected pigs were used as negative controls. Rotavirus antigen ELISA optical density (OD) values were adjusted based on the average OD values of the negative controls from different ELISA plates. Protection rate of diarrhea or shedding upon challenge was calculated using the formula (1 − percentage of AttHRV-inoculated pigs with diarrhea or shedding/percentage of mock-inoculated control pigs with diarrhea or shedding) ×100.
Flow cytometry analysis of frequency of CD4+ CD25− FoxP3+ regulatory T cells
MNCs were stained freshly without in vitro stimulation to determine the frequencies of CD4+CD25−FoxP3+ regulatory T (Treg) cells among MNCs in ileum, intraepithelial lymphocytes (IEL), spleen, and blood of Gn pigs by using intracellular cytokine staining and flow cytometry as we previously described (46). Only CD4+CD25−FoxP3+ Treg cells were included in this study because our previous study (46) found that CD25− Treg cells, but not CD25+ Treg cells, were the predominant responding Treg cells after HRV infection or vaccination in Gn pigs. The absolute number of CD4+CD25−FoxP3+ Treg cells per tissue was calculated based on the frequencies of Treg cells and the total number of MNCs isolated from each tissue. SpectralRed™ (SPRD) conjugated mouse antiporcine CD8 (IgG2a; BD Pharmingen) was also included in the staining protocol for examining the efficiency of CD8 depletion by antipig CD8 antibody.
Flow cytometry analysis of frequency of IFN-γ-producing natural killer cells, αβ CD4 and CD8 T cells, and γδ T cells
MNCs were mock stimulated or stimulated with semipurified AttHRV antigen in vitro for 17 h. Brefeldin A was added for the last 5 h to block secretion of cytokines. The frequencies of HRV-specific IFN-γ-producing CD4/CD8 T cells among CD3 cells, IFN-γ-producing CD8+/CD8− γδ T cells among γδ T cells in ileum, IEL, spleen, and blood of Gn pigs were determined by using intracellular cytokine staining and flow cytometry as we previously described (46). Porcine natural killer (NK) cells were defined as CD3−CD8+ cells gated on lymphocytes (13). The frequencies of IFN-γ-producing NK cells were also determined by using flow cytometry. The absolute number of NK cells, CD4 and CD8 T cells, and γδ T cells per tissue was calculated based on their frequencies and the total number of MNCs was isolated from each tissue.
Statistical analysis
Fisher's exact test was used to compare the percentages of pigs with diarrhea or virus shedding among treatment groups. Nonparametric Kruskal–Wallis rank sum test was performed to compare all other parameters among treatment groups at each time point. When differences among the groups were detected, the same test was used in a pairwise fashion to identify the nature of the differences. Statistical significance was assessed at p < 0.05 and the statistical analysis was performed using SAS program 9.2 (SAS Institute, Inc.).
Results
HCKO pigs lack immature B cells and IgSCs and are totally incapable of producing antibodies
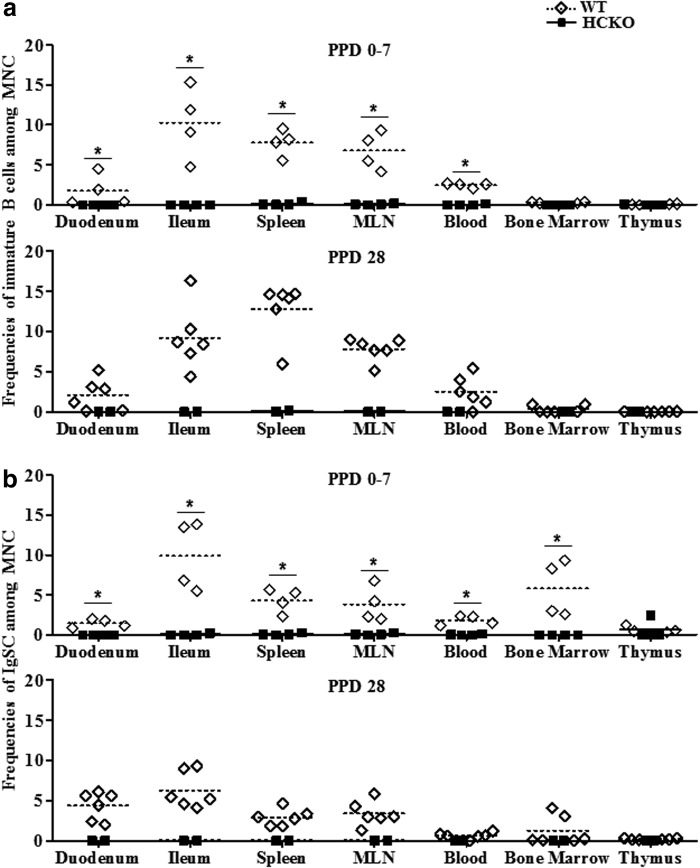
All HCKO pigs had almost no detectable immature B cells and IgSCs in all tissues examined at PPDs 0–7 or PPD 28 with the exception of a few IgSCs in thymus of one pig at PPDs 0–7 (Fig. 1). In contrast, WT pigs had higher or significantly higher frequencies of immature B cells and IgSCs in duodenum, ileum, spleen, MLN, and blood than HCKO pigs at PPDs 0–7 and PPD 28. The lack of B cells is associated with relatively smaller size of spleen of HCKO pigs than that of WT pigs. Rotavirus-specific IgM, IgA, and IgG antibody-secreting cells were also not detected in ileum, spleen, and peripheral blood of any AttHRV/HCKO pigs at PID 28 (AttHRV) and PCD 7 (VirHRV).
FIG. 1.
Frequencies of total immature B cells (CD79a+SWC3a+CD21+CD2+/-IgM+) (a) and IgSCs (CD79a+SWC3a−CD21+/-CD2+/-IgM−) (b) among total gated MNCs. The top figures show the frequencies of immature B cells (a) among MNCs at PPDs 0–7 and PID 28, and the bottom figures show IgSCs (b). Bars indicate group means. The sign “*” indicates significant differences among groups (Kruskal–Wallis test, p < 0.05). See Supplementary Figure S1 legend for staining of B cells. PID, postinoculation day; PPD, postpartum day; WT, wild type; HCKO, heavy chain knockout; MNC, mononuclear cells; IgSCs, immunoglobulin-secreting cells.
Efficiency of CD8 T cell depletion by single injection of antipig CD8 antibody
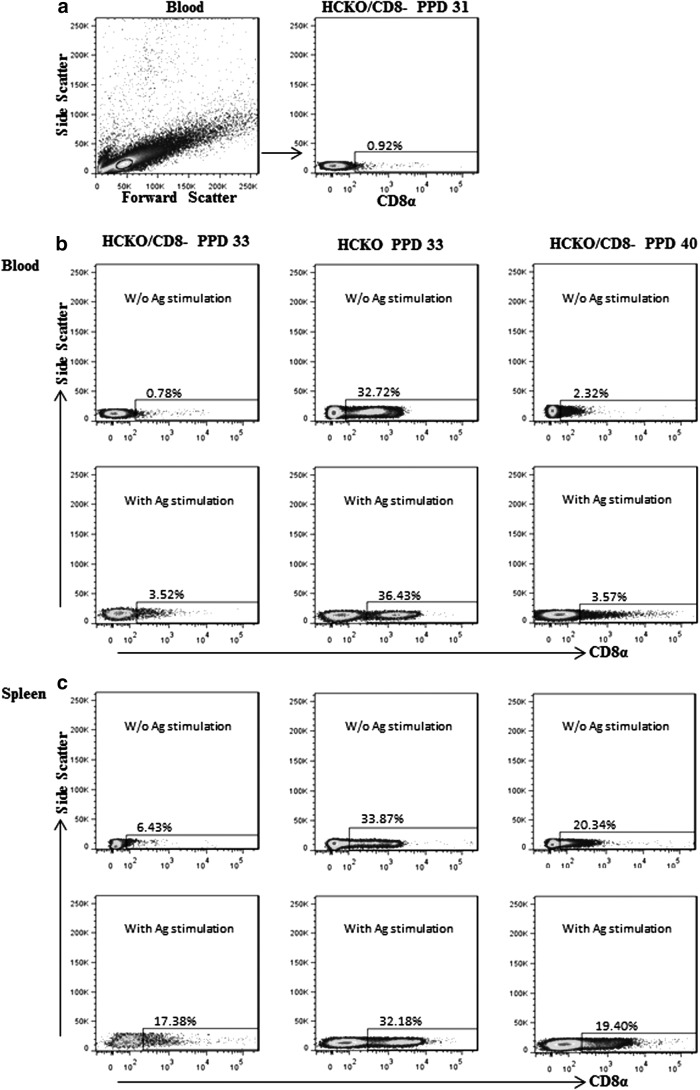
The antipig CD8 monoclonal antibody was intravenously injected in HCKO pigs 5 days before VirHRV challenge on PID 23 (PPD 28). Peripheral blood was collected 3 days later (PPD 31) to examine the efficiency of CD8-cell depletion by using flow cytometry. The representative dot plot in Figure 2a shows the frequencies (0.92%) of CD8 cells among lymphocytes in blood. On PID 28/PCD 0 (PPD 33) and PID 35/PCD 7 (PPD 40), the efficiency of CD8 depletion was examined in blood, spleen, and ileum. The representative dot plots are presented in Figure 2b (blood) and Figure 2c (spleen). As shown, antipig CD8 antibody injection resulted in a 97.6% reduction (32.72–0.78%) of CD8 cells in blood (Fig. 2b upper panel) and an 81% reduction (33.87–6.43%) of CD8 cells in spleen (Fig. 2c upper panel) on the day of VirHRV challenge (PPD 33). Increasing number of cells started to re-express the CD8 marker 12 days after injection by PPD 40; however, the frequencies of CD8 cells are still much lower than those in untreated pigs. Among in vitro semipurified AttHRV antigen-stimulated cells, more cells re-expressed CD8 marker after 17 h of cell culture (Fig. 2b, c lower panels). Our result concurred with the previous report that after a single intravenous injection of the antipig CD8 antibody (clone 76-2-11 as ascites; 0.7 mL/kg), the frequencies of CD8 T cells in blood of the pigs were reduced to the lowest levels (0–5% of total cells) at 4–6 days postinjection (33).
FIG. 2.
Efficiency of transient CD8-cell depletion by single injection of antipig CD8 antibody. Antipig CD8 monoclonal antibody was given at 28 days of age (PPD 28) and, to examine the efficacy of CD8 depletion, peripheral blood was collected from pigs in the AttHRV/HCKO/CD8− group 3 days later (PPD 31) to MNCs. MNCs were also isolated from ileum, spleen, and peripheral blood of pigs euthanized on PID 28 (PPD 33) or PCD 7. (a) Representative dot plots collected from blood at PPD 31. The figures in the top of (b) and (c) show those collected from blood and spleen, respectively, without semipurified AttHRV antigen stimulation, and those in the bottom show the representative dot plots with semipurified AttHRV antigen stimulation for 17 h. The first and second columns in (b) and (c) show the dot plots from HCKO/CD8− and HCKO pigs, respectively, at PPD 33. The last column shows those from HCKO/CD8− pigs at PPD 40. The numbers above the rectangles in dot plots are the frequencies of CD8 cells among lymphocytes. AttHRV, attenuated human rotavirus; PCD, postchallenge day.
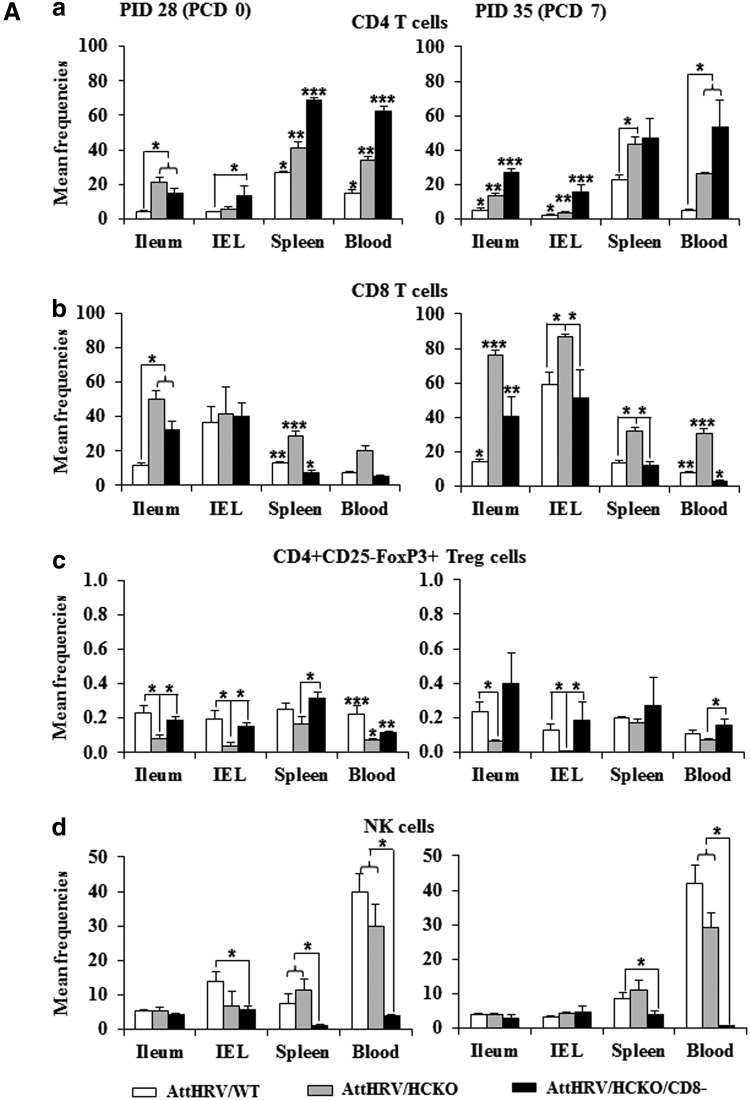
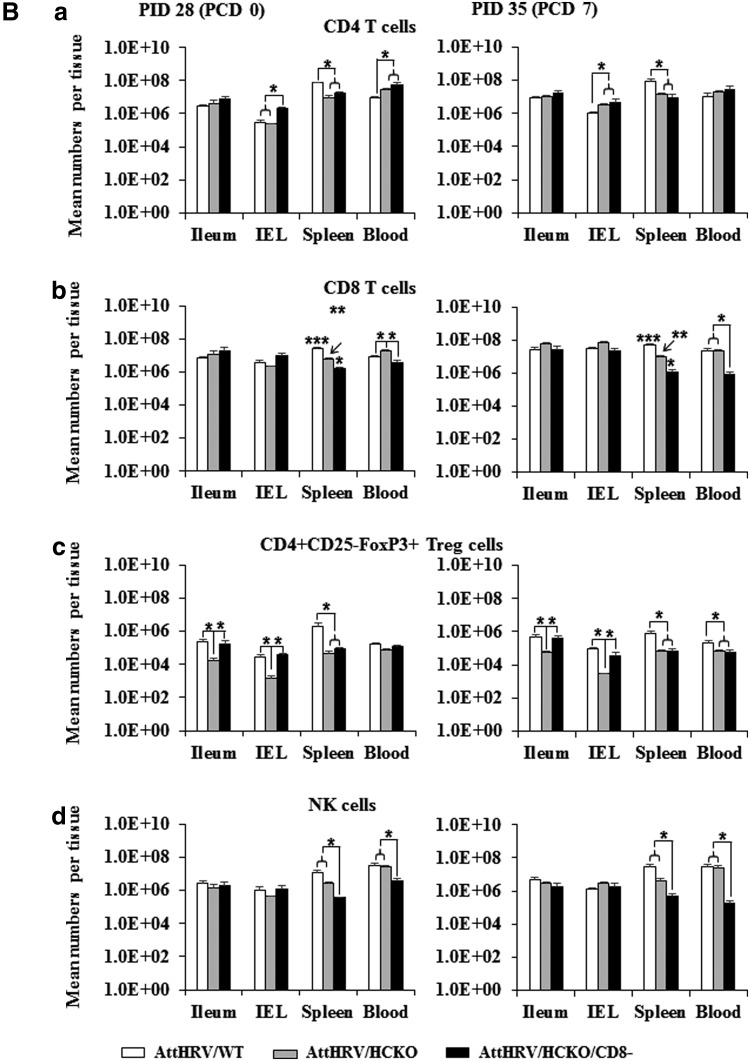
The effects of antipig CD8 antibody injection on the frequencies of CD4 and CD8 T cells, Treg cells, NK cells, and γδ T cells in all the lymphoid tissues examined on PID 28 (PPD 33) and PCD 7 (PPD 40) are summarized (Figs. 3A, 3B, 4 and 5) and compared with AttHRV/WT and/or AttHRV/HCKO pigs in the following sections.
FIG. 3.
Mean frequencies of CD4 T cells (a), CD8 T cells (b), Treg cells (c), and NK cells (d) among lymphocytes (A) and mean numbers per tissue (B). MNCs were mock stimulated for 17 h (Brefeldin A was added for the last 5 h) and then subjected to intracellular staining and flow cytometry for collecting the frequency and number data of CD4 T cells (a), CD8 T cells (b), and NK cells (d) and served as background controls for the data of virus-specific IFN-γ-producing cell frequencies (Fig. 4). MNCs were stained freshly for Treg cells (c) without in vitro stimulation. The absolute number of CD4 and CD8 T cells, Treg cells, and NK cells per tissue was calculated based on their frequencies and the total number of MNCs isolated from each tissue. The left figures show the prechallenge data at PID 28 and those in the right show the postchallenge data at PCD 7. Data are presented as mean frequency/number ± standard error of the mean (n = 3 or 5). The asterisk “*” indicates significant difference in frequencies/numbers among groups for the same cell type and tissue; different number of “*” indicates significant differences among groups (Kruskal–Wallis test, p < 0.05). IFN-γ, interferon-gamma; NK, natural killer; Treg, regulatory T.
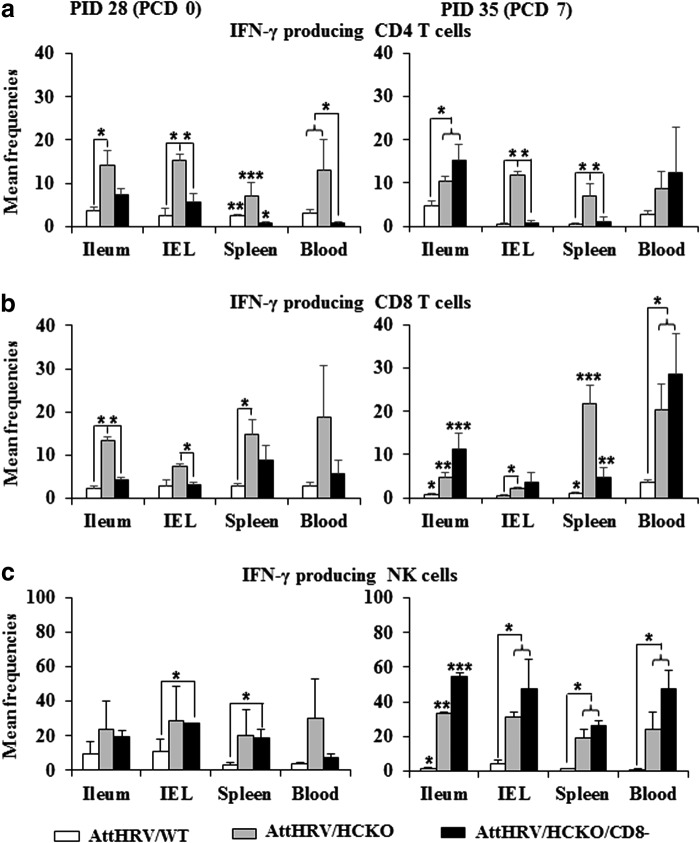
FIG. 4.
IFN-γ-producing CD4 and CD8 T-cell and NK-cell responses. MNCs were stimulated with semipurified AttHRV antigen in vitro for 17 h (Brefeldin A was added for the last 5 h to block IFN-γ secretion) and then subjected to intracellular staining and flow cytometry for collecting the frequency data of IFN-γ-producing CD4 (a) and CD8 (b) T cells and NK cells (c). Data are presented as mean frequency ± standard error of the mean (n = 3 or 5). See Figure 3 legend for panel description and statistical analysis.
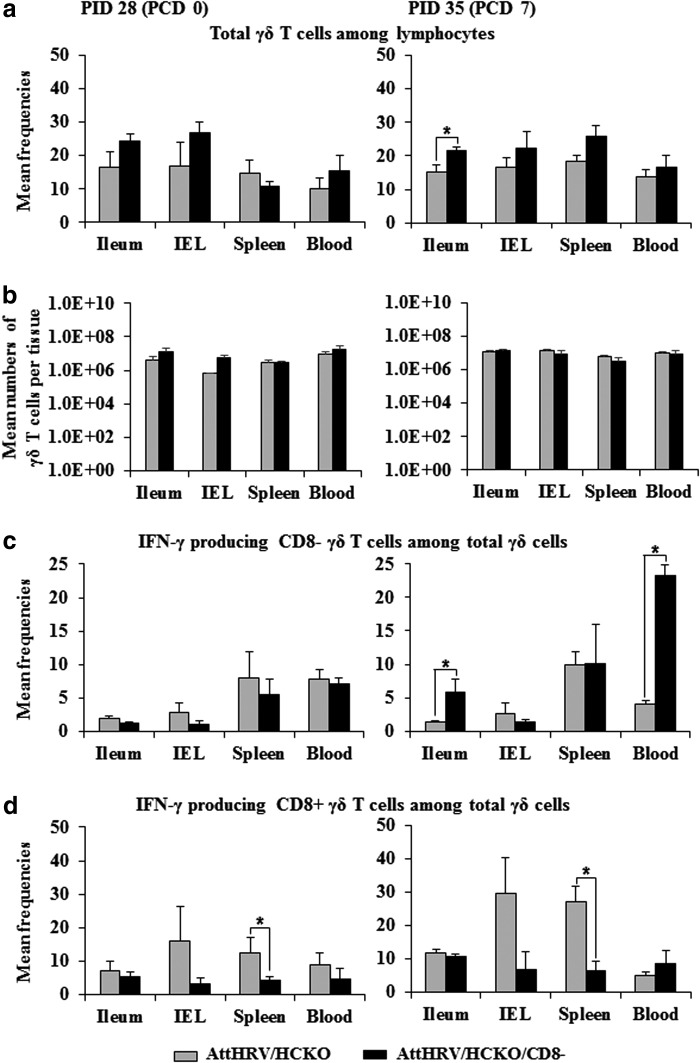
FIG. 5.
Mean frequencies (a) and number of γδ T cells (b) and the IFN-γ production (c and d). Data are presented as mean frequency/number ± standard error of the mean (n = 3). See Figures 3 and 4 legends for detection of rotavirus-specific IFN-γ-producing cells, panel description, and statistical analysis.
HCKO pigs had increased CD4 and CD8 T cell population but decreased Treg-cell population
To assess the influence of B-cell deficiency on other lymphocyte populations, we examined the frequencies and total number of NK- and T-cell subsets by flow cytometry. B-cell deficiency had no significant effect on NK-cell frequencies, but altered the frequencies of CD4 and CD8 T cells and Treg cells (Fig. 3A). AttHRV/HCKO pigs had significantly higher frequencies of CD4 T cells in ileum, spleen, and blood, and significantly higher frequencies of CD8 T cells in ileum and spleen at both PID 28 and PCD 7 than AttHRV/WT pigs (Fig. 3A). In addition, AttHRV/HCKO pigs had significantly higher frequencies of CD4 T cells in IEL and significantly higher frequencies of CD8 T cells in IEL and blood at PCD 7 than AttHRV/WT pigs (Fig. 3A). Changes in the absolute number of the T-cell populations were also observed, although in lesser degree. AttHRV/HCKO pigs had significantly higher number of CD4 and CD8 T cells in blood at PID 28 and significantly higher number of CD4 T cells in IEL at PCD 7 than AttHRV/WT pigs (Fig. 3B). Interestingly, AttHRV/HCKO pigs had significantly lower number of CD4 and CD8 T cells in spleen at both PID 28 and PCD 7 than AttHRV/WT pigs (Fig. 3B).
AttHRV/HCKO pigs had significantly lower frequencies of Treg cells in ileum and IEL at both PID 28 and PCD 7 and significantly lower frequencies of Treg cells in blood at PID 28 than AttHRV/WT pigs (Fig. 3A). AttHRV/HCKO pigs also had lower or significantly lower number of Treg cells in all tissues than AttHRV/WT pigs.
CD8 depletion in B-cell-deficient pigs significantly decreased CD8 T cells and NK cells
As expected, CD8-cell depletion by intravenous injection of antipig CD8 antibody decreased the population of CD8 T cells in AttHRV/HCKO/CD8− pigs. Comparing with non-CD8-depleted pigs, AttHRV/HCKO/CD8− pigs had significantly lower frequencies of CD8 T cells in spleen at PID 28 and in all tissues at PCD 7 than AttHRV/HCKO pigs (Fig. 3A). In addition, AttHRV/HCKO/CD8− pigs had significantly lower number of CD8 T cells in spleen and blood at PID 28 and PCD 7 than AttHRV/HCKO pigs (Fig. 3B).
Comparing with AttHRV/WT pigs, AttHRV/HCKO/CD8− pigs had significantly lower frequencies of CD8 T cells in spleen at PID 28 and in blood at PCD 7 than AttHRV/WT pigs (Fig. 3A). AttHRV/HCKO/CD8− pigs also had significantly lower number of CD8 T cells in spleen at both PID 28 and PCD 7 and in blood at PCD 7 than AttHRV/WT pigs (Fig. 3B). However, AttHRV/HCKO/CD8− pigs still had significantly higher frequencies of CD8 T cells in ileum at both PID 28 and PCD 7 than AttHRV/WT pigs because of the large increase in the total CD8 T-cell frequencies in the B-cell-deficient pigs than the WT pigs (Fig. 3A).
CD8-cell depletion also decreased the population of NK cells because they too express the CD8 surface marker in swine (13). CD8 depletion greatly reduced the NK-cell population in spleen and blood at both PID 28 and PCD 7 (Fig. 3A). AttHRV/HCKO/CD8− pigs had significantly lower frequencies of NK cells in spleen and blood at PID 28 and in blood at PCD 7 than AttHRV/WT pigs and AttHRV/HCKO pigs (Fig. 3A). AttHRV/HCKO/CD8− pigs also had significantly lower frequencies of NK cells in IEL at PID 28 and in spleen at PCD 7 than AttHRV/WT pigs (Fig. 3A). Similar to the reduced frequencies, AttHRV/HCKO/CD8− pigs had significantly lower number of NK cells in spleen and blood at both PID 28 and PCD 7 than AttHRV/WT pigs and AttHRV/HCKO pigs (Fig. 3B).
CD8 depletion in B-cell-deficient pigs further increased CD4 T cells but restored the level of Treg cell population
AttHRV/HCKO/CD8− pigs had overall the highest frequencies (with exception in ileum at PID 28) (Fig. 3A) and the highest number (with exception in spleen) (Fig. 3B) of CD4 T cells among the three groups. CD8-cell depletion in the HCKO pigs significantly increased the frequencies of CD4 T cells in spleen and blood at PID 28 and in ileum and IEL at PCD 7 (Fig. 3A). AttHRV/HCKO/CD8− pigs also had significantly increased number of CD4 T cells in IEL at PID 28 (Fig. 3B). AttHRV/HCKO/CD8− pigs had similar frequencies of Treg cells as in AttHRV/WT pigs (with exception in blood at PID 28) and significantly higher frequencies of Treg cells in all tissues at PID 28 and in IEL and blood at PCD 7 than AttHRV/HCKO pigs (Fig. 3A). AttHRV/HCKO/CD8− pigs also had similar number of Treg cells in ileum and IEL as AttHRV/WT pigs but significantly higher frequencies of Treg cells in ileum and IEL than AttHRV/HCKO pigs (Fig. 3B).
B-cell deficient pigs had higher frequencies of IFN-γ-producing CD4 and CD8 T cells and NK cells
AttHRV/HCKO pigs had overall higher frequencies of IFN-γ-producing CD4 and CD8 T cells, and NK cells in all tissues than AttHRV/WT pigs (Fig. 4). Notably, before challenge, AttHRV/HCKO pigs had significantly higher frequencies of IFN-γ-producing CD4 and CD8 T cells in ileum, spleen, and blood than AttHRV/WT pigs at PID 28 (Fig. 4a, b). AttHRV/HCKO pigs had significantly higher frequencies of IFN-γ-producing NK cells (Fig. 4c) and CD8 T cells in all tissues and IFN-γ-producing CD4 T cells in spleen and blood at PCD 7 (Fig. 4a, b).
CD8 depletion in B-cell-deficient pigs significantly decreased the IFN-γ-producing CD4 and CD8 T cells in most tissues
Compared with AttHRV/HCKO pigs, AttHRV/HCKO/CD8− pigs had significantly lower frequencies of IFN-γ-producing CD8 T cells in ileum, spleen, and blood at PID 28 and in IEL, spleen, and blood at PCD 7 (Fig. 4b). Interestingly, AttHRV/HCKO/CD8− pigs also had significantly lower frequencies of IFN-γ-producing CD4 T cells in spleen and blood at PID 28 and in spleen at PCD 7 than AttHRV/HCKO pigs (Fig. 4a), suggesting that IFN-γ-producing CD4 T cells in pig systemic lymphoid tissues are likely double positive cells (CD4+CD8+). Similar findings that double positive CD4+CD8+ cells are the major IFN-γ-producing T cells in pigs have been reported previously (32).
CD8 depletion in B-cell-deficient pigs further enhanced IFN-γ-producing NK cells in all tissues and IFN-γ-producing CD4 and CD8 T cells in ileum at PCD 7
AttHRV/HCKO/CD8− pigs had the highest frequencies of IFN-γ-producing NK cells in all tissues among all three groups at PCD 7 (Fig. 4c). AttHRV/HCKO/CD8− pigs had significantly higher frequencies of IFN-γ-producing NK cells in ileum at PCD 7 than AttHRV/HCKO pigs (Fig. 4c). In addition, AttHRV/HCKO/CD8− pigs had the highest frequencies of IFN-γ-producing CD4 and CD8 T cells in ileum among all three groups at PCD 7 (Fig. 4a, b). AttHRV/HCKO/CD8− pigs had significantly higher frequencies of IFN-γ-producing CD4 T cells in ileum at PCD 7 than AttHRV/HCKO pigs (Fig. 4a).
CD8 depletion in B-cell-deficient pigs reduced IFN-γ-producing CD8+ γδ T cells but enhanced IFN-γ-producing CD8− γδ T cells
Compared with AttHRV/HCKO pigs, CD8 depletion overall had no effects on the number of γδ T cells but slightly increased γδ T-cell frequencies (one exception in spleen at PID 28) (Fig. 5a, b) and significantly enhanced IFN-γ-producing CD8− γδ T cells in ileum and blood at PCD 7 (Fig. 5c). As expected, CD8 depletion reduced IFN-γ-producing CD8+ γδ T cells in all tissues, with significant reductions in spleen at both PID 28 and PCD 7 (Fig. 5d). Data were not collected for γδ T cells in the AttHRV/WT pigs.
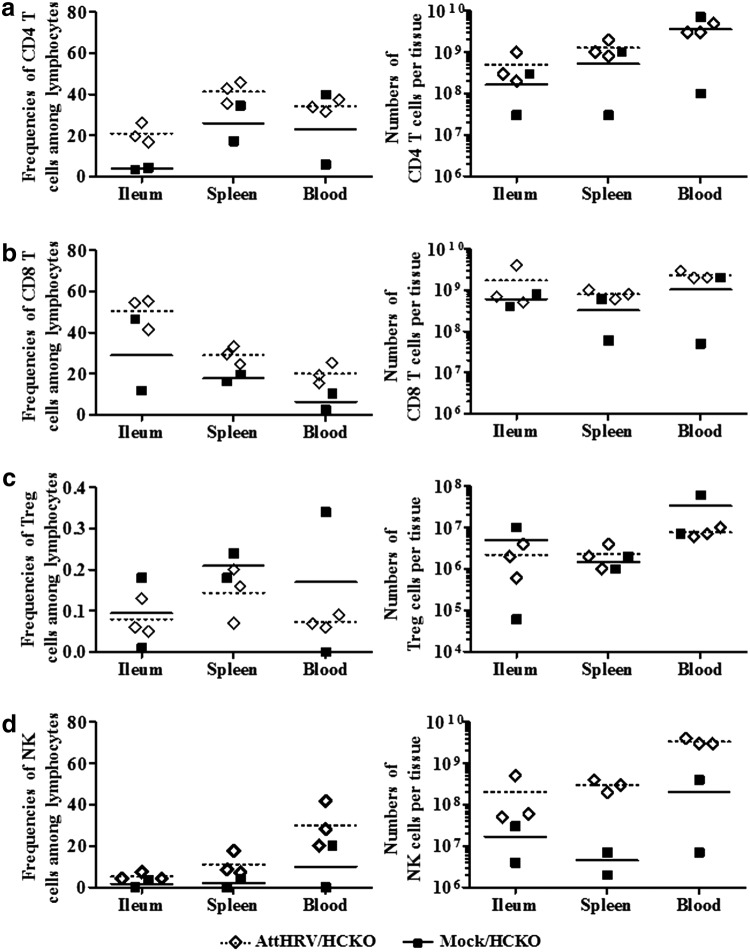
AttHRV vaccination increased CD4 and CD8 T cell and NK-cell population and decreased Treg cell population in HCKO pigs
The MNCs were not stimulated with antigen or mitogen in vitro to investigate the sole effect of AttHRV vaccination on the cell population in vivo. Compared with HCKO pigs without vaccination, AttHRV/HCKO pigs had the overall trend of increased frequencies of CD4 and CD8 T cells and NK cells and their numbers in ileum, spleen, and blood (Fig. 6), suggesting that AttHRV vaccination provided sufficient antigen stimulation in neonatal Gn pigs for activation of immune cells. AttHRV/HCKO pigs had lower frequencies and number of Treg cells (one exception for the Treg cell mean numbers in spleen) than nonvaccinated pigs (Fig. 6c).
FIG. 6.
Frequencies of CD4 T cells (a), CD8 T cells (b), Treg cells (c), and NK cells (d) among gated lymphocytes and the numbers per tissue in vaccinated HCKO pigs and HCKO control pigs at PID 28. See Figure 3 legend for data collection. Because there were only two pigs in the Mock/HCKO group, no statistical comparison between the groups was performed. Bars indicate group means.
B cells contribute to protective immunity against rotavirus infection and diarrhea
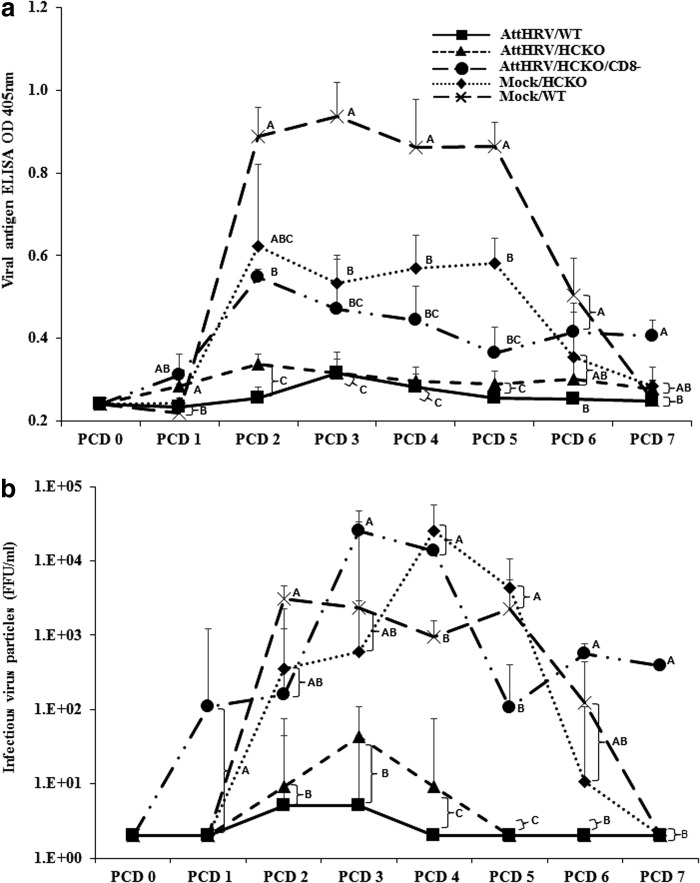
To dissect the protective role of lymphocyte responses elicited by the AttHRV vaccine, we compared clinical signs and rotavirus fecal shedding in immunized WT pigs and B-cell-deficient HCKO pigs. Forty percent (two out of five) AttHRV/WT pigs did not have diarrhea or shed rotavirus after virHRV challenge, whereas all Mock/WT, Mock/HCKO, and AttHRV/HCKO pigs developed diarrhea and virus shedding (Table 1). AttHRV/HCKO pigs had significantly earlier onset of fecal virus shedding (1.7 vs. 5.2 days) and prolonged duration of virus shedding (4.0 vs. 0.8 days) than AttHRV/WT pigs (Table 1). AttHRV/HCKO pigs also had earlier onset of diarrhea (Table 1) and increased daily shedding titers (Fig. 7a) than AttHRV/WT pigs. But these differences were not statistically significant, with one exception (the significantly higher shedding titers of AttHRV/HCKO pigs at PCD 1 shown in Fig. 7a). AttHRV/HCKO pigs also had more infectious virus particles than AttHRV/WT pigs from PCD 2 to PCD 4 (Fig. 7b).
Table 1.
Clinical Signs and Rotavirus Fecal Shedding in Gnotobiotic Pigs After Virulent Human Rotavirus Challenge
| Clinical signs | Fecal virus shedding (by CCIF and/or ELISA) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatments | n | % with diarrhea*,a | Mean days to onset** | Mean duration days**,b | Mean cumulative score**,c | % shedding virus* | Mean days to onset** | Mean duration days** | Mean peak titer (FFU/mL)**,d |
| AttHRV/WT | 5 | 60 (3/5)A | 5.4 (1.3e)A | 1.0 (0.4)C | 8.5 (0.5)B | 60 (3/5)A | 5.2 (1.2)A | 0.8 (0.4)B | 5 (40)C |
| AttHRV/HCKO | 3 | 100 (3/3)A | 3.0 (0.0)A | 1.3 (0.3)BC | 7.5 (1.3)B | 100 (3/3)A | 1.7 (0.3)B | 4.0 (1.2)A | 43 (66)C |
| AttHRV/HCKO/CD8− | 3 | 100 (3/3)A | 3.3 (0.7)A | 3.7 (0.9)AB | 12.0 (1.9)AB | 100 (3/3)A | 1.7 (0.7)AB | 5.3 (0.7)A | 944 (8,666)BC |
| Mock/HCKO | 3 | 100 (3/3)A | 3.0 (0.0)A | 3.3 (0.3)A | 11.2 (0.2)A | 100 (3/3)A | 2.3 (0.3)AB | 5.0 (0.0)A | 71,765 (29,956)A |
| Mock/WT | 4 | 100 (4/4)A | 2.8 (0.8)A | 3.8 (0.9)AB | 11.9 (0.8)A | 100 (4/4)A | 2.0 (0.0)B | 5.3 (0.5)A | 7,601 (2,070)AB |
Pigs with daily fecal scores of ≥2 were considered diarrheic. Fecal consistency was scored as follows: 0, normal; 1, pasty; 2, semiliquid; and 3, liquid.
For durations of diarrhea and virus shedding, if no diarrhea or virus shedding until the euthanasia day (PCD 7), the duration (days) was recorded as 0 and the onset (days) was as 8 for statistical analysis.
Mean cumulative score calculation included all the pigs in each group.
FFU, fluorescent focus-forming units. Geometric mean peak titers were calculated among pigs that shed virus.
Standard error of the mean.
Fisher's exact test or **Kruskal–Wallis rank sum test was used for comparisons. Different letters (A and B, A and BC, etc.) indicate significant differences among treatment groups (p < 0.05), whereas shared letters (AB and A, AB and B, etc.) indicate no significant difference.
AttHRV, attenuated human rotavirus; CCIF, cell culture immunofluorescence; ELISA, enzyme-linked immunosorbent assay; PCD, postchallenge day; WT, wild type.
FIG. 7.
ELISA mean OD values for rotavirus antigen (a) and infectious virus particles measured by cell culture immunofluorescence (b) in fecal samples of WT Gn pigs, HCKO Gn pigs, and CD8-depleted (CD8−) HCKO Gn pigs. Pigs were vaccinated with AttHRV or mock vaccinated and challenged with virulent human rotavirus. Rectal swabs were collected for 7 days after pigs were challenged at PID 28. Fecal samples from mock-infected pigs were used as negative controls. Fecal rotavirus antigen was measured by ELISA and results are expressed as OD units. The OD values were adjusted based on the average OD values of the negative controls from different ELISA plates. Data are presented as mean OD values/FFU/mL ± standard error of the mean (n = 3–5). Different letters on each time point indicate significant differences among groups (Kruskal–Wallis test, p < 0.05), whereas shared letters indicate no significant difference. ELISA, enzyme-linked immunosorbent assay; FFU, fluorescent focus-forming units; Gn, gnotobiotic; OD, optical density.
In the absence of B cells, vaccine-induced adaptive immune responses still provided partial protection
When comparing between AttHRV/HCKO and Mock/HCKO pigs, the vaccinated pigs had significantly shorter mean duration (1.3 vs. 3.3 days) and lower cumulative scores (7.5 vs. 11.2) of diarrhea, as well as significantly lower (1,669-fold) peak titers of virus shed than Mock/HCKO pigs (Table 1). In addition, AttHRV/HCKO pigs had lower (from PCD 2 to PCD 7) or significantly lower (from PCD 3 to PCD 5) virus shedding titers than Mock/HCKO pigs (Fig. 7a). AttHRV/HCKO pigs also had less (from PCD 2 to PCD 6) or significantly less (PCD 4 and PCD 5) infectious virus particles than Mock/HCKO pigs (Fig. 7b). The data suggest that in the absence of B cells, vaccine-induced other lymphocyte responses (T cells) are actively involved in providing partial protection.
Notably, when comparing between Mock/HCKO and Mock/WT pigs, Mock/HCKO pigs shed significantly more infectious rotavirus particles on PCD 4 than the Mock/WT pigs (Fig. 7b). However, Mock/WT pigs shed significantly more rotavirus antigen [measured by ELISA OD value] from PCD 3 to PCD 5 than Mock/HCKO pigs (Fig. 7a).
AttHRV vaccine-induced CD8 T cells played a role in shortening the duration of diarrhea and decreasing virus shedding titers
VirHRV challenge of the AttHRV/HCKO/CD8− pigs was performed at 5 days after the injection of antipig CD8 antibodies when the CD8 cells decreased to the lowest frequencies among lymphocytes. The CD8-cell depletion extended the mean duration of rotavirus diarrhea (3.7 vs. 1.3 days) and increased the fecal cumulative scores (12.0 vs. 7.5) in AttHRV/HCKO/CD8− pigs than in AttHRV/HCKO pigs (Table 1). Notably, pigs in the AttHRV/HCKO/CD8− group had almost the same mean duration of diarrhea (3.7 vs. 3.3 days) and mean cumulative score (12.0 vs. 11.2) as those in the Mock/HCKO control group (Table 1). Depletion of CD8 lymphocytes also substantially increased the peak virus shedding titers (43 vs. 944 FFU/mL) (Table 1), significantly increased viral antigen shedding on PCDs 2 and 7 (Fig. 7a), and significantly increased infectious virus particles shedding from PCD 3 to PCD 7 (Fig. 7b). The data suggest that the CD8 T-cell response induced by the AttHRV vaccine in AttHRV/HCKO pigs was largely responsible for the partial protection against diarrhea and for controlling rotavirus replication after infection.
Vaccine-induced other lymphocytes may have contributed to resolving rotavirus shedding
Compared with Mock/HCKO pigs, pigs in the AttHRV/HCKO/CD8− group had significantly lower peak virus shedding titers (944 vs. 71,765 FFU/mL) (Table 1), shed lower amount of viral antigen from PCD 2 to PCD 5 (Fig. 7a), and shed significantly lower infectious virus particles at PCD 5 (Fig. 7b). Since B cells were absent in pigs of both groups and CD8 cells were mostly depleted in AttHRV/HCKO/CD8− pigs, the results suggest that AttHRV vaccine induced other lymphocytes (mainly CD4 T cells), may have functioned in controlling virus shedding postchallenge. Interestingly, the AttHRV/HCKO/CD8− pigs shed significantly more infectious virus particles at PCD 7 than Mock/HCKO pigs (Fig. 7b).
Discussion
The total B-cell-deficient HCKO pigs offer a unique animal model to elucidate the mechanisms of rotavirus-protective immunity. In this study, we first characterized this model by immune phenotyping and then used the model to address the question “which lymphocyte population is the most critical in protection against rotavirus infection and disease?” The lack of B-cell immune responses increased the incidence of rotavirus diarrhea, demonstrating the critical role of B-cell responses in protection against rotavirus diarrhea. The lack of B cells also increased the incidence and the duration of rotavirus shedding, which is consistent with findings from the previous studies of different gene knockout mice, showing that B-cell-dependent humoral immunity was the main protective arm against rotavirus infection (11,37). However, B-cell deficiency only increased the quantities of rotavirus shedding in a relatively small degree compared with CD8 depletion, suggesting that B cells contribute to, but are not essential for, protection against rotavirus shedding. A previous study using T-cell knockout mice found the exclusive role of B-cell responses in completely resolving rotavirus infection (10).
Depletion of CD8 cells in AttHRV-vaccinated HCKO pigs increased the duration and severity of diarrhea (cumulative fecal score), confirming the critical role of CD8 T cells in protection against rotavirus diarrhea. Moreover, CD8-cell depletion significantly increased virus shedding quantities and resulted in rotavirus shedding beyond PCD 7, suggesting that CD8 T cells are required for completely resolving rotavirus infection. This is consistent with the previous observation in severely immunodeficient mice, in which adoptive transfer of immune CD8 T cells completely cleared rotavirus infection (23). Our study also found that immune CD4 T cells contributed to controlling the quantities of rotavirus shedding because AttHRV/HCKO/CD8− pigs had 76-fold lower mean peak virus shedding titers than nonvaccinated Mock/HCKO pigs. Depletion of CD8 cells led to persistent rotavirus infection and consequently significant increases in IFN-γ-producing NK-cell response in ileum and the IFN-γ-producing CD8− (pro-inflammatory) γδ T-cell response in ileum and blood postchallenge, reflecting enhanced intestinal innate immune responses to the increased viral antigen load in the AttHRV/HCKO/CD8− pigs (45). Hence both arms of the adaptive immune responses are critical in protection against rotavirus diarrhea. Effective rotavirus vaccines should include antigenic epitopes and adjuvants for stimulating and enhancing all adaptive immune response effector cells (B cells, CD4, and CD8 T cells).
The redundancy of vaccine-induced immune responses is well recognized (6). This study also found that B-cell deficiency drastically increased CD4 and CD8 T-cell population, and increased IFN-γ-producing CD4 and CD8 T-cell responses. The increased IFN-γ-producing CD4 and CD8 T-cell responses at PID 28 partially compensated for the lack of B-cell responses in protection against rotavirus challenge. Studies of B-cell-deficient mice (C57BL/6 μMT) infected with Brucella abortus also showed that the μMT mice had significantly increased percentages of CD4+ IFN-γ and CD8+ IFN-γ cells and a reduction in IL-10-producing cells compared with infected WT mice (14).
Injection of anti-CD8 antibody not only temporarily reduced the frequencies of all CD8+ cells (including CD8 T cells, NK cells, and CD8+ γδ T cells), but it also potentially affects other lymphocyte populations (cortical thymocytes and subsets of dendritic cells). Since the direct role of the latter cell populations in protective immunity against rotavirus has not been identified, their potential effects as confounders on the observed results are unknown. CD8 depletion increased the CD4 T-cell population in most lymphoid tissues of the HCKO pigs but downregulated IFN-γ-producing CD4 T-cell responses. CD4 T cells essentially shape the magnitude and quality of CD8 T-cell responses (48); however, CD8 T cells are also believed to reflectively regulate CD4 T-cell responses, although its investigation and understanding have much lagged behind those for the effect of CD4 T cells on CD8 T cells. The cytokines IFN-γ and IL-12 produced by CD8+ T cells mainly stimulate proliferation and differentiation of IFN-γ-producing Th1 CD4 T cells (49). Therefore, depletion of CD8+ cells (CD8 T cells, NK cells, etc.) will inevitably lower the production of IFN-γ and IL-12 and thus downregulate IFN-γ-producing CD4 T cells. It is interesting to note that B-cell-deficient pigs had decreased CD4+CD25− Treg-cell population in all tissues, probably because of the strong IFN-γ cytokine milieu in the HCKO pigs that downregulated Treg-cell population. The reduced IFN-γ cytokine milieu in the CD8-depleted and B-cell-deficient HCKO pigs at PID 28 led to the increased Treg-cell responses and the recovering of the Treg-cell population. The role of Treg cells in downregulating rotavirus-protective immunity has been recognized. As it was reported previously, rotavirus vaccine-induced protective immune responses were associated with reduced number of tissue-residing Treg cells while the vaccine promotes the development of IFN-γ-producing effector T-cell responses (47).
In this study, we had low number of HCKO pigs in some treatment groups because using SCNT and cloning technology to generate genetically modified pigs, such as the HCKO pigs, can be challenging at times. These processes are labor intensive and inefficient. In addition, pigs generated through SCNT often show developmental defects, which is the reason for the high mortality rate at birth observed in HCKO pigs in this study (data not shown) and other studies with genetically modified pigs using SCNT. However, in recent years, the explosive development in porcine gene-editing technologies based on meganucleases (Zinc Finger Nucleases, Tal effector nucleases, CRISPR/Cas9) has provided new tools that may help to get around the need for SCNT for developing genetically modified pigs with a much higher efficiency at lower costs (16,28,44). Genetically modified pigs are becoming more readily available for the study of mechanisms of protective immunity against human viral diseases. In addition to using the B-cell-deficient HCKO Gn pigs, future studies using other genetically modified pigs, such as IgA-deficient, IgG-deficient, CD8-deficient (CD8α−/−), αβ T-cell-deficient (αβ TCR−/−), γδ T-cell-deficient (γδ TLR−/−), αβ and γδ T-cell-deficient (αβ/γδ TLR−/−), and SCID pig (Rag-1−/− and Rag-2−/−), will greatly further advance the understanding of mechanisms of rotavirus-protective immunity and the field of viral immunity research in general. In the past, some of these gene knockout mouse models have been used in previous studies of protective immunity against rotavirus infections (3,8,11,20,37); however, the determinates of rotavirus protection immunity against rotavirus diarrhea in human infants still have not been fully resolved.
Compared with mice and other animals, pigs are the closest relatives to humans (except for nonhuman primates). During thousands of years of domestication, they have developed many similarities to humans. For example, they have developed digestion and absorption systems more similar to humans than to other members in the Artiodactyla clade. They share many diseases with humans and are susceptible to many of the same infectious viral and bacterial pathogens, including rotavirus gastroenteritis. The findings from pig models of human diseases are more relevant for the development of therapeutics, vaccines, and antivirals for humans (5,15,21,34,38–40). The findings from this study provide new knowledge for the understanding of rotavirus vaccine-induced protective immunity.
Supplementary Material
Acknowledgments
This work was supported by a grant (5R01AT004789-04) to L.Y. from the National Center of Complementary and Alternative Medicine, National Institutes of Health, Bethesda, MD. We thank Kimberly Allen and Karen Hall for animal care and Dr. Kevin Pelzer for veterinary service. The attenuated and virulent strains of HRV were from Dr. Linda J. Saif, The Ohio State University.
Author Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Akkina R. New generation humanized mice for virus research: comparative aspects and future prospects. Virology 2013;435:14–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azevedo MS, Yuan L, Jeong KI, et al. Viremia and nasal and rectal shedding of rotavirus in gnotobiotic pigs inoculated with Wa human rotavirus. J Virol 2005;79:5428–5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blutt SE, Miller AD, Salmon SL, Metzger DW, and Conner ME. IgA is important for clearance and critical for protection from rotavirus infection. Mucosal Immunol 2012;5:712–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blutt SE, Warfield KL, Estes MK, and Conner ME. Differential requirements for T cells in viruslike particle- and rotavirus-induced protective immunity. J Virol 2008;82:3135–3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler JE, Lager KM, Splichal I, et al. The piglet as a model for B cell and immune system development. Vet Immunol Immunopathol 2009;128:147–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadevall A, and Pirofski LA. Exploiting the redundancy in the immune system: vaccines can mediate protection by eliciting ‘unnatural’ immunity. J Exp Med 2003;197:1401–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai Y, Vaught TD, Boone J, et al. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol 2002;20:251–255 [DOI] [PubMed] [Google Scholar]
- 8.Dharakul T, Labbe M, Cohen J, et al. Immunization with baculovirus-expressed recombinant rotavirus proteins VP1, VP4, VP6, and VP7 induces CD8+ T lymphocytes that mediate clearance of chronic rotavirus infection in SCID mice. J Virol 1991;65:5928–5932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn CS, Mehtali M, Houdebine LM, Gut JP, Kirn A, and Aubertin AM. Human immunodeficiency virus type 1 infection of human CD4-transgenic rabbits. J Gen Virol 1995;76(Pt 6):1327–1336 [DOI] [PubMed] [Google Scholar]
- 10.Franco MA, and Greenberg HB. Immunity to rotavirus in T cell deficient mice. Virology 1997;238:169–179 [DOI] [PubMed] [Google Scholar]
- 11.Franco MA, and Greenberg HB. Immunity to rotavirus infection in mice. J Infect Dis 1999;179 Suppl 3:S466–S469 [DOI] [PubMed] [Google Scholar]
- 12.Franco MA, and Greenberg HB. Immunity to homologous rotavirus infection in adult mice. Trends Microbiol 2000;8:50–52 [DOI] [PubMed] [Google Scholar]
- 13.Gerner W, Kaser T, and Saalmuller A. Porcine T lymphocytes and NK cells—an update. Dev Comp Immunol 2009;33:310–320 [DOI] [PubMed] [Google Scholar]
- 14.Goenka R, Parent MA, Elzer PH, and Baldwin CL. B cell-deficient mice display markedly enhanced resistance to the intracellular bacterium Brucella abortus. J Infect Dis 2011;203:1136–1146 [DOI] [PubMed] [Google Scholar]
- 15.Kim YB. Gnotobiotic miniature swine for immunobiology and medicine. Microb Ecol Helath Dis 2007;19:251–252 [Google Scholar]
- 16.Lee K, Kwon DN, Ezashi T, et al. Engraftment of human iPS cells and allogeneic porcine cells into pigs with inactivated RAG2 and accompanying severe combined immunodeficiency. Proc Natl Acad Sci U S A 2014;111:7260–7265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L, Li Q, Bao Y, et al. RNAi-based inhibition of porcine reproductive and respiratory syndrome virus replication in transgenic pigs. J Biotechnol 2014;171:17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu F, Wen K, Li G, et al. Dual functions of Lactobacillus acidophilus NCFM as protection against rotavirus diarrhea. J Pediatr Gastroenterol Nutr 2014;58:169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo Y, Lin L, Bolund L, Jensen TG, and Sørensen CB. Genetically modified pigs for biomedical research. J Inherit Metab Dis 2012;35:695–713 [DOI] [PubMed] [Google Scholar]
- 20.McNeal MM, VanCott JL, Choi AH, et al. CD4 T cells are the only lymphocytes needed to protect mice against rotavirus shedding after intranasal immunization with a chimeric VP6 protein and the adjuvant LT(R192G). J Virol 2002;76:560–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meurens F, Summerfield A, Nauwynck H, Saif L, and Gerdts V. The pig: a model for human infectious diseases. Trends Microbiol 2012;20:50–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer RC, Bohl EH, and Kohler EM. Procurement and maintenance of germ-free seine for microbiological investigations. Appl Microbiol 1964;12:295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Offit PA, and Dudzik KI. Rotavirus-specific cytotoxic T lymphocytes passively protect against gastroenteritis in suckling mice. J Virol 1990;64:6325–6328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramsoondar J, Vaught T, Ball S, et al. Production of transgenic pigs that express porcine endogenous retrovirus small interfering RNAs. Xenotransplantation 2009;16:164–180 [DOI] [PubMed] [Google Scholar]
- 25.Reid W, Sadowska M, Denaro F, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A 2001;98:9271–9276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren RB, Costantini F, Gorgacz EJ, Lee JJ, and Racaniello VR. Transgenic mice expressing a human poliovirus receptor: a new model for poliomyelitis. Cell 1990;63:353–362 [DOI] [PubMed] [Google Scholar]
- 27.Saif L, Yuan L, Ward L, and To T. Comparative studies of the pathogenesis, antibody immune responses, and homologous protection to porcine and human rotaviruses in gnotobiotic piglets. 1st International Rushmore Conference on Mechanisms in the Pathogenesis of Enteric Diseases Rapid City, SD: Plenum Press Div Plenum Publishing Corp., 1995 [Google Scholar]
- 28.Sato M, Miyoshi K, Nagao Y, et al. The combinational use of CRISPR/Cas9-based gene editing and targeted toxin technology enables efficient biallelic knockout of the alpha-1,3-galactosyltransferase gene in porcine embryonic fibroblasts. Xenotransplantation 2014;21:291–300 [DOI] [PubMed] [Google Scholar]
- 29.Scott BB, Velho TA, Sim S, and Lois C. Applications of avian transgenesis. ILAR J 2010;51:353–361 [DOI] [PubMed] [Google Scholar]
- 30.Sellin CI, and Horvat B. Current animal models: transgenic animal models for the study of measles pathogenesis. Curr Top Microbiol Immunol 2009;330:111–127 [DOI] [PubMed] [Google Scholar]
- 31.Sinkora M, and Butler JE. The ontogeny of the porcine immune system. Dev Comp Immunol 2009;33:273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stepanova H, Pavlova B, Stromerova N, et al. Cell-mediated immune response in swine infected with Mycobacterium avium subsp. avium. Vet Immunol Immunopathol 2011;142:107–112 [DOI] [PubMed] [Google Scholar]
- 33.Suzuki T, Sundt TM, 3rd, Mixon A, and Sachs DH. In vivo treatment with antiporcine T cell antibodies. Transplantation 1990;50:76–81 [DOI] [PubMed] [Google Scholar]
- 34.Swindle MM, Makin A, Herron AJ, Clubb FJ, Jr., and Frazier KS. Swine as models in biomedical research and toxicology testing. Vet Pathol 2012;49:344–356 [DOI] [PubMed] [Google Scholar]
- 35.Taube S, Kolawole AO, Hohne M, et al. A mouse model for human norovirus. mBio 2013;4:e00450-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tesson L, Cozzi J, Ménoret S, et al. Transgenic modifications of the rat genome. Transgenic Res 2005;14:531–546 [DOI] [PubMed] [Google Scholar]
- 37.VanCott JL, McNeal MM, Flint J, Bailey SA, Choi AH, and Ward RL. Role for T cell-independent B cell activity in the resolution of primary rotavirus infection in mice. Eur J Immunol 2001;31:3380–3387 [DOI] [PubMed] [Google Scholar]
- 38.Verma N, Rettenmeier AW, and Schmitz-Spanke S. Recent advances in the use of Sus scrofa (pig) as a model system for proteomic studies. Proteomics 2011;11:776–793 [DOI] [PubMed] [Google Scholar]
- 39.Vodicka P, Smetana K Jr., et al. The miniature pig as an animal model in biomedical research. Ann N Y Acad Sci 2005;1049:161–171 [DOI] [PubMed] [Google Scholar]
- 40.Walters EM, and Prather RS. Advancing swine models for human health and diseases. Mo Med 2013;110:212–215 [PMC free article] [PubMed] [Google Scholar]
- 41.Ward LA, Rosen BI, Yuan L, and Saif LJ. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol 1996;77(Pt 7):1431–1441 [DOI] [PubMed] [Google Scholar]
- 42.Ward RL. Possible mechanisms of protection elicited by candidate rotavirus vaccines as determined with the adult mouse model. Viral Immunol 2003;16:17–24 [DOI] [PubMed] [Google Scholar]
- 43.Ward RL, McNeal MM, and Sheridan JF. Development of an adult mouse model for studies on protection against rotavirus. J Virol 1990;64:5070–5075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei C, Liu J, Yu Z, Zhang B, Gao G, and Jiao R. TALEN or Cas9- rapid, efficient and specific choices for genome modifications. J Genet Genomics 2013;40:281–289 [DOI] [PubMed] [Google Scholar]
- 45.Wen K, Bui T, Li G, et al. Characterization of immune modulating functions of gamma delta T cell subsets in a gnotobiotic pig model of human rotavirus infection. Comp Immunol Microbiol Infect Dis 2012;35:289–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wen K, Li G, Bui T, et al. High dose and low dose Lactobacillus acidophilus exerted differential immune modulating effects on T cell immune responses induced by an oral human rotavirus vaccine in gnotobiotic pigs. Vaccine 2012;30:1198–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wen K, Li G, Yang X, et al. CD4+ CD25- FoxP3+ regulatory cells are the predominant responding regulatory T cells after human rotavirus infection or vaccination in gnotobiotic pigs. Immunology 2012;137:160–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiesel M, and Oxenius A. From crucial to negligible: functional CD8(+) T-cell responses and their dependence on CD4(+) T-cell help. Eur J Immunol 2012;42:1080–1088 [DOI] [PubMed] [Google Scholar]
- 49.Yamane H, and Paul WE. Early signaling events that underlie fate decisions of naive CD4(+) T cells toward distinct T-helper cell subsets. Immunol Rev 2013;252:12–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan L, and Saif LJ. Induction of mucosal immune responses and protection against enteric viruses: rotavirus infection of gnotobiotic pigs as a model. Vet Immunol Immunopathol 2002;87:147–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan L, Ward LA, Rosen BI, To TL, and Saif LJ. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol 1996;70:3075–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan L, Wen K, Azevedo MS, Gonzalez AM, Zhang W, and Saif LJ. Virus-specific intestinal IFN-gamma producing T cell responses induced by human rotavirus infection and vaccines are correlated with protection against rotavirus diarrhea in gnotobiotic pigs. Vaccine 2008;26:3322–3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.