Abstract
Background. Subjects on suppressive combination antiretroviral therapy (cART) who do not achieve robust reconstitution of CD4+ T cells face higher risk of complications and death. We studied participants in the Women's Interagency HIV Study with good (immunological responder [IR]) or poor (immunological nonresponder [INR]) CD4+ T-cell recovery after suppressive cART (n = 50 per group) to determine whether cytokine levels or low-level viral load correlated with INR status.
Methods. A baseline sample prior to viral control and 2 subsequent samples 1 and 2 years after viral control were tested. Serum levels of 30 cytokines were measured at each time point, and low-level human immunodeficiency virus (HIV) viral load and anti-HIV antibody levels were measured 2 years after viral suppression.
Results. There were minimal differences in cytokine levels between IR and INR subjects. At baseline, macrophage inflammatory protein-3β levels were higher in IR subjects; after 1 year of suppressive cART, soluble vascular endothelial growth factor-R3 levels were higher in IR subjects; and after 2 years of suppressive cART, interferon gamma-induced protein 10 levels were higher in INR subjects. Very low-level HIV viral load and anti-HIV antibody levels did not differ between IR and INR subjects.
Conclusions. These results imply that targeting residual viral replication might not be the optimum therapeutic approach for INR subjects.
Keywords: CD4+ T cells, chemokines, cytokines, cART, HIV
Combination antiretroviral therapy (cART) has transformed human immunodeficiency virus (HIV) from a nearly universally fatal infection to a manageable chronic disease for most of those with access to therapy. Recent estimates of life expectancy for subjects infected with HIV and on cART range from as few as 1 to 8 years of life lost, with the majority of deaths occurring due to non-acquired immune deficiency syndrome (AIDS)-related causes [1]. Patients with suppression of viral replication and CD4+ T-cell counts above 350 cells/µL have normal projected lifespan, whereas those with suboptimal CD4+ T-cell recovery will face considerably shorter life expectancy [2]. Subjects infected with HIV who achieve virologic suppression on cART but have incomplete reconstitution of CD4+ T-cell counts are termed immunological nonresponders (INRs), and they have an approximately 10-fold increased risk of an AIDS-defining event or death compared with immunological responders (IRs) [3]. The INR phenotype has been noted in the latest US Health and Human Services HIV/AIDS treatment guidelines, and due to lack of understanding of the causes and no known cure, no effective treatment or monitoring is available or recommended for those at higher risk of death due to poor CD4+ T-cell recovery [4].
Why some subjects have an incomplete immunological recovery is not entirely understood, and it is clearly important because INR subjects face an increased risk of death in spite of what appears to be effective cART. Risk factors for inadequate CD4+ T-cell reconstitution include older age [5–8], lower nadir CD4+ T-cell count [7, 8], more than 1 year with CD4+ T-cell count <200 before antiretroviral therapy (ART) initiation [5], and longer time from ART initiation to suppression of viral replication [7]. It has long been appreciated that recovery of T-cell counts is not directly linked to suppression of HIV replication, because some subjects with continued viral replication experience robust CD4+ T-cell count gains [9–11] and others with complete suppression by commercially available viral load assays have incomplete or blunted recovery of CD4+ T-cell counts [10]. Some, but not all, studies find that the degree of CD4+ T-cell increase correlates with the degree of viral replication in those with residual viral replication [11, 12]. In those with viral suppression, the presence of viral blips between periods of complete suppression does not predict CD4+ T-cell increase [13].
Potential explanations for the INR phenotype include (1) thymic involution and accelerated immune senescence with an inability to generate new CD4+ T cells and (2) increased immune activation that contributes to immune exhaustion. Consistent with lower nadir CD4+ T-cell count predicting slower recovery, the number of naive CD4+ T cells at cART initiation and after 1 year or more of therapy correlates with the rate of CD4+ T-cell recovery [14–16]. Thymic-derived naive T cells are also low in INR subjects, implying that decreased thymic production of cells is part of the reason for inadequate CD4+ T-cell recovery in these patients [15, 17]. In the current study, we examined whether soluble markers of inflammation would predict the INR phenotype in subjects in the first 2 years of suppressive cART. A panel of 30 soluble markers of inflammation was tested longitudinally before and after suppression of viral replication by cART in 50 IR and 50 INR subjects.
METHODS
Study Participants and Sample Selection
Subjects were participants in the Women's Interagency HIV Study (WIHS), an ongoing multisite cohort study of HIV among US women, which enrolled participants in 1994–1995 and 2001–2002 [18, 19], and all subjects were enrolled with informed consent. Semiannual visits include interview, clinical exam, and collection of biologic specimens. Hepatitis C virus (HCV) serology was performed at study entry, and HCV plasma ribonucleic acid (RNA) quantitation was performed on seropositive women to determine whether infection was ongoing versus resolved. Participants for the current study were chosen from 2961 HIV seroprevalent women in the WIHS cohort who initiated cART. Immunological nonresponder phenotype periods were defined as those with HIV viral load suppressed to below 80 copies/mL and CD4+ T-cell count <500 (the approximate lower limit of the reference range for HIV-uninfected females [20, 21]) for at least 2 years while on suppressive cART. In total, 91 women met the criteria, and the 50 with lowest CD4+ T-cell count slopes were selected as the cases. Immunological responder phenotype periods were defined as those with HIV viral load suppressed to below 80 copies/mL for at least 2 years while on cART and CD4+ T-cell count ≥500 at the end of the period. In total, 231 women met the criteria, and 150 women with highest CD4+ T-cell count slopes were selected as the potential pool to match the 50 selected cases, and the 50 best-matched IR subjects were selected for study. Immunological responder women were matched to INR women based on ethnicity (African-American vs other), age, AIDS status, CD4 T cell, nadir CD4 T cell, and HIV viral load at index visit, HCV antibody status at WIHS study entry, and time of follow up in the cohort (within 1 year), using propensity score. Index visit was defined as the visit with detectable HIV viral load and nadir CD4 T cell <500, prior to the phenotype period. Three serum samples for each subject were tested for cytokine levels, with the samples chosen at the visit prior to phenotype period and at 1 and 2 years on phenotype (Figure 1). In addition, low-level viral load quantitation was performed at the 2-year on phenotype time point. Because this required at least 2 mL plasma, for some subjects the visit before or after the 2-year time point was selected based on available sample volume.
Figure 1.
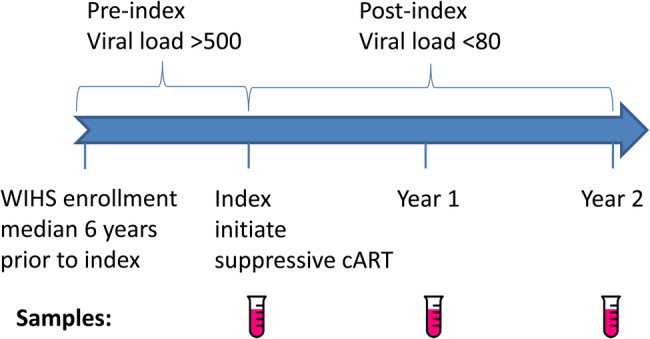
Schematic of study design and sample collection. Abbreviations: cART, combination antiretroviral therapy; WIHS, Women's Interagency HIV Study.
Multiplex Cytokine and Chemokine Analysis
Serum samples were assayed using the MilliPlex kits (Millipore) for the proinflammatory cytokines interleukin [IL]-6, IL-17, and tumor necrosis factor (TNF)-α; the chemoattractants interferon gamma-induced protein 10 ([IP-10] CXCL10), monocyte chemotactic protein (MCP)-1, macrophage-derived chemokine (MDC), macrophage inflammatory protein (MIP)-1α, MIP-1δ, MIP-3β, fractalkine, growth-related oncogene ([GRO] CXCL1), 6Ckine (CCL21), B-cell–activating chemokine (BCA)-1 (CXCL13), cutaneous T-cell-attracting chemokine ([CTACK] CCL27), eotaxin-2, stromal cell-derived factor (SDF)-1, hemofiltrate CC chemokine (HCC-1; CCL14), interferon-inducible T-cell alpha chemoattractant ([I-TAC] CXCL11), monokine induced by interferon ([MIG] CXCL9), and lymphotactin; the anti-inflammatory factors IL-10 and TNF-related apoptosis-inducing ligand (TRAIL); the soluble receptors soluble glycoprotein 130 (sgp130), sIL-1R1, sIL-1RII, sTNFRII, soluble vascular endothelial growth factor (sVEGFRII), and sVEGFRIII; and the growth factors IL-21 and fibroblast growth factor (FGF)-2, following the manufacturer's protocols. Standard curves were run in duplicate, and samples were tested in duplicate. Samples were tested on a Labscan 200 analyzer (Luminex) using Bio-Plex manager 4.1 software (Bio-Rad).
Low-Level Viral Load Testing
Samples from the 2-year post-cART time point were retested to quantify low-level HIV viral load, below the limits of detection of standard commercial assays. The Aptima HIV-1 Quant Dx (Aptima) assay is an in vitro nucleic acid amplified test for the detection and quantitation of HIV type 1 (HIV-1) RNA on the fully automated Panther system (Hologic) [22, 23]. The assay uses 3 main steps, which all take place in a single tube: target capture, target amplification by transcription-mediated amplification, and detection of the amplification products (amplicon) by fluorescent-labeled probes (torches). Detection is achieved using the single-stranded nucleic acid torches that are present during the amplification of the target and hybridize specifically to the amplicon as it is generated in real time. Each reaction has an internal calibrator/internal control (IC) that controls for variations in specimen processing, amplification, and detection. The concentration of a sample is determined by the Panther system software using the HIV-1 and IC signals for each reaction and comparing them to calibration information. The lower limit of quantitation (LLOQ) is 30 copies/mL, and the limit of detection (LOD) is 13 copies/mL [23]. For values below the LLOQ we assigned a value of 21 copies/mL (halfway between 13 and 29), and for values below the LOD we assigned a value of 6 copies/mL (halfway between 0 and 12).
Less-Sensitive Antibody Testing
The less-sensitive Vitros (LS-Vitros) assay is based on the VITROS ECi/ECiQ Immunodiagnostic System—a chemiluminescence assay that gives a quantitative measure of HIV antibodies (Ortho-Clinical Diagnostics). As described previously, samples were diluted 1:400 in Ortho diluent buffer B before placing the sample on the diagnostic system [24]. In 50 minutes, results were reported as signal to cutoff.
Statistical Analysis
Cytokine levels were compared between clinical groups using one-way analysis of variance (ANOVA) and Tukey's honestly significant difference tests. Differences in subject characteristics between groups were evaluated by ANOVA for continuous variables and by Fisher's exact test for ongoing HCV infection. Cytokine and viral load values were log-transformed prior to analysis due to nonnormal distribution of the data. P values were adjusted into false discovery rates (FDR) by the Benjamini-Hochberg controlling procedure, a commonly used method for analysis of large sets of biological data [25]. Statistical significance was defined as P < .05 and FDR < 0.1. R/Bioconductor software was utilized for statistical analyses.
RESULTS
Participant Characteristics
Fifty subjects each from the INR and IR groups were included. The median age was 34- and 35-years-old in the 2 groups, respectively (Table 1), and a significant minority of the subjects was African American (38%). The median viral load was 10 000 RNA copies/mL at index in both groups, in spite of significant prior ART exposure in both groups (74% and 96% in IR and INR groups, respectively; P = .004). The total median duration of prior ART exposure was 4 and 2.75 years in the INR and IR groups, respectively (Table 1). The median CD4+ T-cell count prior to suppressive cART for the 2 groups was 200 and 227, respectively, and the nadir CD4+ T-cell count was 132 in the INR group and 167 in the IR group (P = .1). The proportion of women who were infected with HCV or who had detectable HCV viremia at baseline also did not differ between the INR and IR groups. Overall, there were no significant differences in the subject characteristics on which they were matched.
Table 1.
Demographic and Clinical Characteristics in 100 Included Womena
| Characteristic | INR | IR | P Value |
|---|---|---|---|
| Age (years) | 34 (30–42) | 35 (31–37) | 0.6 |
| Race (%Black) | 38% | 38% | 1 |
| HCV antibody+ | 13 (27%) | 11 (22%) | 0.8 |
| HCV RNA+ | 10 (21%) | 11 (22%) | 1 |
| AIDS at index visit | 9 (18%) | 6 (12%) | 0.4 |
| Pre-index ART (years) | 4 (2–7.1) | 2.75 (0–5.5) | 0.08 |
| CD4 count at index (cells/µL) | 200 (124–265) | 227 (158–266) | 0.4 |
| Nadir CD4 count (cells/µL) | 132 (75–91) | 167 (100–233) | 0.1 |
| Viral load at index (log RNA copies/mL) | 4.2 (3.1–4.6) | 4.6 (3.8–5.0) | 0.07 |
Abbreviations: AIDS, acquired immune deficiency syndrome; ART, antiretroviral therapy; HCV, hepatitis C virus; INR, immunological nonresponder; IQR, interquartile range; IR, immunological responder; RNA, ribonucleic acid.
a Median and IQR values are shown, except race, which is shown as percentage; HCV RNA+ = HCV RNA positive at study entry. Hepatitis C virus antibody data were missing for 2 INR subjects and 1 IR subject. Hepatitis C virus RNA testing was performed on subjects with detectable HCV antibody, and RNA data were missing for 1 INR subject.
Virological and Immunological Responses to Combination Antiretroviral Therapy
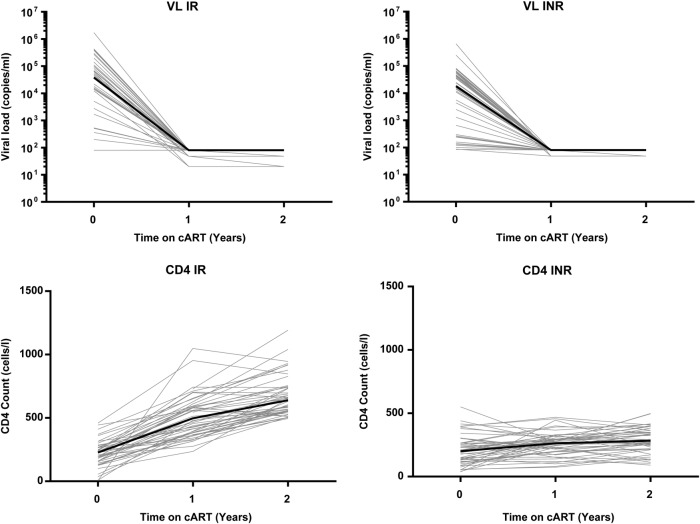
Both the INR and IR groups showed a good virological response to cART, with undetectable levels of viral load at 1 and 2 years after therapy initiation (Figure 2, top panels). There was no difference in the level of viremia detected by commercial assays at any of the time points. CD4+ T-cell counts were higher after 1 and 2 years of therapy in the IR than INR group (640 vs 283, P < .001; Figure 2, bottom panels). The longitudinal study design allowed comparison of cytokine levels within a given individual before and after suppressive cART initiation, which has more power to detect effects of cART than a previous cross-sectional study we performed [26]. Within 1 year of cART initiation levels of TNF-α, IP-10, MIP-3β, BCA-1, I-TAC, MIG, and TRAIL, all fell significantly, and these decreases were maintained at 2 years (Table 2). It is interesting to note that the level of a number of analytes increased after cART was started, including IL-15, FGF-2, sTNFRI, sIL-1RII, sgp130, SDF-1, and eotaxin-2. Of these analytes, IL-15 had previously been compared cross-sectionally between subjects with uncontrolled HIV replication and HIV-seronegative controls, and IL-15 levels were found to be low in the HIV-infected subjects [26]. For at least this analyte, it seems that its increase after cART initiation represents a partial normalization of the systemic cytokine profile. Likewise, TNF-α and IP-10 were previously found to be elevated in the setting of uncontrolled HIV replication, and their decrease with cART represents a move toward levels found in HIV-uninfected subjects [26].
Figure 2.
Human immunodeficiency virus (HIV) viral load (VL) and CD4+ T-cell count after combination antiretroviral therapy (cART) initiation. Baseline and subsequent HIV viral load measurements are shown for each individual in light gray lines (top panels). The bold line shows the median viral load. Baseline and subsequent CD4+ T-cell counts are shown for each individual in light gray lines (bottom panels). The bold line shows the median CD4+ T-cell count. Abbreviations: INR, immunological nonresponder; IR, immunological responder.
Table 2.
Effect of cART on Cytokine Levelsa
| Cytokine | pre-cART |
1 y cART |
2 y cART |
|||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | |
| Proinflammatory | ||||||
| IL-6 | 2.1 | 1.1–4.4 | 2.0 | 1.0–4.2 | 2.7 | 1.2–5.9 |
| IL-17 | 1.7 | 1.6–6.8 | 2.1 | 1.6–10.5 | 2.0 | 1.6–9.9 |
| TNF-α | 15.6 | 10.4–21.1 | 11.0 | 7.2–15.5 | 10.8 | 8.4–15.5 |
| Chemoattractants | ||||||
| IP-10 | 1120 | 793–1640 | 478 | 329–785 | 461 | 302–658 |
| MCP-1 | 562 | 393–850 | 539 | 338–742 | 554 | 431–849 |
| MDC | 1350 | 1060–1750 | 1490 | 1120–1910 | 1660 | 1170–2000 |
| MIP-1α | 9.8 | 9.8–10.8 | 9.8 | 9.8–15.9 | 9.8 | 9.8–18.5 |
| MIP-1δ | 2660 | 1740–3620 | 2870 | 1880–3760 | 2920 | 2030–4270 |
| MIP-3β | 199 | 159–266 | 114 | 85.6–153 | 112 | 84.1–154 |
| Fractalkine | 905 | 590–1650 | 887 | 576–1750 | 951 | 644–1670 |
| GRO | 29.7 | 8.8–54.8 | 32.3 | 16.4–67.1 | 37.2 | 10.9–57.3 |
| 6Ckine | 343 | 244–523 | 348 | 244–513 | 365 | 256–544 |
| BCA-1 | 95.5 | 56.3–150 | 39.5 | 25.8–62.6 | 36.5 | 24.1–55.5 |
| CTACK | 983 | 677–1230 | 1060 | 760–1240 | 1040 | 730–1320 |
| Eotaxin-2 | 398 | 214–810 | 647 | 368–1060 | 619 | 402–1160 |
| SDF1 | 4680 | 3620–5680 | 5080 | 4110–6140 | 5070 | 4130–6090 |
| HCC-1 | 2820 | 2190–3870 | 2860 | 2160–4290 | 3150 | 2120–4610 |
| I-TAC | 339 | 178–555 | 171 | 93.1–316 | 151 | 87.4–274 |
| MIG | 3680 | 1990–5510 | 1100 | 758–1580 | 966 | 653–1520 |
| Lymphotactin | 87.5 | 46.8–118 | 88.0 | 61.9–142 | 93.3 | 60.1–127 |
| Anti-inflammatory/Th2 | ||||||
| IL-10 | 18.6 | 8.8–36.5 | 12.3 | 5.6–24.3 | 9.6 | 5.3–19.4 |
| TRAIL | 73.9 | 53.8–95.9 | 52.4 | 39.1–74.1 | 52.2 | 36.3–75.9 |
| Soluble receptors | ||||||
| sgp130 | 170k | 141k–200k | 200k | 168k–200k | 200k | 174k–200k |
| sIL-1RI | 29.2 | 23.0–38.1 | 30.7 | 23.1–37.5 | 29.8 | 22.7–39.5 |
| sIL-1RII | 4370 | 3440–6670 | 7540 | 5630–8810 | 7580 | 5270–9590 |
| sTNFRII | 10 900 | 8200–12 700 | 7810 | 6140–10 800 | 7880 | 6220–10 800 |
| sVEGFRII | 20 200 | 16 100–25 100 | 18 900 | 15 400–22 400 | 19 000 | 16 000–23 900 |
| sVEGFRIII | 1550 | 818–2870 | 1820 | 932–3460 | 1820 | 1030–3290 |
| Growth factors | ||||||
| IL-21 | 9.8 | 9.8–9.8 | 9.8 | 9.8–9.8 | 9.8 | 9.8–9.8 |
| FGF-2 | 27.2 | 16.7–42.7 | 31.3 | 16.7–53.6 | 28.9 | 16.6–53.7 |
Abbreviations: BCA, B-cell–activating chemokine; cART, combination antiretroviral therapy; CTACK, cutaneous T-cell-attracting chemokine; FDR, false discovery rates; FGF, fibroblast growth factor; gp, glycoprotein; GRO, growth-related oncogene; HCC, hemofiltrate CC chemokine; IL, interleukin; IP-10, interferon gamma-induced protein 10; IQR, interquartile range; I-TAC, interferon-inducible T-cell alpha chemoattractant; MCP, monocyte chemotactic protein; MDC, macrophage-derived chemokine; MIG, monokine induced by interferon; MIP, macrophage inflammatory protein; s, soluble; SDF, stromal cell-derived factor; TNF, tumor necrosis factor; TNFR, TNF receptor; TRAIL, TNF-related apoptosis-inducing ligand; VEGFR, vascular endothelial growth factor receptor.
a Bold numbers denote values different from presuppressive cART levels ( P < .05, FDR < 0.1).
Systemic Immune Profile of Subjects With Incomplete Immune Recovery
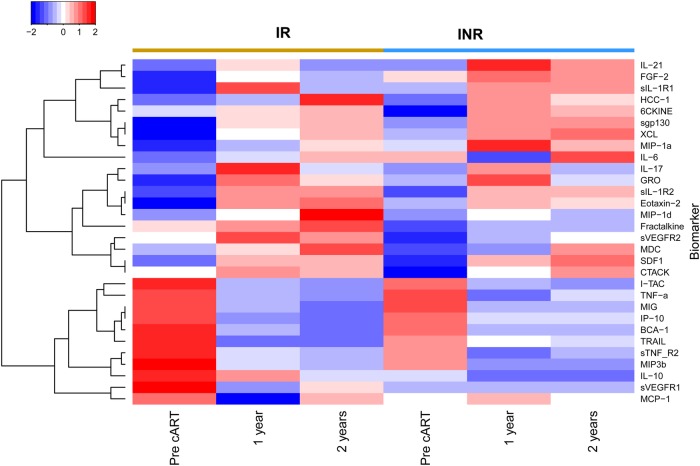
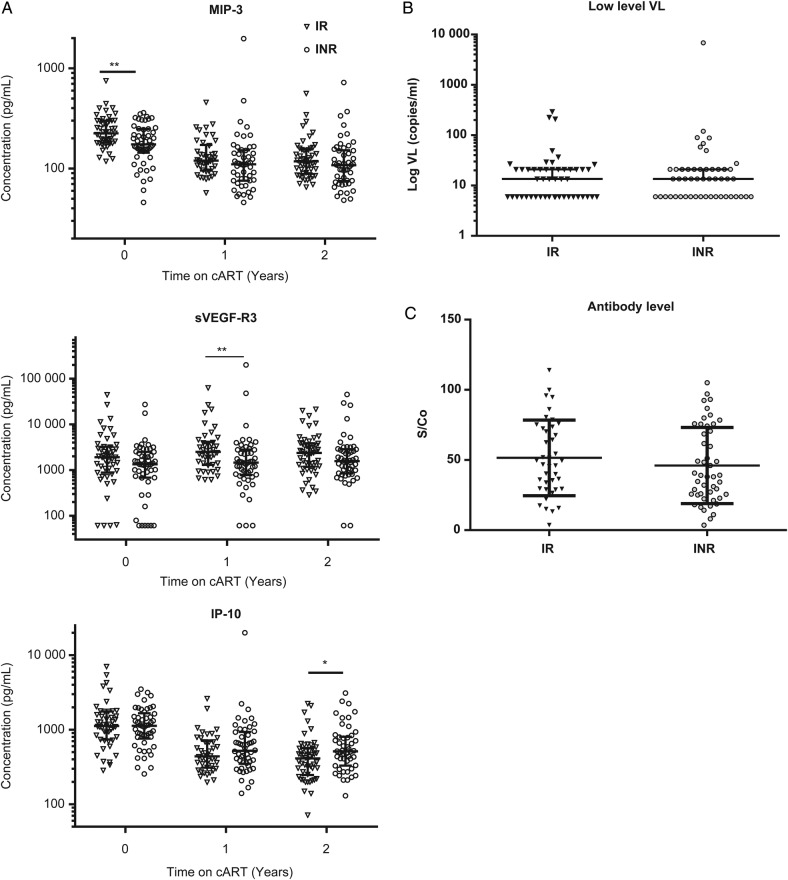
We hypothesized that subjects who had poor CD4+ T-cell recovery in spite of complete virological suppression measured by commercial assays would have a more pronounced proinflammatory profile. To determine whether cART was more effective at normalizing the systemic cytokine profile in IR than INR subjects, these groups were compared. As can be seen from a heat map (Figure 3), and consistent with the data in Table 2, 1 set of analytes showed increasing levels after initiation of cART (top portion of Figure 3), whereas a second set showing decreasing levels after cART clustered together (bottom portion of Figure 3). Of note, the trends for each of the analytes were largely similar between the IR and INR groups. Only 3 analytes showed significant differences between IR and INR subjects at any time point (P < .05, FDR < 0.1). At baseline, MIP-3β was higher in IR than INR subjects (Figure 4A, top panel). One year after cART, sVEGF-R3 levels were higher in IRs, and this change was no longer significant after 2 years of therapy (Figure 4A, middle panel). Finally, IP-10 levels were lower in IRs 2 years after cART was begun (Figure 4A, bottom panel). Overall, cytokine profiles after the initiation of cART were remarkably similar between the IR and INR groups, contrary to our hypothesis.
Figure 3.
Cytokine levels in immunological responder (IR) and immunological nonresponder (INR) subjects before and after combination antiretroviral therapy (cART). Subjects are grouped in columns according to clinical group, with time points displayed below. Cytokine values are shown as the mean log10 value of all subjects within a given group at each time point. Cytokines were clustered in an unsupervised fashion, with 2 main branches forming, analytes with levels that rose after suppressive cART, and below a group of analytes whose levels fell after suppressive cART.
Figure 4.
Comparison of individual cytokines and low-level viral loads (VL) by clinical group. (A) Individual data points over time are shown for each of the cytokines that showed a significant difference between immunological responder (IR) and immunological nonresponder (INR) groups. (B) Samples from the 2-year postsuppressive combination antiretroviral therapy (cART) initiation were retested using a transcription-mediated amplification (TMA) assay for human immunodeficiency virus (HIV) ribonucleic acid (RNA). Results are expressed as log10 viral load based on comparison of 2 replicate TMA results to a standard curve (see Methods). (C) Samples from the 2-year postsuppressive cART initiation were tested using a less-sensitive HIV antibody assay, with results reported as signal to cutoff (S/Co). Horizontal line signifies median, and error bars represent the interquartile range. Abbreviations: IP-10, interferon gamma-induced protein 10; MIP, macrophage inflammatory protein; sVEGFR, vascular endothelial growth factor.
The finding of higher IP-10 levels in INR subjects was of interest, because this cytokine has been shown to correlate with viral load in antiretroviral (ARV)-naive subjects, and importantly it was the only analyte of 32 soluble markers studied to show significant negative correlation with CD4+ T-cell count in a prior study [26]. These findings led us to test whether the higher IP-10 level in INR subjects indicated low-level viral replication taking place in this population that was not detected using commercial HIV RNA detection assays. Accordingly, samples from the 2-year time point were tested using the Aptima assay, with a limit of detection of 13 RNA copies/mL. Most of the samples showed virus load <100 RNA copies/mL, and there was no difference between virus load in the IR and INR groups. Furthermore, in contrast to our findings of a correlation between IP-10 levels in ARV-naive subjects [26], there was no correlation between IP-10 levels and low-level viral load (Figure 4B). In addition to directly measuring virus, examination of antiviral immune responses can provide a sensitive measure of low-level virus replication [27, 28]. Using a less-sensitive anti-HIV antibody test designed to identify individuals with recent HIV infection [29], we were able to measure anti-HIV antibody levels after 2 years of suppressive cART. The assay revealed no difference between antibody levels in the IR and INR groups (Figure 4C). These results indicate that low-level, ongoing viral replication does not appear to be the cause of poor CD4+ T-cell recovery.
DISCUSSION
This study explored the hypothesis that increased levels of systemic inflammation are responsible for a lack of appropriate CD4+ T-cell reconstitution after suppressive cART. Suppression of viral replication with cART significantly altered the cytokine profile of both IR and INR subjects, increasing levels of multiple cytokines and decreasing levels of others such as TNF-α and IP-10, known to be elevated in untreated HIV infection. Although changes in multiple markers of infection were detected after initiation of cART, very few differences were seen in cytokine levels between the IR and INR groups.
Overall, we found very little evidence of differences in soluble inflammatory markers between IR and INR subjects. These results are consistent with the few studies that have measured cytokines in INR subjects, including no differences in IL-1β, IL-6, IL-8, MCP-1, and TNF-α levels in 1 study [30] and sTNFRI, sTNFRII, sCD14, and IL-6 levels in another [31]. Although levels of cytokines were not different between IR and INR subjects, higher levels of sTNFRII and IL-6 were found in INR subjects compared with HIV-uninfected subjects [31]. In addition, IL-7 levels have been variously described as borderline elevated [15] or suppressed [32] in INR subjects. Soluble markers of inflammation presumably reflect activation of circulating immune cells and tissues, although it has been shown that cellular activation can exist, as measured by interferon-stimulated gene expression, in the absence of significant perturbations in systemic cytokine levels [31]. In contrast to the lack of signal for cytokines in predicting the INR phenotype, multiple studies have demonstrated differences in markers of cellular activation between IR and INR subjects. Higher levels of CD38+CD8+ T cells is correlated with poorer CD4+ T-cell recovery [33], although the dependence on CD8+ T-cell activation may not persist after the first year on cART [34]. Some studies have also suggested that HLA-DR+CD8+ T cells correlate better with the INR phenotype than CD38 expression [30]. Lower proportions of CD28+CD8+ T cells have also been associated with the INR state, presumably reflecting a smaller naive T-cell pool [30, 35]. In addition, higher levels of soluble CD14, the lipopolysaccharide receptor, have been associated with the INR phenotype [36].
Of the cytokines found to be different between IR and INR subjects, IP-10 was the most interesting from a pathogenesis perspective. This cytokine has previously been shown to be correlated with higher viral load and lower CD4+ T-cell count [26, 37]. We hypothesized that low-level viral replication might be driving higher IP-10 levels at the 2-year time point in the INR subjects, but using a sensitive HIV RNA detection assay we found no evidence for increased viral replication in this group (Figure 4B). It is possible that IP-10 is serving as a marker of increased HIV-driven inflammation in tissues such as lymph nodes, with virus-infected cells eliminated prior to replication of virus. We did not measure the latent reservoir of infected cells in this study, although a recent study demonstrated that INR subjects did not have increased HIV RNA or DNA levels in colonic biopsy samples [31].
Several trials have been performed to target inflammation or residual HIV replication in INR subjects in an effort to boost CD4+ T-cell counts. It has been shown that strong cytomegalovirus (CMV)-specific T-cell responses are associated with blunted CD4+ T-cell recovery after cART [6]. Targeting CMV with valganciclovir reduced the number of activated CD8+ T cells and C-reactive protein levels but did not change CD4+ T-cell counts or HIV viral load [38]. Hydroxychloroquine was shown to decrease CD4+ T-cell activation and boost CD4+ T-cell percentage but not numbers [39]. Intensification of ARV therapy with raltegravir led to a faster increase in CD4+ T cells but no difference in levels at 48 weeks compared with placebo in a randomized, controlled trial [40]. Taken together, attempts to target residual inflammation or viral replication in INR subjects have not yielded conclusive benefits in terms of CD4+ T-cell count recovery.
CONCLUSIONS
In summary, we found that of a broad array of markers spanning pro- and anti-inflammatory cytokines, chemokines, and growth factors, none of the soluble markers tested proved to be reliable discriminators of the IR versus INR phenotypes, either at baseline before cART or after 1 or 2 years of suppressive cART. Our current results and prior studies demonstrate that cART modulates the cytokine profile of HIV-infected subjects. Moreover, in studies in which healthy control subjects have been included, the direction of change (increase or decrease) in these levels seems to be towards levels found in healthy controls. These data suggest that poor CD4+ T-cell recovery after effective cART may be mediated by cellular activation that does not result in substantial perturbations of systemic cytokine levels, combined with other known risk factors for poor CD4+ T-cell recovery, such as lower nadir CD4+ T-cell count, older age, and a longer exposure to high levels of HIV replication. These findings reinforce arguments for early treatment of HIV and suggest that research into methods of directly boosting T-cell production in INR patients may be more fruitful than indirect therapies targeting residual HIV replication.
Acknowledgments
Disclaimer. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Financial support. Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS). The WIHS (Principal Investigators): Bronx WIHS (K. A.; U01-AI-035004); Brooklyn WIHS (H. L. M. and Deborah Gustafson; U01-AI-031834); Chicago WIHS (Mardge Cohen and Audrey French; U01-AI-034993); Metropolitan Washington WIHS (M. Y.; U01-AI-034994); Connie Wofsy Women′s HIV Study, Northern California (R. M. G., Bradley Aouizerat, and Phyllis Tien; U01-AI-034989); WIHS Data Management and Analysis Center (S. J. G. and E. T. G.; U01-AI-042590); Southern California WIHS (Joel Milam; U01-HD-032632) (WIHS I–WIHS IV).
The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases, with additional cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Mental Health. Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research, the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Deafness and Other Communication Disorders, and the NIH Office of Research on Women's Health. WIHS data collection was also funded by University of California, San Francisco Clinical and Translational Science Awards (grant UL1-TR000004).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
Collaborators: the Women's Interagency HIV Study, Kathryn Anastos, Howard Minkoff, Deborah Gustafson, Mardge Cohen, Audrey French, Mary Young, Ruth Greenblatt, Bradley Aouizerat, Phyllis Tien, Stephen Gange, Elizabeth Golub, and Joel Milam
References
- 1.van Sighem AI, Gras LA, Reiss P et al. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS 2010; 24:1527–35. [DOI] [PubMed] [Google Scholar]
- 2.May MT, Gompels M, Delpech V et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS 2014; 28:1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piketty C, Weiss L, Thomas F et al. Long-term clinical outcome of human immunodeficiency virus-infected patients with discordant immunologic and virologic responses to a protease inhibitor-containing regimen. J Infect Dis 2001; 183:1328–35. [DOI] [PubMed] [Google Scholar]
- 4.AIDSinfo. Poor CD4 cell recovery and persistent inflammation despite viral suppression. Guidelines for the Use Antiretroviral Agents HIV-1-Infected Adults and Adolescents. US Department of Health and Human Services. Available at: http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv-guidelines/470/poor-cd4-recovery Accessed 18 April 2015.
- 5.Engsig FN, Gerstoft J, Kronborg G et al. Long-term mortality in HIV patients virally suppressed for more than three years with incomplete CD4 recovery: a cohort study. BMC Infect Dis 2010; 10:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Appay V, Fastenackels S, Katlama C et al. Old age and anti-cytomegalovirus immunity are associated with altered T-cell reconstitution in HIV-1-infected patients. AIDS 2011; 25:1813–22. [DOI] [PubMed] [Google Scholar]
- 7.Engsig FN, Zangerle R, Katsarou O et al. Long-term mortality in HIV-positive individuals virally suppressed for >3 years with incomplete CD4 recovery. Clin Infect Dis 2014; 58:1312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saison J, Ferry T, Demaret J et al. Association between discordant immunological response to highly active anti-retroviral therapy, regulatory T cell percentage, immune cell activation and very low-level viraemia in HIV-infected patients. Clin Exp Immunol 2014; 176:401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufmann D, Pantaleo G, Sudre P, Telenti A. CD4-cell count in HIV-1-infected individuals remaining viraemic with highly active antiretroviral therapy (HAART). Swiss HIV Cohort Study. Lancet 1998; 351:723–4. [DOI] [PubMed] [Google Scholar]
- 10.Piketty C, Castiel P, Belec L et al. Discrepant responses to triple combination antiretroviral therapy in advanced HIV disease. AIDS 1998; 12:745–50. [DOI] [PubMed] [Google Scholar]
- 11.Deeks SG, Barbour JD, Martin JN et al. Sustained CD4+ T cell response after virologic failure of protease inhibitor-based regimens in patients with human immunodeficiency virus infection. J Infect Dis 2000; 181:946–53. [DOI] [PubMed] [Google Scholar]
- 12.Lu W, Andrieu JM. HIV protease inhibitors restore impaired T-cell proliferative response in vivo and in vitro: a viral-suppression-independent mechanism. Blood 2000; 96:250–8. [PubMed] [Google Scholar]
- 13.Kelley CF, Kitchen CM, Hunt PW et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis 2009; 48:787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hengel RL, Jones BM, Kennedy MS et al. Lymphocyte kinetics and precursor frequency-dependent recovery of CD4(+)CD45RA(+)CD62L(+) naive T cells following triple-drug therapy for HIV type 1 infection. AIDS Res Hum Retroviruses 1999; 15:435–43. [DOI] [PubMed] [Google Scholar]
- 15.Marziali M, De Santis W, Carello R et al. T-cell homeostasis alteration in HIV-1 infected subjects with low CD4 T-cell count despite undetectable virus load during HAART. AIDS 2006; 20:2033–41. [DOI] [PubMed] [Google Scholar]
- 16.Mendez-Lagares G, Garcia-Perganeda A, del Mar del Pozo-Balado M et al. Differential alterations of the CD4 and CD8 T cell subsets in HIV-infected patients on highly active antiretroviral therapy with low CD4 T cell restoration. J Antimicrob Chemother 2012; 67:1228–37. [DOI] [PubMed] [Google Scholar]
- 17.Li T, Wu N, Dai Y et al. Reduced thymic output is a major mechanism of immune reconstitution failure in HIV-infected patients after long-term antiretroviral therapy. Clin Infect Dis 2011; 53:944–51. [DOI] [PubMed] [Google Scholar]
- 18.Barkan SE, Melnick SL, Preston-Martin S et al. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology 1998; 9:117–25. [PubMed] [Google Scholar]
- 19.Bacon MC, von Wyl V, Alden C et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005; 12:1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valiathan R, Deeb K, Diamante M et al. Reference ranges of lymphocyte subsets in healthy adults and adolescents with special mention of T cell maturation subsets in adults of South Florida. Immunobiology 2014; 219:487–96. [DOI] [PubMed] [Google Scholar]
- 21.Uppal SS, Verma S, Dhot PS. Normal values of CD4 and CD8 lymphocyte subsets in healthy indian adults and the effects of sex, age, ethnicity, and smoking. Cytometry B Clin Cytom 2003; 52:32–6. [DOI] [PubMed] [Google Scholar]
- 22.Hopkins M, Hau S, Tiernan C et al. Comparative performance of the new Aptima HIV-1 Quant Dx assay with three commercial PCR-based HIV-1 RNA quantitation assays. J Clin Virol 2015; 69:56–62. [DOI] [PubMed] [Google Scholar]
- 23.Nair SV, Kim HC, Fortunko J et al. Aptima HIV-1 Quant DX -- A fully automated assay for both diagnosis and quantification of HIV-1. J Clin Virol 2016; 77:46–54. [DOI] [PubMed] [Google Scholar]
- 24.Keating SM, Hanson D, Lebedeva M et al. Lower-sensitivity and avidity modifications of the vitros anti-HIV 1+2 assay for detection of recent HIV infections and incidence estimation. J Clin Microbiol 2012; 50:3968–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 1995; 57:289–300. [Google Scholar]
- 26.Keating SM, Golub ET, Nowicki M et al. The effect of HIV infection and HAART on inflammatory biomarkers in a population-based cohort of women. AIDS 2011; 25:1823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yukl SA, Boritz E, Busch M et al. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog 2013; 9:e1003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henrich TJ, Hanhauser E, Marty FM et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med 2014; 161:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kassanjee R, McWalter TA, Welte A. Short communication: defining optimality of a test for recent infection for HIV incidence surveillance. AIDS Res Hum Retroviruses 2014; 30:45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erikstrup C, Kronborg G, Lohse N et al. T-cell dysfunction in HIV-1-infected patients with impaired recovery of CD4 cells despite suppression of viral replication. J Acquir Immune Defic Syndr 2010; 53:303–10. [DOI] [PubMed] [Google Scholar]
- 31.Dunham RM, Vujkovic-Cvijin I, Yukl SA et al. Discordance between peripheral and colonic markers of inflammation during suppressive ART. J Acquir Immune Defic Syndr 2014; 65:133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bai F, Bellistrì GM, Tincati C et al. Reduced CD127 expression on peripheral CD4+ T cells impairs immunological recovery in course of suppressive highly active antiretroviral therapy. AIDS Lond Engl 2010; 24:2590–3. [DOI] [PubMed] [Google Scholar]
- 33.Hunt PW, Martin JN, Sinclair E et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis 2003; 187:1534–43. [DOI] [PubMed] [Google Scholar]
- 34.Benito JM, Lopez M, Lozano S et al. CD4+ T cell recovery beyond the first year of complete suppression of viral replication during highly active antiretroviral therapy is not influenced by CD8+ T cell activation. J Infect Dis 2005; 192:2142–6. [DOI] [PubMed] [Google Scholar]
- 35.Seu L, Ortiz GM, Epling L et al. Higher CD27+CD8+ T cells percentages during suppressive antiretroviral therapy predict greater subsequent CD4+ T cell recovery in treated HIV infection. PLoS One 2013; 8:e84091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lederman MM, Calabrese L, Funderburg NT et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis 2011; 204:1217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts L, Passmore JA, Williamson C et al. Plasma cytokine levels during acute HIV-1 infection predict HIV disease progression. AIDS 2010; 24:819–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunt PW, Martin JN, Sinclair E et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis 2011; 203:1474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piconi S, Parisotto S, Rizzardini G et al. Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders. Blood 2011; 118:3263–72. [DOI] [PubMed] [Google Scholar]
- 40.Massanella M, Negredo E, Puig J et al. Raltegravir intensification shows differing effects on CD8 and CD4 T cells in HIV-infected HAART-suppressed individuals with poor CD4 T-cell recovery. AIDS 2012; 26:2285–93. [DOI] [PubMed] [Google Scholar]