Abstract
The purpose of this study was to evaluate the response of actively growing renal masses to stereotactic body radiation therapy (SBRT). We retrospectively reviewed our institutional review board–approved kidney database and identified 4 patients who underwent SBRT, 15 Gy dose, for their rapidly growing renal masses. Three patients had a decreased tumor size after radiation treatment by 20.8%, 38.1%, and 20%. The other patient had a size gain of 5.6%. This patient maintained a similar tumor growth rate before and after SBRT. Mean follow-up time was 13.8 months. SBRT represents an effective management option in select patients with larger rapidly growing kidney masses.
Keywords: Oncology, Radiotherapy, Kidney, Tumor
Introduction
The detection of small renal masses has increased with the frequency of cross-sectional imaging. Recent data suggest that conservative management may be an attractive option for patients who are poor surgical candidates with small renal masses.1 Stereotactic body radiation therapy (SBRT) represents a novel treatment approach in the setting of renal cell carcinoma (RCC). We retrospectively reviewed the outcomes of 4 patients with rapidly growing renal masses treated with SBRT who were medically unfit for surgical intervention.
Methods
We retrospectively reviewed our prospectively maintained institutional review board–approved kidney database for all patients seen for a renal mass from 1994 to 2012. We identified 4 patients who were found to have rapidly growing renal masses and were treated with SBRT as primary management. We examined patient factors including age at diagnosis, time from diagnosis to initial visit, tumor size, tumor growth rate, treatment dose, time from presentation to treatment, response to therapy, and patient-reported toxicity as well as serum creatinine. Tumor size was measured using the largest transverse dimension on computed tomography (CT) scan.
Simulation, Planning, and Treatment
All patients provided informed consent for SBRT. A stereotactic body immobilization system (BodyFIX, Medical Intelligence, Schwabmuenchen, Germany) was used in all cases. Four-dimensional CT was obtained to assess tumor motion with respiration. Target delineation was carried out in all respiratory phases, creating an internal target volume by contouring gross disease. A 5-mm isotropic expansion was used to create a planning target volume (PTV). Organs at risk segmented for evaluation included kidney minus gross tumor volume, spinal cord, liver, bowel, and stomach. Standard dose constraints were applied for organs at risk.
Eclipse treatment planning software (Varian, Palo Alto, CA) was used for radiation plan development. Inhomogeneity corrections were applied. Guidelines for plan review were akin to that of the contemporaneous RTOG 0915 protocol. Maximum dose was to be located within the PTV. Conformality indices (ie, dose spillage) consistent with the previously mentioned protocol were in compliance.
A 15 Gy dose was prescribed to the PTV. Treatment was carried out with Varian Trilogy linear accelerator. Volumetric modulated arc therapy with 6 MeV photons was used. Cone-beam CT was obtained following setup, and appropriate shifts were made.
Results
Three of 4 patients had a measurable decrease in size of their renal mass. The average change was 0.85 cm decrease in size after treatment (P = .06). Average follow-up was 13.8 months, which included cross-sectional imaging at the last recorded visit. The mean age at diagnosis was 76 years with a mean tumor size of 3.8 cm (Table 1).
Table 1.
Tumor response
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Mean | |
|---|---|---|---|---|---|
| Age at diagnosis, y | 89 | 72 | 73 | 70 | 76 |
| Tumor size at diagnosis, cm | 4.1 | 4.0 | 3.5 | 3.6 | 3.8 |
| Time from diagnosis to initial visit, mo | 14.6 | 0.9 | 23.7 | 32.4 | 17.9 |
| Tumor size at initial visit, cm | 5.1 | 4.8 | 4.2 | 4.0 | 4.53 |
| Tumor growth rate from diagnosis to initial visit, cm/y | 0.69 | 10.67 | 0.36 | 0.14 | 3.0 |
| Approx tumor size at time of treatment, cm | 5.4 | 4.8 | 4.2 | 6.0 | 5.1 |
| SBRT treatment dose, Gy | 15 | 15 | 15 | 15 | 15 |
| Time from visit to treatment completion, mo | 2.17 | 5.83 | 2.5 | 17.8 | 7.07 |
| Tumor size at last follow-up, cm | 5.7 | 3.8 | 2.7 | 4.8 | 4.25 |
| Size reduction (%) | 5.56 | −20.8 | −38.1 | −20 | −18.3 |
| Overall size reduction, cm | 0.3 | −1 | −1.6 | −1.2 | −0.85 |
| Follow-up, mo | 6 | 22 | 21 | 6 | 13.8 |
SBRT, stereotactic body radiation therapy.
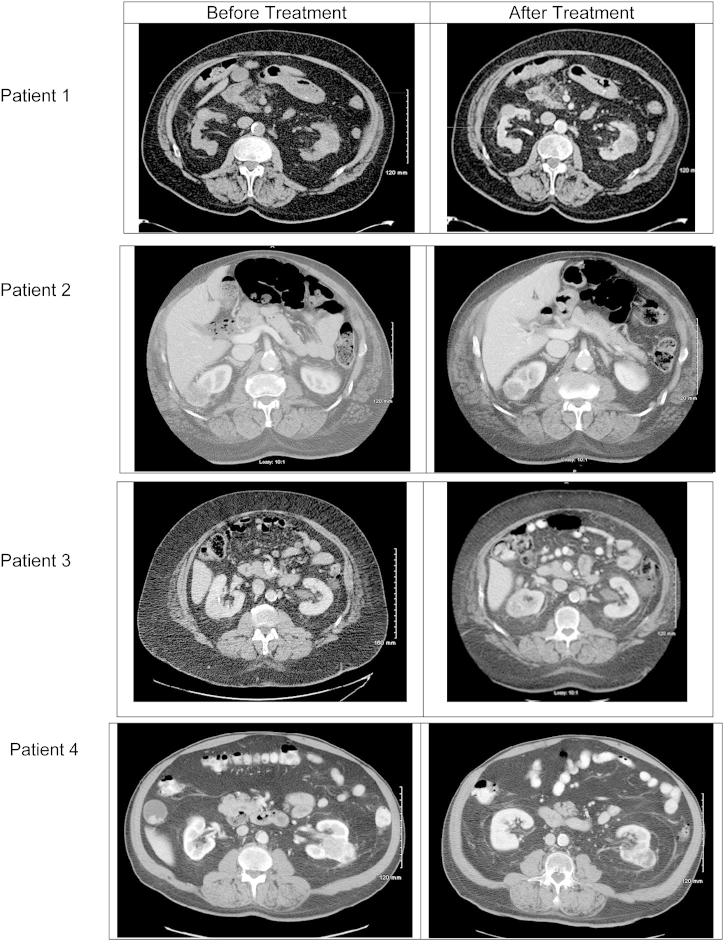
The first patient is an 89-year-old Caucasian man who did not have a successful response to SBRT therapy. The patient was diagnosed with a 4.1-cm lower pole tumor. He re-presented 14.6 months later after the tumor grew to 5.1 cm (Fig. 1), a growth rate of 0.69 cm/y. He underwent treatment with a maximal tumor size of 5.4 cm. At 6-month follow-up, the tumor had grown to 5.7 cm (Fig. 1), an increase of 0.3 cm (5.6%). This tumor failed to respond to SBRT and continued to progress with an increase in the rate of growth to 0.74 cm/y.
Figure 1.
Pretreatment and Post-treatment imaging.
The second patient, a 72-year-old African-American man, had a successful response to therapy. The patient was diagnosed with a 4-cm upper lobe tumor that grew to 4.8 cm (Fig. 1) in 1 month, giving a growth rate of 10.67 cm/y. The patient's most recent follow-up was 22 months after treatment and revealed a 3.8-cm tumor (Fig. 1), a 20.8% reduction.
The third patient, a 73-year-old Caucasian male, had a successful response to therapy. The patient was diagnosed with a 3.5-cm mid to upper pole right renal mass that grew to 4.2 cm (Fig. 1) in 23.7 months, demonstrating a growth rate of 0.36 cm/y. Follow-up CT 21 months after treatment revealed a 2.7-cm tumor (Fig. 1), a 38.1% reduction.
The fourth patient, a 70-year-old Caucasian man, also had a successful response to therapy. The patient was diagnosed with a 3.6-cm tumor. During surveillance, the tumor grew to 6.0 cm with a growth rate calculated 3.0 cm/y. At 6 months follow-up, the tumor decreased in size to 4.8 cm (Fig. 1), a 20% reduction.
In all 4 patients, there was no significant decline in serum creatinine from prior baseline levels after SBRT at most recent follow-up. No adverse events were reported.
Discussion
The limitations of our data are well recognized. Among these are small sample size, inconsistent follow-up, and lack of histologic verification. Given the lack of a tissue diagnosis in our patients, we chose the conservative dose of 15 Gy in one fraction. Besides the high likelihood of sparing organs at risk, there were data to suggest that a threshold exists around 15 Gy for increased cell kill because of effects on endothelial cells.2
There is now an actively growing body of literature for the use of SBRT in RCC. The preliminary results of one of the largest experiences to date revealed 93% local control in patients after a mean 12-month follow-up, using 40 Gy in 5 fractions.3 Ponksy et al4 reported that 16 Gy in 4 fractions achieved a pathologic complete response in only 1 of 3 patients with nephrectomy 8 weeks after radiation. Nevertheless, a modest size reduction was seen in our patients after SBRT treatment. Toxicity rates were low in these series, which was consistent with our results.
The increasing acceptance of SBRT in other disease sites coupled with the common notion that RCC is a radioresistant histology renders SBRT an attractive treatment option in inoperable patients. Our series suggests that SBRT is a safe and feasible treatment option for this patient population. Even with a modest dose of 15 Gy, response was seen in 3 of 4 patients.
Conclusion
SBRT decreases the size of rapidly growing renal masses with even modest doses, and may represent a viable treatment option in the medically inoperable treatment population as it is safe and well tolerated. Future prospective studies are needed with larger numbers, standardized dosing, and histologic confirmation.
Consent
Informed consent was obtained from all patients for being included in the study.
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Available online 28 June 2014
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
References
- 1.Jewett M.A., Mattar K., Basiuk J. Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol. 2011;60:39–44. doi: 10.1016/j.eururo.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Barros M., Paris F., Cordon-Cardo C. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 3.Qian G., Lowry J., Silverman P. Stereotactic extra-cranial radiosurgery for renal cell carcinoma. Int J Radiat Oncol Biology Phys. 2003;57(Supplement):S283. [Google Scholar]
- 4.Ponsky L.E., Mahadevan A., Gill I.S. Renal radiosurgery: initial clinical experience with histological evaluation. Surg Innov. 2007;14:265–269. doi: 10.1177/1553350607310546. [DOI] [PubMed] [Google Scholar]