Abstract
The aim of the present study was to examine the effects of testosterone (T) and estradiol-17β (E2) on the production of progesterone (P4) by granulosa cells, and of the E2 on the production of P4 and T by theca internal cells. In the first experiment, granulosa cells isolated from the largest (F1) and third largest (F3) preovulatory follicle were incubated for 4 h in short-term culture system, P4 production by granulosa cells of both F1 and F3 was increased in a dose-dependent manner by ovine luteinizing hormone (oLH), but not T or E2. In the second experiment, F1 and F3 granulosa cells cultured for 48 h in the developed monolayer culture system were recultured for an additional 48 h with increasing doses of various physiological active substances existing in the ovary, including T and E2. Basal P4 production for 48 h during 48 to 96 h of the cultured was about nine fold greater by F1 granulosa cells than by F3 granulosa cells. In substances examined oLH, chicken vasoactive intestinal polypeptide (cVIP) and T, but not E2, stimulated in a dose-dependent manner P4 production in both F1 and F3 granulosa cells. In addition, when the time course of P4 production by F1 granulosa cells in response to oLH, cVIP, T and E2 was examined for 48 h during 48 to 96 h of culture, although E2 had no effect on P4 production by granulosa cells of F1 during the period from 48 to 96 h of culture, P4 production with oLH was found to be increased at 4 h of the culture, with a maximal 9.14 fold level at 6 h. By contrast, P4 production with cVIP and T increased significantly (p<0.05) from 8 and 12 h of the culture, respectively, with maximal 6.50 fold response at 12 h and 6, 48 fold responses at 36 h. Furthermore, when F1 granulosa cells were precultured with E2 for various times before 4 h culture with oLH at 96 h of culture, the increase in P4 production in response to oLH with a dose-related manner was only found at a pretreatment time of more than 12 h. In the third experiment, theca internal cells of F1, F2 and the largest third to fifth preovulatory follicles (F3-5) were incubated for 4 h in short-term culture system with increasing doses of E2. The production of P4 and T by theca internal cells were increased with the addition of E2 of 10−6 M. These increases were greater in smaller follicles. These results indicate that, in granulosa cells of the hen, T may have a direct stimulatory action in the long term on P4 production, and on E2 in long-term action which may enhance the sensitivity to LH for P4 production, and thus, in theca internal cells, E2 in short term action may stimulate the production of P4 and T.
Keywords: Preovulatory Follicles, Steroids Hormone, Granulosa, Theca Internal and External Cells
INTRODUCTION
In most avian species only the left ovary becomes functional. Within this ovary there are several clearly recognizable groups of follicles, though each merges into the other in order of increasing size. The ovary in the domestic fowl (Gallus domesticus) contains five or six largest preovulatory follicles arranged in a hierarchy. The ovum within each of the four largest follicles is surrounded by a single layer of granulosa cells. The granulosa layer is an avascular cell layer that produces primarily progestins and small amounts of androgens. The basement membrane is a multilayered laminar sheet that grows from approximately 0.2 to 1 m in thickness during stage 2 of follicular development. This membrane serves as a substratum for granulosa cells and separates the granulosa and theca layers (Rothwell and Salomon, 1977; Perry et al., 1978). The theca layer is composed of vascular, neural, connective and steroidogenic tissues and divided into theca internal and external layers. The theca internal layer is situated adjacent to the basement membrane and characterized by the presence of a discontinuous layer of fibroblast cells and theca cells, in small follicles, the theca cells may be grouped together into so-called theca glands. The theca external layer represents the major proportion of the follicles walls and is composed of sheets of fibroblast-like cells, numerous microfilaments, collagen fibers, and actin, but relatively few theca cells.
In the domestic fowl, progesterone (P4) being produced in granulosa cells of the larger preovulatory follicles plays a key role in the endocrine control of the hypothalamic-hypophysial-ovarian axis. The production of P4 has been known to be stimulated mainly by luteinizing hormone (LH) (Schally et al., 1971; Hammond et al., 1980; Wells et al., 1981; Marrone and Hertelendy, 1983; Robinson and Renema, 1999; Hyang et al., 2007). However, physiological active substances existing in the ovary, including testosterone (T) and estradiol-17β (E2), were recently shown to influence P4 production by granulosa cells of the hen (Johnson and Tilly, 1988; Johnson et al., 1988; Porter et al., 1989; Kamiyoshi et al., 1992; Von Engelhard and Groothuis, 2005). Porter et al. (1989), who examined P4 production in hen granulosa cells treated with androgens for 2 days, found that the treatment with androgens alone enhances the production of P4. Johnson et al. (1988) studied the influence of androgens and E2 on P4 production by hen granulosa cells in short-term culture, and they showed that androgens and E2 suppress basal and LH-stimulated P4 production. Further, P4 production by hen granulosa cells in response to LH has been reported to be increased by the pretreatment with E2 for 48 h (Kamiyoshi et al., 1992; Groothuis et al., 2005). However, the influence of androgens and estrogens on the production of P4 in hen granulosa cells is not fully understood. Also, to the best of our knowledge, there are no reports that examined the effect of E2 on the production of P4 and T by theca internal cells.
Johnson and Tilly (1988), who examined the effect of vasoactive intestinal polypeptide (VIP) on the production of P4 and cAMP in hen granulosa cells, found that the action of VIP on the P4 production had been delayed, because a significant increase in P4 production caused by VIP was not found until after 8h of culture. However, in most reports studying a physiological effect of VIP on P4 production by hen granulosa cells in vitro, the effect has been examined by short-term culture system in floating condition of cells. Generally, it is virtually impossible to examine the long-term actions of hormones in a cell suspension culture.
Therefore, to evaluate the ability of T and E2 to affect P4 production in granulosa cells, and to study whether E2 affects the production of P4 and T in theca internal cells, the objectives of the present study were i) to determine whether or not the short-term cultures for 4 h with T and E2 affect the P4 production by granulosa cells isolated from the largest (F1) and third largest (F3) preovulatory follicle of the hen; ii) to determine, by using the developed monolayer culture system, whether or not the long term cultures for 48 h with T, E2 prostaglandin E1 (PGE1), prostaglandin F2α (PGF2α), epinephrine (E), norepinephrine (NE) mesotocin (MT), arginine vasotocin (AVT) and chicken vasoactive intestinal peptide (cVIP) which has been reported to exist in the follicles of the hen, or with ovine gonadotropins, affect the P4 production by F1 and F3 granulosa cells of the hen; iii) to examine the time course of the P4 production by F1 granulosa cells cultured with ovine luteinizing hormone (oLH), cVIP and T, which stimulated P4 production by F1 granulosa cells in substances examined, and furthermore whether or not the pretreatment with E2 affects the responsiveness of granulosa cells for P4 production and the effectiveness depends on the time of pretreatment; iv) to determine whether E2 for short term affects the productions of P4 and T by theca internal cells of F1 and F3-5 of the hen.
MATERIALS AND METHODS
Birds and collection of follicles
Birds used were White Leghorn hens laying more than 4 eggs in a sequence of one-day pause between sequences. They had been kept in individual cages with feed and water provided ad libitum. In each experiment, 4 to 10 laying hens were killed 1 to 2 h after oviposition, and F1 and F3 in the experiments for granulosa cells or F1, F2 and F3-5 in the experiments for theca internal cells were immediately excised, and granulosa layer and theca layer were separated from the excised follicle according to the method of Huang and Nalbandov (1979).
Hormones and reagents
The National Hormones and Pituitary Program kindly provided the oLH (NIH-oLH-S26, NIDDK) and (ovine follicle stimulating hormone (oFSH) (NIH-oFSH-S20, NIDDK). P4, T, E2, E, NE, PGF2α, PGE1, corticosterone (B), bovine transferrin, porcine insulin and bovine serum albumin (BSA, fraction V) were purchased from sigma Chemical Co. (St. Louis, MO, USA). cVIP was obtained from Peninsula Laboratories, Inc. (Belmont, CA, USA). The AVT and MT were purchased from Biochemist Inc. (Bubendorf, Switzerland). Antisera to P4 and T were the generous gift of RIA Center of Gunma University. (1, 2, 6, 7-3H) P4, (1, 2, 6, 7-3H) T and ASC-II scintillators were obtained from Amersham International plc. (Buckinghamshire, UK). Mc Coy’s 5a medium without serum and Ham’s F12 medium were obtained from Gibco Life Technologies Inc. (Grand Island, NY, USA). Fetal calf serum was purchased from Boëhringer Mannheim (Mannheim, Germany).
Dispersion and culture of granulosa cells for short-term culture
After removing the outer fibrous tissues and the separation of the granulosa layer according to the method of Huang and Nalbandov (1979), pooled granulosa layers were placed in 10 mL Medium 199 supplemented with 10 mM Hepes and 0.08% collagenase in a 50 mL plastic centrifuge tube, and then incubated for 5 min while shaking at 120 cycle/min in a bath kept at 37°C. After the incubation, the dispersion was aspirated and expelled for approximately 30 s with a plastic syringe, and centrifuged at 250×g for 5 min at 4°C. The pellet was redispersed in 10 mL Medium 199 supplemented with 10 mM Hepes, gently stirred for 1 to 2 min, filtered through a nylon gauze (mesh size, 60 m) into a sterile plastic tube, and centrifuged at 250×g for 5 min at 4°C. The cell pellet was washed twice with 20 mL Medium 199 supplemented with 10 mM Hepes and then once with 20 mL of the culture medium containing 10 mM Hepes and 0.4% BSA in Medium 199. The final washed pellet was suspended in 10 mL of the culture medium, and the number of living and dead cells in the suspension was counted on a hemocytometer following trypan blue exclusion. The viability of the cells was more than 95%. After the cell count, the cells at the density of 2×105 cells/mL/tube were incubated for 4 h at 37°C with or without increasing doses (5 to 160 ng/mL) of ovine LH (oLH; NIH-oLH-S26, NIDDK) or ovine FSH (oFSH; NIH-oFSH-S20, NIDDK). After culture, the granulosa cells were stored −20°C.
Dispersion and culture of theca internal cells for short-term culture
After removing the outer fibrous tissues and the separation of the granulosa layer, the remainder of each follicle (theca folliculi) was inverted, and incubated for 30 min in Medium 199 containing 10 mM Hepes and 0.2% collagenase. Theca internal layer was gently scraped off with a scalpel blade until the color of tissue changed from pink to near white, and returned to Medium 199 containing 10 mM Hepes and 0.2% collagenase, then further incubated for 25 min. During the incubation with collagenase, mechanical dispersion was performed every 5 min with a syringe. The remaining theca external layer was minced into about 2 mm square pieces, and incubated for 60 min in Medium 199 containing 10 mM Hepes and 0.2% collagenase with mechanical disruption of tissue every 15 min of interval with a syringe. The cell suspensions of theca internal and theca external were filtrated though a nylon gauze ( mesh size, 60 m) and were centrifuged twice instead of once at 400×g for 10 min at 4°C. The cell pellet was resuspended in Medium 199 containing 40% Percoll and centrifuged at 400×g for 20 min. Top layer containing theca cell was removed and pelleted by centrifugation at 400×g for 10 min.
The cell pellet was suspended in culture medium containing 10 mM Hepes and 0.2% BSA in Medium 199, and centrifuged at 400×g 10 min. After the cell count on a hemocytometer following a trypan blue dye exclusion, suspensions of living cells of the theca internal and theca external were diluted to the cell density of 2×105 cells/0.5 mL with culture medium. oLH (NIH-oLH-S26, NIDDK) and oFSH (NIH-oFSH-S20, NIDDK) were diluted in culture medium at the concentrations of 0.1, 1, 10, and 100 ng/0.5 mL and added to the cell suspensions. The cells with an incubation volume of 1 mL were incubated for 4 h at 37°C. After incubation, the theca cells were stored at −20°C.
Dispersion of granulosa cells for long-term culture
Granulosa layers were separated from the excised follicles according to the method of Huang and Nalbandov (1979). After washing with Hepes buffer (25 mM Hepes; 137 mM NaCl; 5 mM KCl; 0.7 mM Na2HPO4·2H2O; 10 mM glucose; 360 mM CaCl2·2H2O; pH 7.4) supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin sulfate, the pooled granulosa layers were placed in 10 mL Hepes buffer containing 0.08% collagenase in a 50 mL plastic centrifuge tube, and then incubated for 5 min while shaking at 120 cycle/min in a bath kept at 37°C. After the incubation, the dispersion was aspirated and expelled for approximately 30 s with a plastic syringe, and centrifuged at 250×g for 5 min at 4°C. The pellet was redispersed in 10 mL Hepes buffer, gently stirred for 1 to 2 min, filtrated though nylon gauze (mesh size, 60 μm) into a sterile plastic tube, and centrifuged at 250×g for 5 min at 4°C. The cell pellet was washed twice with 20 mL Hepes buffer and once with 20 mL of desired medium. The final washed pellet was suspended in 10 mL of desired medium and the number of living and dead cells in the suspension was counted on a hemocytometer following the trypan blue exclusion. The viability of cells was always more than 95%.
Experiment 1: Effect of oLH, T, and E2 for short term P4 production of granulosa cells
To examine the effect of oLH, T, and E2 for short term on P4 production of granulosa cells, F1 and F3 granulosa cells (2×105 cells/mL/tube) were incubated for 4 h with or without increasing concentrations of oLH (10, 20, 40, 80, and 160 ng/mL), T (10−9, 10−8, 10−7, and 10−6 M) and E2 (10−9, 10−8, 10−7, and 10−6 M). After the incubation, the cultured medium was collected and stored at −20°C.
Experiment 2: Monolayer cultured method of granulosa cells
To select an optimum fined medium for the long-term culture of hen granulosa cells, the cell density of F1 granulosa cells was adjusted to 7.5×105 cells/mL with the following media supplemented with 10 mM Hepes, 2 mM L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin sulfate: Mc Coy’s 5a medium, Ham’s F12 medium, basic medium consisting of a 1:1 mixture of Mc Coy’s 5a medium and Ham’s F12 medium, serum- containing medium supplemented with 10% (vol/vol) fetal calf serum to the basic medium or serum-free medium supplemented with insulin (20 mU/mL), transferrin (5 μg/mL), BSA (4 mg/mL) and B ( 20 ng/mL) to the basic medium. Aliquots (0.4 mL) of the cell suspension containing 3×105 living cells were placed in wells of the 24-well plastic culture dishes (Nunc Int. Med., Sigma-Aldrich, Inc. St. Louis, Mo, USA) and cultured at 38°C under a water-saturated atmosphere of 95% air and 5% CO2. Medium was changed every 48 h. At 48 and 96 h of the culture, after morphological appearance of cell was observed, the medium was collected and stored at −20°C, and the cell number was counted on a hemocytometer after the treatment with 0.05% trypsin–0.02% ethylenediaminetetra-acetic acid (EDTA)-Hepes buffer.
To examine the responsiveness to oLH of P4 production by hen granulosa cells cultured for 96 h in serum-containing medium and serum-free medium described above, F1 granulosa cells (3×10 5 cells/well) were cultured for 96 h in serum-containing medium or serum-free medium. Medium was renewed every 48 h. After removal of the medium at 96 h of the culture, fresh serum-free medium containing oLH (100 ng/mL) nothing else was added and then cultured for 4 h. After the culture, the medium was collected and stored at −20°C, and the number of cells were counted as described above.
Experiment 3: P4 production by granulosa cells in long-term culture with physiological active substances
To evaluate the P4 production by hen granulosa cells cultured for a long-term, granulosa cells (3×105 cells/well) of the F1 and F3 cultured for 48 h in serum-free medium were cultured for an additional 48 h with serum-free medium containing one of the following materials: oLH and oFSH at the concentrations of 0.1, 1, 10, and 100 ng/mL, respectively, and cVIP, AVT, MT, T, E2, E, NE, PGE1, and PGF2α at the concentrations of 10−9, 10−8, 10−7, and 10−6 M, respectively. After the culture, the medium was collected and stored at −20°C, and the cell number in cultures with oLH, cVIP, and T, which were shown to enhance P4 production by granulosa cells in preliminary experiments, was counted as described above.
Experiment 4: Time course of P4 production by granulosa cells with oLH, cVIP, T, and E2
In order to evaluate the time course of P4 production in culture with oLH, cVIP, T, and E2, after F1 granulosa cells (3×105 cells/well) were cultured for 48 h in serum-free medium, the cells were washed and cultured in serum-free medium with or without oLH (100 ng/mL), cVIP (10−6 M), T (10−6 M) and E2 (10−6 M) for 4, 6, 8, 12, 24, 36, or 48 h, respectively. After the culture, the medium was frozen and stored at −20°C.
Experiment 5: Effect of E2 pretreatment on LH-stimulated P4 production by granulosa cells
To know whether or not the pretreatment with E2 affects the responsiveness of granulosa cells to LH for P4 production, after the preculture for 48 h with or without increasing doses (10−9, 10−8, 10−7, and 10−6 M) of E2, the F1 granulosa cells (3×105 cells/well) were washed and recultured again with or without oLH (100 ng/mL) for 4 h. After the culture, the medium was stored at −20°C.
To examine whether or not the enhancement of LH-stimulated P4 production with E2 is dependent on the time of pretreatment, F1 granulosa cells (3×105 cells/well) cultured for 48 h were pretreated with E2 (10−6 M) in different period of the time for 6, 12, 24, 36, or 48 h, respectively, before 96 h of culture. After this pretreatment, the cells were washed and then recultured again with or without oLH (100 ng/mL) for 4h. After the 4 h cultures, the medium was stored at −20°C.
Experiment 6: Effect of E2 in short-term culture on P4 and T production by theca internal cells
To examine whether E2 affects P4 and T production by theca internal cells of F1, F2, and F3-5, theca internal cells (2×105 cells/mL/tube) were incubated for 4h with or without E2 at the concentrations of 10−9, 10−8, 10−7, and 10−6 M and oLH (100 ng/mL) and oFSH (100 ng/mL), respectively. After the incubation, the culture medium was collected and stored at −20°C.
Progesterone and testosterone radioimmunoassay
In this assay, The cross-reaction for the antisera was as follows: P4 antiserum <0.1% with 17α-hydroxy-P4, <0.06% with T, 0.006% with E2, <0.02% with dehydroepiandrosterone and negligible with other steroids: T antiserum <0.0032% with 20α-dihydro-P6, 0.0032% with 17α-hydroxy-P4, 0.0097% with P4, 0.0032% with pregnenolone, 0.0094% with cortisol, 0.0107% with deoxycorticosterone, 1.25% with androstenedione, 0.0057% with dehydroepiandrosterone, 0.004% with E2, 0.0032% estrone, 0.0032% with 17α-hydroxypregnenolone, 0.0093% with 20β-hydroxy-P6, E2 antiserum −3.2% with estrone, 1.77% with estriol, 0.8% with estradiol-17α, 0.44% with androstenedione, 0.19% with T and negligible with other steroid. Radioactive [1, 2, 6, 7- 3H] P4, [1, 2, 6, 7-3H] T were obtained from Amersham Corp. (UK). P4 in the medium was measured without extraction. In brief, 100 μL of the sample dissolved in 1% BSA – 0.1 M phosphate saline buffer (PBS), 100 μL of the steroid antiserum diluted with 1% normal rabbit serum-0.05 M EDTA – 0.1 M PBS and 100 μL of 1% BSA – 0.1 M PBS containing the 3H steroid of about 25.000 cpm were mixed in glass tubes, and incubated for 24 h at 4°C. For the separation of bound and unbound steroid, 0.2 mL of dextran-coated charcoal suspension consisting of 6.5 g charcoal Norit A and 0.65 g dextran T-70 (Pharmacia, Uppsala, Sweden) per liter of 0.01 M PBS was added, and the tubes were kept in an ice bath for exactly 30 min. After centrifugation at 1,500×g for 15 min at 4°C, the supernatant was decanted into a vial containing 4 mL of ACS-II scintillator (Amersham Corp, UK) and vortexed for about 10 s. On the following day, the radioactivity was counted for 5 min. All samples were measured in duplicate. The assay sensitivity of P4 and T (more than a 2SD different from zero bound) were 2.5 pg per tube in P4 and T. Intra and interassay coefficients of variations of P4 and T were 6.0% and 12.5%; 10.2% and 15.2%, respectively.
Statistical analyses
At least three independent replicates in each experiment were performed. Data was analyzed by analysis of variance, and followed by Duncan’s new multiple range tests among more than two means and by Student’s t test between two means.
RESULTS
Experiment 1: Effect of LH, T, and E2 in short-term culture on P4 production by granulosa cells
As shown in Figure 1 and 2, basal production of P4 for 4 h in short-term culture system was about five folds greater in F1 granulosa cells than in F3 granulosa cells. P4 productions by the F1 and F3 granulosa cells were increased by addition of oLH from 20 and 80 ng/mL, respectively, in a dose-related manner, but not by that of T and E2 in doses used.
Figure 1.
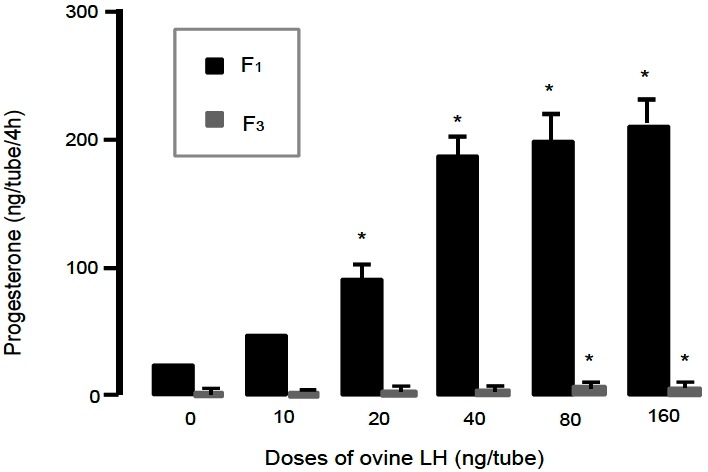
Progesterone production by granulosa cells of the largest and third largest preovulatory follicles cultured with ovine LH for 4 h in short-term culture system. LH, luteinizing hormone.
Figure 2.
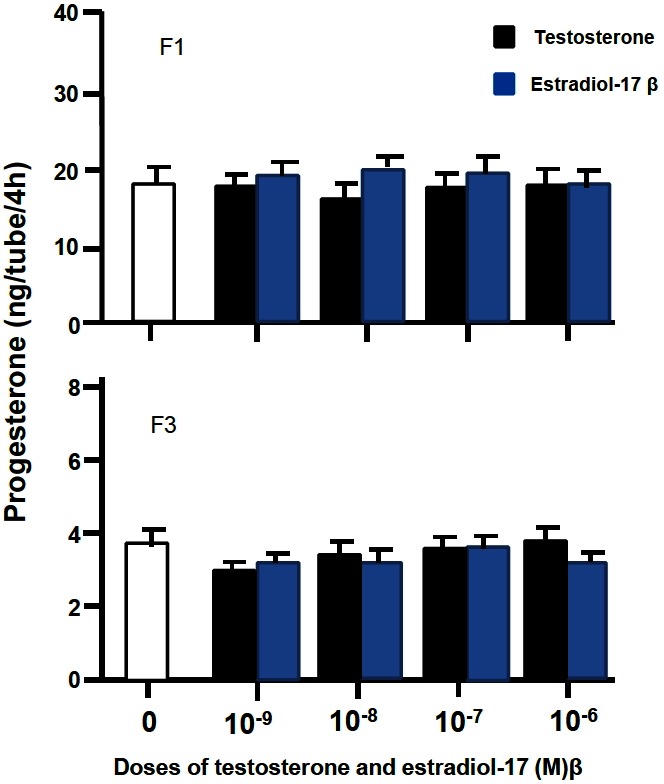
Progesterone production by granulose cells of the largest and third largest preovulatory follicles incubated with testosterone or estradiol-17β for 4 h in short-term culture system.
Experiment 2: Monolayer culture of granulosa cells
When F1 granulosa cells of the hen were cultured for 96 h in Mc Coy’s 5a medium without serum, Ham’s F12 medium or basic medium consisting of a 1:1 mixture of Mc Coy’s 5a and Ham’s F12 medium, no surviving cells were observed after the cultures for 96 h. However, when the cells were cultured in serum-containing medium, the basic medium supplemented with 10% fetal calf serum, or serum-free medium supplemented with insulin, transferrin, B and BSA, cells were alive and looked healthy under a microscope until the end of 96 h-culture (Figure 3). Granulosa cells (3×105 cells/well) of the largest preovulatory follicles were cultured for 96 h in serum-free medium (Figure 3A-up side) supplemented with insulin 20 mU/mL, transferrin 5 μg/mL, corticosterone 20 ng/mL, and BSA 4 mg/mL, the basal medium consisting of a 1:1 mixture of Mc Coy’s 5a (without serum) and Hams-12F medium or serum-containing medium. (Figure 3B-down side) supplemented with 10% vol/vol of fetal calf serum to the basal medium. The medium was renewed every 48 h. At 48 and 96 h of the culture, morphological appearance of cells was observed, cell number was counted and P4 in the medium was measured. During the cultures the number of cells increased, although the latter was less in the serum-free medium than in the serum containing medium (p<0.05; Figure 4). In contrast, basal P4 production was significantly greater in the cells cultured in serum-free medium than in those cultured in serum-containing medium (p<0.05; Figure 5).
Figure 3.
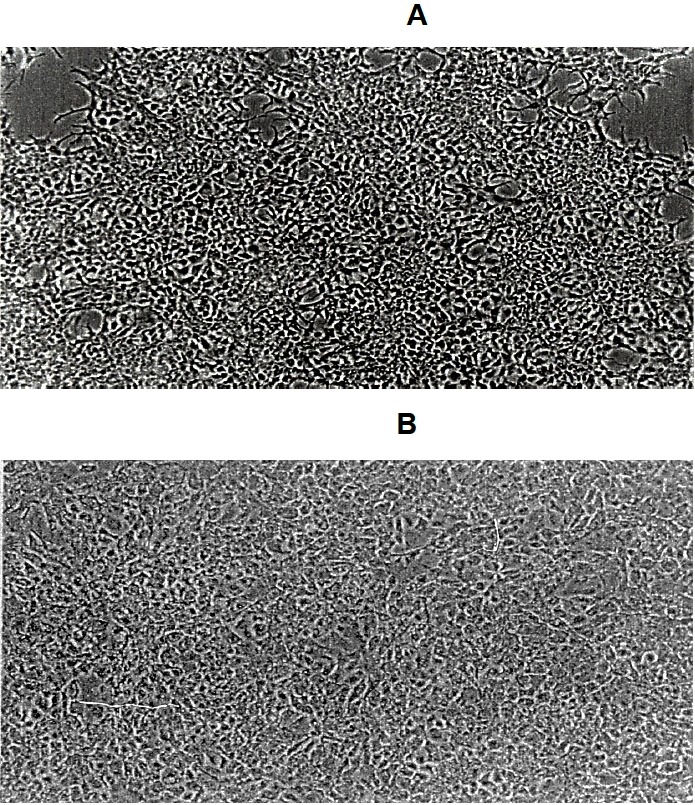
Morphological appearance (×100) of hen granulosa cells cultured for 96 h in serum-free medium and serum-containing medium.
Figure 4.
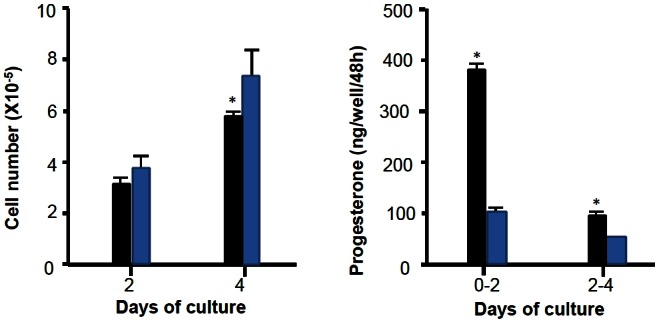
Cell proliferation and progesterone production of granulosa cells cultured in serum-free medium and serum-containing medium and their progesterone production.
Figure 5.
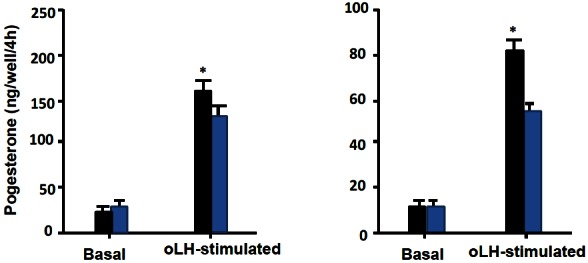
Basal and LH-stimulated progesterone production by granulosa cells cultured in serum-free medium and serum-containing medium. LH, luteinizing hormone.
Furthermore, the responsiveness of the cells to oLH for P4 production, which was examined by measuring P4 in the medium after exposing to oLH (100 ng/mL) for 4 h following the 96 h of the culture, was significantly greater in the culture in serum-free medium than in that in serum-containing medium (p<0.05; Figure 4). Based on the results described above, the serum-free medium supplemented with insulin, transferrin, B and BSA to the basic medium was used in the following experiments.
Experiment 3: P4 production by granulosa cells in long-term culture
As shown in Figure 6 and 7, basal P4 production for 48 h during 48 to 96 h of the culture was about nine folds greater in F1 granulosa cells than F3 granulosa cells. In both F1 and F3 granulosa cells, P4 production was increased in a dose dependent manner by oLH, cVIP, and T (p<0.05) but not by other substances (p>0.05), without affecting the number of cells. However, the rate of increase in P4 production compared with each basal P4 production was about half in F3 granulosa cells than in F1 granulosa cells. Also only in F3 granulosa cells, P4 production was stimulated 1.46 and 1.95 fold by oFSH at the concentrations of 10 and 100 ng/mL, respectively.
Figure 6.
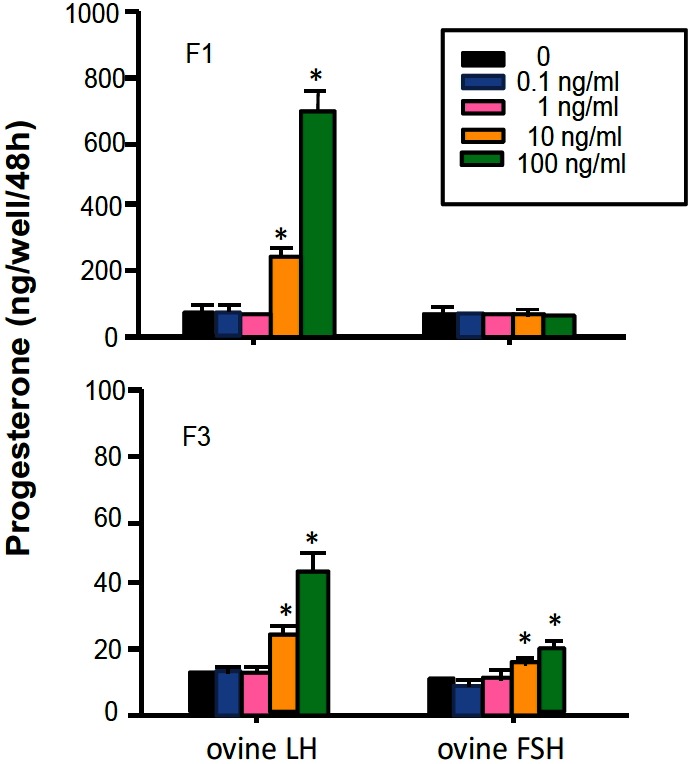
Progesterone production by granulosa cells of the largest and third largest preovulatory follicles cultured with ovine LH or FSH for 48 h in monolayer culture system. LH, luteinizing hormone; FSH, follicle stimulating hormone.
Figure 7.
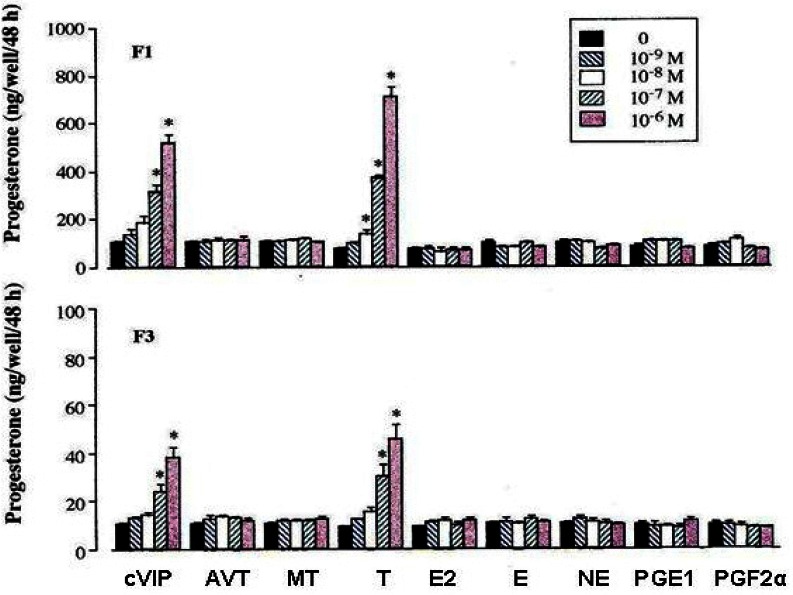
Progesterone production by granulosa cells of the largest and third largest preovulatory follicles cultured with various physiological substances for 48 h in monolayer system. cVIP, chicken vasoactive intestinal peptide; AVT, arginine vasotocin; MT, mesotocin testosterone; T, testosterone; E2, estradiol-17β; E, epinephrine; NE, norepinephrine; PGE1, prostaglandin E1; PGF2α, prostaglandin F2α.
Experiment 4: Time course of P4 production by granulosa cells with oLH, cVIP, T, and E2
When the time course of P4 production by oLH, cVIP, T, and E2 in F1 granulosa cells was examined for 48 h during 48 to 96 h of culture (Figure 8), although E2 had no effect on P4 production by granulosa cells of F1 during the period from 48 until 96 h of the culture, the increase in P4 production by oLH was found at 4 h of the culture with the maximal 9.14-fold level at 6 h. By contrast, the increases in P4 production by cVIP and T were found between 8 to 12 h of the culture (p<0.05) with the maximal 6.50-fold level at 12 h and the 6.48-fold level at 36 h, respectively.
Figure 8.
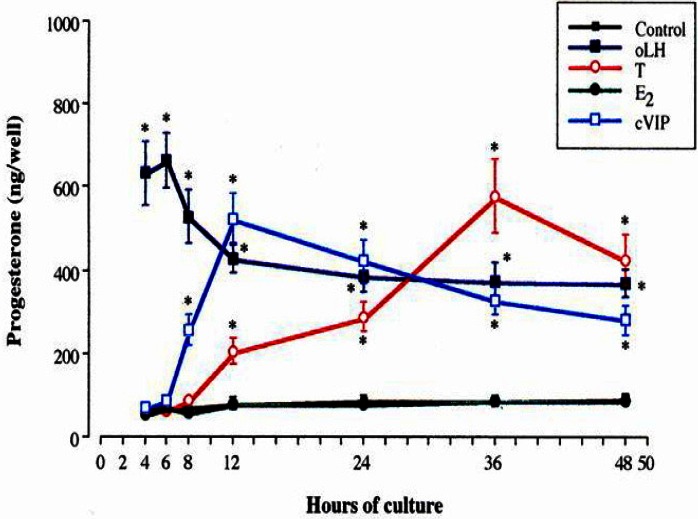
Time-course of progesterone production by granulosa cells of the largest preovulatory follicle with oLH, cVIP, testosterone and estradiol-17β. oLH, ovine luteinizing hormone; cVIP, chicken vasoactive intestinal peptide.
Experiment 5: Effect of pretreatment with E2 on LH-stimulated P4 production by granulosa cells
As shown in Figure 9, P4 production in response to oLH for 4 h at or with 96 h of culture was increased with the pretreatment of E2 for 48 h without affecting the cell number. This increase was greater as the dose of E2 was increased. In addition, the enhancement of the responsiveness to oLH with E2 was found at more than 12 h of pretreatment (p<0.05, Figure 10).
Figure 9.
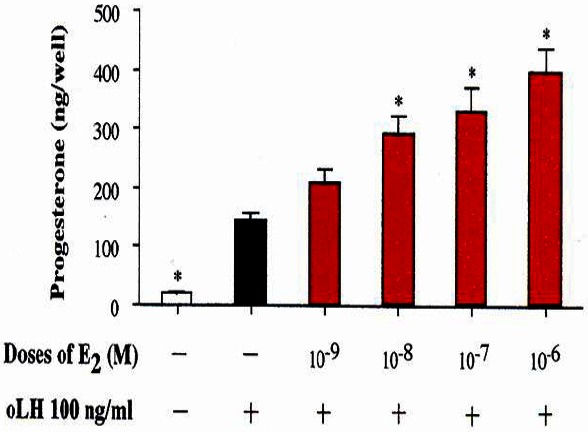
Effect of pretreatment with estradiol-17β on LH-stimulated progesterone production. LH, luteinizing hormone.
Figure 10.
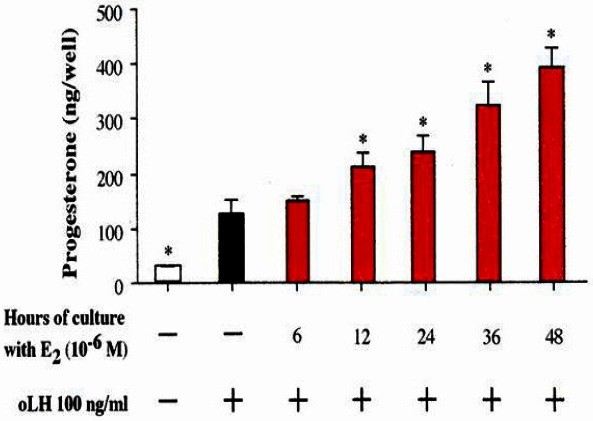
Time dependence for the enhancing effect of estradiol–17β on LH-stimulated progesterone production. LH, luteinizing hormone.
Experiment 6: Effect of E2 and gonadotropins for short-term on P4 production by theca internal cells
As shown in Figure 11 and 12, E2 at the concentration of 10−6 M was found to enhance the productions of P4 and T by theca internal cells. In addition, these increases were greater in smaller follicles in both steroid hormones. Also, oLH and oFSH stimulated the productions of P4 and T by theca internal cells with greater response in smaller follicles.
Figure 11.
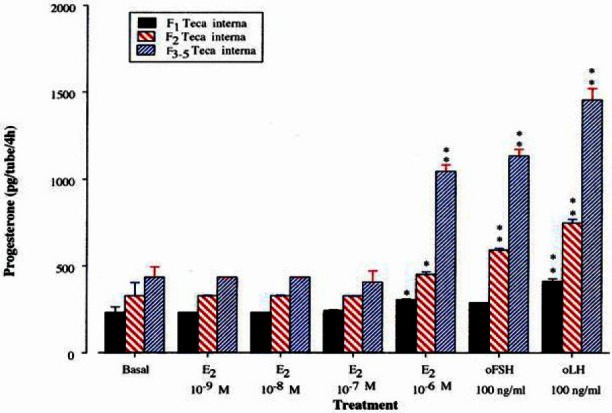
Progesterone production by theca interna cells of the largest, second and third-fifth preovulatory follicles incubated with estradiol-17β for 4 h.
Figure 12.
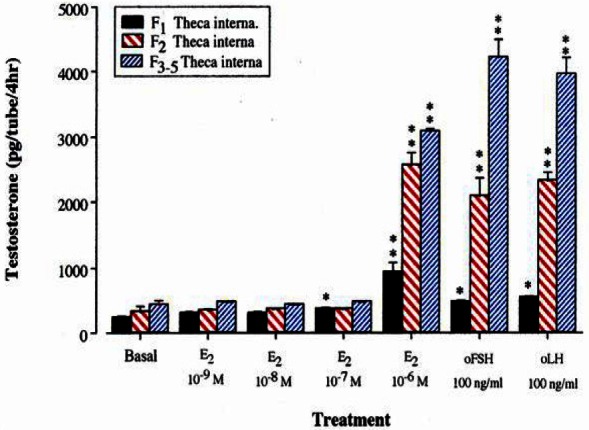
Testosterone production by theca interna cells of the largest, second and third-fifth preovulatory follicles incubated with estradiol-17β for 4 h.
DISCUSSION
In the present experiments, a 1:1 mixture of Mc Coy’s 5a medium and Ham’s F12 medium was used as a basic medium. Mc Coy’s 5a medium contains large amounts of amino acids (Mikami, 1980), while Ham’s F12 medium is composed of a number of components (Ham, 1965). Mc Coy’s 5a medium, Ham’s F12 medium, and the basic medium did not support the survival of the cultured hen granulosa cells during 96 h of the cultures. However, when the combination of insulin, transferrin, B and BSA or fetal calf serum, which is known to enhance growth and functions of the cells (Heuson et al., 1967; Griffith, 1970; Channing et al., 1976; Barnes and Hammond, 1980; Orly et al., 1980), were added to the basic medium, healthy cells were maintained for at least 96 h and the cultured cells attached to the bottom of the wells, proliferated and responded to LH for P4 production. Although the rate of cell proliferation was lower than in cells cultured in serum-containing medium, the cells cultured in serum-free medium showed greater basal and LH-stimulated P4 production, than those cultured in serum-containing medium. Thus, the serum-free medium is much more suitable for the study of the regulation mechanism of P4 production by hen granulosa cells, than the serum-containing medium. Similar results have been report in the culture of mammalian granulosa cells (Orly et al., 1980; Barano and Hammond, 1985; Chin and Abayasekara, 2004). The use of serum-free medium supplemented with insulin, transferrin, B and BSA to the basic medium, enable to study the long action of hormones on P4 production by hen granulosa cells. Therefore, in the present experiments, the developed monolayer culture system using the chemically defined serum-free medium was used as the long-term culture system in the subsequent experiments.
In mammals, granulosa cells have been shown to metabolize androgens into estrogens (Evans et al., 1981; Wang and Bahr, 1983), but in birds, aromatase enzymes, capable of catalyzing the conversion of androgens into estrogens are not present in granulosa cells of preovulatory follicles (Armstrong, 1984). The present experiments showed that basal P4 production for 48 h during 48 to 96 h of culture is about nine-greater in F1 granulosa cells, than in F3 granulosa cells. Furthermore, LH, cVIP, and T, but not E2, stimulated P4 production without affecting the cell number in both F1 and F3 granulosa cells of hen granulosa cells with the treatment of these hormones for 48 h. However, the rate of increase in P4 production by these hormones compared to the basal P4 production of each follicle was about half in F3 granulosa cells, than F1 granulosa cells. In addition, FSH was found to stimulated P4 production by F3 granulosa cells, but not by F1 granulosa cells. These results are in agreement with those of many investigators in short-term culture system, showing that the production of P4 by hen granulosa cells in culture is dependent on follicular maturation (Asem and Hertelendy, 1986; Johnson, 1990; Johnson and Bridghan, 2001; Woods et al., 2007).
Johnson and Tilly (1988), who examined the effect of VIP on P4 production in F1 granulosa cells, have shown that a significant increase in P4 production in response to VIP is not detectable until after 8 h of the culture. Johnson et al. (1994), who found that immune-reactive VIP is located within the theca internal and theca external layers of nonhierarchical follicles in the hen, but not within granulosa cells of these follicles, reported that granulosa cells from 9 to 12 mm small follicles responded to cVIP with increased P4 production in 3 h-short incubation, whereas the P4 production in F1 granulosa cells was not increased with the treatment of cVIP for 3 h. Although the present experiment, did not examine the time course of P4 production in response to cVIP in F3 granulosa cells, because basal P4 production by F3 granulosa cells were less than by F1 granulosa cells, P4 production in F1 granulosa cells treated with cVIP began to increase from 8 h of the treatment with the maximal increase at 12 h. This is consistent with previous findings (Johnson and Tilly, 1988), that the action of VIP for stimulation of P4 production in F1 granulosa cells is required for long-term treatment. Recently, VIP receptors were found that exist in granulosa cells of the hen (Kikushi and Ishii, 1992). Therefore, the present results, together with previous results, indicate that VIP diffused from the theca layer of follicles have a direct stimulatory effect in a long-term action on P4 production by granulosa cells of the hen via receptors of VIP.
The present results, showed that the P4 production by hen granulosa cells is enhanced with the treatment of T alone for 48 h, as reported by (Porter et al., 1989), who examined the production of P4 in hen granulosa cells treated with androgens for 2 days in 199 medium, supplemented with 5% porcine serum. However, in their report (Porter et al., 1989), the cultured cells were not attached to the bottom of the wells, and, moreover, the effect of androgens on P4 production were not examined except that with the treatment for 2 days, during the present experiments, the culture cells attached to the bottom of the wells showed the responsiveness to LH. Furthermore, the enhanced effect of T on the production of P4 was found to be necessary for treatment lasting more than 12 h, with a maximal enhancement at 36 h of treatment. These results indicate that the long-term action of T clearly stimulate the production of P4 by hen granulosa cells, although hen granulosa cells maintained for several days in cultured have been report to metabolize P4 to a number of steroids, the principal of which is 3α-hydroxy-5β-pregnan-20one (Williams and Sharp 1978; Von Engelhard and Groothuis, 2005).
The present results showed that LH-stimulated P4 production in hen granulosa cells is enhanced by pretreatment with E2 for 48 h as in the previous report (Kamiyoshi et al., 1992). In addition, the enhancement of the LH-stimulated P4 production with E2 was found only when the granulosa cells were pretreated with E2 for more than 12 h, indicating that E2 in long-term action may prime the hen granulosa cells to enhance the responsiveness to LH for P4 production.
In contrast, to the present results and of the Porter et al. (1989) and Johnson et al. (1988), who studied the influence of androgens and E2 on P4 production by hen granulosa cells in short-term culture, showed that androgens and E2 suppress basal and LH-stimulated P4 production. Subsequently, they suggested that androgens primarily act at the conversion site of cholesterol to pregnenolone (P5) to suppress P4 production, while E2 for inhibition of P4 production acts at the conversion of P5 to P4 (Lee and Bahr, 1989; Caicedo et al., 2005). However, in their experiments on short-term culture of F1 granulosa cells, inhibitory effects of androgens and E2 on P5 and P4 biosynthesis, respectively, are caused at high concentrations of 10−6 to 10−5 M of these steroids. On the other hand, in the present experiments in long-term culture system, stimulatory effects of T and E2 on P4 production and responsiveness to LH were discovered from the concentration of 10−8 M, respectively, and further required for the treatment with those hormones for more than 12 h. Therefore, disparities in the effect of T and E2 on P4 production, between the present and their experiments may be attributed to differences in concentrations of these hormones used and/or during of treatment.
In the present experiments, maximal, stimulatory effects of LH, VIP, and T on P4 production by hen granulosa cells were obtained at 6, 12, and 36 h of the respective treatments, and enhancement of the response to LH was greatest at 48 h of E2 treatment, indicating that the action mechanism of these hormones may be different among these hormones. LH has been reported to promote Ca2+ mobilization and phosphoinositide hydrolysis, and to participate in P4 production by granulosa cells of the hen (Hertelendy et al., 1987; Krzysik-Walker et al., 2007). Although, the production of P4 by granulosa cells is known to be mediated via the adenylyl cyclase/cAMP second messenger system (Calvo and Bahr, 1983; Johnson and Tilly, 1988; Wu et al., 2003; Woods and Johnson, 2005), reported that VIP stimulates the production of cAMP, at a lower rate than LH because the effects of VIP on cAMP accumulation and P4 production in granulosa cells are not detectable, until after 8 h of the culture, while, LH significantly enhances cAMP accumulation and P4 production, after 4 h of the culture. As mentioned above, androgens and E2 in short-term culture is reported to suppress P4 production by hen granulosa cells (Johnson et al., 1988). According to the reports of Lee and Bahr (1989), androgens and E2 suppress P4 production in hen granulosa cells by inhibiting activities of cytochrome P-450 cholesterol site chain cleavage (P-450scc) and 3β-hydroxysteroid dehydrogenase, respectively. Moreover, they suggested that the inhibitory effects of androgens and E2 may not be mediated by these receptors, because E2 has been found to act as a competitive inhibitor of 3β-hydroxysteroid dehydrogenase in isolated microsomes as well as in the presence of an estrogen receptor agonist (Freeman, 1985). In contrast to inhibitory effects of androgens and E2 on P4 production in short-term culture, the present experiments showed that T might has a facilitate action on P4 synthesis by granulosa cells in long-term culture. There are reports of androgen receptors in avian (Yoshimura et al., 1995) and mammalian granulosa cells (Hsueh et al., 1983). Nuclear estrogen receptor has been reported to be present in granulosa cells of the hen (Zarrow and Bastian, 1953; Kamiyoshi et al., 1986). Action of steroid hormones, via nuclear receptors is required for long duration to induce de novo synthesis of protein (Knecht et al., 1985). Also, estrogens have been known to induce the production of LH receptors in mammalian granulosa cells (Richards et al., 1976; Ritzhaup and Bahr, 1987). Therefore, the present finding, that over a long term T stimulate P4 production in hen granulosa cells and E2 enhances the responsiveness to LH for P4 production by hen granulosa cells, would indicate that T and E2 may act via a receptor-mediated mechanism, and induce synthesis of enzymes participating in the production of P4 and synthesis of LH receptors, respectively.
In the present experiments it was found that E2 for short-terms, enhance the production of P4 and T by theca internal cells. These finding suggest that estrogens produced in theca external cells, may act in a paracrine manner, not only on granulosa cells, but also on theca internal cells. Furthermore, E2 advance the production of P4 and T in the theca internal cells, not only by gonadotropins, but estrogens as well. Although, the mechanism by which estrogens stimulate the production of P4 and T in the theca internal cells is unclear, estrogens may enhance the production of these hormones, by activating the enzymes participating in the synthesis of P4 and T, because the stimulatory action of E2 for the production of P4 and T by theca internal cells occurred in a short term, compared with the stimulatory action of T and E2 for the production of P4 by granulosa cells (Li et al., 2001; Caicedo, 2004). However, further investigations are necessary to verify these concepts.
IMPLICATIONS
The results in vivo and in vitro, this study may suggest that T and E2 began to be produced in theca cells, granulosa acting on paracrine manner and is involved in the induction of LH surge before ovulation, and this is motivated by the increased sensitivity to LH in granulosa cells, respectively. Finally, based on this study and previous studies on the interaction of gonadotropin hormones and steroids on steroidogenesis in ovarian follicles of the domestic hen, in this study a new scheme of the mechanism of hormonal interplay of steroid hormones and gonadotropin hormones is proposed in the ovary of the domestic hen (Figure 13).
Figure 13.
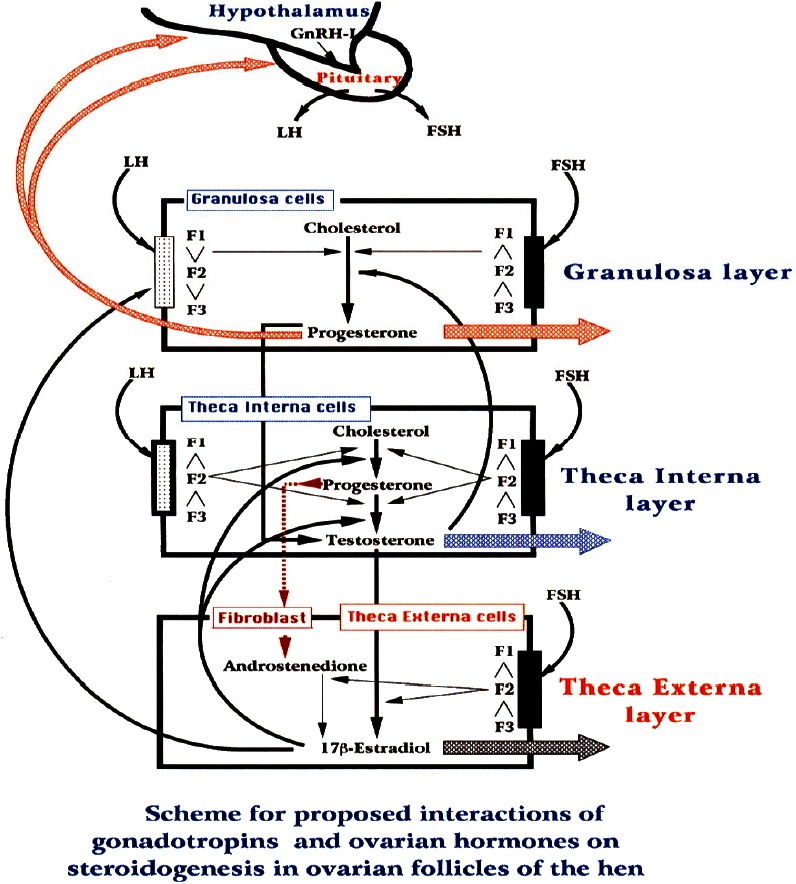
Scheme proposed by the interaction of gonadotropins and steroids hormones in ovarian follicles of domestic fowl.
ACKNOWLEDGMENTS
The authors would like to thanks Fukaya Masonori, Nonobe Yukiko and Yoshida Hitoshi for technical assistance and Kawashima Mitsuo for your advices; Drs. K. Wakabayashi, and M. Hattori, (Gunma University) kindly donated antisera to P4 and T. This research was supported by Japanese Minister Education, Science and Culture, Japan.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- Armstrong DG. Ovarian aromatase activity in the domestic fowl (Gallus domesticus) J Endocrinol. 1984;100:81–86. doi: 10.1677/joe.0.1000081. [DOI] [PubMed] [Google Scholar]
- Asem EK, Hertelendy F. Steroidogenesis and cAMP production in isolated avian granulosa cells during follicular maturation: Lack of positive correlation. Acta Endocrimol. 1986;113:289–297. doi: 10.1530/acta.0.1130289. [DOI] [PubMed] [Google Scholar]
- Barano JL, Hammond JM. Serum-free medium enhances growth and differentiation of cultured pig granulosa cells. Endocrinology. 1985;116:51–58. doi: 10.1210/endo-116-1-51. [DOI] [PubMed] [Google Scholar]
- Barnes JLS, Hammond JM. Serum-free cell culture: A unifying approach. Cell. 1980;22:649–655. doi: 10.1016/0092-8674(80)90540-1. [DOI] [PubMed] [Google Scholar]
- Caicedo RE. Steroidogenesis in theca cells of chicken follicles. Tecnociencia. 2004;6:71–83. [Google Scholar]
- Caicedo RE, Zumaquero JL, Quintero JD. Effect of estrogen in the progesterone production in granulosa cells. Scientia. 2005;17:47–56. [Google Scholar]
- Calvo FO, Bahr JM. Adenylyl cyclase system of the small preovulatory follicles of the domestic hen: Responsiveness to follicle-stimulating hormone and luteinizing hormone. Biol Reprod. 1983;29:542–547. doi: 10.1095/biolreprod29.3.542. [DOI] [PubMed] [Google Scholar]
- Channing CP, Tsai V, Sachs D. Role of insulin, cortisol in luteinization of porcine granulosa cells growth in chemically defined media. Biol Reprod. 1976;15:235–247. doi: 10.1095/biolreprod15.2.235. [DOI] [PubMed] [Google Scholar]
- Chin EC, Abayasecara DRE. Progesterone secretion by luteinizing human granulosa cells: A possible cAMP-dependent but PKA-independent mechanism involved in its regulation. J Endocrinol. 2004;183:51–60. doi: 10.1677/joe.1.05550. [DOI] [PubMed] [Google Scholar]
- Erickson GF, Wang C, Hsuech AJW. FSH induction of functional LH receptors in granulosa cells cultured in a chemically defined medium. Nature. 1979;279:336–338. doi: 10.1038/279336a0. [DOI] [PubMed] [Google Scholar]
- Evans G, Dobias M, King GJ, Armstrong DT. Estrogen, androgen, and progesterone biosynthesis by theca and granulosa of preovulatory follicles in the pig. Biol Reprod. 1981;25:673–682. doi: 10.1095/biolreprod25.4.673. [DOI] [PubMed] [Google Scholar]
- Freeman DA. Estradiol acts as a competitive inhibitor of the 3β-hidroxysteroid dehydrogenase/Δ5-Δ4 isomerase enzyme of cultured Leydig tumor cells. Endocrinology. 1985;117:2127–2133. doi: 10.1210/endo-117-5-2127. [DOI] [PubMed] [Google Scholar]
- Griffith JB. The effect of insulin on the growth and metabolism of the human diploid cell, WI-38. J Cell Sci. 1970;7:575–585. doi: 10.1242/jcs.7.2.575. [DOI] [PubMed] [Google Scholar]
- Groothuis TG, Müller W, von Engelhardt N, Carere C, Eising C. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci Biobehav Rev. 2005;29:329–352. doi: 10.1016/j.neubiorev.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Ham RG. Clonal growth of mammalian cells in a chemically defined, synthetic medium. Proc Natl Acad Sci. 1965;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond RW, Olson DW, Frenkel RB, Biellier HV, Hertelendy F. Prostaglandins and steroid hormones in plasma and ovarian follicles during the ovulation cycle of the domestic hen (Gallus domesticus) Gen Comp Endocrinol. 1980;42:195–202. doi: 10.1016/0016-6480(80)90188-4. [DOI] [PubMed] [Google Scholar]
- Hertelendy F, Nemecz B, Asem EK. Influence of follicular maturation on LH-promoted Ca2+ mobilization phosphoinositide hydrolysis in granulosa cells of the domestic fowl. Biol Reprod Suppl. 1987;1:125. [Google Scholar]
- Heuson JC, Coune A, Heimann R. Cell proliferation induced by insulin in organ culture of rat mammary carcinoma. Exp Cell Res. 1967;45:351–360. doi: 10.1016/0014-4827(67)90185-1. [DOI] [PubMed] [Google Scholar]
- Hsueh AJW, Jones PBC, Adashi EY, Wang C, Zhuang LZ, Welsh THJ. Intraovarian mechanisms in the hormonal control of granulosa cell differentiation in rats. J Reprod Fertil. 1983;69:325–342. doi: 10.1530/jrf.0.0690325. [DOI] [PubMed] [Google Scholar]
- Huang ESR, Nalbandov AV. Steroidogenesis of chicken granulosa theca cells: in vitro incubation system. Biol Reprod. 1979;20:442–453. doi: 10.1095/biolreprod20.3.442. [DOI] [PubMed] [Google Scholar]
- Johnson AL. Steroidogenesis and actions of steroids in the hen ovary. Cri Rev Poult Biol. 1990;2:319–346. [Google Scholar]
- Johnson AL, Bridghan JT. Regulation of steroidogenic acute regulatory protein and luteinizing hormone receptor messenger ribonucleic acid in hen granulosa cells. Endocrinology. 2001;142:3116–3124. doi: 10.1210/endo.142.7.8240. [DOI] [PubMed] [Google Scholar]
- Johnson AL, Li Z, Gibney JA, Malamed S. Vasoactive intestinal peptide-induced expression of cytochrome P-450 cholesterol side-chain cleavage 17α-hydroxylase enzyme activity in hen granulosa cells. Biol Reprod. 1994;51:327–333. doi: 10.1095/biolreprod51.2.327. [DOI] [PubMed] [Google Scholar]
- Johnson AL, Tilly JL. Effects of vasoactive intestinal peptide on steroid secretion and plasminogen activator activity in granulosa cells of the hen. Biol Reprod. 1988;38:296–303. doi: 10.1095/biolreprod38.2.296. [DOI] [PubMed] [Google Scholar]
- Johnson PA, Green C, Lee HT, Bahr JM. Inhibition of progesterone secretion from granulosa cells by estradiol and androgens in the domestic hen. Endocrinology. 1988;123:473–477. doi: 10.1210/endo-123-1-473. [DOI] [PubMed] [Google Scholar]
- Kamiyoshi M, Kawashima M, Tanaka K. Estrogens promote cultured granulosa cells of the hen for progesterone production in response to LH. Jpn Poult Sci. 1992;29:91–97. [Google Scholar]
- Kamiyoshi M, Niwa T, Tanaka K. Nuclear estrogen receptor bindings in granulosa cells estradiol-17β in follicular membranes of the ovary of the hen during the ovulatory cycle. Gen Comp Endocrinol. 1986;61:428–435. doi: 10.1016/0016-6480(86)90229-7. [DOI] [PubMed] [Google Scholar]
- Kikushi M, Ishii S. Changes in luteinizing hormone receptors in the granulosa and theca layers of the ovarian follicle during follicular maturation in the Japanese quail. Gen Comp Endocrinol. 1992;85:124–137. doi: 10.1016/0016-6480(92)90180-r. [DOI] [PubMed] [Google Scholar]
- Knecht M, Morris CHT, Catt KJ. Estrogen dependence of luteinizing hormone receptor expression in cultured rat granulosa cells. Inhibition of granulosa cell development by the antiestrogens tamoxifen and keoxifene. Endocrinology. 1985;116:1771–1777. doi: 10.1210/endo-116-5-1771. [DOI] [PubMed] [Google Scholar]
- Krzysik-Walker SM, Ocón-Grove Olga M, Maddineni SB, Hendricks GL, III, Ramachandran R. Identification of calcitonin expression in the chicken ovary: Influence of follicular maturation and ovarian steroids. Biol Reprod. 2007;4:626–635. doi: 10.1095/biolreprod.106.054957. [DOI] [PubMed] [Google Scholar]
- Lee HT, Bahr JM. Inhibitory sites of androgens and estradiol in progesterone biosynthesis in granulosa cells of the domestic hen. Endocrinology. 1989;125:760–765. doi: 10.1210/endo-125-2-760. [DOI] [PubMed] [Google Scholar]
- Li X, Peegel H, Menon KMJ. Regulation of high density lipoprotein receptor messenger ribonucleic acid expression and cholesterol transport in theca-interstitial cells by insulin and human chorionic gonadotropin. Endocrinology. 2001;142:174–181. doi: 10.1210/endo.142.1.7865. [DOI] [PubMed] [Google Scholar]
- Marrone BL, Hertelendy F. Steroidogenesis by avian ovarian cells: Effects of luteinizing hormone and substrate availability. Am J Physiol. 1983;244:E487–E493. doi: 10.1152/ajpendo.1983.244.5.E487. [DOI] [PubMed] [Google Scholar]
- Mikami S. Hypothalamic control of the avian adenohypophysis. In: Tanabe Y, Tanaka K, Ookawa T, editors. Biological Rhythmus in Birds: Neural Endocrine Aspects. Jp Sc Soc Press, Springer-Verlag; Heidelberg, Berlin, Germany: 1980. pp. 17–32. [Google Scholar]
- Orly J, Sato G, Erickson GF. Serum suppresses the expression of hormonally induced functions in cultured granulosa cells. Cell. 1980;20:817–827. doi: 10.1016/0092-8674(80)90328-1. [DOI] [PubMed] [Google Scholar]
- Perry MM, Gilbert AB, Evans AJ. Electron microscope observations on the ovarian follicle of the domestic fowl during the rapid growth phase. J Anat. 1978;125:481–497. [PMC free article] [PubMed] [Google Scholar]
- Porter TE, Hargis BM, Silsby JL, El Halawani ME. Differential steroid production between theca interna and theca externa cells: A three-cell model for follicular steroidogenesis in avian species. Endocrinology. 1989;125:109–116. doi: 10.1210/endo-125-1-109. [DOI] [PubMed] [Google Scholar]
- Richards JS, Ireland JJ, Rao MC, Bernath GA, Midgley ARJ, Reichert LEJ. Ovarian follicular development in the rat: Hormone receptor regulation by estradiol, follicle stimulating hormone and luteinizing hormone. Endocrinology. 1976;99:1562–1570. doi: 10.1210/endo-99-6-1562. [DOI] [PubMed] [Google Scholar]
- Ritzhaup LK, Bahr JM. A decrease in FSH receptors of granulosa cells during follicular maturation in the domestic hen. J Endocrinol. 1987;115:303–310. doi: 10.1677/joe.0.1150303. [DOI] [PubMed] [Google Scholar]
- Robinson FE, Renema RA. Principios del manejo de los foto períodos en Reproductoras de engorde. Editorial Cobb-Vantress Incorporation, Arkansas, USA. Boletin Técnico. 1999;7:1–6. [Google Scholar]
- Rothwell B, Salomon SE. The ultrastructure of the follicle wall of the domestic fowl during the phase of rapid growth. Br Poult Sci. 1977;18:605–610. doi: 10.1080/00071667708416409. [DOI] [PubMed] [Google Scholar]
- Schally AV, Nair RM, Redding TW, Arimura A. Isolation of the luteinizing hormone and follicle-stimulating hormone-releasing hormone from porcine hypothalamus. J Biol Chem. 1971;23:7230–7236. [PubMed] [Google Scholar]
- Seol HS, Sato K, Matsubara Y, Schneider WJ, Akiba Y. Modulation of sterol regulatory element binding protein-2 in response to rapid follicle development in chickens. Comp Biochem Physiol Part B: Biochem Mol Biol. 2007;147:698–703. doi: 10.1016/j.cbpb.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Von Engelhard NK, Groothuis TGG. Measuring steroid hormones in avian eggs. Ann NY Acad Sci. 2005;1046:181–192. doi: 10.1196/annals.1343.015. [DOI] [PubMed] [Google Scholar]
- Wang SC, Bahr JM. Estradiol secretion by theca cells of the domestic hen during the ovulatory cycle. Biol Reprod. 1983;28:618–624. doi: 10.1095/biolreprod28.3.618. [DOI] [PubMed] [Google Scholar]
- Wells JW, Dick HR, Gilbert AB. The biosynthesis of progesterone by fowl granulosa cells in vitro from 14C-labelled substrates. J Steroid Biochem. 1981;14:651–656. doi: 10.1016/0022-4731(81)90376-9. [DOI] [PubMed] [Google Scholar]
- Williams JB, Sharp PJ. Control of the preovulatory surge of luteinizing hormone in the hen (Gallus domesticus): The role of progesterone and androgens. J Endocrinol. 1978;77:57–65. doi: 10.1677/joe.0.0770057. [DOI] [PubMed] [Google Scholar]
- Woods DC, Johnson AL. Regulation of follicle-stimulating hormone-receptor messenger RNA in hen granulosa cells relative to follicle selection. Biol Reprod. 2005;72:643–650. doi: 10.1095/biolreprod.104.033902. [DOI] [PubMed] [Google Scholar]
- Woods DC, Haugen MJ, Johnson AL. Actions of epidermal growth factor receptor/mitogen-activated protein kinase and protein kinase C signaling in granulose cells from Gallus gallus are dependent upon stage of differentiation. Biol Reprod. 2007;77:61–70. doi: 10.1095/biolreprod.106.059394. [DOI] [PubMed] [Google Scholar]
- Wu Q, Sucheta S, Azhar A, Menon KMJ. Lipoprotein enhancement of ovarian theca-interstitial cell steroidogenesis: Relative contribution of scavenger receptor class B (type I) and adenosine 5- triphosphate-binding cassette (type A1) transporter in high-density lipoprotein-cholesterol transport and androgen synthesis. Endocrinology. 2003;144:2437–2445. doi: 10.1210/en.2002-221110. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Okamoto T, Tamura T. Effects of luteinizing hormone follicle-stimulating hormone on the progesterone receptor induction in chicken granulosa cells in vivo. Poult Sci. 1995;74:147–151. doi: 10.3382/ps.0740147. [DOI] [PubMed] [Google Scholar]
- Zarrow MX, Bastian JW. Blockade of ovulation in the hen with adrenolytic and parasympatholytic drugs. Proc Soc Exp Biol Med. 1953;84:457–459. doi: 10.3181/00379727-84-20676. [DOI] [PubMed] [Google Scholar]


