SECTION 1
An 11-year-old, right-handed girl with normal development, no significant medical history, and no known epilepsy risk factors presented to the clinic for evaluation of new-onset epilepsy.
Her first unprovoked seizure began with the symptom of “blurry vision” and an “inability to see right.” Minutes later, she started to turn clockwise, her right arm pointing in the air, in a nonpurposeful manner, before her body “became very limp” and she collapsed. Subsequently, she had several convulsive seizures requiring IV benzodiazepines and temporary intubation.
Over the next 2 months, the patient continued to have stereotyped seizures. All seizures were preceded by sensory auras of the right arm and occasional foot numbness and tingling paresthesias, as well as by frequently simultaneous complaints of “blurry vision” or complete “loss” of vision. Then there would be a forced head version with tonic gaze deviation to the right, and finally a hemi-tonic-clonic activity of the right arm > leg, with full-body version to the right.
Question for consideration:
To which brain region does the patient's seizure semiology localize best?
SECTION 2
The complaint of “blurry vision” and “inability to see right” is vague and could include anything from diplopia to nystagmus over distinct visual field deficits, vs scotomas to complete blindness, vs a neglect phenomenon.
Ictal nystagmus can be an isolated presentation of focal seizures and usually localizes to the posterior quadrant of the cerebral hemisphere (i.e., occipital, parietal, or posterior temporal lobe). The fast phase of the nystagmus is directed away from the seizure focus. Focal seizures originating from the occipital lobes can be responsible for elementary or complex visual hallucinations. They can also present as “negative symptoms” with various degrees of vision loss, including small focal scotomas, partial, or complete visual field loss (“ictal amaurosis”) affecting the visual hemifield or quadrant contralateral to the seizure focus.1
Somatosensory symptoms are the most common auras associated with parietal lobe seizures followed by affective, vertiginous, and visual auras.2
Both the occipital and parietal lobe are relatively small lobes that are well connected to the ipsilateral frontal and temporal lobe, thalamus, and the supplementary sensorimotor areas (SSMAs).2 Motor representation in the SSMAs is bihemispheric and these regions are well connected with subcortical structures, which can result in seizures involving unilateral asymmetric tonic posturing of extremities, complex limb movements (e.g., bicycling), and truncal rotation.3
The visual phenomena of the patient's seizures could be the result of an occipital seizure and the right sensory aura of a left parietal lobe seizure. Both seizures could subsequently evolve either into an asymmetric tonic posturing and/or complex motor movements of the right arm and body (e.g., pointing or truncal rotation) as they spread to the ipsilateral SSMA or into a right versive head turn, tonic gaze deviation, and hemi-clonic activity through involvement of the left frontal lobe.
Initial and further evaluation.
The initial evaluation of the patient included a neurologic examination, urine toxicology, basic labs, a noncontrasted head CT and brain MRI, which were unrevealing. A lumbar puncture (LP) was unremarkable with the exception of a mild lymphocytic predominant leukocytosis (7 leukocytes, 85% lymphocytes).
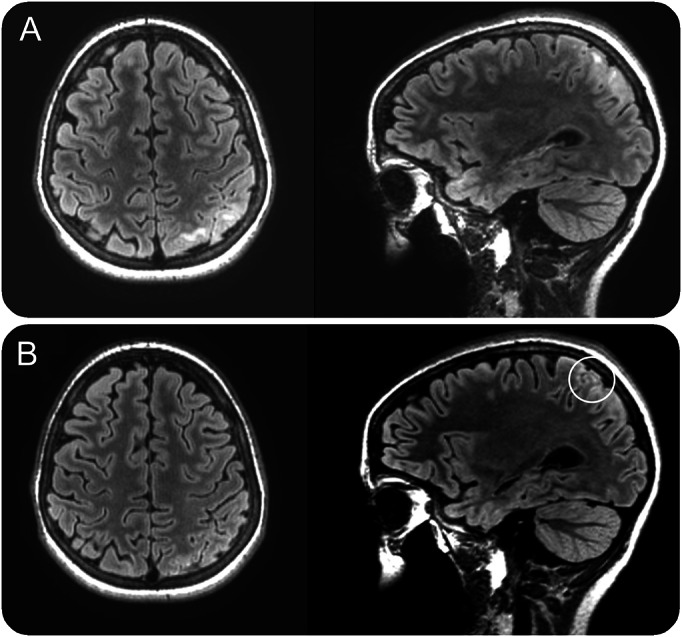
Over the course of the following 10 months, the patient was admitted multiple times for cluster of seizures. The video-EEG monitoring (vEEG) tracing during one of these admissions is shown in figure e-1, A–C, on the Neurology® Web site at Neurology.org. Parts of the writing and reading tasks of a formal neuropsychological (NP) evaluation between admissions are shown in figure e-2 and video 1. Video 2 shows a motor task from a second NP testing session conducted with concordant vEEG 2 months into optimized treatment with anticonvulsants. A 3-tesla (3T) MRI with epilepsy protocol and gadolinium early (A) and 2 months into treatment (B) is shown in figure 1.
Figure 1. Snapshots of axial and sagittal T2 fluid-attenuated inversion recovery sequence of an MRI early (A) and 2 months (B) into optimized seizure treatment.
Questions for consideration:
What are the abnormal findings on the vEEG and the NP testing?
How do they tie in with our suspected localization?
SECTION 3
The vEEG tracing in figure e-1 shows continuous focal slowing with epileptiform discharges over the left centroparietal region (maximal at C3 and P3) in wakefulness (A) and non-REM sleep (B). Figure e-1C shows a focal seizure arising from C3.
The writing sample in figure e-2 shows 3 aspects: (1) the patient starts to use only the left half of the space to complete the sentence; (2) her handwriting changes and becomes less legible; and (3) there are numerous spelling errors at the end of the page. As the figure shows, the right-sided neglect resolves while the dysgraphia worsens over the course of the test.
A profound hemineglect is a relatively rare ictal symptom and has been more commonly described as a postictal deficit affecting the nondominant parietal lobe.4 Among the group of the so-called “negative seizures,” speech arrest and retrograde amnesia seem to be the most common ictal phenomena, but various types of apraxia, aphasia, homonymous hemianopsia, and acute-onset hemiparesis have also been described as rare seizure semiologies.5
A writing disorder in which orthographic movements are diminished despite normal sensorimotor function of the hand and with preserved oral spelling and typing have been discussed as “apractic agraphia,” which might result from a loss of representation for movements of writing due to interruption of associative fibers from the angular gyrus to the superior parietal lobule within the dominant (left) hemisphere.6
The reading sample in video 1 is considered to be an easy text given the patient's age, but she has significant difficulties reading it. In particular, she appears to be dysfluent and hesitant, omits the initial letters of words, has omissions, transpositions, and substitutions of function words, and at one point she actually stops reading halfway across the page, ignoring the right half of the sentence. Just minutes later, the patient was capable of reading a more complex text more fluently and with significantly less mistakes.
A dysfluent and hesitant reading style could be related to a right hemifield cut (hemianopic alexia). Hemianopic alexia is characterized by reduced reading speed. As the visual field region used for reading is asymmetric, requiring a larger perceptual span to the right, patients with right-sided hemifield deficits are more impaired in their reading fluency, while patients with a left hemifield deficit often have more problems with finding the next line of the text.7
Video 2 shows part of a follow-up NP evaluation with simultaneous vEEG: it demonstrates the patient's initial difficulties with performing a motor sequence, followed by resolution of the impairment coincident with cessation of the seizure activity on the vEEG. Localization of this impairment includes the left superior parietal lobule, which is thought to be involved in controlling visual-to-motor transformations and specifying the spatial and temporal aspects of motor acts (particularly motor sequences), including moment-to-moment updating of the representation of the body.8
Overall, the deficits during NP testing are best localized to the dominant (left) parietal lobe. Their fluctuating quality indicates a dynamic process interfering with these higher cortical functions that fits with frequent focal seizures arising from the left centroparietal region recorded on the vEEG.
Question for consideration:
What is the differential diagnosis for the MRI lesion in the context of the previous workup?
SECTION 4
Figure 1A shows T2 hyperintensities in the subcortical white matter of several superior left parietal gyri along with surrounding sulcal effacement but no restricted diffusion, reduced susceptibility, or parenchymal enhancement.
Based on epidemiologic studies, the most common etiology for parietal lobe seizures is mass lesions (e.g., gliomas or astrocytomas) followed by birth and postnatal trauma and postinflammatory gliosis.2 The sulcal effacement in this case could represent a subtle mass effect of a low-grade glioma given the lack of contrast enhancement. An inflammatory (e.g., autoimmune or paraneoplastic) process in the setting of a mild lymphocytic predominant leukocytosis in the initially obtained CSF is possible. Furthermore, the differential diagnosis includes a focal cortical dysplasia (FCD) with surrounding peri- or postictal edema of the cortex and subcortical white matter.
Repeated LP and further imaging studies.
A repeated LP showed a normal cell count, glucose, and protein. A paraneoplastic antibody panel in the CSF was negative. As such, the mild CSF leukocytosis was thought to be the result of a seizure cluster rather than an inflammatory process. Two months after improved seizure control, a repeated 3T MRI showed a significant interval decrease of the subcortical white matter T2 hyperintensity in the left superior parietal lobe. There was no well-defined “transmantle sign” (white circle in figure 1B). Hence, most of the initial MRI changes were most likely due to frequent seizure activity.
Peri- and postictal MRI changes can range from T2 hyperintensities and restricted diffusion to meningeal enhancement. They can be multifocal, affect cortical and subcortical structures, and are usually reversible.9
FCDs are malformations of cortical development that are classified into type I and II and are a common etiology for childhood-onset epilepsy that becomes rapidly refractory to medical treatment. In general, type II FCDs are isolated extratemporal lesions that have a characteristic appearance on MRI including blurring of the gray–white junction, cortical thickening, T2 or fluid-attenuated inversion recovery abnormality, and bottom-of-sulcus dysplasia (“transmantle sign”). In contrast, type I FCDs, which tend to affect the parietal, temporal, and occipital lobes, often appear normal on MRI.10
The most likely diagnosis is focal, localization-related epilepsy in the context of an FCD within the superior left parietal lobe. The distinct features of the FCD might be beyond the resolution of a 3T MRI. The patient is currently being evaluated for epilepsy surgery.
Supplementary Material
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Wolfgang Muhlhofer: manuscript concept and authorship, acquisition, analysis, and interpretation of data. Brianna Paul: acquisition, analysis, and interpretation of data, critical revision of manuscript for intellectual content. George Lin: acquisition, analysis, and interpretation of data, critical revision of manuscript for intellectual content. Nilika Singhal: critical revision of manuscript for intellectual content.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Bajwa R, Jay WM, Asconape J. Neuro-ophthalmologic manifestations of epilepsy. Semin Ophthalmol 2006;21:255–261. [DOI] [PubMed] [Google Scholar]
- 2.Sveinbjornsdottir S, Duncan JS. Parietal and occipital lobe epilepsy: a review. Epilepsia 1993;34:493–521. [DOI] [PubMed] [Google Scholar]
- 3.Laich E, Kuzniecky R, Mountz J, et al. Supplementary sensorimotor area epilepsy: seizure localization, cortical propagation and subcortical activation pathways using ictal SPECT. Brain 1997;120:855–864. [DOI] [PubMed] [Google Scholar]
- 4.Prilipko O, Seeck M, Mermillod B, Landis T, Pegna AJ. Postictal but not interictal hemispatial neglect in patients with seizures of lateralized onset. Epilepsia 2006;47:2046–2051. [DOI] [PubMed] [Google Scholar]
- 5.Meador KJ, Moser E. Negative seizures. J Int Neuropsychol Soc 2000;6:731–733. [DOI] [PubMed] [Google Scholar]
- 6.Alexander MP, Fischer RS, Friedman R. Lesion localization in apractic agraphia. Arch Neurol 1992;49:246–251. [DOI] [PubMed] [Google Scholar]
- 7.Gall C, Sabel BA. Reading performance after vision rehabilitation of subjects with homonymous visual field defects. PM R 2012;4:928–935. [DOI] [PubMed] [Google Scholar]
- 8.Rushworth MFS, Krams M, Passingham RE. The attentional role of the left parietal cortex: the distinct lateralization and localization of motor attention in the human brain. J Cogn Neurosci 2001;13:698–710. [DOI] [PubMed] [Google Scholar]
- 9.Cianfoni A, Caulo M, Cerase A, et al. Seizure-induced brain lesions: a wide spectrum of variably reversible MRI abnormalities. Eur J Radiol 2013;82:1964–1972. [DOI] [PubMed] [Google Scholar]
- 10.Spreafico R, Blümcke I. Focal cortical dysplasias: clinical implication of neuropathological classification systems. Acta Neuropathol 2010;120:359–367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


