Abstract
Objective:
To describe the clinical features, etiology, findings from neuroimaging, and treatment results in a series of 29 patients with Holmes tremor (HT).
Methods:
A retrospective study was performed based on review of medical records and videos of patients with HT diagnosis.
Results:
A total of 16 women and 13 men were included. The mean age at the moment of CNS insult was 33.9 ± 20.1 years (range 8–76 years). The most common causes were vascular (48.3%), ischemic, or hemorrhagic. Traumatic brain injury only represented 17.24%; other causes represented 34.5%. The median latency from lesion to tremor onset was 2 months (range 7 days–228 months). The most common symptoms/signs associated with HT were hemiparesis (62%), ataxia (51.7%), hypoesthesia (27.58%), dystonia (24.1%), cranial nerve involvement (24.1%), and dysarthria (24.1%). Other symptoms/signs were vertical gaze disorders (6.8%), bradykinesia/rigidity (6.8%), myoclonus (3.4%), and seizures (3.4%). Most of the patients had lesions involving more than one area. MRI showed lesions in thalamus or midbrain or cerebellum in 82.7% of the patients. Levodopa treatment was effective in 13 out of 24 treated patients (54.16%) and in 3 patients unilateral thalamotomy provided excellent results.
Conclusions:
The most common causes of HT in our series were vascular lesions. The most common lesion topography was mesencephalic, thalamic, or both. Treatment with levodopa and thalamic stereotactic lesional surgery seems to be effective.
Holmes tremor (HT) was first described in 1904 by Gordon Holmes1 as a 3–4 Hz flexor-extension oscillation, present at rest and exacerbated with posture and additionally intensified with action. There is an older description made by Benedikt in 1889, who described the tremor as secondary to a mesencephalic infarction. This tremor is also known as rubral, mesencephalic, or thalamic tremor, but these terms are no longer used because typical cases have been described with lesions located in other areas, and red nucleus experimental lesions fail to induce persistent tremor.2–5
The current definition of HT is derived from the Consensus Statement of the Movement Disorder Society on Tremor from 1998.6 It is described as a rest and intention tremor with sometimes irregular amplitude. However, a postural tremor is also present in many patients.6 It is a slow frequency tremor, usually less than 4.5 Hz.6 As it is a symptomatic tremor, imaging studies are usually abnormal, although in some cases no lesion can be demonstrated.7,8
Tremor commonly develops between 1 and 24 months after a CNS insult. This delayed onset might be due to neuronal plastic changes.5
It is assumed that a double lesion is required to develop HT, including the dopaminergic nigrostriatal system and the cerebello-thalamo-cortical or dentate-rubro-olivary pathways.3,4,6,9–11
Pharmacologic treatment is usually unsuccessful, although levodopa treatment has provided benefit in some cases.12–16 Surgery can be an option for drug-resistant cases.
We report on a series of 29 patients with HT describing their etiologies, associated neurologic manifestations, lesion localization, and response to treatment.
METHODS
A retrospective study from July 1996 to November 2014 was performed, based on our medical records, patient MRI studies, CT images, and videotapes from the Movement Disorders Centre, Hospital de Clínicas, University of Buenos Aires, Argentina Hospital Español, Buenos Aires, Argentina; Hospital Británico, Buenos Aires, Argentina; Instituto Trelles Montes, Lima, Peru; and Hospital Civil de Guadalajara “Fray Antonio Alcalde,” Guadalajara, Mexico.
Standard protocol approvals, registrations, and patient consents.
Ethical committee approval was obtained in every center.
Twenty-nine patients with HT were identified. HT diagnosis was made according to the Consensus Statement of the Movement Disorder Society.
The following items were analyzed: sex, age at the moment of CNS lesion, latency to the moment of tremor onset, etiology and localization of the CNS lesion, characteristics of HT, associated neurologic symptoms, and the results of the different treatments.
Regarding the analysis of the images, we somewhat arbitrarily divided the involved territories into brainstem, thalamus, cerebellum, and nonthalamus supratentorial regions.
The patients were retrospectively evaluated based on medical records and pretreatment and posttreatment video recordings.
RESULTS
General findings.
Twenty-nine patients were identified (16 women and 13 men) with a mean age at the time of CNS injury of 33.9 ± 20.1 years (range 8–76 years). The median latency between the time of the CNS injury and HT development, in 25 out of 29 patients, was 2 months (range 7 days–228 months). In 4 cases, the latency could not be determined: case 1 had an arteriovenous malformation (AVM) with a mass effect (figure 1); in cases 5 and 22, there were demyelinating lesions and the exact time of demyelinating plaques development could not be determined; and in case 20, the patient began with tremor and had several brain cystic lesions of unknown nature despite having a brain biopsy.
Figure 1. MRI from patient 1 shows an arteriovenous malformation acting as a mass.
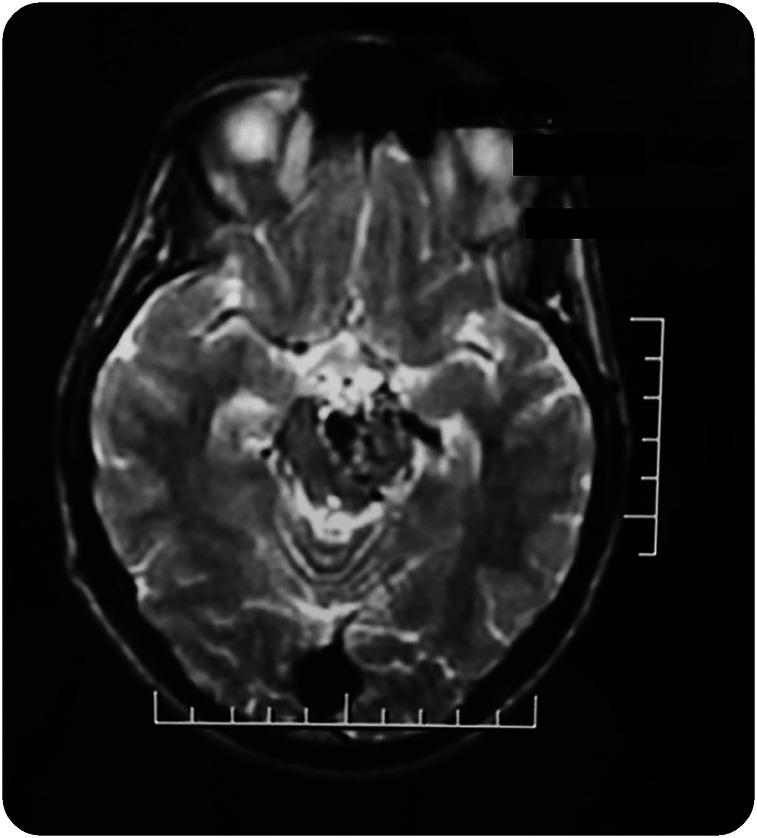
Patients with HT secondary to vascular and traumatic lesions had similar associated findings regarding hemiparesis and latency from the CNS lesion to the development of HT.
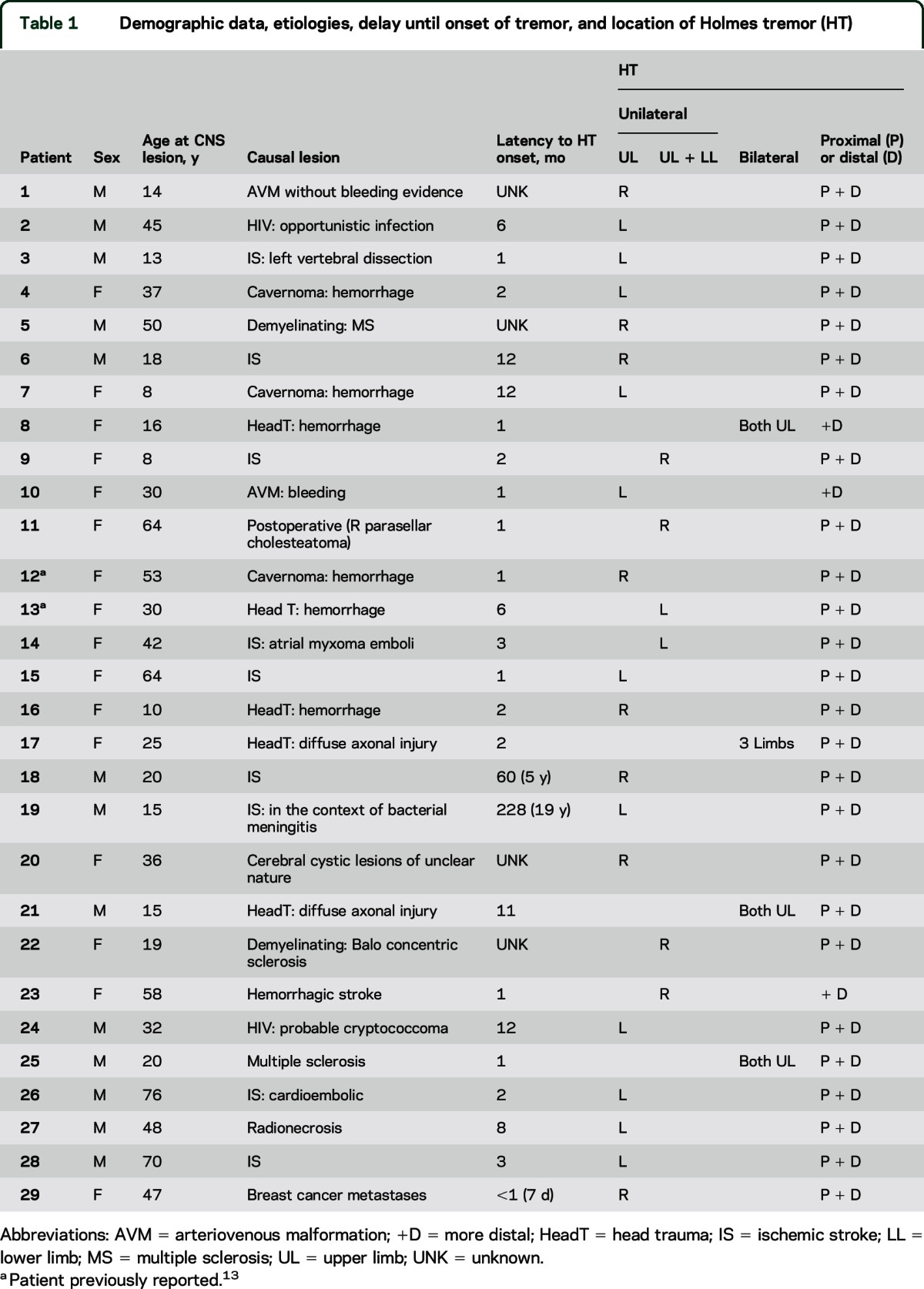
Tremor involved only one upper limb in 19 patients (65.5%), the upper and lower limb in 6 (20.7%), bilateral involvement in 4 (13.7%), both upper limbs in 3,8,21,25 and both upper limbs and one lower limb in case 17. The postural component was present in all patients. The severity of the tremor was similar for both proximal and distal segments of the limbs, except in 3 patients in whom the distal component was more severe (table 1).
Table 1.
Demographic data, etiologies, delay until onset of tremor, and location of Holmes tremor (HT)
Etiology.
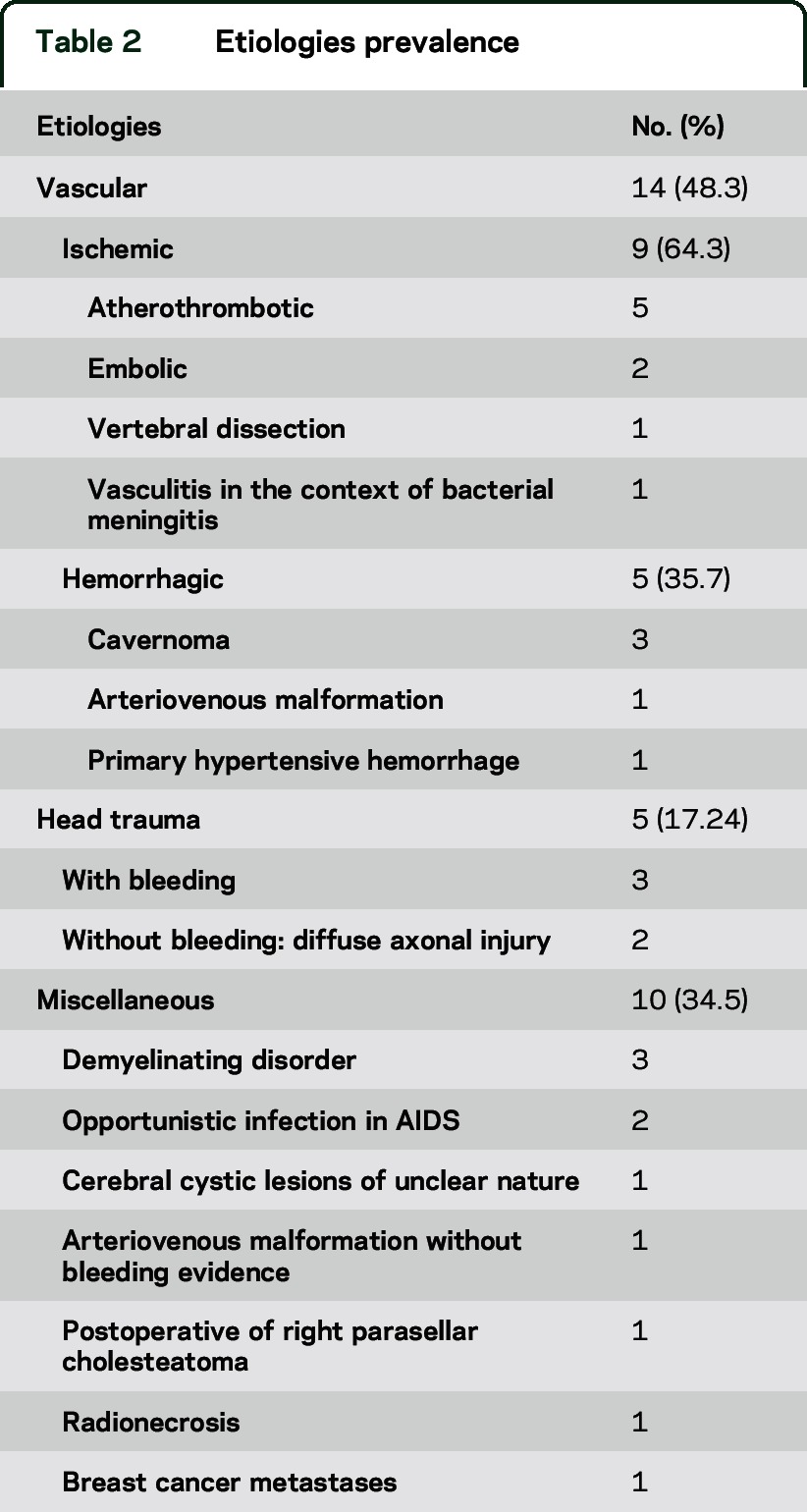
The most common etiologies were stroke in 14 cases (48.3%) and head trauma in 5 (17.2%) (figure e-1 on the Neurology® Web site at Neurology.org). The other 10 cases (34.5%) included 3 with demyelinating diseases, 2 with AIDS with associated opportunistic infections, 1 with an AVM without evidence of bleeding, 1 with cerebral cystic lesions of unclear origin, 1 with a postoperative sequelae of a parasellar cholesteatoma surgery, 1 with radionecrosis with a diencephalic-mesencephalic AVM, and 1 with breast cancer metastases (table 2).
Table 2.
Etiologies prevalence
Neurologic manifestations associated with HT.
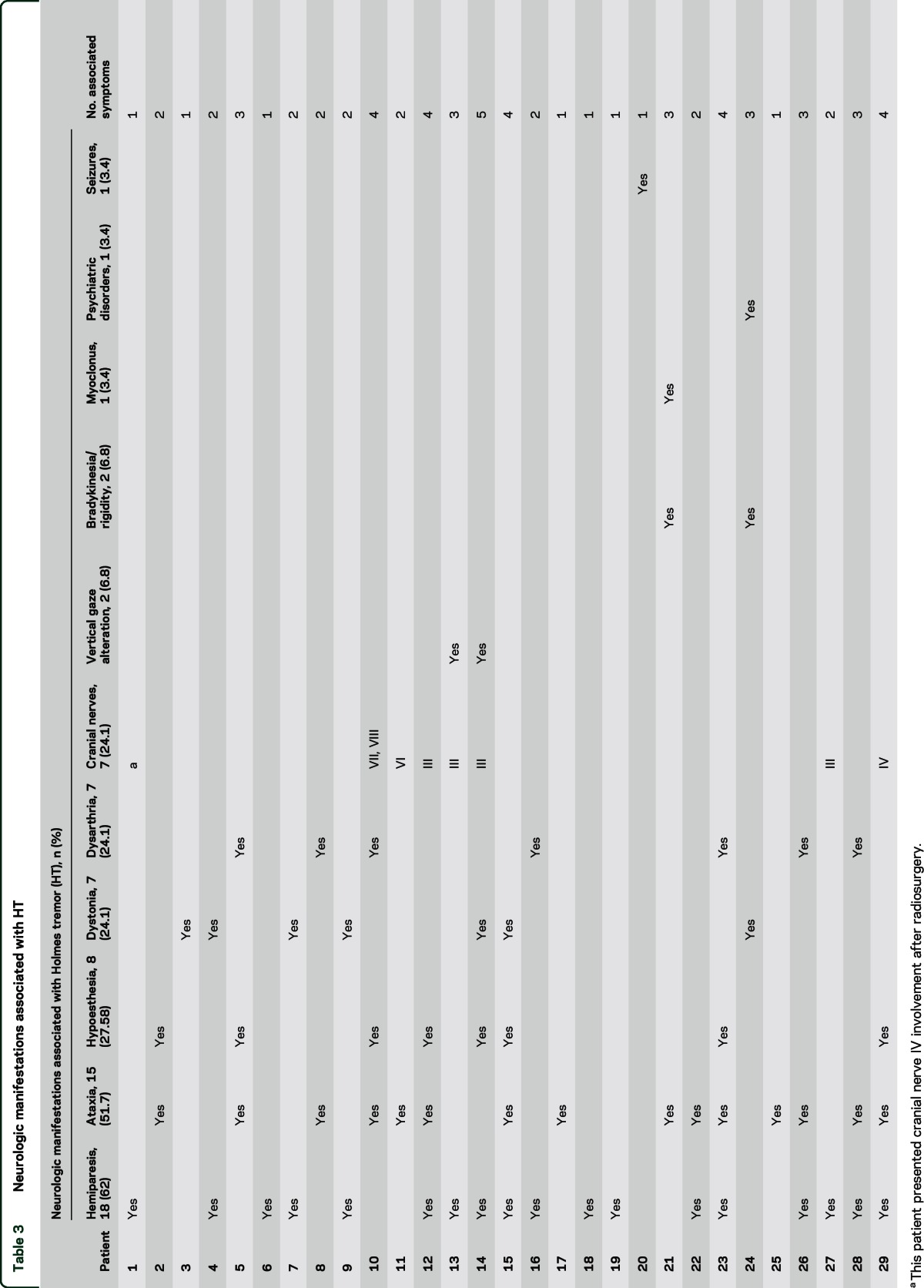
All patients had at least 1 associated neurologic manifestation. The most common was hemiparesis in 18 (62%), ataxia in 15 (51.7%), hypoesthesia in 8 (27.58%), and dystonia in 7 (24.1%) involving the same body region as the tremor. Of the 7 cases with dystonia, 3 had dystonic jerks. We found 7 patients (24.1%) with cranial nerve involvement and the III cranial nerve was the most commonly affected. Two patients had vertical gaze palsy. The number of associated neurologic manifestations in each patient ranged from 1 to 5, taking into account that 58.62% (17 patients) of them had only 1 or 2 associated manifestations. Patient 1 was treated with radiosurgery for his AVM, and presented secondary IV cranial nerve involvement (table 3).
Table 3.
Neurologic manifestations associated with HT
Neuroimaging data.
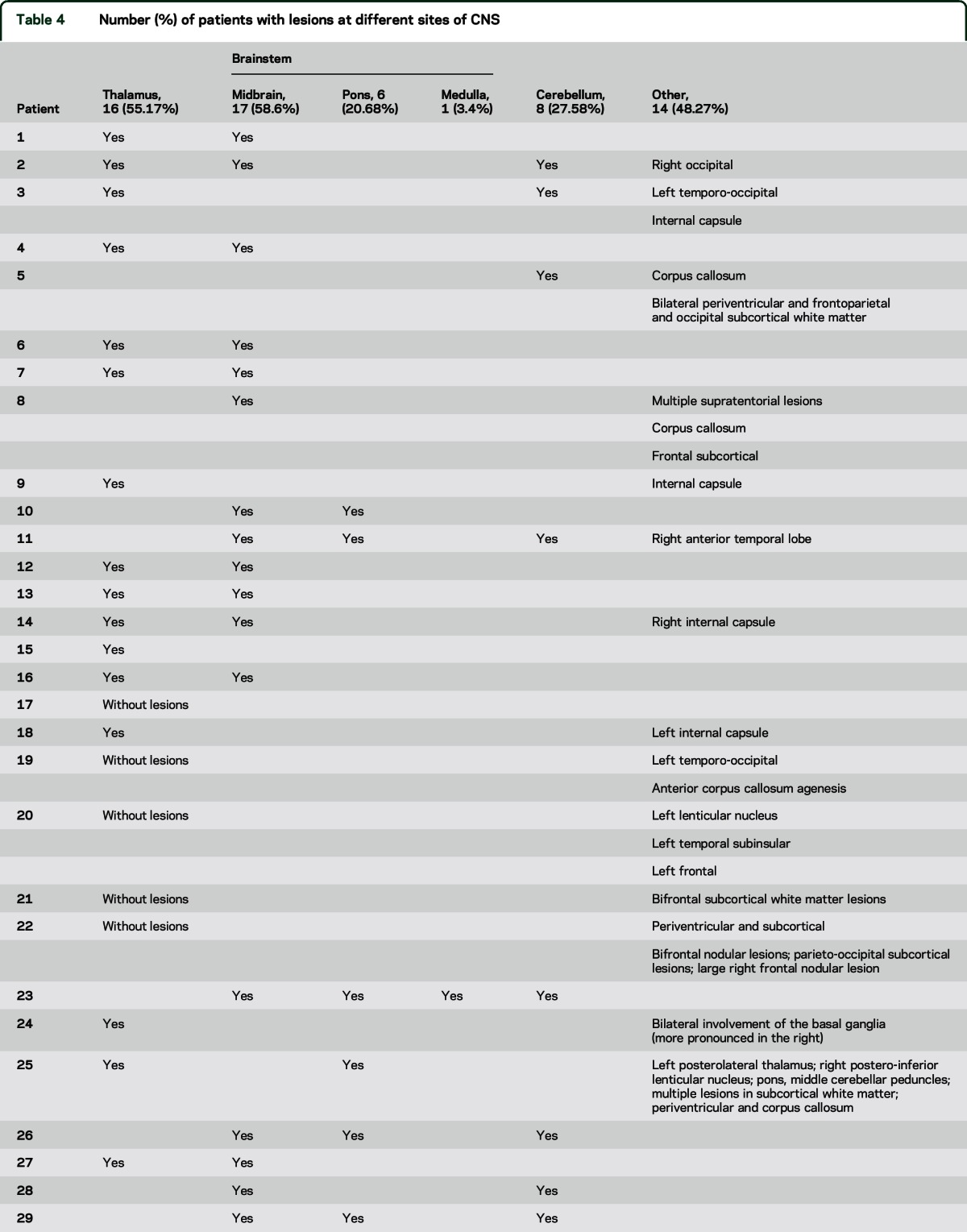
An MRI study was performed in every patient, except for patient 19, in whom only CT could be performed because he had a ventriculoperitoneal shunt that prevented him from being exposed to MRI. Neuroimages were abnormal in 28 out of 29 patients (96.5%). Patient 17 had head trauma with diffuse axonal injury with normal MRI studies. We found structural lesions in thalamus or brainstem or cerebellum in 24 of 29 patients (82.7%). Most patients had lesions involving more than one region. The most common sites affected were midbrain in 17 (58.6%), thalamus in 16 (55.17%), and the cerebellum in 8 (27.58%). The pons was affected in 6 (20.68%) and the medulla in 1 (3.4%). Nonthalamic supratentorial lesions were found in 14 cases (48.27%). In 10 patients, these lesions were associated with injuries in the thalamus or brainstem or cerebellum, while in 4 of them it was the only finding (cases 19, 20, 21, and 22) (table 4).
Table 4.
Number (%) of patients with lesions at different sites of CNS
Clinical–radiologic correlation.
There was no correlation between the extent of the lesions and the number and severity of associated neurologic manifestations. Patient 11 underwent extensive surgery to remove a parasellar cholesteatoma that involved the anterior pole of right temporal lobe, right midbrain and pons, right inferior and middle cerebellar peduncles, and right anterior cerebellum. She presented only gait ataxia and a right VI cranial nerve palsy associated with right HT. Patient 20 had cerebral cystic lesions in the left lenticular nucleus, subinsular region of the left temporal lobe, and the left frontal region, and the only associated symptoms were tonic-clonic seizures, effectively controlled with levetiracetam 3,000 mg/d. Patient 15 had left hemiparesis and hypoesthesia, dysarthria, and dystonia and had only a small right lateral thalamic lesion.
Of the 16 patients with thalamic involvement, either as a single lesion or as part of more widespread lesions, 12 (75%) had hemiparesis, 7 (43.7%) dystonia, 4 (25%) hypoesthesia, and 4 (25%) cerebellar symptoms. All patients with dystonia (n = 7) had thalamic involvement. Case 17 had ataxia with a normal brain MRI. All the patients with cranial nerve involvement had brainstem lesions.
Treatment.
Levodopa therapy was tried in 24 out of 29 patients (82.8%). There was an improvement of HT in 13 (54.16%) of these cases, whereas the remaining 11 (45.84%) had no benefit. Among those who improved, 7 had an almost complete control of HT. In 16 cases, the average dose was 697.65 mg ± 215 mg/d (range 300–1,000 mg/d), whereas for the remaining 8 patients, the dose could not be determined. It is interesting to note that there were no significant differences in the doses of levodopa employed in the group presenting a marked improvement when compared with the group with no response (678.5 ± 257.9 vs 662.5 ± 214 mg, respectively; p = 0.755). Patients who responded positively to levodopa improved in the 3 components of HT (kinetic, postural, and resting). Patient 4 exhibited limited improvement with pramipexole 1.5 mg/d, but she then received levodopa with positive response of the 3 components of HT. No other symptom/sign improved with levodopa, except in patient 8, whose postural trunk control was alleviated enough so that she could sit. Patient 17 had an additional improvement with topiramate 100 mg/d on postural and kinetic tremor, although with levodopa she had a partial improvement of the 3 components of tremor.
Onabotulinumtoxin A was tested in 5 patients. One patient showed mild improvement (case 1) and 2 had moderate improvement (patients 10 and 16); they remain on onabotulinumtoxin A injections every 3 months as the only treatment. Two patients showed no improvement (cases 4 and 24).
Patients 5, 6, and 9 underwent a left ventral intermediate nucleus (VIM) thalamotomy, exhibiting significant benefit. They all had a marked improvement. Patient 9 did not have improvement in the dystonic posture of her right hand, although the tremor was almost completely controlled. She regained some functions; she could write again with her right hand and use cutlery. In cases 5 and 6, the 3 components of tremor, proximal and distal, were abolished.
Patient 26, who developed HT after an embolic ischemic stroke with hemorrhagic transformation, received levodopa, phenobarbital, amantadine, and clonazepam with no response, but improved with quetiapine 100 mg/d. When quetiapine was discontinued, there was a marked worsening of HT, which improved after reintroducing the drug.
A variety of other drugs, including pramipexole, levetiracetam, lamotrigine, clonazepam, clozapine, flunarizine, carbamazepine, trihexyphenidyl, propranolol, topiramate, baclofen, gabapentin, valproic acid, and piracetam, were used with poor or no response.
DISCUSSION
To our knowledge, this is the largest series of HT cases reported so far. The major limitation of our study is that it is retrospective, but the advantage is the number of patients collected.
On comparing previous publications on HT, we found a predominance of male patients, yet in our series we found the opposite,1,3–5,7,8,10–40,e1–e52 with similar findings regarding the etiologies,1,3–5,7,8,10–40,e1–e52 with the vascular disorders, either ischemic or hemorrhagic, being the most frequent (around 50%), reaching 48.3% in our series. Head trauma accounted for almost 20% of the cases in previous publications,1,3–5,7,8,10–40,e1–e52 while we found 17.24% traumatic cases in our series (table e-1).
Miscellaneous cases represented approximately 30% of previously reported cases,1,3–5,7,8,10–40,e1–e52 while we found that 34.5% of our cases had different etiologies. The age at onset is variable, depending on the moment of CNS lesion and the delay until the moment of HT onset.
As aforementioned, there is a variable delay between CNS lesion and HT onset.6,e47
In our series, the median latency was within the previously described range by other authors.
A long delay of 5 years was evident in case 18, with HT secondary to an ischemic stroke, and case 19, with 19 years of delay after an ischemic stroke secondary to bacterial meningitis. The published research also describes long latencies between the moment of the CNS lesion and the appearance of HT. It was reported that a patient had a delay of 23 years between the initial CNS insult and HT development,e32 and Deuschl et al.e19 reported a patient who had a right hemicerebellectomy and 17 years later he developed a right HT and a typical parkinsonian tremor on his left side, when the patient developed Parkinson disease.
Pathophysiologic heterogeneity is supported by some DaTSCAN SPECT studies as well as with different therapeutic responses to levodopa.3,7 In one of them, 2 blind raters could not find asymmetry in DaTSCAN SPECT, and the quantitative measurements of radiotracer uptake demonstrated only minimal differences between both sides, with slightly worse uptake contralateral to HT.7 In 6 of their 10 patients, levodopa was administered, and only 2 improved: one had a mild response and the other moderate improvement.7 The authors concluded that the dopaminergic deficit on DaTSCAN SPECT could be a predictor of levodopa responsiveness.7
In another report,3 the authors mentioned that all their patients improved to varying degrees with levodopa therapy, and they found that 18F-dopa uptake showed values that were markedly and significantly decreased on the lesion side when compared to healthy controls and the contralateral side. However, some values were lower than those observed in parkinsonian patients. Given the fact that some patients fail to respond to levodopa, it has been speculated whether variations in pathophysiology could explain the different responses to drugs.3,7,34 Our findings suggest that treatment with levodopa should be tested in every patient with HT because some of them can achieve marked improvement, as was previously reported by other authors.3,5,7,11–17,19,25,26,33,36,e25,e33
In addition, some degree of improvement can be achieved with botulinum toxin injections. We have 2 patients with good response and they continue only on this treatment, one of them for more than 8 years.
Many other medications were reported to improve HT, such as dopaminergic agonists, anticholinergic drugs, clonazepam, levetiracetam, and carbamazepine, although in our patients these medications did not show any clinically relevant benefit.5,e11,e18,e20,e25,e38,e48
Although deep brain stimulation (DBS) for other tremors appears generally less effective than for Parkinson disease or essential tremor, for patients with refractory HT, DBS or thalamotomy of VIM can provide good HT control.4,10,40,e2,e5,e7,e8,e13,e15,e18,e23,e31,e34,e35,e37,e50 In 3 patients from our series, thalamotomy of VIM showed excellent improvement. In some cases, it has been necessary to make a combined DBS of VIM and subthalamic nucleus to effectively control HT.e20,e22,e34,e36 There is also a report of a combined DBS of VIM and globus pallidus internus to control HT and another report of a patient who underwent a posteroventral pallidotomy to relieve HT since VIM high-frequency electrical stimulation had failed to control the tremor.40,e23 A double ipsilateral stimulation of VIM and ventralis oralis anterior and ventralis oralis posterior has also been reported (table e-2).e51
The present series of patients with HT showed that the most common cause of HT was vascular, the anatomic regions most frequently affected were mesencephalon and thalamus, and the majority of cases presented other neurologic manifestations associated with HT such as hemiparesis and ataxia. Levodopa therapy may be useful in some cases, while most other pharmacologic agents are generally unsuccessful for HT control. Functional surgery appears to be the most effective treatment in the management of HT.
Supplementary Material
GLOSSARY
- AVM
arteriovenous malformation
- DBS
deep brain stimulation
- HT
Holmes tremor
- VIM
ventral intermediate nucleus
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Drs. Raina and Cersosimo: study concept and design, analysis and interpretation. Dr. Micheli: critical revision of the manuscript for important intellectual content, study supervision. All authors participated in acquisition of data.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Holmes G. On certain tremors in organic cerebral lesions. Brain 1904;27:327–375. [Google Scholar]
- 2.Benedikt M. Tremblement avec paralysie croisée du moteur oculaire commun. Bull Med 1889;3:547–548. [Google Scholar]
- 3.Remy P, de Recondo A, Defer G, et al. Peduncular “rubral” tremor and dopaminergic denervation: a PET study. Neurology 1995;45:472–477. [DOI] [PubMed] [Google Scholar]
- 4.Rieder CR, Reboucas RG, Ferreira MP. Holmes tremor in association with bilateral hypertrophic olivary degeneration and palatal tremor: chronological considerations: case report. Arq Neuropsiquiatr 2003;61:473–477. [DOI] [PubMed] [Google Scholar]
- 5.Samie MR, Selhorst JB, Koller WC. Post-traumatic midbrain tremors. Neurology 1990;40:62–66. [DOI] [PubMed] [Google Scholar]
- 6.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorders Society on Tremor Ad Hoc Scientific committee. Mov Disord 1998;13(suppl 3):2–23. [DOI] [PubMed] [Google Scholar]
- 7.Gajos A, Bogucki A, Schinwelski M, et al. The clinical and neuroimaging studies in Holmes tremor. Acta Neurol Scand 2010;122:360–366. [DOI] [PubMed] [Google Scholar]
- 8.Biary N, Cleeves L, Findley L, Koller W. Post-traumatic tremor. Neurology 1989;39:103–106. [DOI] [PubMed] [Google Scholar]
- 9.Vidailhet M, Jedynak CP, Pollak P, Agid Y. Pathology of symptomatic tremors. Mov Disord 1998;13(suppl 3):49–54. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd GM, Tauböll E, Bakke SJ, Nyberg-Hansen R. Midbrain tremor and hypertrophic olivary degeneration after pontine hemorrhage. Mov Disord 1997;12:432–437. [DOI] [PubMed] [Google Scholar]
- 11.Defer GL, Remy P, Malapert D, Ricolfi F, Samson Y, Degos JD. Rest tremor and extrapyramidal symptoms after midbrain haemorrhage: clinical and 18F-dopa PET evaluation. J Neurol Neurosurg Psychiatry 1994;57:987–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velez M, Cosentino C, Torres L. Levodopa responsive rubral (Holmes) tremor. Mov Disord 2002;17:741–742. [DOI] [PubMed] [Google Scholar]
- 13.Raina GB, Velez M, Fernandez Pardal M, Micheli F. Holmes tremor secondary to brainstem hemorrhage responsive to levodopa: report of two cases. Clin Neuropharmacol 2007;30:95–100. [DOI] [PubMed] [Google Scholar]
- 14.Fujieda T, Yamauchi T, Takahashi S, Moroji T. Letter: effect of levodopa on tremor in Benedikt's syndrome. Br Med J 1974;1:456–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Findley LJ, Gresty MA. Suppression of “rubral” tremor with levodopa. Br Med J 1980;281:1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woo JH, Hong BY, Kim JS, et al. Holmes tremor after brainstem hemorrhage, treated with levodopa. Ann Rehabil Med 2013;37:591–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boelmans K, Gerloff C, Münchau A. Long-lasting effect of levodopa on Holmes' tremor. Mov Disord 2012;27:1097–1098. [DOI] [PubMed] [Google Scholar]
- 18.Lekoubou A, Njouoguep R, Kuate C, Kengne AP. Cerebral toxoplasmosis in acquired immunodeficiency syndrome (AIDS) patients also provides unifying pathophysiologic hypotheses for Holmes tremor. BMC Neurol 2010;10:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pezzini A, Zavarise P, Palvarini L, Viale P, Oladejl O, Padovani A. Holmes' tremor following midbrain Toxoplasma abscess: clinical features and treatment of a case. Parkinsonism Relat Disord 2002;8:177–180. [DOI] [PubMed] [Google Scholar]
- 20.Mattos JP, Rosso AL, Correa RB, Novis SA. Movement disorders in 28 HIV-infected patients. Arq Neuropsiquiatr 2002;60:525–530. [PubMed] [Google Scholar]
- 21.Koppel BS, Daras M. “Rubral” tremor due to midbrain Toxoplasma abscess. Mov Disord 1990;5:254–256. [DOI] [PubMed] [Google Scholar]
- 22.Kipfer S, Frigerio SB. Post-ischemic stroke Holmes' tremor of the upper limb. Mov Disord 2013;28:1347. [DOI] [PubMed] [Google Scholar]
- 23.Coumou AD, van Dijk JM, De Vries JJ, Leenders KL, Kuijlen JM. Successful surgical treatment of an arachnoid cyst inducing a Holmes' tremor. Mov Disord 2012;27:964. [DOI] [PubMed] [Google Scholar]
- 24.Castrop F, Jochim A, Berends LP, Haslinger B. Sustained suppression of Holmes tremor after cessation of thalamic stimulation. Mov Disord 2013;28:1456–1457. [DOI] [PubMed] [Google Scholar]
- 25.Harmon RL, Long DF, Shirtz J. Treatment of post-traumatic midbrain resting-kinetic tremor with combined levodopa/carbidopa and carbamazepine. Brain Inj 1991;5:213–218. [DOI] [PubMed] [Google Scholar]
- 26.Bolen RD, Balkrishnan N. Palatal myoclonus, eight-and-a-half syndrome, and Holmes tremor in a patient from a single brainstem lesion. J Neurol Sci 2014;347:411–412. [DOI] [PubMed] [Google Scholar]
- 27.Strecker K, Schneider JP, Sabri O, et al. Responsiveness to a dopamine agent in Holmes tremor-case report. Eur J Neurol 2007;14:e9–e10. [DOI] [PubMed] [Google Scholar]
- 28.Kremer W, Russell WR, Smyth GE. A mid-brain syndrome following head injury. J Neurol Neurosurg Psychiatry 1947;10:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong J, Li ST, Xu SQ, Wan L. Holmes' tremor caused by midbrain cavernoma. Chin Med J 2007;120:2059–2061. [PubMed] [Google Scholar]
- 30.Walker M, Kim H, Samii A. Holmes-like tremor of the lower extremity following brainstem hemorrhage. Mov Disord 2007;22:272–274. [DOI] [PubMed] [Google Scholar]
- 31.Paviour DC, Jäger HR, Wilkinson L, Jahanshahi M, Lees AJ. Holmes tremor: application of modern neuroimaging techniques. Mov Disord 2006;21:2260–2262. [DOI] [PubMed] [Google Scholar]
- 32.Menon B, Sasikala P, Agrawal A. Giant middle fossa epidermoid presenting as holmes' tremor syndrome. J Mov Disord 2014;7:22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim DG, Koo YH, Kim OJ, Oh SH. Development of Holmes' tremor in a patient with Parkinson disease following acute cerebellar infarction. Mov Disord 2009;24:463–464. [DOI] [PubMed] [Google Scholar]
- 34.Yuill GM. Suppression or “rubral” tremor with levodopa. Br Med J 1980;281:1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrews J, Fowler CJ, Harrison MJG. Tremor after head injury and its treatment by stereotaxic surgery. J Neurol Neurosurg Psychiatry 1982;45:815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Recondo A, Rondot P, Loc'h C, et al. Positron-emission tomographic study of the dopaminergic system in a case of secondary unilateral tremor after mesencephalic hematoma. Rev Neurol 1993;149:46–49. [PubMed] [Google Scholar]
- 37.Fahn S, Koller WC, Hallet M. What is it? Mov Disord 1986;1:299–308.3504254 [Google Scholar]
- 38.Mossuto-Agatiello L, Puccetti G, Castellano AE. “Rubral tremor” after thalamic haemorrhage. J Neurol 1993;241:27–30. [DOI] [PubMed] [Google Scholar]
- 39.Follett MA, Torres-Russotto D, Follett KA. Bilateral deep brain stimulation of the ventral intermediate nucleus of the thalamus for post traumatic midbrain tremor. Neuromodulation 2014;17:289–291. [DOI] [PubMed] [Google Scholar]
- 40.Aydin S, Abuzayed B, Kiziltan G, Gunduz A, Yagci S. Unilateral thalamic Vim and GPi stimulation for the treatment of Holmes' tremor caused by midbrain cavernoma: case report and review of the literature. J Neurol Surg A Cent Eur Neurosurg 2013;74:271–276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


