Abstract
Studies on rodent models and rare human disorders of estrogen production or response have revealed an increased complexity of the actions of estrogen on bone. ERα disruption in human males results in delayed epiphyseal maturation, tall stature, trabecular thinning, marked cortical thinning, genu valgum and significantly reduced cortical vBMD, but trabecular number is preserved and there is normal to increased periosteal expansion. Aromatase deficiency results overall in a similar phenotype, although less is known about skeletal architecture. Importantly, estrogen replacement in these individuals, even if provided late in the third decade, may normalize aBMD. Less certain is whether there is complete recovery of normal skeletal architecture and strength. Rodent models, in general, are consistent with the human phenotype but are confounded by inherent differences between mouse and human physiology and issues regarding the completeness of the different knock-out lines. Both human and rodent studies suggest that residual effects of estrogen through ERβ, truncated ERα forms or nonclassical estrogen receptors might account for different phenotypes in the hERKO man, aromatase deficient subjects and rodents. Importantly, androgen, particularly by preserving trabecular number and augmenting both periosteal and epiphyseal growth, also has significant actions on bone.
Keywords: Estrogen, Androgen, Estrogen receptor, Bone, Epiphysis, Osteoporosis, Aromatase
1. Introduction
Estrogen is well known to be important in females for bone growth and metabolism. However, evidence has emerged from rodent models [1] and rare disorders of estrogen production or response in humans [2,3] that the physiologic role for estrogen is far more complicated, involving actions in males as well as females [4]. Estrogen actions are further complicated by the presence of two distinct but related nuclear receptors, ERα [4] and ERβ [5], both with different splice variants [6] and the recent discovery of a membrane bound functional ER, the G protein-coupled receptor GPR30 [7–9]. For this review, we will describe recent experimental evidence from human and animal models that increase understanding of the role of estrogen relative to androgen in bone.
2. Production and cellular mechanism of action of estrogen
Circulating estrogens are produced from the aromatization of androgens by the cytochrome P450 enzyme, aromatase [10]. In males, unlike females in which the ovaries are the primary source of estrogen, the majority of the estrogen in the circulation is derived from extragonadal tissues [11]. The regulation of aromatase activity in different tissues such as breast, adipose and gonads is complex and it is increasingly clear that in addition to classical hormonal pathways there are important local regulatory mechanisms in place that regulate estrogen production in a tissue specific manner [12–14]. Estrogen produced by androgen aromatization induces cellular changes by several different mechanisms [15]. The major signal transducers are two distinct receptor proteins, ERα and ERβ, which have distinct tissue expression patterns [16] in both humans and rodents. ERα and ERβ are encoded by unique genes from separate chromosomal locations. Estrogens are thought to diffuse into target cells and are bound by the ER, which is located primarily in the nucleus, but can also be associated with the plasma membrane [17]. Recently, the membrane form [18] has been demonstrated to be unable to rescue a deficiency in the nuclear located receptor. The nuclear ER-estrogen complex can regulate genes, positively or negatively, by binding directly to specific DNA sequences in the promoter region of regulated genes involving the recruitment of coregulatory proteins (coactivators or corepressors) to the promoter, producing increased or decreased mRNA levels and associated protein production, and resulting physiological response. Gene-targeted knock-out mouse models lacking these receptors exhibit distinct phenotypes [19].
An alternative mode of action of estrogen involves an indirect mechanism by which the ER does not bind directly to the DNA but interacts with existing transcription factors; this is referred to as the tethered mechanism of nuclear receptor gene regulation [20,21]. To elicit many actions of the hormone this “genomic” mechanism typically occurs over the course of hours in most tissues. In contrast, non-genomic mechanisms, can also occur with estrogen, either through the ER located in or adjacent to the plasma membrane involving adaptor proteins such as caveolin-1 or Shc, or through other non-ER plasma membrane–associated estrogen-binding proteins, such as GPR30 [22], resulting in cellular responses such as activation of kinases which then acts to prime the genomic actions. Finally, a third aspect of ER activity involves the ligand independent activation of the receptor protein. Such an action has been shown experimentally in cells and animal models, involving the activation of kinase cascades by growth factors or other membrane signaling agents [23]. The extent to which any of these specific mechanisms are involved in mediating the physiological actions of estrogen still requires considerable study in order to develop effective biomedical understanding and therapeutic treatment.
3. Control of longitudinal growth and final stature
The most visible action of estrogen on the human skeleton for both males and females is control of body proportion and determination of final stature [2,3]. The male identified with ERα point mutation (hERKO), described throughout this review, is a unique individual and clinical case. He presented at age 28 with tall stature (204 cm), eunuchoid body proportions (0.83 (average for men, 0.96)), unfused epiphyses (bone age ~15 years) and moderate to severe genu valgum. Unlike normal males, in which there is a self-limited surge of estrogen coinciding with a growth spurt [24], in the hERKO man there was likely a prolonged phase of elevated estrogens associated with sustained linear growth into his third decade. There was a minimal bone age progression despite normal testosterone and insulin-like growth factor-I (IGF-I) levels. Tall stature ensued but with marked eunuchoid body proportions and debilitating genu valgum. This is strikingly similar to reports in 7 aromatase deficient (AD) men all whom presented either in their 3rd or 4th decade with tall stature ranging from 183 to 204 cm, bone ages ranging from 14.5 to 16.5 years, genu valgum and eunuchoid body proportions (see Table 1) [2,25–31]. By contrast, a 8th case of aromatase deficiency presented at age 17.1 years with a bone age of 12, normal stature and apparently normal body proportions [32] and an 9th case was reported as a male infant [33]. Importantly, in the aromatase deficient cases in which estrogen treatment was initiated [30,34–37], there was epiphyseal maturation, but only in the adolescent-aged individual was a significant growth acceleration observed [32]. AD has been reported in 11 females to date [2] but are readily identified early in life secondary to virilization; estrogen therapy is instituted before a bone phenotype becomes manifest.
Table 1.
hERKO man and ArKO individuals: skeletal findings at presentation and aBMD response to estrogen treatment.
| Chronological age (year) | Bone age (year) | Height (cm) | Genu valgum | Spine aBMD
|
||
|---|---|---|---|---|---|---|
| At presentation | After estrogen | |||||
| ArKO | ||||||
| Morishima et al. [2,34] | 24 | 14 | 204 | Moderate | 0.931 (T = −1.96) | 1.123 (T = +0.08) (12 months of estrogen) |
| Carani et al. [26,35] | 31 39 |
14.8 14.8 |
187 190 |
Severe | 0.933 (T = −2.07) | 1.18 (T = −0.20) (~2 years of estrogen) |
| Herrmann et al. [37] | 27 | 16.5 | 197 | Severe | 0.971 (T = −2.24) | 1.150 (T = −1.0) (6 months of estrogen) |
| Maffei et al. [27,36] | 29 | 15 | 183.5 | Severe | 0.773 (T = −2.2) | 0.813 (T = −1.9) (12 months of estrogen) |
| Maffei et al. [28] | 25 | 15.3 | 191.8 | Severe | 0.352 (T = −1.8) (ultradistal forearm) | Not reported |
| Bouillon et al. [32] | 17 | 12 | 172 | No | 0.837 (T = −2.31) | 1.031 (T = −0.55) (~3 years of estrogen) |
| Lanfranco et al. [30] | 26.8 | 15.5 | 193 | Marked | 0.87 (T = −3.0) | 0.980 (T = −2.2) (14 months of estrogen) |
| Pura et al. [31] | 26.8 | ? | 190 | ? | ? | ? |
| Deladoey et al. [33] | Infant | ? | ? | N/A | N/A | N/A |
| hERKO | ||||||
| Smith et al. [3] | 28 | 15 | 204 | Severe | 0.745 (T = −3.1) | No change; near complete fusion by age 35 without treatment |
Consistent with the longitudinal growth observations, ERα and ERβ [38–42], and more recently with the GPR30 [22,43], a G protein membrane estrogen signaling receptor, have been localized to human growth plate chondrocytes. In the rabbit, which resembles the human with respect to epiphyseal fusion in response to estrogen, ERα is readily detectable in resting, proliferative and hypertrophic chondrocytes [44]. However, animal models other than the rabbit and human have manifested different phenotypes, perhaps in part because of fundamental differences in growth dynamics [45–49]. Notably, in normal mice, the epiphyses do not completely fuse during the equivalence of puberty [24,50,51]. Further complicating interpretation is a sexually dimorphic age dependent pattern in the mouse knock-out models [52] and differences related to the degree of completeness of the knock-out [1,21,53,54]. For example, putatively complete ERKO mice [55] appear to have femur bone length similar to wild type in both males and females, while other reports indicate shorter bones in both males and females [56,57]. ERβknock-out (bERKO) male and young and old female mice display unchanged femur lengths whereas in intermediate aged female mice with naturally occurring high estrogen levels have longer bones [49]. However, bERKO female mice do not have abnormal estrogen levels compared to wild type [55,58,59]. Aromatase deficient mice (ArKO) phenotype reveals a shortened femur length in males with less effect in females [45,46]. GRP30 knock-out female mice are smaller than normal where as male knock-out animals appear similar to wild type controls [60]; subsequent analysis of the effect of ovariectomy on female GRP30−/− animals reveals decreased femur length compared to wild type ovariectomized with reduced impact of estrogen replacement [61]. Finally, androgen receptor knock-out (ARKO) mouse models show shorter femurs accompanied by osteopenia only in males and not in females [62].
As a result of the disparate phenotypes, the primary reason for difference in phenotypes between rodents and man remains largely speculative. Does ERβ account for some estrogen-mediated actions on epiphyseal maturation? Do the shorter femurs in female GRP30−/− animals coupled with localization of GRP30 in growth plates indicate an important function for GRP30 mediated estrogen signaling in bone growth? If so, why only in female mice and why are femurs shorter rather than longer? An intriguing potential explanation has been suggested from the observation that hERKO man possessed a mutation in exon 2 consisting of a cytosine-to-thymine transition at codon 157 of both alleles, resulting in a premature stop codon that likely produced a truncated protein [3,52,53]. There is in vitro evidence that this truncated product may sustain some residual estrogenic activity [52,63]. Chagin and Savendahl [52] have speculated that this residual truncated N-terminal fragment [6,52,53,64] in the hERKO man may have served to retard epiphyseal closure until the 4th decade of life by acting as a dominant negative inhibitor of ERβ. However, no experimental studies have indicated such a mechanism to date. In fact, cultured bone cells from the ERKO man, despite having both ERβ mRNA and protein, failed to elicit any estrogen responsiveness relative to control wild type cells [65,66]. The fused epiphyses ultimately occurred in the hERKO man at age 35.5 without the benefit of significant estrogen intervention [65] but with elevated endogenously produced estrogen. Epihyseal fusion could be explained by a prolonged action of modestly high estrogens through either ERβ, GRP30 or truncated ERα but there remains no definitive evidence for any of these possibilities.
Although the ArKO cases and hERKO man clearly demonstrate that estrogen is critical for final epiphyseal maturation, a closer examination of their “pubertal” increment in growth does not implicate conclusively which sex steroid, androgen or estrogen, in addition to the prerequisite contribution of other hormone systems such as the GH-IGF-I axis [67–69], is the primary mediator of the pubertal growth spurt that is typically about 15–20% of final height [67,70,71]. Bone ages in the hERKO man and male aromatase deficient patients at presentation were substantially more advanced though not fused, and stature was greater, than in late presenting hypogonadal individuals [72,73]. Interestingly, the available longitudinal growth data in the hERKO case and the aromatase deficient patients suggest that a pubertal growth spurt occurred since the documented statures at age 18 matched the predicted height based on parental target range. This was followed during the third decade by tall stature greater than observed in isolated hypogonadism [69,74], eunuchoid body proportions similar to isolated hypogonadism and genu valgum seemingly more severe than reported with hypogonadism [72–74]. Although androgen insensitivity (AI) syndromes argue strongly that estrogen in the absence of a functional androgen receptor (AR) can induce both a growth spurt and epiphyseal maturation [75–77], there is controversy in these rare individuals regarding whether estrogen can fully compensate for the lack of androgen action [75,78]. Do the available data support the notion that androgen can promote growth without inordinate bone age maturation under some circumstances? The growth data of hypogonadal individuals, particularly with intact adrenal androgen levels and normal growth hormone secretion [72,73], reveal an apparent maintenance of prepubertal height velocity during the first half of the second decade. Bone age is delayed but a full growth increment characteristic of puberty does not generally occur. This suggests that the differences in the growth observed in the hERKO case and the aromatase deficiency patients compared with hypogonadal individuals is related to combined androgen exposure and effectively deficient estrogen state. Seemingly, despite the well-documented actions of nonaromatizable androgen to advance bone age [79–82], under some circumstances androgen levels in the pubertal range can promote pubertal growth without rapid maturation of the epiphyses in the absence of full estrogen action.
What precise sex steroid environment promotes linear growth without inordinately advancing bone age is an important question to address in future clinical studies. Modulation of estrogen exposure, while simultaneously maintaining normal circulating androgens, has a number of intriguing potential benefits. Age-appropriate masculinization can be achieved while suppressing bone age maturation. In addition, maintenance of androgen concentrations allows for greater increments in height per unit of bone age advancement and, as mentioned later in this review, may result in simultaneous periosteal growth of the skeleton and could lead to a greater peak bone mass. As already indicated, this is a markedly different outcome from the tapered height velocity observed in longstanding hypogonadism [24] and may result in a larger and stronger skeleton less susceptible to fractures.
A tentative overall conclusion is that normal to increased stature can ultimately evolve over time without estrogen and that bone age maturation tends to arrest at about 15 years. Final epiphyseal fusion, coupled with a growth spurt, is, however, observed only if estrogen exposure occurs at younger bone ages. This conclusion suggests that manipulation of estrogen production and/or signaling has potential for augmenting final height. Recent studies using aromatase inhibitors confirm the potential for increasing stature in a variety of clinical conditions by modulating estrogen concentrations [83]. For example, Mauras et al. [84], in a study involving 52 short adolescent males randomized to receive either anastrozole or placebo for up to 36 months, demonstrated similar linear growth with a decrease in the rate of bone age advancement. The resulting increase in predicted height at 36 months was +6.7 ± 1.4 cm for the anastrozole group. Importantly, there was no significant difference in the spine BMD Z-score. However, the authors and a recent position statement by the Lawson Wilkins Pediatric Endocrine Society [85] stress that long-term follow-up is needed before aromatase inhibitors are used in children for stature manipulation outside of a controlled trial setting.
A final important caveat, however, to the prospects for sex steroid manipulation of final height is that there are senescence factors, intrinsic to the epiphysis, that serve to define an overall limit on final stature [24]. The near final epiphyseal fusion in the hERKO subject in particular supports this notion. Not only was linear growth without ERα activity largely completed by the middle to end of the 3rd decade in this patient and in the aromatase deficient cases, but the increased stature was accompanied by abnormalities of the body proportion, marked genu valgum, and osteopenia. The role of estrogen appears to be accelerate senescence within a growth plate that has an internal program limiting the number of chondrocyte divisions [24,86]. Though both high doses of estrogen and early estrogen exposure will reduce final height [87], estrogen exposure within the physiologic range is not associated with significantly compromised final height and reduced estrogen production in boys with constitutional delay may lead to improved height prediction. The rapid fusion observed in the estrogen treated aromatase patients [26] and the progressive epiphyseal maturation observed in young children with precocious puberty [88] all point to a program of senescence within the growth plate that estrogen is able to modulate in humans and in some mammals. Stature increments, substantially beyond the normal genetic potential, are not likely to be achieved safely by manipulating estrogen action for more than a few years.
4. Bone mineral density and architectural integrity in human models
The next most consistent clinical phenotype of human models of estrogen deficiency and/or resistance is marked decreased areal measures of bone mineral density (aBMD). The decreased spine aBMD in the 7 reported aromatase patients improved significantly within 6 months of estrogen treatment (Table 1). The decreased aBMD is presumably a manifestation of decreased trabecular volume from increased bone resorption coupled with decreased bone formation, a predictable outcome of the severe disruption in estrogen action [89,90] on trabeculae, a well-documented site for ERα [38,40] and ERβ [38,40] receptor expression in both osteoblasts and osteoclasts. Interestingly, many studies on the relationship between bone and circulating sex steroids in men suggest a greater correlation with estradiol than testosterone [91–94]. However, with the hERKO man, the only case with a detailed histomorphometric analysis [65], preservation of trabecular number is documented. This is not characteristic of male and female hypogonadism, and is more typical of the aging skeleton with normal androgen concentrations [95]. The preserved trabecular number may be explained, in part, by residual actions of estrogen through ERβ [55], known to be expressed in human osteoblasts [38] and osteoclasts [39,40], and normal androgen levels [96,97]. Studies involving supplementation with testosterone [98] to standard estrogen/progesterone replacement in postmenopausal women and analysis of AI individuals [75] strongly suggest important actions of androgens on trabecular bone. Androgen receptors have been reported in human osteoblasts [99,100]. Finally, in the detailed longitudinal follow-up of the aromatase subject described by Rochira et al. [36]; following a window of estrogen replacement for approximately 2 years, androgen was added to the regimen with a further increase in spine aBMD from 1.018 g/cm2 to 1.134 g/cm2. The authors’ interpretation is that the full anabolic action of estrogen requires androgen. Regardless, a potential clinical implication is that interventions that augment ERα pathways and preserve the anabolic actions of androgen may be particularly effective in maintaining and/or augmenting trabecular bone.
It is interesting to compare these observations with the impact of estrogen on human bone among lactating women. Decreases of 3–9% in spine and femoral neck aBMD have been reported among lactating women [101–103]. This decrease occurs rapidly within the first 3–6 months of lactation, reaching rates of bone loss of 1% per month. However, the amount of bone lost during lactation is variable and the length of postpartum amenorrhea is an important determinant. Although the length of postpartum amenorrhea is associated with the length of lactation, some women resume menses while still breastfeeding. Several studies have found that the net change in spine aBMD is less in women who resumed menses during lactation compared to women who do not resume menses [103,104]. Importantly and particularly relevant for this review, spine aBMD increases on average 5.2% between 6 and 12 months postpartum, the majority of this increase occurring following weaning [103] and women with high parity who breast-fed their children have greater bone cross-sectional area and bone bending strength later in life [105]. These findings underscore the importance of estrogen in regulating bone loss during lactation and suggest there are inherent mechanisms for skeletal recovery from periods of lowered estrogen.
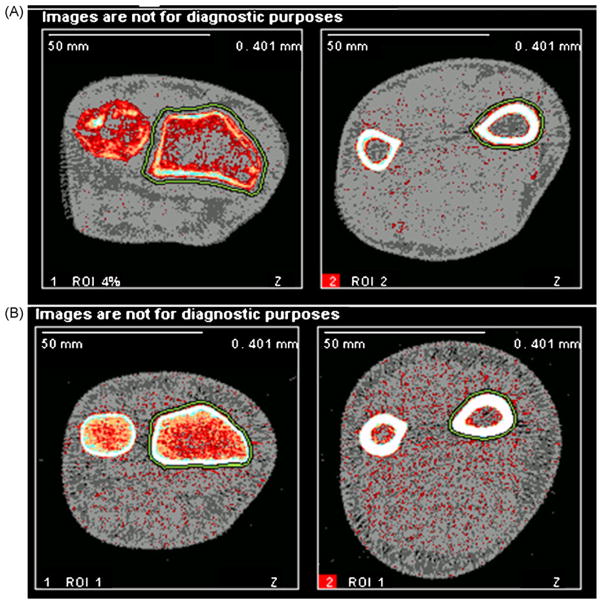
Another remarkable set of findings observed in 2 of the aromatase patients by pQCT and by both pQCT and histomorphometry in the hERKO males is a marked thinning of the cortex with low cortical vBMD and increased trabecularization by histomorphometry [65] (Table 2). The decreased cortical thickness was present with normal to increased periosteal circumference or cross-sectional bone area (Fig. 1). The ArKO subject described by Rochira, who was also hypogonadal with low testosterone concentrations, showed increases in cortical thickness and periosteal expansion with testosterone plus estrogen replacement [36]. Bone cross-sectional area is greater in men and is considered to be secondary to the increased periosteal apposition rate induced by androgens [106–109] and a possible inhibition of resorption on endosteal surfaces by estrogen [110]. Postmenopausal women are susceptible to increased endosteal bone loss relative to men, exhibit less periosteal apposition of new bone, and display greater cortical porosity [111].
Table 2.
Comparison of bone phenotype in males with different conditions.
| Parameter | Aging | Hypogonadism | Estrogen resistance | Aromatase deficiency |
|---|---|---|---|---|
| aBMD | Decreased | Decreased | Decreased | Decreased |
| vertebral Tb bone mineral loss | 40% decrease (from 20 to 80 years) [123] | Similar to females | Markedly decreased | Markedly decreased |
| Tb thickness (μm) (iliac crest) | 129 ± 28 [124] | ~124 | 76.2 | ? |
| Tb number/mm (iliac crest) | 1.23 ± 0.37 [124] | 1.161 ± 0.230 [125] | 1.4 | ? |
| Tb structural integrity (iliac crest) | Thinned but intact | Thinned with some decreased integrity | Thinned but intact | ? |
| Cortical thickness (iliac crest and wrist) | Decreased | Markedly decreased | Modestly decreased | Modestly decreased (wrist) |
| Bone turnover | Active | Modestly increased | Markedly quiescent | ? |
| Skeletal size | Larger relative to women | Increased in length but not width | Increased in length and width | Increased in length and width |
Fig. 1.
pQCT images of hERKO subject and normal man. This figure depicts pQCT images at the 4% (left) and 20% (right) distal radius in the hERKO subject (top row, A) and a man with similar arm length (bottom row, B). Decreased cortical thickness is apparent at both sites, as well as increased periosteal circumference and decreased trabecular vBMD, at the 4% distal radius.
The greater cortical vBMD that is observed in women of reproductive age has been hypothesized to be a result of estrogen-driven packing of excess bone for needs related to pregnancy and lactation [112]. Although estrogen is considered by some to exert primarily inhibitory actions on periosteal expansion, there is recent evidence from animals models that many of the actions of estrogen on periosteum may be to stimulate expansion through ERα, but in a dosage sensitive manner, and potentially, in collaboration with androgen actions [76,76,97,113,114] on periosteally located AR [114]. However, recent studies have found that estrogen does not have an independent effect on periosteal expansion and that the action of estrogen and bone loading on bone structure are independent and additive [115]. In the aromatase deficient subject presenting at age 17 years described by Bouillon et al., estrogen replacement alone appeared to induce modest linear growth but skeletal size was proportionately augmented; total vBMD and bone mineral apparent density (BMD adjusting for bone size) at the 4% distal radius measured by pQCT were unchanged, yet aBMD at the same location was increased, indicating that bone size and not true volumetric density were affected [32]. However, longitudinal measures at the 4% distal radius are difficult to compare due to large differences in bone diameter that occur at this location within very short distances. Because of the continued growth of this individual, it would have been impossible to measure the same location repeatedly over time, making the results difficult to interpret.
Related to the above description of the periosteum, bone width is affected in both the hERKO and aromatase deficient subjects. Specifically, wrist breadth at time of presentation of the hERKO man was greater than normal at 7.2 cm (normal adult males: 6.0 ± 0.5 [mean ± SD]; normal adult females: 5.3 ± 0.3 cm) and the width of the distal femurs were both estimated to be 113 mm (normal adult male 90 ± 7 mm). The skeletal “frame size” as measured at the wrist and knee is distinctly different from the ectomorphic appearing skeleton of longstanding hypogonadism [116] and this phenotype may be due to important actions of androgen in these individuals [79,97,113]. The ArKO subjects have normal to slightly elevated androgen prior to intervention and this may serve to maintain and/or augment bone size. In the ArKO patient described by Bouillon et al. [32], the initiation of estrogen replacement, which was followed by reduction but relative maintenance androgen concentrations, resulted in significant increase in skeletal size based on the observation that despite increases in cortical cross-sectional area, vBMD remained unchanged [32]. This notion that androgen enlarges the skeleton is supported by studies demonstrating that femoral area of androgen resistant rats is lower compared to normal males [117]. In addition, it is well established that androgen stimulates periosteal bone growth [79,118–120] and different skeletal domains such as the mandible [121,122] display sexual dimorphism.
5. Conclusions
Detailed analysis of homozygous-affected hERKO patient and aromatase individuals, as well as appropriate experimental animal models, suggest a complex contribution of estrogen to bone growth, aBMD, and skeletal structural integrity (Fig. 2). ERα disruption specifically results in delayed epiphyseal maturation, tall stature, trabecular thinning, marked cortical thinning, significantly reduced cortical vBMD, and normal to increased periosteal expansion. Of significant clinical relevance, debilitating genu valgum evolves. This supports an important role for ERα in mediating the actions of estrogen on the male skeleton, a result supported from the animal models as well. Whether this would hold true for female subjects is not known at the current time, since no hERKO female patients have been identified and the findings in the female mice are not as revealing as expected. Lack of estrogen due to aromatase deficiency results in a similar phenotype though less is known about skeletal architecture. Estrogen replacement, even if provided late in third decade may normalize aBMD, although it is less clear if there is complete recovery of normal skeletal architecture and strength. It also is not clear whether the residual effect of estrogen through ERβ, truncated ERα or GPR30 might manifest a different phenotype in the hERKO man than aromatase deficient subjects. Androgen, particularly by preserving trabecular number and augmenting both periosteal and epiphyseal growth, has important actions on bone. Targeting both ER and AR and the production of their respective activating hormones and/or mimetics will provide complimentary strategies to the management of sex hormone-regulated diseases such as osteoporosis and short stature.
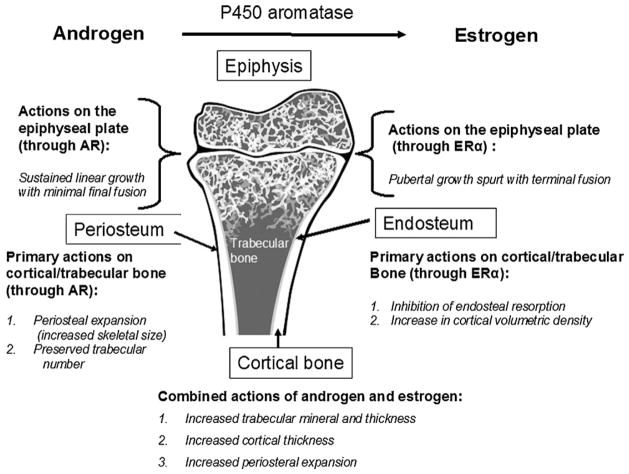
Fig. 2.
Summary of the action of estrogen and androgen on bone. This figure depicts a rendition of a human distal femur. The principle actions of the sex steroids on bone are demonstrated based on the clinical findings in ArKO male subjects and the hERKO man. The action of androgen are listed on the left; the effects of estrogen on the right. The combined actions are shown on the lower middle.
Acknowledgments
The authors thank Sarah E. Smith for providing an artist’s rendition of a distal femur. This research was supported by the Clinical Research Center, CCHMC and the Division of Internal Research at the NIEHS project Z01 ES70065 to KSK.
Footnotes
Special Issue selected article from the IX International Aromatase Conference (Aromatase 2008) held at Shanghai, China, on October 13th–16th, 2008.
References
- 1.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90(23):11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab. 1995;80(12):3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- 3.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331(16):1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 4.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116(3):561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93(12):5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schreihofer DA, Rowe DF, Rissman EF, Scordalakes EM, Gustafsson JJ, Shupnik MA. Estrogen receptor-alpha (ERalpha), but not ERbeta, modulates estrogen stimulation of the ERalpha-truncated variant, TERP-1. Endocrinology. 2002;143(11):4196–4202. doi: 10.1210/en.2002-220353. [DOI] [PubMed] [Google Scholar]
- 7.Prossnitz ER, Oprea TI, Sklar LA, Arterburn JB. The ins and outs of GPR30: a transmembrane estrogen receptor. J Steroid Biochem Mol Biol. 2008;109(3–5):350–353. doi: 10.1016/j.jsbmb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146(2):624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 9.Filardo EJ, Thomas P. GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab. 2005;16(8):362–367. doi: 10.1016/j.tem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, Speed C, Jones M. Aromatase—a brief overview. Annu Rev Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- 11.Gennari L, Nuti R, Bilezikian JP. Aromatase activity and bone homeostasis in men. J Clin Endocrinol Metab. 2004;89(12):5898–5907. doi: 10.1210/jc.2004-1717. [DOI] [PubMed] [Google Scholar]
- 12.Simpson ER, Clyne C, Speed C, Rubin G, Bulun S. Tissue-specific estrogen biosynthesis and metabolism. Ann NY Acad Sci. 2001;949:58–67. doi: 10.1111/j.1749-6632.2001.tb04002.x. [DOI] [PubMed] [Google Scholar]
- 13.Demura M, Reierstad S, Innes JE, Bulun SE. Novel promoter I.8 and promoter usage in the CYP19 (Aromatase) gene. Reprod Sci. 2008;15(10):1044–1053. doi: 10.1177/1933719108322441. [DOI] [PubMed] [Google Scholar]
- 14.Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, Tamura M, Langoi D, Deb S. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57(3):359–383. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- 15.Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276(40):36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- 16.Mueller SO, Korach KS. Estrogen receptors and endocrine diseases: lessons from estrogen receptor knockout mice. Curr Opin Pharmacol. 2001;1(6):613–619. doi: 10.1016/s1471-4892(01)00105-9. [DOI] [PubMed] [Google Scholar]
- 17.Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER. Plasma membrane estrogen receptors exist and functions as dimers. Mol Endocrinol. 2004;18(12):2854–2865. doi: 10.1210/me.2004-0115. [DOI] [PubMed] [Google Scholar]
- 18.Pedram A, Razandi M, Kim JK, O’Mahony F, Lee EY, Luderer U, Levin ER. Developmental phenotype of a membrane only estrogen receptor alpha (MOER) mouse. J Biol Chem. 2009;284(6):3488–3495. doi: 10.1074/jbc.M806249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20(3):358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 20.Hewitt SC, Deroo BJ, Korach KS. Signal transduction. A new mediator for an old hormone? Science. 2005;307(5715):1572–1573. doi: 10.1126/science.1110345. [DOI] [PubMed] [Google Scholar]
- 21.Pendaries C, Darblade B, Rochaix P, Krust A, Chambon P, Korach KS, Bayard F, Arnal JF. The AF-1 activation-function of ERalpha may be dispensable to mediate the effect of estradiol on endothelial NO production in mice. Proc Natl Acad Sci USA. 2002;99(4):2205–2210. doi: 10.1073/pnas.042688499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heino TJ, Chagin AS, Savendahl L. The novel estrogen receptor G-protein-coupled receptor 30 is expressed in human bone. J Endocrinol. 2008;197(2):R1–R6. doi: 10.1677/JOE-07-0629. [DOI] [PubMed] [Google Scholar]
- 23.Curtis SW, Washburn T, Sewall C, DiAugustine R, Lindzey J, Couse JF, Korach KS. Physiological coupling of growth factor and steroid receptor signaling pathways: estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. Proc Natl Acad Sci USA. 1996;93(22):12626–12630. doi: 10.1073/pnas.93.22.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilsson O, Marino R, De LF, Phillip M, Baron J. Endocrine regulation of the growth plate. Horm Res. 2005;64(4):157–165. doi: 10.1159/000088791. [DOI] [PubMed] [Google Scholar]
- 25.Zirilli L, Rochira V, Diazzi C, Caffagni G, Carani C. Human models of aromatase deficiency. J Steroid Biochem Mol Biol. 2008;109(3–5):212–218. doi: 10.1016/j.jsbmb.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 26.Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, Korach KS, Simpson ER. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med. 1997;337(2):91–95. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 27.Maffei L, Murata Y, Rochira V, Tubert G, Aranda C, Vazquez M, Clyne CD, Davis S, Simpson ER, Carani C. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. J Clin Endocrinol Metab. 2004;89(1):61–70. doi: 10.1210/jc.2003-030313. [DOI] [PubMed] [Google Scholar]
- 28.Maffei L, Rochira V, Zirilli L, Antunez P, Aranda C, Fabre B, Simone ML, Pignatti E, Simpson ER, Houssami S, Clyne CD, Carani C. A novel compound heterozygous mutation of the aromatase gene in an adult man: reinforced evidence on the relationship between congenital oestrogen deficiency, adiposity and the metabolic syndrome. Clin Endocrinol (Oxf) 2007;67(2):218–224. doi: 10.1111/j.1365-2265.2007.02864.x. [DOI] [PubMed] [Google Scholar]
- 29.Herrmann BL, Saller B, Janssen OE, Gocke P, Bockisch A, Sperling H, Mann K, Broecker M. Impact of estrogen replacement therapy in a male with congenital aromatase deficiency caused by a novel mutation in the CYP19 gene. J Clin Endocrinol Metab. 2002;87(12):5476–5484. doi: 10.1210/jc.2002-020498. [DOI] [PubMed] [Google Scholar]
- 30.Lanfranco F, Zirilli L, Baldi M, Pignatti E, Corneli G, Ghigo E, Aimaretti G, Carani C, Rochira V. A novel mutation in the human aromatase gene: insights on the relationship among serum estradiol, longitudinal growth and bone mineral density in an adult man under estrogen replacement treatment. Bone. 2008;43(3):628–635. doi: 10.1016/j.bone.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Pura M, Mittre H, Carreau S, Kottler ML. Clinical findings in an adult man with a novel mutation in the aromatase gene. Program of the 85th Annual Meeting of the Endocrine Society. 2003:P1-467-243. ref type: abstract. [Google Scholar]
- 32.Bouillon R, Bex M, Vanderschueren D, Boonen S. Estrogens are essential for male pubertal periosteal bone expansion. J Clin Endocrinol Metab. 2004;89(12):6025–6029. doi: 10.1210/jc.2004-0602. [DOI] [PubMed] [Google Scholar]
- 33.Deladoey J, Fluck C, Bex M, Yoshimura N, Harada N, Mullis PE. Aromatase deficiency caused by a novel P450arom gene mutation: impact of absent estrogen production on serum gonadotropin concentration in a boy. J Clin Endocrinol Metab. 1999;84(11):4050–4054. doi: 10.1210/jcem.84.11.6135. [DOI] [PubMed] [Google Scholar]
- 34.Bilezikian JP, Morishima A, Bell J, Grumbach MM. Increased bone mass as a result of estrogen therapy in a man with aromatase deficiency. N Engl J Med. 1998;339(9):599–603. doi: 10.1056/NEJM199808273390905. [DOI] [PubMed] [Google Scholar]
- 35.Rochira V, Faustini-Fustini M, Balestrieri A, Carani C. Estrogen replacement therapy in a man with congenital aromatase deficiency: effects of different doses of transdermal estradiol on bone mineral density and hormonal parameters. J Clin Endocrinol Metab. 2000;85(5):1841–1845. doi: 10.1210/jcem.85.5.6583. [DOI] [PubMed] [Google Scholar]
- 36.Rochira V, Zirilli L, Madeo B, Aranda C, Caffagni G, Fabre B, Montangero VE, Roldan EJ, Maffei L, Carani C. Skeletal effects of long-term estrogen and testosterone replacement treatment in a man with congenital aromatase deficiency: evidences of a priming effect of estrogen for sex steroids action on bone. Bone. 2007;40(6):1662–1668. doi: 10.1016/j.bone.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 37.Herrmann BL, Janssen OE, Hahn S, Broecker-Preuss M, Mann K. Effects of estrogen replacement therapy on bone and glucose metabolism in a male with congenital aromatase deficiency. Horm Metab Res. 2005;37(3):178–183. doi: 10.1055/s-2005-861292. [DOI] [PubMed] [Google Scholar]
- 38.Kusec V, Virdi AS, Prince R, Triffitt JT. Localization of estrogen receptor-alpha in human and rabbit skeletal tissues. J Clin Endocrinol Metab. 1998;83(7):2421–2428. doi: 10.1210/jcem.83.7.4981. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson O, Chrysis D, Pajulo O, Boman A, Holst M, Rubinstein J, Martin RE, Savendahl L. Localization of estrogen receptors-alpha and -beta and androgen receptor in the human growth plate at different pubertal stages. J Endocrinol. 2003;177(2):319–326. doi: 10.1677/joe.0.1770319. [DOI] [PubMed] [Google Scholar]
- 40.Bord S, Horner A, Beavan S, Compston J. Estrogen receptors alpha and beta are differentially expressed in developing human bone. J Clin Endocrinol Metab. 2001;86(5):2309–2314. doi: 10.1210/jcem.86.5.7513. [DOI] [PubMed] [Google Scholar]
- 41.van der Eerden BC, Gevers EF, Lowik CW, Karperien M, Wit JM. Expression of estrogen receptor alpha and beta in the epiphyseal plate of the rat. Bone. 2002;30(3):478–485. doi: 10.1016/s8756-3282(01)00703-7. [DOI] [PubMed] [Google Scholar]
- 42.Egerbacher M, Helmreich M, Rossmanith W, Haeusler G. Estrogen receptor-alpha and estrogen receptor-beta are present in the human growth plate in childhood and adolescence, in identical distribution. Horm Res. 2002;58(2):99–103. doi: 10.1159/000064661. [DOI] [PubMed] [Google Scholar]
- 43.Chagin AS, Savendahl L. GPR30 estrogen receptor expression in the growth plate declines as puberty progresses. J Clin Endocrinol Metab. 2007;92(12):4873–4877. doi: 10.1210/jc.2007-0814. [DOI] [PubMed] [Google Scholar]
- 44.Nilsson O, Abad V, Chrysis D, Ritzen EM, Savendahl L, Baron J. Estrogen receptor-alpha and -beta are expressed throughout postnatal development in the rat and rabbit growth plate. J Endocrinol. 2002;173(3):407–414. doi: 10.1677/joe.0.1730407. [DOI] [PubMed] [Google Scholar]
- 45.Oz OK, Hirasawa G, Lawson J, Nanu L, Constantinescu A, Antich PP, Mason RP, Tsyganov E, Parkey RW, Zerwekh JE, Simpson ER. Bone phenotype of the aromatase deficient mouse. J Steroid Biochem Mol Biol. 2001;79(1–5):49–59. doi: 10.1016/s0960-0760(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 46.Oz OK, Zerwekh JE, Fisher C, Graves K, Nanu L, Millsaps R, Simpson ER. Bone has a sexually dimorphic response to aromatase deficiency. J Bone Miner Res. 2000;15(3):507–514. doi: 10.1359/jbmr.2000.15.3.507. [DOI] [PubMed] [Google Scholar]
- 47.Vidal O, Lindberg MK, Hollberg K, Baylink DJ, Andersson G, Lubahn DB, Mohan S, Gustafsson JA, Ohlsson C. Estrogen receptor specificity in the regulation of skeletal growth and maturation in male mice. Proc Natl Acad Sci USA. 2000;97(10):5474–5479. doi: 10.1073/pnas.97.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moverare S, Venken K, Eriksson AL, Andersson N, Skrtic S, Wergedal J, Mohan S, Salmon P, Bouillon R, Gustafsson JA, Vanderschueren D, Ohlsson C. Differential effects on bone of estrogen receptor alpha and androgen receptor activation in orchidectomized adult male mice. Proc Natl Acad Sci USA. 2003;100(23):13573–13578. doi: 10.1073/pnas.2233084100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chagin AS, Lindberg MK, Andersson N, Moverare S, Gustafsson JA, Savendahl L, Ohlsson C. Estrogen receptor-beta inhibits skeletal growth and has the capacity to mediate growth plate fusion in female mice. J Bone Miner Res. 2004;19(1):72–77. doi: 10.1359/JBMR.0301203. [DOI] [PubMed] [Google Scholar]
- 50.Weise M, De-Levi S, Barnes KM, Gafni RI, Abad V, Baron J. Effects of estrogen on growth plate senescence and epiphyseal fusion. Proc Natl Acad Sci USA. 2001;98(12):6871–6876. doi: 10.1073/pnas.121180498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abad V, Meyers JL, Weise M, Gafni RI, Barnes KM, Nilsson O, Bacher JD, Baron J. The role of the resting zone in growth plate chondrogenesis. Endocrinology. 2002;143(5):1851–1857. doi: 10.1210/endo.143.5.8776. [DOI] [PubMed] [Google Scholar]
- 52.Chagin AS, Savendahl L. Oestrogen receptors and linear bone growth. Acta Paediatr. 2007;96(9):1275–1279. doi: 10.1111/j.1651-2227.2007.00415.x. [DOI] [PubMed] [Google Scholar]
- 53.Perry RJ, Farquharson C, Ahmed SF. The role of sex steroids in controlling pubertal growth. Clin Endocrinol (Oxf) 2008;68(1):4–15. doi: 10.1111/j.1365-2265.2007.02960.x. [DOI] [PubMed] [Google Scholar]
- 54.Chen M, Wolfe A, Wang X, Chang C, Yeh S, Radovick S. Generation and characterization of a complete null estrogen receptor alpha mouse using Cre/LoxP technology. Mol Cell Biochem. 2009;321(1–2):145–153. doi: 10.1007/s11010-008-9928-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sims NA, Dupont S, Krust A, Clement-Lacroix P, Minet D, Resche-Rigon M, Gaillard-Kelly M, Baron R. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-beta in bone remodeling in females but not in males. Bone. 2002;30(1):18–25. doi: 10.1016/s8756-3282(01)00643-3. [DOI] [PubMed] [Google Scholar]
- 56.McCauley LK, Tozum TF, Rosol TJ. Estrogen receptors in skeletal metabolism: lessons from genetically modified models of receptor function. Crit Rev Eukaryot Gene Exp. 2002;12(2):89–100. doi: 10.1615/critreveukaryotgeneexpr.v12.i2.10. [DOI] [PubMed] [Google Scholar]
- 57.Korach KS, Taki M, Kimbro KS. Proceedings 2nd International Symposium on Women’s Health and Menopause: Risk Reduction Strategies. Kluwer Academics Publishing; Dordrecht, The Netherlands: 1997. The effects of estrogen receptor gene disruption on bone; pp. 69–74. [Google Scholar]
- 58.Chagin AS, Savendahl L. Estrogens and growth: review. Pediatr Endocrinol Rev. 2007;4(4):329–334. [PubMed] [Google Scholar]
- 59.Simm PJ, Bajpai A, Russo VC, Werther GA. Estrogens and growth. Pediatr Endocrinol Rev. 2008;6(1):32–41. [PubMed] [Google Scholar]
- 60.Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, szkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grande PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150(2):687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- 61.Windahl SH, Andersson N, Chagin AS, Martensson UE, Carlsten H, Olde B, Swanson C, Moverare-Skrtic S, Savendahl L, Lagerquist MK, Leeb-Lundberg LM, Ohlsson C. The role of the G protein-coupled receptor GPR30 in the effects of estrogen in ovariectomized mice. Am J Physiol Endocrinol Metab. 2009;296(3):E490–E496. doi: 10.1152/ajpendo.90691.2008. [DOI] [PubMed] [Google Scholar]
- 62.Kawano H, Sato T, Yamada T, Matsumoto T, Sekine K, Watanabe T, Nakamura T, Fukuda T, Yoshimura K, Yoshizawa T, Aihara K, Yamamoto Y, Nakamichi Y, Metzger D, Chambon P, Nakamura K, Kawaguchi H, Kato S. Suppressive function of androgen receptor in bone resorption. Proc Natl Acad Sci USA. 2003;100(16):9416–9421. doi: 10.1073/pnas.1533500100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flouriot G, Brand H, Denger S, Metivier R, Kos M, Reid G, Sonntag-Buck V, Gannon F. Identification of a new isoform of the human estrogen receptor-alpha (hER-alpha) that is encoded by distinct transcripts and that is able to repress hER-alpha activation function. EMBO J. 2000;19(17):4688–4700. doi: 10.1093/emboj/19.17.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jakacka M, Ito M, Weiss J, Chien PY, Gehm BD, Jameson JL. Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J Biol Chem. 2001;276(17):13615–13621. doi: 10.1074/jbc.M008384200. [DOI] [PubMed] [Google Scholar]
- 65.Smith EP, Specker B, Bachrach BE, Kimbro KS, Li XJ, Young MF, Fedarko NS, Abuzzahab MJ, Frank GR, Cohen RM, Lubahn DB, Korach KS. Impact on bone of an estrogen receptor-alpha gene loss of function mutation. J Clin Endocrinol Metab. 2008;93(8):3088–3096. doi: 10.1210/jc.2007-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dieudonne SC, Xu T, Chou JY, Kuznetsov SA, Satomura K, Mankani M, Fedarko NS, Smith EP, Robey PG, Young MF. Immortalization and characterization of bone marrow stromal fibroblasts from a patient with a loss of function mutation in the estrogen receptor-alpha gene. J Bone Miner Res. 1998;13(4):598–608. doi: 10.1359/jbmr.1998.13.4.598. [DOI] [PubMed] [Google Scholar]
- 67.Pescovitz OH. The endocrinology of the pubertal growth spurt. Acta Paediatr Scand Suppl. 1990;367:119–125. doi: 10.1111/j.1651-2227.1990.tb11646.x. [DOI] [PubMed] [Google Scholar]
- 68.Delemarre-van de Waal HA, Van Coeverden SC, Rotteveel J. Hormonal determinants of pubertal growth. J Pediatr Endocrinol Metab. 2001;14(Suppl 6):1521–1526. [PubMed] [Google Scholar]
- 69.Tanner JM, Whitehouse RH, Hughes PC, Carter BS. Relative importance of growth hormone and sex steroids for the growth at puberty of trunk length, limb length, and muscle width in growth hormone-deficient children. J Pediatr. 1976;89(6):1000–1008. doi: 10.1016/s0022-3476(76)80620-8. [DOI] [PubMed] [Google Scholar]
- 70.Tanner JM, Whitehouse RH, Marubini E, Resele LF. The adolescent growth spurt of boys and girls of the Harpenden growth study. Ann Hum Biol. 1976;3(2):109–126. doi: 10.1080/03014467600001231. [DOI] [PubMed] [Google Scholar]
- 71.Ritzen EM, Nilsson O, Grigelioniene G, Holst M, Savendahl L, Wroblewski J. Estrogens and human growth. J Steroid Biochem Mol Biol. 2000;74(5):383–386. doi: 10.1016/s0960-0760(00)00116-3. [DOI] [PubMed] [Google Scholar]
- 72.Van DC, Burstein S, Conte FA, Grumbach MM. Isolated gonadotropin deficiency in boys: clinical characteristics and growth. J Pediatr. 1987;111(5):684–692. doi: 10.1016/s0022-3476(87)80243-3. [DOI] [PubMed] [Google Scholar]
- 73.Kaushanski A, Laron Z. Growth pattern of boys with isolated gonadotropin deficiency. Isr J Med Sci. 1979;15(6):518–521. [PubMed] [Google Scholar]
- 74.Tanner JM. The growth and development of the Annals of Human Biology: a 25-year retrospective. Ann Hum Biol. 1999;26(1):3–18. doi: 10.1080/030144699282949. [DOI] [PubMed] [Google Scholar]
- 75.Marcus R, Leary D, Schneider DL, Shane E, Favus M, Quigley CA. The contribution of testosterone to skeletal development and maintenance: lessons from the androgen insensitivity syndrome. J Clin Endocrinol Metab. 2000;85(3):1032–1037. doi: 10.1210/jcem.85.3.6428. [DOI] [PubMed] [Google Scholar]
- 76.Venken K, De GK, Boonen S, Ophoff J, Bouillon R, Swinnen JV, Verhoeven G, Vanderschueren D. Relative impact of androgen and estrogen receptor activation in the effects of androgens on trabecular and cortical bone in growing male mice: a study in the androgen receptor knockout mouse model. J Bone Miner Res. 2006;21(4):576–585. doi: 10.1359/jbmr.060103. [DOI] [PubMed] [Google Scholar]
- 77.Papadimitriou DT, Linglart A, Morel Y, Chaussain JL. Puberty in subjects with complete androgen insensitivity syndrome. Horm Res. 2006;65(3):126–131. doi: 10.1159/000091592. [DOI] [PubMed] [Google Scholar]
- 78.Munoz-Torres M, Jodar E, Quesada M, Escobar-Jimenez F. Bone mass in androgen-insensitivity syndrome: response to hormonal replacement therapy. Calcif Tissue Int. 1995;57(2):94–96. doi: 10.1007/BF00298426. [DOI] [PubMed] [Google Scholar]
- 79.Vanderschueren D, Gaytant J, Boonen S, Venken K. Androgens and bone. Curr Opin Endocrinol Diabetes Obes. 2008;15(3):250–254. doi: 10.1097/MED.0b013e3282fe6ca9. [DOI] [PubMed] [Google Scholar]
- 80.Limbeck GA, Ruvalcaba RH, Mahoney CP, Kelley VC. Studies on anabolic steroids. IV. The effects of oxandrolone on height and skeletal maturation in uncomplicated growth retardation. Clin Pharmacol Ther. 1971;12(5):798–805. doi: 10.1002/cpt1971125798. [DOI] [PubMed] [Google Scholar]
- 81.Bareille P, Massarano AA, Stanhope R. Final height outcome in girls with Turner syndrome treated with a combination of low dose oestrogen and oxandrolone. Eur J Pediatr. 1997;156(5):358–362. doi: 10.1007/s004310050614. [DOI] [PubMed] [Google Scholar]
- 82.Schroor EJ, van Weissenbruch MM, Knibbe P, Delemarre-van de Waal HA. The effect of prolonged administration of an anabolic steroid (oxandrolone) on growth in boys with constitutionally delayed growth and puberty. Eur J Pediatr. 1995;154(12):953–957. doi: 10.1007/BF01958637. [DOI] [PubMed] [Google Scholar]
- 83.Dunkel L. Update on the role of aromatase inhibitors in growth disorders. Horm Res. 2009;71(Suppl 1):57–63. doi: 10.1159/000178040. [DOI] [PubMed] [Google Scholar]
- 84.Mauras N, Gonzalez de PL, Hsiang HY, Desrosiers P, Rapaport R, Schwartz ID, Klein KO, Singh RJ, Miyamoto A, Bishop K. Anastrozole increases predicted adult height of short adolescent males treated with growth hormone: a randomized, placebo-controlled, multicenter trial for one to three years. J Clin Endocrinol Metab. 2008;93(3):823–831. doi: 10.1210/jc.2007-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shulman DI, Francis GL, Palmert MR, Eugster EA. Use of aromatase inhibitors in children and adolescents with disorders of growth and adolescent development. Pediatrics. 2008;121(4):e975–e983. doi: 10.1542/peds.2007-2081. [DOI] [PubMed] [Google Scholar]
- 86.Nilsson O, Baron J. Impact of growth plate senescence on catch-up growth and epiphyseal fusion. Pediatr Nephrol. 2005;20(3):319–322. doi: 10.1007/s00467-004-1689-4. [DOI] [PubMed] [Google Scholar]
- 87.Binder G, Grauer ML, Wehner AV, Wehner F, Ranke MB. Outcome in tall stature. Final height and psychological aspects in 220 patients with and without treatment. Eur J Pediatr. 1997;156(12):905–910. doi: 10.1007/s004310050740. [DOI] [PubMed] [Google Scholar]
- 88.Eugster EA. Peripheral precocious puberty: causes and current management. Horm Res. 2009;71(Suppl 1):64–67. doi: 10.1159/000178041. [DOI] [PubMed] [Google Scholar]
- 89.Falahati-Nini A, Riggs BL, Atkinson EJ, O’Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106(12):1553–1560. doi: 10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Specker BL. Influence of rapid growth on skeletal adaptation to exercise. J Musculoskelet Neuronal Interact. 2006;6(2):147–153. [PubMed] [Google Scholar]
- 91.Lapauw B, Taes Y, Bogaert V, Vanbillemont G, Goemaere S, Zmierczak H, De BD, Kaufman J. Serum estradiol is associated with volumetric bone mineral density and modulates the impact of physical activity on bone size at the age of peak bone mass; a study in healthy male siblings. J Bone Miner Res. 2008 doi: 10.1359/jbmr.081260. [DOI] [PubMed] [Google Scholar]
- 92.Paller CJ, Shiels MS, Rohrmann S, Basaria S, Rifai N, Nelson W, Platz EA, Dobs A. Relationship of sex steroid hormones with bone mineral density (BMD) in a nationally representative sample of men. Clin Endocrinol (Oxf) 2009;70(1):26–34. doi: 10.1111/j.1365-2265.2008.03300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mellstrom D, Vandenput L, Mallmin H, Holmberg AH, Lorentzon M, Oden A, Johansson H, Orwoll ES, Labrie F, Karlsson MK, Ljunggren O, Ohlsson C. Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. J Bone Miner Res. 2008;23(10):1552–1560. doi: 10.1359/jbmr.080518. [DOI] [PubMed] [Google Scholar]
- 94.Amin S, Zhang Y, Sawin CT, Evans SR, Hannan MT, Kiel DP, Wilson PW, Felson DT. Association of hypogonadism and estradiol levels with bone mineral density in elderly men from the Framingham study. Ann Intern Med. 2000;133(12):951–963. doi: 10.7326/0003-4819-133-12-200012190-00010. [DOI] [PubMed] [Google Scholar]
- 95.Mosekilde L. Sex differences in age-related loss of vertebral trabecular bone mass and structure—biomechanical consequences. Bone. 1989;10(6):425–432. doi: 10.1016/8756-3282(89)90074-4. [DOI] [PubMed] [Google Scholar]
- 96.Notini AJ, McManus JF, Moore A, Bouxsein M, Jimenez M, Chiu WS, Glatt V, Kream BE, Handelsman DJ, Morris HA, Zajac JD, Davey RA. Osteoblast deletion of exon 3 of the androgen receptor gene results in trabecular bone loss in adult male mice. J Bone Miner Res. 2007;22(3):347–356. doi: 10.1359/jbmr.061117. [DOI] [PubMed] [Google Scholar]
- 97.Callewaert F, Venken K, Ophoff J, De GK, Torcasio A, van Lenthe GH, Van OH, Boonen S, Bouillon R, Verhoeven G, Vanderschueren D. Differential regulation of bone and body composition in male mice with combined inactivation of androgen and estrogen receptor-alpha. FASEB J. 2009;23(1):232–240. doi: 10.1096/fj.08-113456. [DOI] [PubMed] [Google Scholar]
- 98.Raisz LG, Wiita B, Artis A, Bowen A, Schwartz S, Trahiotis M, Shoukri K, Smith J. Comparison of the effects of estrogen alone and estrogen plus androgen on biochemical markers of bone formation and resorption in post-menopausal women. J Clin Endocrinol Metab. 1996;81(1):37–43. doi: 10.1210/jcem.81.1.8550780. [DOI] [PubMed] [Google Scholar]
- 99.Orwoll ES, Stribrska L, Ramsey EE, Keenan EJ. Androgen receptors in osteoblast-like cell lines. Calcif Tissue Int. 1991;49(3):183–187. doi: 10.1007/BF02556115. [DOI] [PubMed] [Google Scholar]
- 100.Colvard DS, Eriksen EF, Keeting PE, Wilson EM, Lubahn DB, French FS, Riggs BL, Spelsberg TC. Identification of androgen receptors in normal human osteoblast-like cells. Proc Natl Acad Sci USA. 1989;86(3):854–857. doi: 10.1073/pnas.86.3.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ritchie LD, Fung EB, Halloran BP, Turnlund JR, Van L, Cann CE, King JC. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr. 1998;67(4):693–701. doi: 10.1093/ajcn/67.4.693. [DOI] [PubMed] [Google Scholar]
- 102.Karlsson C, Obrant KJ, Karlsson M. Pregnancy and lactation confer reversible bone loss in humans. Osteoporos Int. 2001;12(10):828–834. doi: 10.1007/s001980170033. [DOI] [PubMed] [Google Scholar]
- 103.Kalkwarf HJ, Specker BL. Bone mineral loss during lactation and recovery after weaning. Obstet Gynecol. 1995;86(1):26–32. doi: 10.1016/0029-7844(95)00083-4. [DOI] [PubMed] [Google Scholar]
- 104.Polatti F, Capuzzo E, Viazzo F, Colleoni R, Klersy C. Bone mineral changes during and after lactation. Obstet Gynecol. 1999;94(1):52–56. doi: 10.1016/s0029-7844(99)00236-7. [DOI] [PubMed] [Google Scholar]
- 105.Specker B, Binkley T. High parity is associated with increased bone size and strength. Osteoporos Int. 2005;16(12):1969–1974. doi: 10.1007/s00198-005-1978-1. [DOI] [PubMed] [Google Scholar]
- 106.Seeman E, Bianchi G, Khosla S, Kanis JA, Orwoll E. Bone fragility in men—where are we? Osteoporos Int. 2006;17(11):1577–1583. doi: 10.1007/s00198-006-0160-8. [DOI] [PubMed] [Google Scholar]
- 107.Orwoll ES. Toward an expanded understanding of the role of the periosteum in skeletal health. J Bone Miner Res. 2003;18(6):949–954. doi: 10.1359/jbmr.2003.18.6.949. [DOI] [PubMed] [Google Scholar]
- 108.Rauch F. Bone growth in length and width: the Yin and Yang of bone stability. J Musculoskelet Neuronal Interact. 2005;5(3):194–201. [PubMed] [Google Scholar]
- 109.Seeman E. The periosteum—a surface for all seasons. Osteoporos Int. 2007;18(2):123–128. doi: 10.1007/s00198-006-0296-6. [DOI] [PubMed] [Google Scholar]
- 110.Horsman A, Jones M, Francis R, Nordin C. The effect of estrogen dose on postmenopausal bone loss. N Engl J Med. 1983;309(23):1405–1407. doi: 10.1056/NEJM198312083092301. [DOI] [PubMed] [Google Scholar]
- 111.Brockstedt H, Kassem M, Eriksen EF, Mosekilde L, Melsen F. Age- and sex-related changes in iliac cortical bone mass and remodeling. Bone. 1993;14(4):681–691. doi: 10.1016/8756-3282(93)90092-o. [DOI] [PubMed] [Google Scholar]
- 112.Jarvinen TL, Kannus P, Sievanen H. Estrogen and bone—a reproductive and locomotive perspective. J Bone Miner Res. 2003;18(11):1921–1931. doi: 10.1359/jbmr.2003.18.11.1921. [DOI] [PubMed] [Google Scholar]
- 113.Ophoff J, Venken K, Callewaert F, Boonen S, Bouillon R, Vanderschueren D. Sex steroids during bone growth: a comparative study between mouse models for hypogonadal and senile osteoporosis. Osteoporos Int. 2009;20(10):1749–1757. doi: 10.1007/s00198-009-0851-z. [DOI] [PubMed] [Google Scholar]
- 114.Vanderschueren D, Venken K, Ophoff J, Bouillon R, Boonen S. Clinical review: sex steroids and the periosteum—reconsidering the roles of androgens and estrogens in periosteal expansion. J Clin Endocrinol Metab. 2006;91(2):378–382. doi: 10.1210/jc.2005-1766. [DOI] [PubMed] [Google Scholar]
- 115.Pajamaki I, Sievanen H, Kannus P, Jokihaara J, Vuohelainen T, Jarvinen TL. Skeletal effects of estrogen and mechanical loading are structurally distinct. Bone. 2008;43(4):748–757. doi: 10.1016/j.bone.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 116.Vanderschueren D, Vandenput L, Boonen S. Reversing sex steroid deficiency and optimizing skeletal development in the adolescent with gonadal failure. Endocr Dev. 2005;8:150–165. doi: 10.1159/000084100. [DOI] [PubMed] [Google Scholar]
- 117.Vanderschueren D, Van HE, Geusens P, Suiker A, Visser W, Chung K, Bouillon R. Androgen resistance and deficiency have different effects on the growing skeleton of the rat. Calcif Tissue Int. 1994;55(3):198–203. doi: 10.1007/BF00425875. [DOI] [PubMed] [Google Scholar]
- 118.Vanderschueren D, Boonen S, Bouillon R. Action of androgens versus estrogens in male skeletal homeostasis. Bone. 1998;23(5):391–394. doi: 10.1016/s8756-3282(98)00131-8. [DOI] [PubMed] [Google Scholar]
- 119.Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29(4):441–464. doi: 10.1210/er.2008-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Orwoll ES. Androgens: basic biology and clinical implication. Calcif Tissue Int. 2001;69(4):185–188. doi: 10.1007/s00223-001-1062-6. [DOI] [PubMed] [Google Scholar]
- 121.Maor G, Segev Y, Phillip M. Testosterone stimulates insulin-like growth factor-I and insulin-like growth factor-I-receptor gene expression in the mandibular condyle—a model of endochondral ossification. Endocrinology. 1999;140(4):1901–1910. doi: 10.1210/endo.140.4.6618. [DOI] [PubMed] [Google Scholar]
- 122.Verdonck A, De RL, Kuhn R, Darras V, Carels C, de ZF. Effect of testosterone replacement after neonatal castration on craniofacial growth in rats. Arch Oral Biol. 1998;43(7):551–557. doi: 10.1016/s0003-9969(98)00030-2. [DOI] [PubMed] [Google Scholar]
- 123.Clarke BL, Ebeling PR, Jones JD, Wahner HW, O’Fallon WM, Riggs BL, Fitzpatrick LA. Changes in quantitative bone histomorphometry in aging healthy men. J Clin Endocrinol Metab. 1996;81(6):2264–2270. doi: 10.1210/jcem.81.6.8964862. [DOI] [PubMed] [Google Scholar]
- 124.Schnitzler CM, Pettifor JM, Mesquita JM, Bird MD, Schnaid E, Smyth AE. Histomorphometry of iliac crest bone in 346 normal black and white South African adults. Bone Miner. 1990;10(3):183–199. doi: 10.1016/0169-6009(90)90261-d. [DOI] [PubMed] [Google Scholar]
- 125.Jackson JA, Kleerekoper M, Parfitt AM, Rao DS, Villanueva AR, Frame B. Bone histomorphometry in hypogonadal and eugonadal men with spinal osteoporosis. J Clin Endocrinol Metab. 1987;65(1):53–58. doi: 10.1210/jcem-65-1-53. [DOI] [PubMed] [Google Scholar]