Abstract
Mechanistic insight into the pathway of the Bischler-Mohlau indole formation reaction is provided by isotopic labeling utilizing judicious incorporation of a 13C atom within the α-bromoacetophenone analogue reactant. The resulting rearranged 2-aryl indole, isolated as the major product, located the 13C isotope label at the methine carbon of the fused five-membered heterocyclic ring, which suggested that the mechanistic pathway of cyclization, in this specific example, required two equivalents of the aniline analogue reactant partner and proceeded through an imine intermediate rather than by direct formation of the corresponding 3-aryl indole accompanied by a concomitant 1,2-aryl shift rearrangement.
Keywords: Bischler-Mohlau Indole Synthesis, Indole Synthesis, Mechanistic Investigation, 13C Atom Incorporation
Introduction
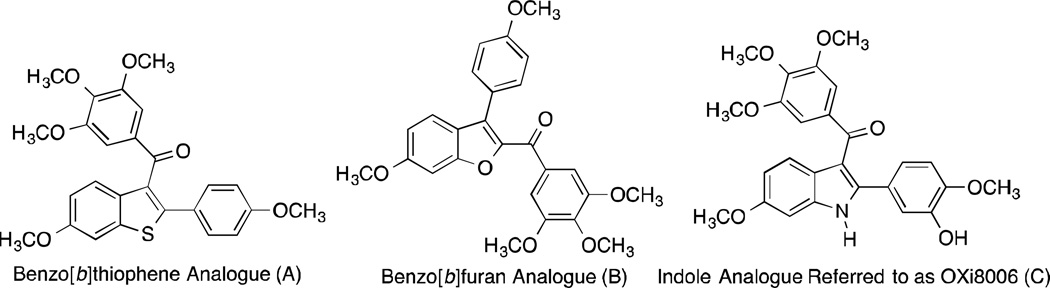
In the context of a long-standing program focused on the design, synthesis, and biological evaluation of diversely functionalized small-molecule anticancer agents, a series of benzo[b]thiophene,1–3 benzo[b]furan,4–5 and indole-based6–8 analogues emerged as promising potential pre-clinical candidates (Fig. 1). These compounds were designed to function as inhibitors of tubulin polymerization (assembly) that bind to the colchicine site on the tubulin heterodimer and certain of these compounds demonstrated a dualistic mechanism of action functioning both as potent antiproliferative agents and as pronounced vascular disrupting agents (VDAs).9–10 In each case, the heterocyclic fused ring was introduced synthetically through an efficient ring-closing step, and it proved intriguing to consider whether this reaction in the indole series of analogues mirrored that of the benzo[b]thiophene series or was mechanistically distinct.
Figure 1.
Representative Examples of Inhibitors of Tubulin Polymerization Incorporating Fused Heterocyclic Ring Systems: Benzo[b]thiophene (A);1–3 Benzo[b]furan (B);4–5 and Indole (C) 6–8
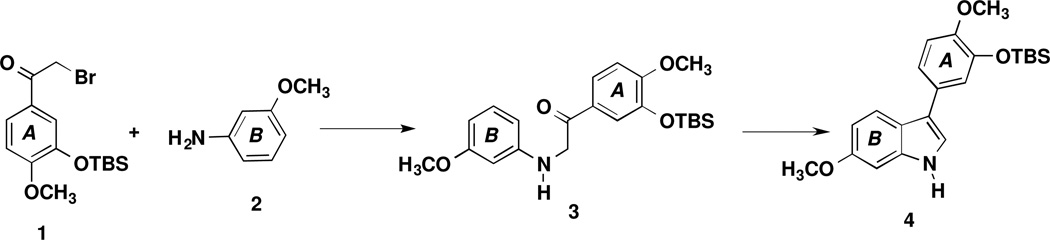
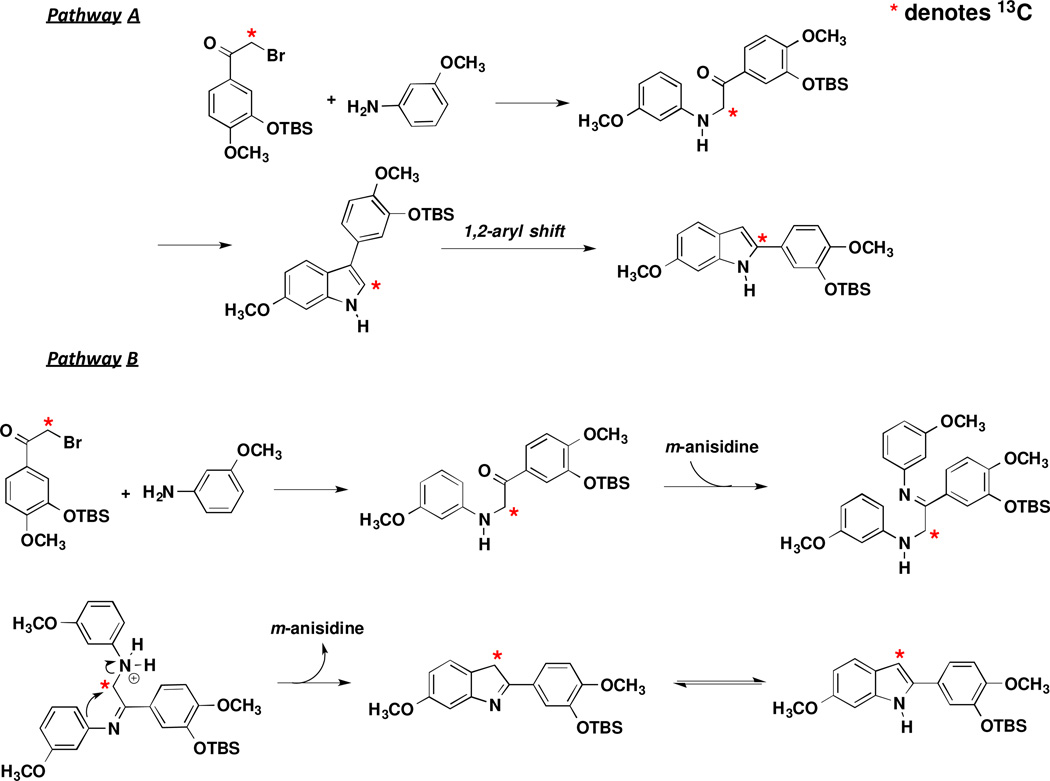
The indole core ring system continues to be utilized as an abundant molecular scaffold in medical chemistry11 and represent an important class of heterocycles.12 The mechanistic pathways and synthetic routes available towards the indole platform are well established and vary greatly.13 One established approach, referred to as the Fischer indole synthesis, involves a [3,3]-sigmatropic rearrangement followed by closure to the fused fivemembered ring. This method was first pioneered by Fischer in 188314 and continues to be explored today.15 Another frequently utilized approach is the Bischler-Mohlau indole synthesis16–18 that involves the reaction between aniline analogues and α-halogenated ketone analogues and results in both 2-aryl and 3-aryl indole regioisomers. While the Bischler-Mohlau reaction19–24 accommodates a wide-range of functionalized α-bromoketones and aniline analogues as starting materials, low isolated yields and unpredictable regiochemistry25–26 remain potentially problematic. The reaction is heavily substrate dependent (in terms of yield and regiochemical outcome) and modifications in reaction conditions, including microwave heating,25 can dramatically influence the overall process.26 The perceived simplicity of the Bischler-Mohlau reaction somewhat disguises the complex mechanistic pathways which can lead to both 2-aryl and 3-aryl indole analogues. The indole product (2-aryl or 3-aryl) is dependent on one of several potential mechanistic pathways. These pathways were further investigated by Vara and co-workers by assessing the activation energies associated with intermediates and transition states.26 Pathway A (Scheme 1) involves initial displacement of a bromine atom from 2-bromoacetophenone or an appropriately functionalized 2-bromoacetophenone analogue by aniline or an analogous aniline analogue. The pathway proceeds through intramolecular cyclization and subsequent re-aromatization to afford non-rearranged 3-aryl indole 4.
Scheme 1.
Mechanistic Pathway A Associated with the Bischler-Mohlau Reaction
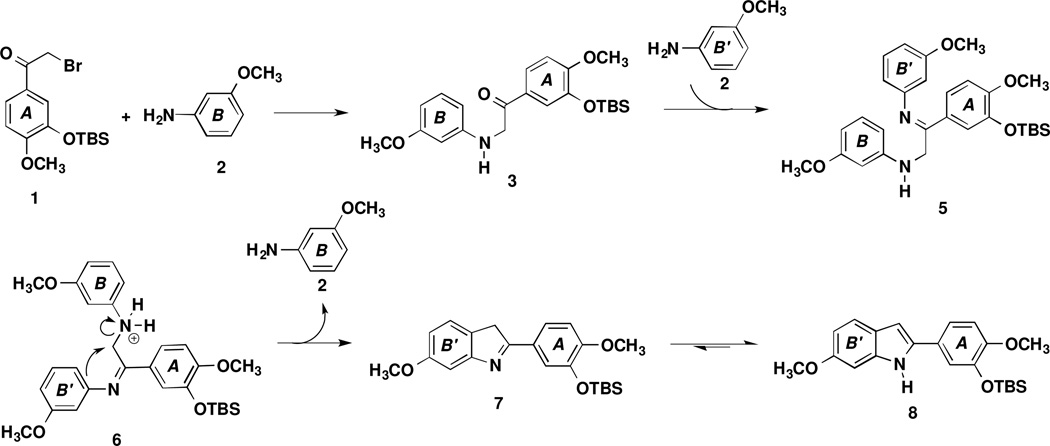
A well-recognized competing Pathway B (Scheme 2), which most often is invoked as being predictive of the major product of the Bischler-Mohlau indole synthesis, initiates in a similar fashion with the aniline analogue (3-methoxyaniline in this example) displacing the bromine atom on the alpha-bromoacetophenone analogue (compound 1 in this example). Condensation with a second molecule of 3-methoxyaniline results in the formation of imine intermediate 5, which upon intramolecular cyclization involving displacement of the initial aniline molecule and subsequent tautomerization of 2-aryl indole 7, generates the stable 2-aryl indole tautomer 8. Previous computational and experimental studies have suggested that pathway B is preferred when an excess of aniline is used.26 It should be noted that Vara and co-workers have evaluated another mechanistic possibility leading to formation of rearranged indole products (such as 8), through an interesting carbonyl-shift rearrangement of a ketone (such as 3) to an aldehyde prior to cyclization.26–28
Scheme 2.
Mechanistic Pathway B Associated with the Bischler-Mohlau Reaction.
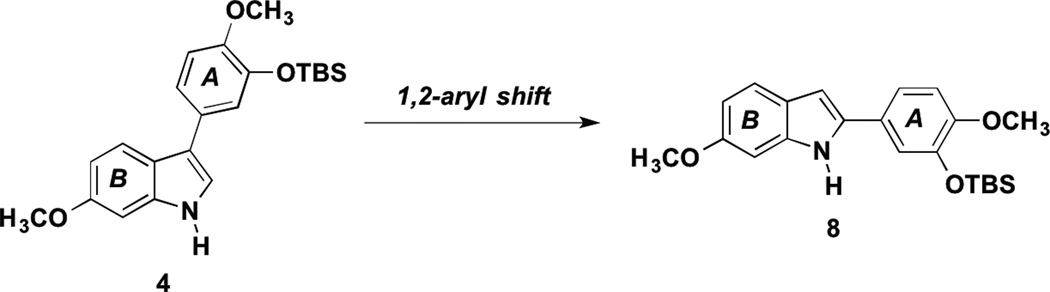
We were intrigued by the possibility that a 3-aryl-indole analogue formed through pathway A could perhaps undergo a subsequent 1,2 aryl shift (pH dependent) resulting in a rearranged 2-aryl-indole analogue as the thermodynamic sink under these reaction conditions (Scheme 3).29–30
Scheme 3.
Postulated 1,2-Aryl Shift Resulting in Rearranged Indole Analogue.
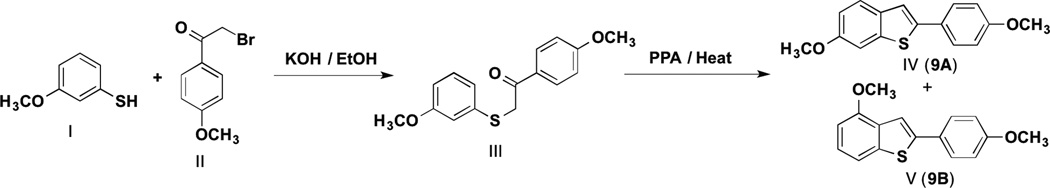
This postulated methodology (1,2-aryl shift in the indole system under Bischer-Mohlau conditions) is somewhat reminiscent of previous studies with related benzo[b]thiophene ring systems in which alpha-thio-ketone III (Scheme 4), for example, was converted to benzo[bthiophene regioisomers IV and V upon treatment with PPA.1,31–32 The mechanism is widely thought to involve concomitant cyclization and 1,2 aryl ring migration. The regioisomers in this example result from initial cyclization occurring either ortho or para to the methoxy group on the aryl ring of the sulfide. 1,31–32
Scheme 4.
Formation of Benzo[b]thiophene Regioisomers Via Cyclization and Concomitant 1,2-Aryl Ring Migration.
In an effort to further explore the Bischler-Mohlau reaction pathways a 13C isotopic labeleling strategy was developed in which the α-carbon (to the carbonyl) was selectively labeled with 13C. This labeled carbon atom can be readily traced through the identification of key distinct 13C NMR signatures, specifically 13C DEPT NMR, thus providing evidence for which mechanistic pathway predominates in this specific indole-forming reaction sequence (Scheme 5).
Scheme 5.
Potential Mechanistic Pathways Leading to 13C Labeled Indole Analogues
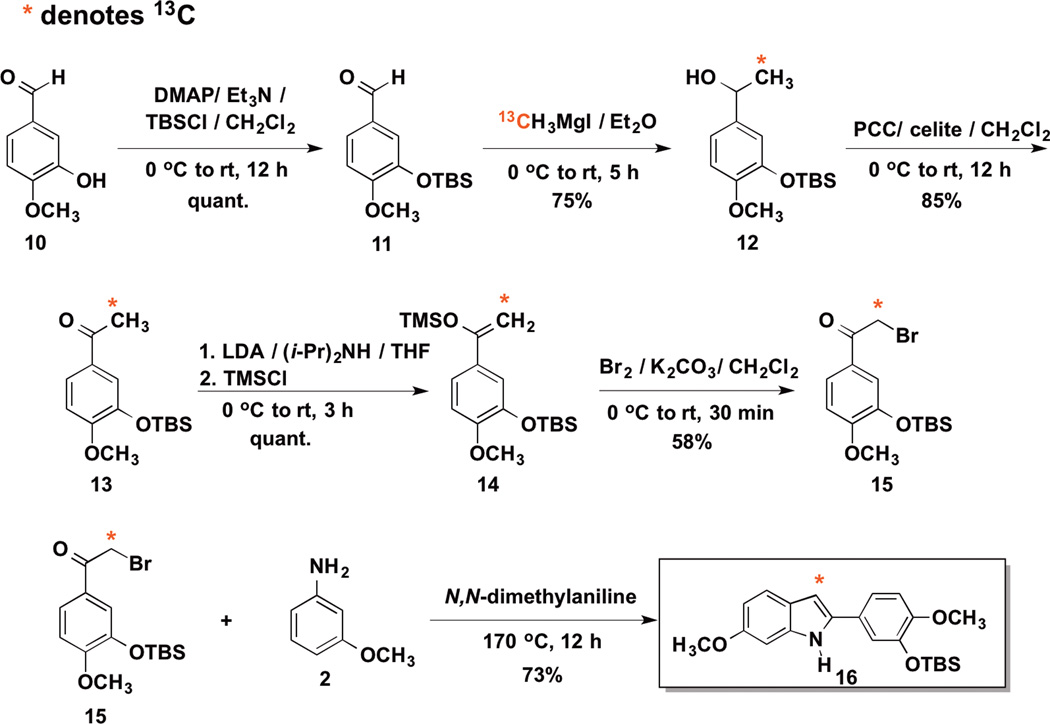
The synthetic route to 13C-labeled bromoacetophenone intermediate 16 followed a similar sequence as the non-labeled bromoacetophenone intermediate from our previous studies with the indole based vascular disrupting agent (VDA) OXi80066–7,33 along with related work by von Angerer and co-workers,34 and simply replaces the traditional methylation step reagents to install the 13C carbon atom at the alpha position (Scheme 6).
Scheme 6.
Synthesis of 13C Labeled bromoacetophenone 15 and 2-aryl indole 16
Protection of 3-hydroxy-4-methoxybenzaldehyde (isovanillin) with TBSCl in the presence of Et3N and catalytic DMAP afforded TBS-aldehyde 11 which was subsequently treated with in situ generated 13CH3MgI (from commercially available 13CH3I) to yield 13C-labeled secondary alcohol 12. PCC mediated oxidation generated 13C-labeled acetophenone 13, which after enolization was trapped as its corresponding silyl enol ether 14 upon reaction with TMSCl. Bromination of 13C-labled enol ether 14 afforded requisite 13C-labeled bromoacetophenone intermediate 15, which was treated with greater than three molar equivalents of 3-methoxyaniline (m-anisidine) 2 in N,N-dimethylaniline at 170 °C (Bischler-Mohlau conditions) for 12 h to afford rearranged 13C-labeled 2-aryl indole 16 (Scheme 6).
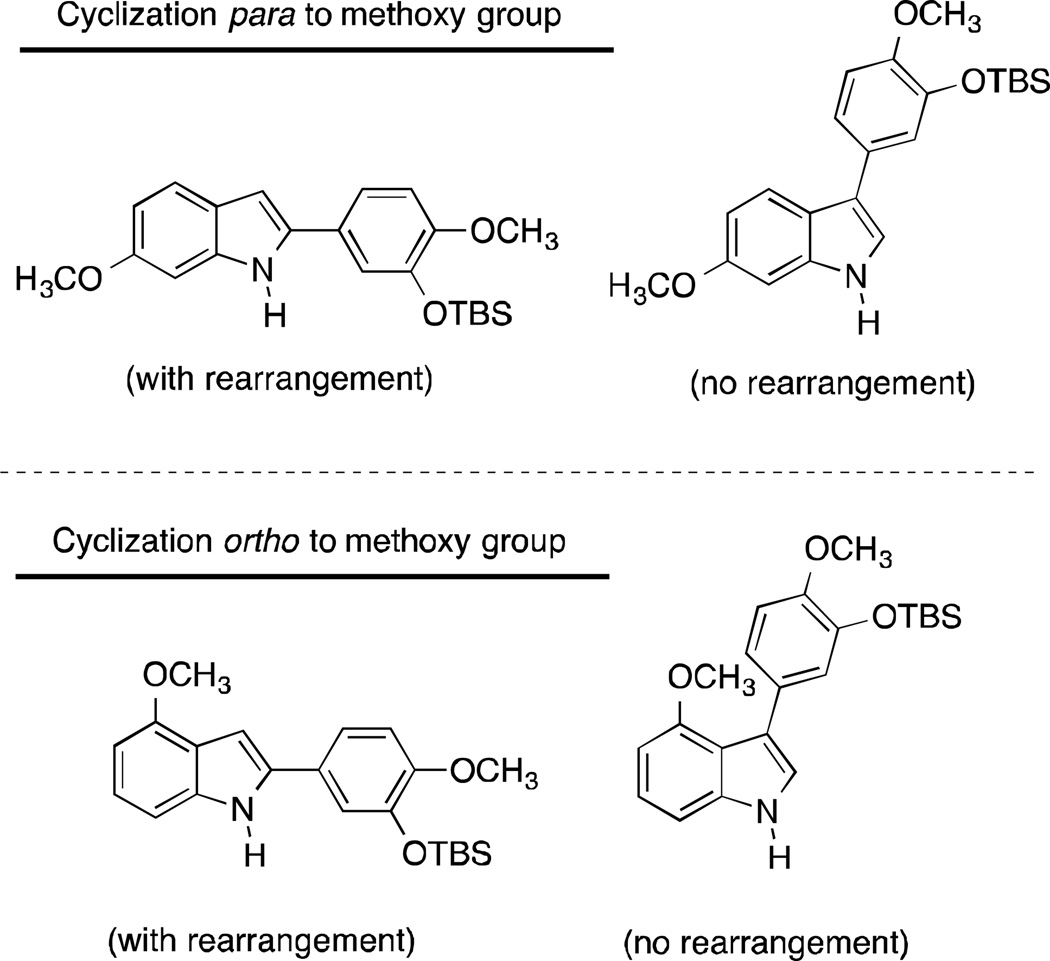
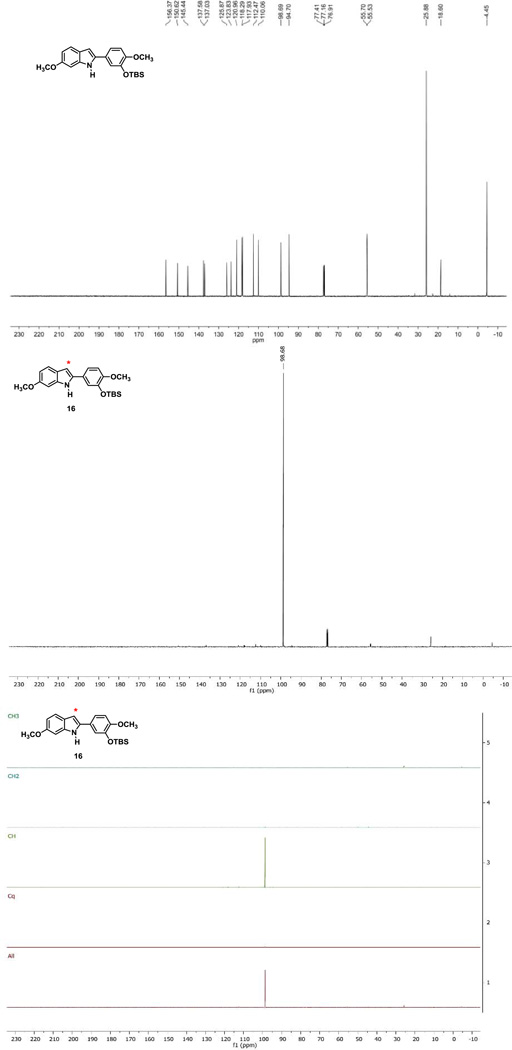
It is important to note that there are four possible indole regioisomers that can result from this transformation (Fig. 2) depending on whether the initial cyclization takes place para or ortho to the methoxy group, with or without rearrangement. In our hands, with this specific set of reactants and these reaction conditions, only one regioisomer was isolated and it was identified as the regioisomer in which the 13C atom label was located at the methine carbon (C-3 position of the indole core), suggesting that the system proceeded mechanistically through pathway B (imine intermediate formation) to generate the rearranged 2-aryl indole analogue 16. The Bischer-Mohlau reaction to form indole analogue 16 was repeated twice for verification. In the first case, the isolated/purified yield of indole 16 was somewhat low (23%), however in the repeated experiment this yield rose to 73% (91% pure by HPLC), and a subsequent recrystallization afforded a pure sample (see Supplementary data for pertinent spectra). Initial comparison of the 13C-NMR of indole regioisomer 16 (Fig. 3) with the predicted spectra (ChemBioDraw Ultra, Version 13.0.2.3020) for each of the four regioisomers, also taking into account the differences in the 1H-NMR coupling patterns in the A-ring between the para and ortho ring closed possible products, strongly suggested regioisomer 16 as the major (and only identified and characterized) product of this reaction (both the intial reaction and the repeated reaction). DEPT 13C NMR analysis (Fig. 3) confirmed that the 13C atom label was located on a methine carbon, thus providing further evidence in support of regioisomer 16. X-ray crystallographic analysis35 of indole regioisomer 16 provided unequovical confirmation of its structural assignment (see Supplementary data).
Figure 2.
Four Possible Indole Regioisomers from Representative Bischler-Mohlau Reaction
Figure 3.
13C-NMR of Unlabeled Indole Analogue 8, 13C-NMR of 13C Labeled Indole Analogue 16 (same as indole 8 but incorporating 13C label), DEPT NMR of 13C Labeled Indole Analogue 16.
Judicious incorporation of a 13C label provided compelling evidence that the indole ring closure occurred (at least in this example) through a Bischler-Mohlau pathway rather than a Friedel-Crafts type ring closure, re-aromatization, accompanied by a concomitant aryl ring migration sequence that was envisioned as a potential competing pathway based on early studies suggesting that certain benzo[b]thiophene systems undergo ring-closure through this pathway under polyphosphoric acid (PPA) conditions. These results suggest that further inquiry into these and related systems may prove fruitful in delineating and predicting mechanistic pathways based (perhaps) on functional group incorporation and choice of reaction conditions, thus expanding the canopy of indole, benzo[b]thiophene, benzo[b]furan, and related small-molecule anticancer agents accessible under these synthetic protocols.
Supplementary Material
Acknowledgments
The authors are grateful to the National Cancer Institute of the National Institutes of Health (grant no. 5R01CA140674 to K.G.P.) and OXiGENE Inc. (grant to K.G.P.) for their financial support of this project, and to the NSF for funding the Varian 500 MHz NMR spectrometer (grant no. CHE- 0420802). The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health. The authors also thank Dr. Alejandro Ramirez (Mass Spectrometry Core Facility, Baylor University), and Dr. Kevin Klausmeyer (X-ray analysis at Baylor University).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data.
Supplementary data (Experimental procedures, 1H NMR, 13C NMR, DEPT NMR, HRMS, and HPLC data, along with X-ray crystallographic data) associated with this article can be found, in the online version, at {to be completed later}.
References Cited
- 1.Pinney KG, Bounds DA, Dingeman KM, Mocharla VP, Pettit GR, Bai R, Hamel E. Bioorg. Med. Chem. Lett. 1999;9:1081. doi: 10.1016/s0960-894x(99)00143-2. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z, Mocharla VP, Farmer JM, Pettit GR, Hamel E, Pinney KG. J. Org. Chem. 2000;65:8811. doi: 10.1021/jo0004761. [DOI] [PubMed] [Google Scholar]
- 3.Mullica DF, Pinney KG, Mocharla VP, Dingeman KM, Bounds AD, Sappenfield EL. J. Chem. Crystallogr. 1998;28:289. [Google Scholar]
- 4.Mocharla VP. Ph.D. Dissertation. Waco, TX: Baylor University; 1999. Design, Synthesis, and Biological Evaluation of Diverse Small Molecular Frameworks as Tubulin Binding Ligands. [Google Scholar]
- 5.Kessler RJ. M.S.Thesis. Waco, TX: Baylor University; 2002. Synthesis and Evaluation of New Inhibitors of Tubulin Polymerization and Their Corresponding Prodrugs as Potential Vascular Targeting Agents. [Google Scholar]
- 6.Hadimani MB, Kessler RJ, Kautz JA, Ghatak A, Shirali R, O’Dell H, Garner CM, Pinney KG. Acta Crystallogr. 2002;C58:330. doi: 10.1107/s0108270102003669. [DOI] [PubMed] [Google Scholar]
- 7.Hadimani MB, MacDonough MT, Ghatak A, Strecker TE, Lopez R, Sriram M, Nguyen BL, Hall JJ, Kessler RJ, Shirali AR, Liu L, Garner CM, Pettit GR, Hamel H, Chaplin DJ, Mason RP, Trawick ML, Pinney KG. J. Nat. Prod. 2013;76:1668. doi: 10.1021/np400374w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacDonough MT, Strecker TE, Hamel E, Hall JJ, Chaplin DJ, Trawick ML, Pinney KG. Bioorg. Med. Chem. Lett. 2013;21:6831. doi: 10.1016/j.bmc.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinney KGIn. Ch.6. In: Siemann D, editor. Vascular-Targeted Therapies in Oncology. London, UK: John Wiley & Sons; 2006. pp. 95–121. [Google Scholar]
- 10.Mason RP, Zhao D, Liu L, Trawick ML, Pinney KG. Integr. Biol. 2011;3:375. doi: 10.1039/c0ib00135j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roughley SD, Jordan AM. J. Med. Chem. 2011;54:3451. doi: 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]
- 12.Sundberg RJ. In: Comprehensive Heterocyclic Chemistry II. Katritzky AR, Rees CW, Scriven EFV, Bird CW, editors. Oxford, UK: Pergamon Press; 1992. [Google Scholar]
- 13.Taber DF, Tirunahari PK. Tetrahedron. 2011;67:7195. doi: 10.1016/j.tet.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer E, Jourdan F. Chem. Ber. 1883;16:2241. [Google Scholar]
- 15.Downing RS, Kunkeler PJ. In: The Fischer Indole Synthesis. Sheldon RA, Bekkum H, editors. New York: Wiley-VCH: Weinheim; 2001. [Google Scholar]
- 16.Bischler A, Brion H. Chem. Ber. 1892;25:2860. [Google Scholar]
- 17.Bischler A, Firemann P. Chem. Ber. 1893;26:1336. [Google Scholar]
- 18.Mohlau R. Chem. Ber. 1881;14:173. [Google Scholar]
- 19.Bunescu A, Piemontesi C, Wang Q, Zhu J. Chem. Commun. 2013;49:10284. doi: 10.1039/c3cc46361c. [DOI] [PubMed] [Google Scholar]
- 20.Bigot P, Saint-Ruf G, Buu-Hoi NP. J.C.S. Perkin I. 1972:2573. doi: 10.1039/p19720002573. [DOI] [PubMed] [Google Scholar]
- 21.Bancroft KCC, Ward TJ. J.C.S. Perkin I. 1974:1852. [Google Scholar]
- 22.Henry JR, Dodd JH. Tetrahedron Lett. 1998;39:8763. [Google Scholar]
- 23.Laube M, Tondera C, Sharma SK, Bechmann N, Pietzsch F-J, Pigorsch A, Kockerling M, Wuest F, Pietzsch J, Kniess T. RSC Adv. 2014;4:38726. [Google Scholar]
- 24.Li JJ. Name Reactions. Berlin Heidelberg: Springer-Verlag; 2009. pp. 46–47. [Google Scholar]
- 25.Sridharan V, Perumal S, Avendano C, Menendez JC. Synlett. 2006:91. [Google Scholar]
- 26.Vara Y, Aldaba E, Arrieta A, Pizarro JL, Arriortua MI, Cossio FP. Org. Biomol. Chem. 2008;6:1763. doi: 10.1039/b719641e. [DOI] [PubMed] [Google Scholar]
- 27.Nelson KL, Seefeld R. J. Am.Chem. Soc. 1958;80:5957. [Google Scholar]
- 28.Nelson KL, Robertson J, Duvall JJ. J. Am.Chem. Soc. 1963;86:684. [Google Scholar]
- 29.MacDonough MT, Zhe S, Pinney KG. Abstracts of Papers; 69th Southwest Regional Meeting of the American Chemical Society; November 16- 19, 2013; Waco, TX. SWRM-229. [Google Scholar]
- 30.MacDonough MT. Ph.D. Dissertation. Waco, TX: Baylor University; 2013. The Design, synthesis, and Biological Evaluation of Indole-based Anticancer Agents. [Google Scholar]
- 31.Jones CD, Jevnikar MG, Pike AJ, Peters MK, Black LJ, Thompson AR, Falcone JF, Clemens JA. J. Med. Chem. 1984;27:1057. doi: 10.1021/jm00374a021. [DOI] [PubMed] [Google Scholar]
- 32.Kost AN, Budylin VA, Matveeva ED, Sterligov DO. Zh. Org. Khim. 1970;6:1503. [Google Scholar]
- 33.Pinney KG, Wang F, Del Pilar Mejia M. PCT Int. Appl. 2001 WO 2001019794 A2 20010322. [Google Scholar]
- 34.Kaufmann D, Pojarova M, Vogel S, Liebl R, Gastpar R, Gross D, Nishino T, Pfaller T, von Angerer E. Bioorg. Med. Chem. 2007;15:5122. doi: 10.1016/j.bmc.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 35.Crystallographic data for13C labeled indole regioisomer 16 presented in this paper have been deposited with the Cambridge Crystallographic Data Centre (CCDC deposition number 1041417). Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK, (fax: +44-(0)1223-336033 or deposit@ccdc.cam.ac.Uk).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.