Abstract
Background:
Preclinical studies suggest that chemotherapy may enhance the immune response against pancreatic cancer.
Methods:
The levels of granulocyte macrophage-colony-stimulating factor (GM-CSF) and interleukin-6 (IL-6) and the associated inflammatory marker C-reactive protein (CRP) were assessed in 38 patients receiving gemcitabine and capecitabine combination chemotherapy for advanced pancreatic cancer within the TeloVac trial. Apoptosis (M30) and total immune response (delayed-type hypersensitivity and/or T-cell response) were also assessed and levels of apoptosis induction correlated with immune response. The telomerase GV1001 vaccine was given either sequentially (n=18) or concomitantly (n=24) with the combination chemotherapy.
Results:
There were no differences between baseline and post-treatment levels of CRP (P=0.19), IL-6 (P=0.19) and GM-CSF (P=0.71). There was a positive correlation between post-chemotherapy CRP and IL-6 levels (r=0.45, P=0.005) and between CRP with carbohydrate antigen-19-9 (CA19-9) levels at baseline (r=0.45, P=0.015) and post treatment (r=0.40, P=0.015). The change in CRP and IL-6 levels was positively correlated (r=0.40, P=0.012). Hazard ratios (95% CI) for baseline CA19-9 (1.30 (1.07–1.59), P=0.009) and CRP (1.55 (1.00–2.39), P=0.049) levels were each independently predictive of survival. The M30 mean matched differences between pre- and post-chemotherapy showed evidence of apoptosis in both the sequential (P=0.058) and concurrent (P=0.0018) chemoimmunotherapy arms. Respectively, 5 of 10 and 9 of 20 patients had a positive immune response but there was no association with apoptosis.
Conclusions:
Combination gemcitabine and capecitabine chemotherapy did not affect circulating levels of GM-CSF, IL-6 and CRP. Chemotherapy-induced apoptosis was not associated with the immunogenicity induced by the GV1001 vaccine in advanced pancreatic cancer.
Keywords: pancreatic adenocarcinoma, chemotherapy, inflammatory cytokines, apoptosis, immunogenicity
Immunotherapy is transforming the management of many cancers. Although single-agent checkpoint inhibition has been disappointing in pancreatic ductal adenocarcinoma (Topalian et al, 2012), it is clear that whole-cell vaccines can positively modulate microenvironmental immunity in this disease, upregulate PD-L1 expression (Lutz et al, 2014) and synergise with checkpoint blockade (Le et al, 2013; Soares et al, 2015). Gemcitabine and 5-fluorouracil (5-FU) are commonly used drugs in the management of advanced pancreatic cancer. A number of studies have demonstrated that instead of inhibiting an immune response against cancer cells as would be classically expected, these drugs can enhance certain facets of the anticancer immune response. Hence, combination treatment with immunotherapy to effect therapeutic synergy has been proposed and clinically tested (Middleton et al, 2014; Emens and Middleton, 2015).
The ultimate outcome of microenvironmental immunity is in part dependent on the balance between antigen-specific T cells and resident immunosuppressive cells, chief of these being the myeloid-derived suppressor cells (MDSCs). In mice harbouring a variety of different tumors, a single dose of gemcitabine significantly reduced the number of splenic MDSCs with no effect on the number of CD4+, CD8+ or B cells (Suzuki et al, 2005). In another preclinical study the only other drug, besides gemcitabine, that significantly reduced the number of MDSCs in the spleens and tumor beds of mice was 5-FU (Vincent et al, 2010). 5-Fluorouracil triggered dose-dependent apoptosis of MDSCs: thymidylate synthase levels in MDSCs were lower than in splenocytes or tumor cells. Adoptive transfer of MDSCs 1 day after 5-FU blunted the antitumour response.
The importance of tumor-infiltrating MDSCs in PDA was assessed in the KPC model, where a marked increase in Gr1+CD11b+ cells (the murine MDSC phenotype) was seen during progression from pancreatic ductal intraepithelial neoplasia (PanIN) to invasive adenocarcinoma (Stromness et al, 2014). Granulocyte macrophage-colony-stimulating factor (GM-CSF) was upregulated by cancer cells and was a critical survival factor for MDSCs. MDSCs suppressed T-cell proliferation and induced apoptosis in activated T cells, whereas antibody-mediated depletion of MDSCs significantly increased the numbers of proliferating peritumoural CD8+ T cells. Depletion of MDSCs also affected the tumor stroma, resulting in areas of extracellular matrix depletion and enhanced vascular patency. These immunomodulatory and stromal effects were accompanied by a significant increase in tumor cell apoptosis. Knockdown of GM-CSF in an orthotopic model significantly reduced the growth of pancreatic adenocarcinoma, reduced Gr1+CD11b+ cell number and caused a pronounced accumulation of CD8+ T cells that drove tumour cell apoptosis (Pylayeva-Gupta et al, 2012). In a study examining the key cytokines in the generation of human MDSCs, the mixture of GM-CSF and interleukin-6 (IL-6) consistently generated MDSCs with the greatest capacity to inhibit autologous T-cell proliferation and IFN-γ production (Lechner et al, 2010). Pancreatic stellate cells (PSCs) secrete little GM-CSF but large quantities of IL-6, causing STAT-3 phosphorylation in human peripheral blood mononuclear cells (PBMCs) and thus inducing highly suppressive MDSCs, principally of granulocytic type (Mace et al, 2013). Inhibition of STAT-3 significantly reduced the generation of MDSCs when PBMCs were cultured with either PSC supernatants or IL-6/GM-CSF.
GM-CSF also activates JAK2 resulting in STAT-3 activation (Quelle et al, 1994). STAT3 is critical in mutant KRAS-induced PanIN formation and progression and the growth of established tumours (Corcoran et al, 2011). Both GM-CSF and IL-6 upregulate the inflammatory marker C-reactive protein (CRP) that contains a STAT-3 response element (Deng et al, 2006; Nishikawa et al, 2008). C-reactive protein is an independent prognostic marker in pancreatic cancer (Szkandera et al, 2014). The clinical importance of STAT-3 activation in advanced pancreatic cancer as a result of cancer-induced inflammation was recently demonstrated in a randomised study of second-line capecitabine with or without the JAK2 inhibitor ruxolitinib (Hurwitz et al, 2015). Using a cutoff CRP level at the median, there was a significant survival advantage for the addition of ruxolitinib in patients with CRP >13 mg l−1, but no benefit in those with CRP <13 mg l−1. The ongoing phase III study is including only patients with CRP >10 mg l−1. Given the interest in combining chemotherapy with immunotherapy, we thus investigated the impact of the combination of gemcitabine and the oral fluoropyrimidine capecitabine (GemCap) on CRP, GM-CSF and IL-6 levels to investigate whether these two agents modulate the immunosuppressive milieu of pancreatic adenocarcinoma.
We also analysed the relationship between the induction of apoptosis by GemCap as measured using the Apoptosense M30 assay and the development of an immune response to a class II telomerase peptide vaccine (GV1001), given either concomitantly with GemCap chemotherapy or in a sequential approach where 7 weeks of GemCap was followed by GV1001 in the TeloVac Study (Middleton et al, 2014). In preclinical models, gemcitabine-induced apoptosis increases antigen cross-presentation and primes the immune system (Nowak et al, 2003). Antigen released by apoptosis is available for cross-presentation rather than sequestered from the cross-presentation pathway. The induction of apoptosis is necessary as mice bearing gemcitabine-resistant cells demonstrated no significant difference in the proliferative activity of adoptively transferred antigen-specific lymphocytes when treated with gemcitabine compared with control. 5-Fluorouracil also enhances antigen cross-presentation (Galetto et al, 2003). The immunological impact of gemcitabine and 5-FU induced apoptosis in humans is unknown.
Materials and methods
Patients
Patients with advanced pancreatic cancer were recruited for translational studies participating in the TeloVac study (ISRCTN 43482138) (Middleton et al, 2014). Venous blood was collected from a subset of patients randomised to chemotherapy with sequential chemoimmunotherapy using the telomerase peptide vaccine GV1001 (Kael Gemvax, Seoul, Korea) or chemotherapy with concurrent chemoimmunotherapy using GV1001 for whom suitable samples were available for T-cell proliferation, CRP, IL-6, GM-CSF and M30 Apoptosense assays (Peviva AB, Strőskarlsva, Sweden). Objective tumor response was measured using RECIST (Therasse et al, 2000).
Chemoimmunotherapy
All patients were treated with combination gemcitabine and capecitabine. Gemcitabine was given intravenously at 1000 mg m−2 weekly × 3 every 4 weeks with orally administered capecitabine at 1660 mg m−2 per day (830 mg m−2 twice daily) for 3 weeks followed by 1 week's rest. In the sequential chemoimmunotherapy arm, patients received an initial 7 weeks of chemotherapy followed by vaccination with GV1001 and GM-CSF (Penn Pharmaceutical Services, Tredegar, UK) as adjuvant as described previously (Middleton et al, 2014). In the concurrent chemoimmunotherapy arm, patients received chemotherapy together with vaccine delivered concomitantly from day 1 of therapy. All subjects provided written informed consent for the trial and inclusion criteria stipulated there was no history of autoimmune disease or recent steroid therapy.
DTH testing
Delayed-type hypersensitivity (DTH) testing was undertaken by giving a second intradermal injection of 0.105 mg of GV1001 given simultaneously at the contralateral site on the lower abdomen without concomitant GM-CSF. The site was examined 48 h later and a positive or negative response was recorded.
Blood sample collections
For patients in the sequential chemoimmunotherapy arm, blood was drawn just before the sixth and final gemcitabine infusion before vaccination and 48 h afterwards for the Apoptosense assay. Blood for CRP, IL-6 and GM-CSF assays was drawn before the start of therapy and before the sixth gemcitabine infusion. Peripheral blood mononuclear cells were drawn at week 18 for T-cell proliferation assays after 10 weeks of GV1001 and GM-CSF administration.
For patients in the concurrent chemoimmunotherapy arm, blood was drawn just before the first gemcitabine infusion at the very start of therapy and 48 h afterwards for the Apoptosense assay. PBMCs were drawn at 10 weeks for T-cell proliferation assays, an equivalent period of vaccination before immunogenicity testing in patients receiving chemotherapy preceding vaccination in the sequential chemoimmunotherapy arm.
Storage and analyses of samples
Blood was drawn into CPT tubes (BD Biosciences, Oxford, UK) and spun at site to isolate PBMCs. The PBMCs were shipped to the biomarker repository at the Cancer Research UK Liverpool Clinical Trials Unit-GCP Laboratory Facility, UK. Viability and cell count of the PBMCs was measured using a ChemoMetec (Allerod, Denmark) NucleoCounter NC-100 before freezing in 90% DMSO and 10% human serum to −80 °C overnight. The aliquots were then stored at −150 °C for subsequent batch analysis. Blood for serum was collected into SST tubes (BD Biosciences) centrifuged at 1500 g for 10 min, aliquoted and stored at −80 °C.
IL-6 and GM-CSF analyses
The levels of IL-6 and GM-CSF along with 25 other analytes were measured in triplicate in patient serum using a commercially available Bio-Plex Pro 27 Plex Human Cytokine, Chemokine and Growth Factor Assay (Bio-Rad Laboratories Ltd, Hercules, CA, USA) on the Bio-Plex 200 System. We report here the analysis of the immunosuppressive cytokines pertinent to MDSC biology and CRP, a surrogate read-out for signalling by these cytokines. Details on the trajectory of the other cytokines have been previously presented (Neoptolemos et al, 2014) and a separate manuscript investigating the predictive value of these is in preparation. Initial data analysis was undertaken using Bio-Plex Manager 5.0 Software to determine concentrations. Serially diluted standards (50 μl) and test serum, diluted 1 in 4 in sample diluent (50 μl), was added to a plate containing magnetic antibody-coupled beads for each of the 27 analytes. The samples were incubated at room temperature on a plate shaker at 900 r.p.m. for 1 min followed by 300 r.p.m. for 30 min. Following washing with the Bio Plex Pro Magnetic Plate Washer, the secondary antibodies (25 μl) were added to the plate and incubated as before. The plate was washed again and streptavidin-PE (50 μl) was added and the plate incubated at room temperature on a plate shaker at 900 r.p.m. for 1 min followed by 300 r.p.m. for 15 min. Assay buffer (125 μl) was added to each well of the plate before being analysed on the Bio-Plex 200 machine. Fluorescent intensities obtained for the test samples were read from the standard curve to give pg ml−1 values for each of the 27 analytes.
CRP analysis
C-reactive protein was measured in serum samples at the Department of Clinical Biochemistry and Metabolic Medicine at the Royal Liverpool University Hospital (Liverpool, UK). The limit of detection for this assay is 5 mg l−1.
M30 apoptosense analysis
Serum levels of M30 were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) (Peviva AB), and the results were expressed as U l−1 (see Dive et al, 2010 for details of CK18 evaluation in pancreatic ductal adenocarcinoma).
T-cell proliferation assay
Thawed PBMCs were seeded in X-VIVO 15 (Lonza, Slough, UK) supplemented with 10% pooled human serum (Innovative Research, Paisely, UK) at 2 × 106 cells per well in 48-well plates (Thermo Fisher Scientific, Asheville, NC, USA) and 20 μg ml−1 GV1001 peptide. Media were changed following 3 days of culture and interleukin-2 (Peprotech, London, UK) added to a final concentration of 10 units ml−1. Restimulation was performed on day 11, and the GV1001-enriched cells were harvested and aliquoted into a round-bottom 96-well plate (50 μl, 1 × 105 cells per well). To the GV1001-enriched cells, irradiated (45 Gy) autologous PBMCs (50 μl, 1 × 105cells per well) were added to act as antigen-presenting cells. The GV1001-specific proliferation was tested for by the addition of 100 μl of control media, media containing 20 μg ml−1 GV1001 or 5 μg ml−1 phytohaemagglutinin (PHA). After 48 h of incubation, 3H-thymidine (1 μCi per well) was added for 16 h before harvesting and counting. A stimulation index above 1·8 with a significant difference in counts per min from four replicates was defined as a positive proliferative response to GV1001.
Positive total immune response
This was defined as a positive DTH test and/or a positive proliferation assay.
Statistical methods
Survival analyses were undertaken using Cox proportional hazards regression and the Kaplan–Meier method. Univariate analyses were carried out for patient characteristics, baseline levels of carbohydrate antigen 19-9 (CA19-9), CRP, IL-6 and GM-CSF dichotomised at median values; GM-CSF was dichotomised into present and absent. Multivariate analysis was carried out using a stepwise method. The Mann–Whitney test was used to analyse dichotomised continuous variables and Fisher's exact for categorical data, and the Wilcoxon test was used for paired analysis.
Results
The trajectory of serum CRP, IL-6 and GM-CSF levels during treatment with GemCap chemotherapy was determined in 38 patients for whom the appropriate longitudinal blood samples were available, and who were treated on the sequential chemoimmunotherapy arm of the TeloVac trial, where patients received 7 weeks of chemotherapy before vaccination with GV1001. The level of these analytes was assessed at baseline and after 7 weeks of chemotherapy, before vaccination with GV1001, at a time that coincided with the first assessment of response by CT scan. The demographics of this subgroup of patients (Table 1) were representative of the patients randomly allocated to sequential chemoimmunotherapy in the TeloVac trial (n=350, median age 64, proportion of males 58%, proportion of locally advanced patients 30%, patients with ECOG performance status 0, 1 and 2, 29%, 60% and 11% respectively; Middleton et al, 2014). The median overall survival of the 38 patient subgroup was 7.6 months and baseline median CA19.9 was 961 kU l−1, and of all 350 patients randomised to sequential chemoimmunotherapy, it was 6.9 months and 933 kU l−1 respectively (Middleton et al, 2014). Table 2 shows the levels of CRP (mg l−1), IL-6 (pg ml−1) and GM-CSF (pg ml−1) pre- and post-GemCap chemotherapy, the percentage change and the radiological response to treatment.
Table 1. Demographic features of 38 patients including summary of the IL-6, CRP and GM-CSF levels pre- and post-chemotherapy.
| Characteristic | N=38 |
|---|---|
|
Age | |
| (years, median, range) | 64.0 (44–79) |
|
Gender | |
| (male/female) | 24 : 14 (63% : 37%) |
|
Survival (based on KM) | |
| (days, median, IQR) | 232 (150–366) |
|
Stage | |
| Locally advanced | 16 (42%) |
| Metastatic | 22 (58%) |
|
ECOG performance status | |
| 0 | 12 (32%) |
| 1 | 21 (55%) |
| 2 | 5 (13%) |
|
Baseline CA19-9 | |
| (kU l−1, median, range) | 961 (10–53 064) |
|
Baseline CRP | |
| (mg l−1, median, range) | 7 (5–238) |
|
Post-treatment CRP | |
| (mg l−1, median, range) | 10 (5–175) |
|
Baseline IL-6 | |
| (pg ml−1, median, range) | 23 (9–1163) |
|
Post-treatment IL-6 | |
| (pg ml−1, median, range) | 18 (3–914) |
|
Baseline GM-CSF | |
| (pg ml−1, median, range) | 0 (0–549) |
|
Post-treatment GM-CSF | |
| (pg ml−1, median, range | 0 (0–427) |
Abbreviations: CA19-9=carbohydrate antigen-19-9; CRP=C-reactive protein; ECOG=Eastern Cooperative Oncology Group; GM-CSF=granulocyte macrophage-colony-stimulating factor; IL-6=interleukin 6; IQR=interquartile range; KM=Kaplan–Meier.
Table 2. Serum CRP, IL-6 and GM-CSF levels in advanced pancreatic cancer patients pre- and post-chemotherapy treatment and radiological response (n=38).
|
CRP (mg l−1) |
CRP |
IL-6 (pg ml−1) |
IL-6 |
GM-CSF (pg ml−1) |
GM-CSF | ||||
|---|---|---|---|---|---|---|---|---|---|
| Radiological response | Pre | Post | % Change | Pre | Post | % Change | Pre | Post | % Change |
| PD | 6 | 38 | 533.33 | 23.52 | 33.07 | 40.6 | 0 | 0 | 0 |
| PD | 27 | 16 | −40.74 | 25.59 | 14.98 | −41.46 | 0 | 0 | 0 |
| PD | 5 | 5 | 0 | 20.98 | 21.91 | 4.43 | 0 | 0 | 0 |
| PD | 38 | 31 | −18.42 | 22.88 | 56.91 | 148.73 | 0 | 0 | 0 |
| PD | 60 | 48 | −20 | 10.52 | 13.95 | 32.6 | 0 | 0 | 0 |
| PD | 8 | 11 | 37.5 | 36.53 | 17.91 | −50.97 | 4.51 | 0 | −100 |
| PD | 16 | 5 | −68.75 | 21.15 | 7.96 | −62.36 | 72.43 | 34.12 | −52.89 |
| PD | 5 | 24 | 380 | 30.49 | 50.11 | 64.35 | 0 | 34.96 | 0 |
| PD | 238 | 175 | −26.47 | 34.19 | 33.27 | −2.69 | 0 | 0 | 0 |
| PR | 8 | 25 | 212.5 | 9.44 | 2.88 | −69.49 | 0 | 0 | 0 |
| SD | 5 | 20 | 300 | 28.91 | 19.03 | −34.18 | 0 | 0 | 0 |
| SD | 11 | 7 | −36.36 | 23.84 | 22.63 | −5.08 | 0 | 51.61 | 100 |
| SD | 5 | 9 | 80 | 41.9 | 23.39 | −44.18 | 0 | 0 | 0 |
| SD | 5 | 5 | 0 | 20.06 | 17.87 | −10.92 | 0 | 0 | 0 |
| SD | 7 | 60 | 757.14 | 37.56 | 57.93 | 54.23 | 0 | 0 | 0 |
| SD | 7 | 27 | 285.71 | 21.56 | 403.25 | 1770.36 | 0 | 0 | 0 |
| SD | 34 | 21 | −38.24 | 1163.43 | 914.06 | −21.43 | 549.41 | 426.57 | −22.36 |
| SD | 5 | 10 | 100 | 9.49 | 13.05 | 37.51 | 2.88 | 0 | −100 |
| SD | 13 | 29 | 123.08 | 36.55 | 17.85 | −51.16 | 30.4 | 0 | −100 |
| SD | 5 | 17 | 240 | 35.82 | 50.34 | 40.54 | 0 | 0 | 0 |
| SD | 10 | 8 | −20 | 24.17 | 20.11 | −16.8 | 0 | 0 | 0 |
| SD | 25 | 6 | −76 | 29.31 | 11.81 | −59.71 | 0 | 0 | 0 |
| SD | 92 | 37 | −59.78 | 49.65 | 24.72 | −50.21 | 0 | 0 | 0 |
| SD | 5 | 5 | 0 | 10.12 | 10.28 | 1.58 | 0 | 0 | 0 |
| SD | 5 | 5 | 0 | 50.89 | 125.54 | 146.69 | 0 | 109.01 | 0 |
| SD | 5 | 5 | 0 | 10.28 | 15.74 | 53.11 | 0 | 0 | 0 |
| SD | 41 | 5 | −87.8 | 19.63 | 12.29 | −37.39 | 0 | 0 | 0 |
| SD | 27 | 33 | 22.22 | 24.3 | 38.6 | 58.85 | 0 | 0 | 0 |
| SD | 5 | 5 | 0 | 20.39 | 12.45 | −38.94 | 0 | 0 | 0 |
| SD | 13 | 13 | 0 | 24.91 | 12.61 | −49.38 | 11.08 | 2.41 | −78.25 |
| SD | 6 | 9 | 50 | 10.73 | 14.38 | 34.02 | 0 | 0 | 0 |
| SD | 5 | 28 | 460 | 14.01 | 19.4 | 38.47 | 6.28 | 10.27 | 63.54 |
| SD | 5 | 5 | 0 | 21.08 | 19.73 | −6.4 | 24.34 | 7.92 | −67.46 |
| SD | 11 | 10 | −9.09 | 19.4 | 7.23 | −62.73 | 1.68 | 0.28 | −83.33 |
| SD | 5 | 5 | 0 | 22.9 | 11.4 | −50.22 | 0 | 0 | 0 |
| SD | 5 | 26 | 420 | 15.36 | 17.75 | 15.56 | 0 | 17.73 | 0 |
| SD | 9 | 5 | −44.44 | 23.45 | 18.71 | −20.21 | 0 | 0 | 0 |
| SD | 5 | 5 | 0 | 14.09 | 9.37 | −33.5 | 0 | 0 | 0 |
| PD median (IQR) | 16 (5.5 to 49) | 24 (8 to 43) | −18.4 (−33.6 to 209) | 23.5 (21 to 32.3) | 21.9 (14.5 to 41.7) | 4.4 (−46 to 52.5) | Yes=2 No=7 | Yes=2 No=7 | Yes=2 No=7 |
| SD median (IQR) | 5.5 (5 to 12) | 9 (5 to 23.5) | 0 (−14.5 to 111) | 23.2 (17.4 to 32.6) | 18.3 (12.5 to 24.1) | −13.9 (−41.6 to 38) | Yes=7 No=21 | Yes=8 No=20 | Yes=8 No=20 |
| All median (IQR) | 7 (5 to 16) | 10.5 (5 to 27) | 0 (−20 to 123) | 23.2 (19.4 to 30.4) | 18.3 (12.6 to 33.1) | −8.66 (−8.7 to 38.5) | Yes=9 No=29 | Yes=10 No=28 | Yes=10 No=28 |
Abbreviations: CRP=C-reactive protein; GM-CSF=granulocyte macrophage-colony-stimulating factor; IL-6=interleukin 6; IQR=interquartile range; PD=progressive disease; PR=partial response; SD=stable disease.
Paired analysis of the log-transformed data showed that there were no significant differences between baseline and post-treatment CRP levels (Wilcoxon signed rank test P=0.19), IL-6 (Wilcoxon signed rank test P=0.19) and GM-CSF levels (McNemar's test P=0.71). There was a significant positive correlation (Spearman's rho) between post-chemotherapy CRP and IL-6 levels (r=0.45, P=0.005) and between both baseline and post-treatment CRP with CA19-9 levels (r=0.45, P=0.015 and r=0.40, P=0.015 respectively). The change in CRP and IL-6 levels was positively correlated (r=0.40, P=0.012). When analysing only the 28 patients with stable disease, again there was no significant difference between pre- and post-chemotherapy CRP levels (Wilcoxon signed rank test P=0.4) and IL-6 (Wilcoxon signed rank test P=0.32). The change in CRP and IL-6 levels in patients with stable disease was also positively correlated (r=0.40, P=0.033).
Table 2 shows that the CRP levels were at or below the limit of detection (5 mg l−1) in 16 out of 38 (42.1%) patients before chemotherapy and in 12 out of 38 patients (31.6%) following 7 weeks of chemotherapy. The majority of patients with a normal CRP level after chemotherapy had normal CRP levels to start with, but in just under a half of patients with initially normal CRP levels the level went up with chemotherapy. Nine out of the 12 (75%) patients with CRP levels at or below the limit of detection after chemotherapy also had had CRP levels at or below the limit of detection before chemotherapy. In 7 of the 16 patients (43.8%) who had CRP levels at or below the limit of detection before chemotherapy, the CRP level increased on chemotherapy.
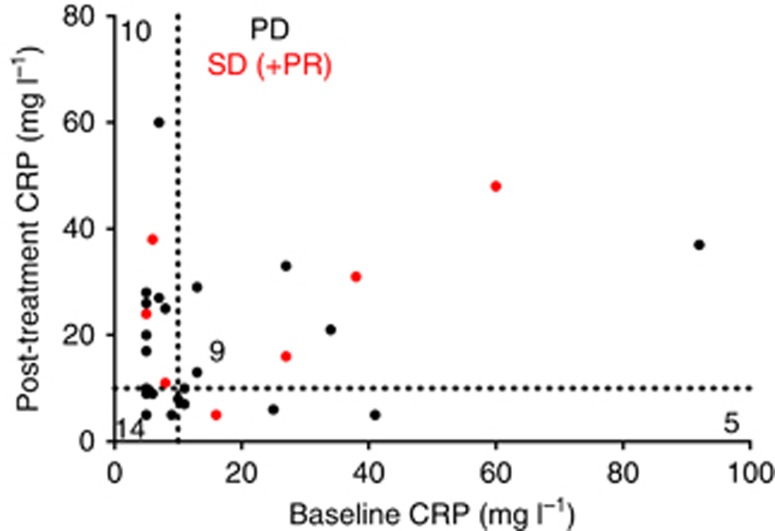
A total of 14 patients had a CRP level >10 mg l−1 at baseline and this increased to 19 patients after chemotherapy. There were 10 patients with a CRP level ⩽10 mg l−1 at baseline that increased to >10 mg l−1 following chemotherapy and there were 9 patients with a CRP level >10 mg l−1 both pre- and post-chemotherapy (Figure 1). Seven out of 10 patients with a CRP level ⩽10 mg l−1 at baseline and which had increased to >10 mg l−1 during chemotherapy had partial response or stable disease as objective response to chemotherapy. In only 4 patients was the CRP level >10 mg l−1 at baseline and ⩽10 mg l−1 after chemotherapy, and in all of these the IL-6 level fell. If the cut-point of CRP >13 mg l−1 was applied as used previously in a randomised study (Brandt et al, 2010), 10 patients had elevated CRP levels before chemotherapy and this increased to 17 patients after chemotherapy.
Figure 1.
The CRP baseline levels plotted against the post-GemCap CPR levels. Indicated are the number of patients with >10 mg l−1 or <10 mg l−1 levels pre- and post-GemCap treatment. PD=progressive disease; PR=partial response; SD=stable disease.
The GM-CSF was detectable in the serum of only 9 out of 38 patients at baseline and 10 out of 38 after chemotherapy. Investigation of positive or negative GM-CSF at baseline and post treatment showed no significant association with response (progressive disease vs stable disease) or stage (locally advanced vs metastatic).
In summary, there was no evidence that gemcitabine and capecitabine combination chemotherapy reduced the level of clinically meaningful inflammatory markers in patients with advanced pancreatic ductal adenocarcinoma.
Univariate analysis of the impact on survival of clinical features along with logged CRP, IL-6 and GM-CSF levels is shown in Table 3. Objective tumor response (P=<0.0001), log CA19-9 (P=0.004) and log baseline CRP (P=0.006) were associated with survival. In a multivariate model, logged CA19-9 and CRP both at baseline were each independently predictive of survival with hazard ratios (95% CI) for baseline CA19-9 levels of 1.30 (1.07–1.59), (P=0.009) and CRP levels of 1.55 (1.00–2.39) (P=0.049).
Table 3. Univariate and multivariate analyses of the impact on survival of clinical features along with logged CRP, IL-6 and GM-CSF levels.
| Univariate | Hazard ratio (95% CI) | P-value |
|---|---|---|
| Response: PD v SD/PR | 8.61 (2.97–24.98) | <0.0001 |
| Age | 0.98 (0.95–1.02) | 0.40 |
| Stage: locally advanced vs metastatic | 0.66 (0.32–1.37) | 0.26 |
| ECOG 0 v 1 | 1.02 (0.45–2.28) | |
| ECOG 1 v 2 | 0.42 (0.14–1.26) | 0.22 |
| ECOG 0 v 2 | 0.41 (0.15–1.17) | |
| Log CA19-9: baseline | 1.33 (1.10–1.61) | 0.004 |
| Log CRP: baseline | 1.76 (1.18–2.65) | 0.006 |
| Log CRP: post treatment | 1.38 (0.89–2.13) | 0.16 |
| Log CRP: difference | 0.75 (0.46–1.23) | 0.25 |
| Log IL-6: baseline | 0.73 (0.47–1.14) | 0.16 |
| Log IL-6: post treatment | 0.84 (0.61–1.15) | 0.27 |
| Log Il-6: difference | 1.05 (0.64–1.75) | 0.84 |
| GM-CSF: baseline | 0.75 (0.33–1.71) | 0.50 |
| GM-CSF: post treatment | 0.81 (0.36–1.81) | 0.60 |
|
Multivariate | ||
| Log CA19-9: baseline | 1.30 (1.07–1.59) | 0.009 |
| Log CRP: baseline | 1.55 (1.00–2.39) | 0.049 |
Abbreviations: CA19-9=carbohydrate antigen-19-9; CI=confidence interval; CRP=C-reactive protein; ECOG=Eastern Cooperative Oncology Group; GM-CSF=granulocyte macrophage-colony-stimulating factor; IL-6=interleukin 6; PD=progressive disease; PR=partial response; SD=stable disease. The bold values are statistically significant.
The results of the M30 Apoptosense assay before and 48 h after chemotherapy, along with percentage change and immune response status, is shown for 42 patients in Table 4. Three out of 18 (16.7%) patients in the sequential chemoimmunotherapy arm and 7 out of 24 (29.2%) patients in the concurrent chemoimmunotherapy had >30% change in the M30 assay (two-tailed Fisher's exact P=0.473). If a more conservative 10% cutoff is used to infer induction of apoptosis, this increased to 9 out of 18 (50%) and 17 out of 24 (70.8%) respectively (two-tailed Fisher's exact P=0.209). The logged M30 data for mean matched differences between pre- and post-chemotherapy for the sequential and concurrent chemoimmunotherapy treatment arms showed evidence of apoptosis (P=0.058 and P=0.0018, respectively).
Table 4. Serum levels of M30 (apoptosense) in patients pre- and post-chemotherapy along with the percentage change and immune response status: sequential chemoimmunotherapy n=18, concurrent chemo-immunotherapy (n=24).
| Pre-chemotherapy M30 (U l−1) | Post-chemotherapy M30 (U l−1) | M30 % change | Immune response |
|---|---|---|---|
|
Sequential chemoimmunotherapy | |||
| Median (IQR)=230.12 (185.3–406.1) | Median (IQR)=236.88 (188.3–481.5) | Median (IQR)=11.77 (−6.7–23.5) | |
| 406.09 | 373.59 | −8 | Negative |
| 159.42 | 188.32 | 18.13 | NA |
| 185.27 | 219.15 | 18.29 | Negative |
| 133.71 | 131.53 | −1.63 | Positive |
| 176.79 | 186.36 | 5.41 | Negative |
| 182.35 | 218.93 | 20.06 | Positive |
| 249.66 | 232.95 | −6.69 | Positive |
| 236.27 | 240.81 | 1.92 | Positive |
| 264.74 | 660.32 | 149.42 | Positive |
| 451.13 | 388.79 | −13.82 | NA |
| 505.93 | 745.08 | 47.27 | Negative |
| 337.78 | 417.17 | 23.50 | Negative |
| 411.02 | 504.14 | 22.66 | NA |
| 193.31 | 481.53 | 149.10 | NA |
| 188.39 | 158.97 | −15.62 | NA |
| 219.87 | 214.43 | −2.47 | NA |
| 223.96 | 184.02 | −17.83 | NA |
| 651.78 | 832.69 | 27.76 | NA |
|
Concurrent chemoimmunotherapy | |||
|---|---|---|---|
| Median (IQR)=235.81 (184.7–352.0) | Median (IQR)=336.66 (517.3–228.1) | Median (IQR)=14.34 (5.4–36.0) | |
| 214.54 | 243.00 | 13.26 | Negative |
| 1046.50 | 1199.07 | 14.58 | NA |
| 340.53 | 326.21 | −4.21 | Positive |
| 284.29 | 312.52 | 9.93 | Positive |
| 239.17 | 240.03 | 0.36 | Negative |
| 293.62 | 347.11 | 18.22 | Negative |
| 568.56 | 788.15 | 38.62 | Negative |
| 346.60 | 411.60 | 18.75 | Positive |
| 166.10 | 487.64 | 193.58 | Positive |
| 184.64 | 209.04 | 13.22 | Negative |
| 148.43 | 164.12 | 10.57 | Negative |
| 539.93 | 525.25 | −2.72 | NA |
| 357.34 | 360.64 | 0.92 | Negative |
| 184.70 | 223.56 | 21.04 | Negative |
| 171.26 | 509.29 | 197.37 | Positive |
| 176.86 | 226.19 | 27.89 | Positive |
| 180.47 | 634.77 | 251.73 | NA |
| 203.97 | 195.89 | −3.96 | Positive |
| 232.44 | 265.19 | 14.09 | Positive |
| 205.12 | 229.96 | 12.11 | Positive |
| 650.12 | 1072.13 | 64.91 | Negative |
| 281.48 | 375.23 | 33.31 | NA |
| 806.24 | 1193.86 | 48.08 | Negative |
| 191.15 | 177.42 | −7.18 | Negative |
Abbreviations: IQR=interquartile range; NA=not available.
The median (95% CI) survival for the 10 patients with apoptosis defined as an M30 >30% rise was 253 (92–304) days compared with 344 (216–443) days for those 32 patients with an M30 <30% rise (log-rank χ2=3.4015, P=0.065) with a hazard ratio (95% CI) of 0.50 (0.23–1.06) (χ2=3.26, P=0.071). The median (95% CI) survival for the 26 patients with apoptosis defined as an M30 >10% rise was 295 (216–399) days compared with 344 (187–527) days for those with an M30 <10% rise (log-rank χ2=0.707, P=0.401) with a hazard ratio (95% CI) of 0.75 (0.38–1.47) (χ2=0.70, P=0.403).
In all, 5 of 10 patients in the sequential chemoimmunotherapy arm and 9 of 20 patients in the concurrent chemoimmunotherapy had a positive immune response (Table 5). There was no association between an apoptotic response 48 h following chemotherapy and a positive immune response irrespective of whether a cutoff of >30% or >10% increase in M30 levels was used (Table 5). Thus, 7 out of 9 immune responders in the concurrent chemoimmunotherapy arm and 4 out of 5 responders in the sequential chemoimmunotherapy arm had no evidence of apoptosis induction using a 30% cutoff on the M30 Apoptosense assay.
Table 5. Number of immune responders in sequential and concurrent chemoimmunotherapy split by apoptosis.
|
Sequential chemoimmunotherapy arm |
Concurrent chemoimmunotherapy arm |
||||
|---|---|---|---|---|---|
|
Immune responsea |
Immune responsea |
||||
| Apoptosisb | Yes (n=5) | No (n=5) | Apoptosisb | Yes (n=9) | No (n=11) |
| Yes (n=2) | 1 | 1 | Yes (n=5) | 2 | 3 |
| No (n=8) | 4 | 4 | No (n=15) | 7 | 8 |
| Apoptosisc | Yes (n=5) | No (n=5) | Apoptosisc | Yes (n=9) | No (n=11) |
| Yes (n=5) | 2 | 3 | Yes (n=14) | 6 | 8 |
| No (n=5) | 3 | 2 | No (n=6) | 3 | 3 |
| Two-tailed Fisher's exact test | P=1.00 | P=1.00 | |||
Defined as T-cell proliferative response or positive delayed-type hypersensitivity (DTH).
Defined as ⩾30% increase in M30 value at 48 h.
Defined as ⩾10% increase in M30 value at 48 h.
Discussion
This study has shown that combination gemcitabine and capecitabine therapy did not reduce CRP, IL-6 or GM-CSF levels in patients with advanced pancreatic cancer. Moreover, apoptosis secondary to chemotherapy did not correlate with enhanced immunogenicity of GV1001. We have previously shown that a combination of gemcitabine and the oral fluoropyrimidine capecitabine (GemCap) failed to reduce the levels of circulating MDSCs in patients with advanced pancreatic cancer independent of response (Annels et al, 2014). We now show that serum levels of the two main cytokines that drive the production of MDSCs in pancreatic cancer, GM-CSF and IL-6, did not significantly fall during treatment with GemCap. The accompanying lack of a fall in CRP is to be expected given that CRP is a transcriptional target of GM-CSF and IL-6 signalling (Deng et al, 2006; Nishikawa et al, 2008). Thus, the use of gemcitabine and fluoropyrimidines as positive immunomodulators alongside immunotherapies in pancreatic cancer must take into account the failure of these chemotherapy agents to affect these key immunosuppressive cytokines, although caution is needed in interpreting our results, as we do not know whether circulating IL-6 and GM-CSF levels reflect levels in the tumor microenvironment. We examined the effect of combined gemcitabine and capecitabine on IL-6, GM-CSF and CRP. Two other regimens are now commonly used in advanced pancreatic cancer: gemcitabine and nab-paclitaxel and FOLFIRINOX. We are not aware of any data looking at the sequential effects of cytokines with these regimens. However, the cytokines we investigated were selected based on their importance to MDSC biology in pancreatic cancer, and there are data on the impact of the other chemotherapy agents used in these regimens on MDSCs. Unlike gemcitabine and 5-FU, paclitaxel and oxaliplatin had no effect on tumoural MDSC numbers in preclinical models (Vincent et al, 2010). Indeed, there was a numerical increase in MDSCs with oxaliplatin. Irinotecan increased MDSC number, increased MDSC NO− and ROS production and blocked the apoptotic effect of 5-FU on MDSCs in a colorectal cancer model (Kanterman et al, 2014). These data suggest that these other combination regimens are unlikely to have a beneficial effect on MDSCs independent of any significant reduction in tumour volume and in the case of FOLFIRINOX may be detrimental. We plan to prospectively examine the effects of FOLFIRINOX on MDSC number and function to test this hypothesis.
The number of patients included was relatively small but the demographics and outcomes of this subset was similar to the entire group of patients treated with initial chemotherapy alone before vaccination and there was no suggestion of a possible tendency towards reduction in the analytes during chemotherapy. The median CRP levels were numerically higher after chemotherapy even in patients with stable disease and there were more patients with elevated CRP after treatment than before. Although there was no control group to investigate changes independent of chemotherapy, obtaining sufficient sequential samples from patients with advanced pancreatic cancer is clearly problematic.
The biological basis of our clinical observation that gemcitabine and fluoropyrimidines do not reduce IL-6 and GM-CSF has been recently demonstrated (Takeuchi et al, 2015). Gemcitabine and 5-FU treatment of pancreatic cancer cells significantly increased the production of IL-6 and GM-CSF by these cells. Human monocytes differentiated into monocytoid MDSCs when conditioned media from pancreatic cancer cells were added to the culture medium and conditioned media from pancreatic cancer cells treated with either gemcitabine or 5-FU reduced HLA-DR expression and enhanced Arginase1 and NOS2 expression even further. The MDSCs derived from chemotherapy-treated cell conditioned media were even more suppressive than those derived using untreated conditioned media. The expression of HLA-DR on infiltrating CD14+ cells was much lower in the pancreatic cancers of patients receiving preoperative chemotherapy as compared with those going straight to surgery.
Immunomonitoring in the TeloVac trial involved both DTH responses and T-cell proliferation. In all, 19 (12%) of 154 patients who had sequential chemoimmunotherapy were positive to DTH, and 47 (20%) of 233 patients who had concurrent chemoimmunotherapy (Middleton et al, 2014). T-cell proliferation was positive in 10 (31%) of 32 patients given sequential chemoimmunotherapy and 10 (15%) of 68 patients given concurrent chemoimmunotherapy. Both DTH and T-cell responses were not predictive of survival. Chemotherapy-mediated apoptosis induction was not significantly associated with the immune responsiveness to the GV1001 peptide vaccine. It may not be possible to enhance immunogenicity through apoptosis-mediated increased antigen cross-presentation if the microenvironment remains unfavourable with high levels of immunosuppressive cytokines such as IL-6 and GM-CSF.
Caspase-mediated apoptosis will cause cleavage of cytokeratin 18 (released following necrosis of malignant and normal epithelial cells) producing the M30 fragment. This cleavage product is not specific to cancer cells undergoing apoptosis either as part of tumor biology or induced by chemotherapy (Dive et al, 2010). Previously, we found no association with survival and circulating M30 levels in a range of patients with early, locally advanced and metastatic pancreatic cancer and similar findings were made in the present study (Dive et al, 2010). A two-fold increase over the baseline spread of M30 assay values has been proposed (Cummings et al, 2006) but we only had a single baseline sample. Others have proposed lower level changes as important given the association between changes and objective response based on ROC characteristics (Brandt et al, 2010).
The majority of the patients for whom we had samples for the M30 assay were in the group receiving concomitant chemotherapy at the time of vaccination, and this may have reduced the level of circulating antigen-specific T cells as has been described with gemcitabine therapy preclinically (Bauer et al, 2014). In addition, any intratumoral release of telomerase peptides secondary to apoptosis induction may also have been insufficient to immunologically synergise with the exogenously administered antigen in the vaccine.
Using a variety of chemotherapies and tumour antigens (both peptides and proteins), Kang et al (2013) demonstrated therapeutic synergy and enhanced numbers of intratumoural and systemic antigen-specific CD8+ T cells when chemotherapy and vaccination were combined in comparison with single-agent therapy. However, antigen density within the tumour was critical: synergy only occurred when antigen was delivered directly into the tumour and did not occur when antigen was delivered by the standard subcutaneous route. Chemotherapy alone enhanced intratumoural dendritic cell density but this effect was only therapeutically and immunologically relevant if there were very high levels of antigen available for uptake and this could only be achieved by direct intratumoural delivery. Chemotherapy-induced apoptosis and the development of an immunologically more favourable microenvironment were insufficient to synergise with antigen delivery by a standard route.
In conclusion, we have shown that combination gemcitabine and capecitabine chemotherapy did not reduce the levels of the immunosuppressive cytokines IL-6 and GM-CSF or the inflammatory marker CRP. Furthermore, there was no evidence that apoptosis induction secondary to this chemotherapy significantly enhanced the immunogenicity of an intradermally administered peptide vaccine. These observations may have implications for the use of gemcitabine and fluoropyrimidines as immunomodulatory agents in pancreatic cancer. Alternative methods to block the action of immunosuppressive cytokines are required. Both IL-6 and GM-CSF function via activation of JAK2/STAT3, and the direct inhibition of JAK2 using ruxolitinib has improved outcome in pancreatic cancer patients treated with gemcitabine precisely in those with an elevated CRP reflecting STAT3 activation (Hurwitz et al, 2015). The phase III confirmation of these data is eagerly awaited.
Acknowledgments
Professor John P Neoptolemos, FMedSci, The Owen and Ellen Evans Chair of Surgery, is a NIHR Senior Investigator. The Royal Liverpool and Broadgreen University Hospitals NHS Trust, Liverpool, UK, sponsored the trial. The protocol of the TeloVac trial may be viewed on the following link: https://www.lctu.org.uk/trial/trial_links.asp?id=42∓tgcode=4∓menuid=43. Cancer Research UK and KAEL-GemVax funded the study. The GM-SF and GV1001 vaccines were supplied by KAEL-GemVax Co., Limited, and capecitabine was supplied by Roche.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Professor Gary Middleton is funded by CR-UK and has received grants from Astra Zeneca, Merck Sharp Dhome, Novartis and KAEL Gemvax. Professor JP Neoptolemos reports none other than grants from Roche, Cancer Research UK, NIHR, Pancreas Cancer UK, North West Cancer Research, Pancreas Cancer Research Fund, the Royal College of Surgeons of England, Pharma Nord and Asrazeneca, and grants and personal fees from KAEL Gemvax during the conduct of the study; personal fees from Oxford Biomedica (UK) Ltd, Pfizer, Novartis, Astellas, Abbott (Mylan), Boehringer Ingelheim Pharma and NUCANA. Professor John Neoptolemos is part funded by the NIHR Biomedical Research Centre at the Royal Liverpool University, Liverpool. Professor Paula Ghaneh reports none other than grants from Cancer Research UK and the NIHR. Dr Eithne Costello reports none other than funding by grants from Cancer Research UK, the European Union, Pancreas Cancer UK, North West Cancer Research and Pancreas Cancer Research Fund. Dr William Greenhalf reports none other than funding by grants from Cancer Research UK, the NIHR, the European Union and the Pancreatic Cancer Research Fund. Professor Daniel Palmer reports none other than funding from Cancer Research UK, North West Cancer Research and the NIHR, and personal fees from Bayer-Schering Pharma, Boehringer-Ingelheim, Celgene and Baxter. Dr Victoria Shaw declares no conflict of interest.
References
- Annels NE, Shaw VE, Gabitass RF, Billingham L, Corrie P, Eatock M, Valle J, Smith D, Wadsley J, Cunningham D, Pandha H, Neoptolemos JP, Middleton G (2014) The effects of gemcitabine and capecitabine combination chemotherapy and of low-dose adjuvant GM-CSF on the levels of myeloid-derived suppressor cells in patients with advanced pancreatic cancer. Cancer Immunol Immunother 63: 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C, Sterzik A, Bauernfeind F, Duewell P, Conrad C, Kiefl R, Endres S, Eigler A, Schnurr M, Dauer M (2014) Concomitant gemcitabine therapy negatively affects DC vaccine-induced CD8(+) T-cell and B-cell responses but improves clinical efficacy in a murine pancreatic carcinoma model. Cancer Immunol Immunother 63: 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt D, Volkmann X, Anstatt M, Langer F, Manns MP, Schulze-Osthoff K, Bantel H (2010) Serum biomarkers of cell death for monitoring therapy response of gastrointestinal carcinomas. Eur J Cancer 46: 1464–1473. [DOI] [PubMed] [Google Scholar]
- Corcoran RB, Contino G, Deshpande V, Tzatsos A, Conrad C, Benes CH, Levy DE, Settleman J, Engelman JA, Bardeesy N (2011) STAT3 plays a critical role in KRAS-induced pancreatic cancer tumorigenesis. Cancer Res 71: 5020–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J, Ranson M, Lacasse E, Ganganagari JR, St-Jean M, Jayson G, Durkin J, Dive C (2006) Method validation and preliminary qualification of pharmacodynamic biomarkers employed to evaluate the clinical efficacy of an antisense compound (AEG35156) targeted to the X-linked inhibitor of apoptosis protein XIAP. Br J Cancer 95: 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z, Yang C, Deng H, Yang A, Geng T, Chen X, Ma A, Liu Z (2006) Effects of GM-CSF on the stem cells mobilization and plasma C-reactive protein levels in patients with acute myocardial infarction. Int J Cardiol 113: 92–96. [DOI] [PubMed] [Google Scholar]
- Dive C, Smith RA, Garner E, Ward T, George-Smith SS, Campbell F, Greenhalf W, Ghaneh P, Neoptolemos JP (2010) Considerations for the use of plasma cytokeratin 18 as a biomarker in pancreatic cancer. Br J Cancer 102: 577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emens LA, Middleton G (2015) The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res 3: 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galetto A, Buttiglieri S, Forno S, Moro F, Mussa A, Matera L (2003) Drug- and cell-mediated antitumor cytotoxicities modulate cross-presentation of tumor antigens by myeloid dendritic cells. Anticancer Drugs 14: 833–843. [DOI] [PubMed] [Google Scholar]
- Hurwitz HI, Uppal N, Wagner SA, Bendell JC, Beck JT, Wade SM 3rd, Nemunaitis JJ, Stella PJ, Pipas JM, Wainberg ZA, Manges R, Garrett WM, Hunter DS, Clark J, Leopold L, Sandor V, Levy RS (2015) Randomized, double-blind, phase II study of ruxolitinib or placebo in combination with capecitabine in patients with metastatic pancreatic cancer for whom therapy with gemcitabine has failed. J Clin Oncol 33: 4039–4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TH, Mao CP, Lee SY, Chen A, Lee JH, Kim TW, Alvarez RD, Roden RB, Pardoll D, Hung CF, Wu TC (2013) Chemotherapy acts as an adjuvant to convert the tumor microenvironment into a highly permissive state for vaccination-induced antitumor immunity. Cancer Res 73: 2493–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanterman J, Sade-Feldman M, Biton M, Ish-Shalom E, Lasry A, Goldshtein A, Hubert A, Baniyash M (2014) Adverse immunoregulatory effects of 5FU and CPT11 chemotherapy on myeloid-derived suppressor cells and colorectal cancer outcomes. Cancer Res 74: 6022–6035. [DOI] [PubMed] [Google Scholar]
- Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA Jr, Donehower RC, Jaffee EM, Laheru DA (2013) Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother 36: 382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner MG, Liebertz DJ, Epstein AL (2010) Characterization of cytokine induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol 185: 2273–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, Solt S, Dorman A, Wamwea A, Yager A, Laheru D, Wolfgang CL, Wang J, Hruban RH, Anders RA, Jaffee EM, Zheng L (2014) Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res 2: 616–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace TA, Ameen Z, Collins A, Wojcik S, Mair M, Young GS, Fuchs JR, Eubank TD, Frankel WL, Bekaii-Saab T, Bloomston M, Lesinski GB (2013) Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res 73: 3007–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton G, Silcocks P, Cox T, Valle J, Wadsley J, Propper D, Coxon F, Ross P, Madhusudan S, Roques T, Cunningham D, Falk S, Wadd N, Harrison M, Corrie P, Iveson T, Robinson A, McAdam K, Eatock M, Evans J, Archer C, Hickish T, Garcia-Alonso A, Nicolson M, Steward W, Anthoney A, Greenhalf W, Shaw V, Costello E, Naisbitt D, Rawcliffe C, Nanson G, Neoptolemos J (2014) Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomised, phase 3 trial. Lancet Oncol 15: 829–840. [DOI] [PubMed] [Google Scholar]
- Neoptolemos J, Greenhalf W, Cox T, Costello E, Shaw V, Valle J, Coxon F, Wadsley J, Propper D, Ross P, Madhusudan S, Roques T, Cunningham D, Eatock M, Iveson T, Patel K, Garcia-Alonso A, Nanson G, Middleton G (2014) Predictive cytokine biomarkers for survival in patients with advanced pancreatic cancer randomized to sequential chemoimmunotherapy comprising gemcitabine and capecitabine (GemCap) followed by the telomerase vaccine GV1001 compared 'to concurrent chemoimmunotherapy in the TeloVac phase III trial. J Clin Oncol 32: 5s 4121. [Google Scholar]
- Nishikawa T, Hagihara K, Serada S, Isobe T, Matsumura A, Song J, Tanaka T, Kawase I, Naka T, Yoshizaki K (2008) Transcriptional complex formation of c-Fos, STAT3, and hepatocyte NF-1 alpha is essential for cytokine-driven C-reactive protein gene expression. J Immunol 180: 3492–3501. [DOI] [PubMed] [Google Scholar]
- Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, Frelinger JA, Robinson BW (2003) Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol 170: 4905–4913. [DOI] [PubMed] [Google Scholar]
- Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D (2012) Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell 21: 836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelle FW, Sato N, Witthuhn BA, Inhorn RC, Eder M, Miyajima A, Griffin JD, Ihle JN (1994) JAK2 associates with the beta c chain of the receptor for granulocyte-macrophage colony-stimulating factor, and its activation requires the membrane-proximal region. Mol Cell Biol 14: 4335–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares KC, Rucki AA, Wu AA, Olino K, Xiao Q, Chai Y, Wamwea A, Bigelow E, Lutz E, Liu L, Yao S, Anders RA, Laheru D, Wolfgang CL, Edil BH, Schulick RD, Jaffee EM, Zheng L (2015) PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother 38: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromnes IM, Brockenbrough JS, Izeradjene K, Carlson MA, Cuevas C, Simmons RM, Greenberg PD, Hingorani SR (2014) Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut 63: 1769–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM (2005) Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 11: 6713–6721. [DOI] [PubMed] [Google Scholar]
- Szkandera J, Stotz M, Absenger G, Stojakovic T, Samonigg H, Kornprat P, Schaberl-Moser R, Alzoughbi W, Lackner C, Ress AL, Seggewies FS, Gerger A, Hoefler G, Pichler M (2014) Validation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. Br J Cancer 110: 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi S, Baghdadi M, Tsuchikawa T, Wada H, Nakamura T, Abe H, Nakanishi S, Usui Y, Higuchi K, Takahashi M, Inoko K, Sato S, Takano H, Shichinohe T, Seino K, Hirano S (2015) Chemotherapy-derived inflammatory responses accelerate the formation of immunosuppressive myeloid cells in the tissue microenvironment of human pancreatic cancer. Cancer Res 75: 2629–2640. [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–216. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366: 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C, Ghiringhelli F (2010) 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 70: 3052–3061. [DOI] [PubMed] [Google Scholar]