Abstract
Background:
To measure the uptake of first invitation to cervical screening by vaccine status in a population-based cohort offered HPV immunisation in a national catch-up campaign.
Methods:
A retrospective observational study of routinely collected data from the Scottish Cervical Screening Programme. Data were extracted and linked from the Scottish Cervical Call Recall System, the Scottish Population Register and the Scottish Index of Multiple Deprivation. Records from 201 023 women born between 1 January 1988 and 30 September 1993 were assessed. Women born in or after 1990 were eligible for the national catch-up programme of HPV immunisation. Attendance for screening was within 12 months of the first invitation at age 20 years.
Results:
There was a significant decline in overall attendance from the 1988 cohort to the 1993 cohort with the adjusted attendance ratio of the 1988 cohort being 1.49 times (95% CI 1.46–1.52) that of the 1993 cohort. Immunisation compensated for this decrease in uptake with unvaccinated individuals having a reduced ratio of attendance compared with those fully vaccinated (RR=0.65, 95% CI 0.64–0.65). Not taking up the opportunity for HPV immunisation was associated with an attendance for screening below the trend line for all women before the availability of HPV immunisation.
Conclusions:
HPV immunisation is not associated with the reduced attendance for screening that had been feared. Immunised women in the catch-up cohorts appear to be more motivated to attend than unimmunised women, but this may be a result of a greater awareness of health issues. These results, while reassuring, may not be reproduced in routinely immunised women. Continued monitoring of attendance for the first smear and subsequent routine smears is needed.
Keywords: cervical cancer screening, screening uptake, HPV immunisation
Countries with organised cytology-based cervical screening programmes have shown a considerable decrease in the incidence of cervical cancer. Data from the United Kingdom and the Republic of Ireland demonstrate the temporal relationship between the central organisation of cervical screening in 1988 and the subsequent decrease in the incidence of invasive cervical carcinoma (Comber and Gavin, 2004). In Scotland, women are currently screened between the ages of 20 and 60 years. Uptake over 5.5 years for the years 2013–2014 was 77.3% overall, with 53.8% for those aged 20–24 years (www.isdscotland.org/health-topics/cancer/cervicalscreening/).
Uptake of cervical screening is affected by a number of factors, including deprivation, accessibility and acceptability of the test, educational attainment and information about cervical cancer and hence perception of risk (Waller et al, 2009; Everett et al, 2011; Waller et al, 2012). Uptake is improved by a systematic approach to call and recall of women. There is a concern that women who have been vaccinated against HPV perceive themselves to be at low risk of developing cervical cancer and hence do not attend for screening when invited (Price et al, 2011; Paynter et al, 2015). Low uptake rates will make the screening programme increasingly ineffective, no matter which test is used and affect the benefits anticipated from vaccination.
Continued attendance for cervical screening is important for many reasons. The HPV types employed in the two vaccines currently account for ∼75% of cancers, depending upon the population. Cross-protection for HPV 31, 33 and 45 would increase the percentage of tumours potentially covered to between 75 and 80% (Smith et al, 2007; Cuschieri et al, 2010). However, this leaves between 20 and 25% of tumours for which regular screening is still the only prevention. The duration of immunity is thought to be extensive on the basis of serological- and population-based studies, and there is emerging evidence of herd protection in countries with high uptake of vaccine (Tabrizi et al, 2014; Drolet et al, 2015; Cameron et al, 2016). There are, however, still several areas that require to be elucidated, including the effect of HPV immunisation at a population level in the long-term and possible HPV genotype replacement. Although preliminary population-based data suggest that type replacement may not be important clinically, at least in the short-term, there is a need for continued surveillance of both immunised and non-immunised women, for which adequate attendance at screening is required (Kavanagh et al, 2014).
Scotland both screens from an early age (currently age 20 years) and has a highly organised and effective school-based immunisation programme. Uptake of vaccine in the catch-up cohorts (catch-up programme ran from September 2008 to end of 2011 and targeted girls from their 13th birthday until their 18th birthday) was 65% overall, varying between 40% in school leavers and 80% in those still at school (Information Services Division, 2012). Routine immunisation in school at age 12–13 years continues to achieve >90% uptake of all three doses (Information Services Division, 2014). In addition, Scotland has the advantage of direct linkage between immunisation status and cervical screening data through the use of a unique personal identifier, the Community Health Index (CHI) number that is used on all health-care systems and records (Bhopal et al, 2012). It enables linkage of a wide variety of systems, allowing correlation of health interventions with disease and a variety of socio-economic and demographic factors. This enables direct examination of the effects of HPV immunisation on several aspects of service delivery. In this paper, we quantify the association between the uptake of first invitation to cervical screening with the uptake of HPV vaccination in the catch-up programme.
Methods
Data selection and extraction
The Scottish Cervical Call Recall System (SCCRS) is a nationwide, population register-based computer system, populated with demographic data from the population register, in use since 2007 whose function is to manage all aspects of call and recall. It incorporates immunisation status, acts as a requesting and reporting system for cytology and records relevant histology and HPV results. It includes in its reports recommended management and refers women directly for colposcopy. The dates of screening invitations and reminders are recorded, as are the reasons for exclusion from screening—for example, pregnancy, no cervix, severe inter-current illness or a formal declaration to opt out. Invitations are sent to all eligible women at their current recorded address by GP registration.
The screening attendance of all women born between 1 January 1988 and 30 September 1993 in the year after their 20th birthday was obtained from SCCRS. This was based upon an extract in Q1 2015 that had validated data up to the end of Q3 2014. Consequently, the 1993 birth cohort is truncated to ensure this cohort has at least 12 months follow-up. The information included:
date invited for screening,
date attended/reminded/defaulted as appropriate,
if excluded from screening, and reason for exclusion,
CHI,
postcode of current residence recorded by registered general practitioner,
number of doses of vaccine administered.
Women in the data set were classified as those eligible for the catch-up vaccination campaign and those not (those born before September 1990) according to their date of birth.
Data linkage
The CHI registry data set was used to identify the population in SCCRS that were resident in Scotland at age 20 years and to eliminate any duplicate CHI records created in error, to record attendance for the same individual. Once duplicate records had been merged with retention of relevant data, women with legitimate exclusions were removed in order to obtain an accurate denominator for the eligible population. These exclusions included ‘Not clinically appropriate', death, transferred out of Scotland, and temporarily excluded for a co-morbidity or for being pregnant.
The postcode of residence was used to generate a deprivation code (Scottish Index of Multiple Deprivation SIMD 2012 version), and indices of rurality (Scottish Government Scottish Index of Multiple Deprivation http://www.scotland.gov.uk/Topics/Statistics/SIMD) Deprivation is divided into quintiles, with SIMD1 being the most deprived and SIMD5 being the least deprived. Rurality is divided into three categories, urban (population of >10 000), accessible remote (30–60 min travel time from an urban centre) and very remote (>60 min travel time from an urban centre). Following data linkage, the data were anonymised by replacing the CHI number with a unique study number.
Statistical analysis
The influence of characteristics of 20-year-old women on their likelihood of attending for screening was estimated through logistic regression, with a log link. The unadjusted and adjusted risk ratios of attendance by year of birth cohort, SIMD, number of vaccine doses (0–3) and rurality were estimated. The primary data analysis was based upon all women resident in Scotland at age 20 years and who were eligible for invitation to screening. We analysed attendance at screening over the subsequent 12 months so that all women had the same time opportunity to attend for screening. In a secondary analysis, we investigated the effect of age on attendance for first screen by devising a time-dependent analysis to properly account for the length of time that the earlier cohorts have to attend for screening compared with the younger cohorts. The results from this analysis were indistinguishable for the primary one, and so are not presented. In a sensitivity analysis, we analysed only those who were eligible for vaccination, that is, born after September 1990.
Potential interactions between the birth cohort and the number of doses, and between number of doses and deprivation, on the uptake of screening were explored. As none of the interactions were prespecified, we use a Bonferroni adjustment in model selection. For the dose and deprivation interaction, further stratification was conducted to compare the uptake rates split by those eligible for the catch-up vaccination campaign and those not. All statistical modelling was conducted in IBM SPSS Statistics version 15 (Chicago, IL, USA) and graphics produced in Microsoft Excel (Microsoft Corporation, Seattle, WA, USA).
Results
Study population
A total of 201 023 women were identified of whom 94 460 (47%) had attended for screening within 12 months of their 20th birthday. The demographic characteristics of all women are shown in Table 1.
Table 1. Demographics of women born between 1 January 1988 and September 1993 invited for screening.
|
Univariate |
Multivariate -all women |
Multivariate -eligible for HPV vaccination (1990 onwards) only |
||||||
|---|---|---|---|---|---|---|---|---|
| No. of women | Attendance % | Risk ratio | 95% CI | Risk ratio | 95% CI | Risk ratio | 95% CI | |
|
Year of birth | ||||||||
| 1988 | 34 506 | 48.7 | 1.100 | 1.081–1.119 | 1.494 | 1.464–1.524 | – | – |
| 1989 | 33 886 | 47.7 | 1.077 | 1.059–1.097 | 1.462 | 1.433–1.492 | – | – |
| 1990 | 35 333 | 47.5 | 1.073 | 1.055–1.092 | 1.330 | 1.306–1.354 | 1.201 | 1.172–1.231 |
| 1991 | 35 510 | 47.7 | 1.077 | 1.058–1.096 | 1.111 | 1.092–1.130 | 1.115 | 1.096–1.134 |
| 1992 | 35 578 | 45.6 | 1.029 | 1.011–1.048 | 1.019 | 1.001–1.037 | 1.018 | 1.000–1.035 |
| 1993 | 26 210 | 44.3 | 1 | 1 | 1 | |||
|
Doses of vaccine | ||||||||
| 0 | 128 629 | 43.6 | 0.807 | 0.799–0.815 | 0.645 | 0.637–0.654 | 0.592 | 0.582–0.602 |
| 1 | 3285 | 44 | 0.815 | 0.784–0.848 | 0.791 | 0.761–0.822 | 0.796 | 0.765–0.829 |
| 2 | 6343 | 48.1 | 0.891 | 0.868–0.915 | 0.863 | 0.841–0.886 | 0.891 | 0.845–0.893 |
| 3 | 54 | 1 | 1 | 1 | ||||
|
SIMD | ||||||||
| 1 (Most deprived) | 45 007 | 46.4 | 1.038 | 1.023–1.054 | 1.040 | 1.025–1.055 | 1.039 | 1.019–1.060 |
| 2 | 41 655 | 47.6 | 1.064 | 1.049–1.080 | 1.058 | 1.043–1.073 | 1.045 | 1.025–1.067 |
| 3 | 38 969 | 47.5 | 1.062 | 1.047–1.078 | 1.049 | 1.034–1.065 | 1.034 | 1.013–1.056 |
| 4 | 34 243 | 49.1 | 1.097 | 1.080–1.114 | 1.070 | 1.054–1.086 | 1.044 | 1.023–1.066 |
| 5 (Least deprived) | 41 149 | 44.7 | 1 | 1 | 1 | |||
|
Urban rural | ||||||||
| Urban | 187 191 | 46.8 | 1.003 | 0.975–1.031 | 1.019 | 0.991–1.048 | 0.988 | 0.951–1.026 |
| Accessible remote | 8142 | 51.5 | 1.104 | 1.066–1.143 | 1.087 | 1.051–1.125 | 1.027 | 0.980–1.076 |
| Very remote | 5690 | 46.7 | 1 | 1 | 1 | |||
Abbreviations: CI=confidence interval; SIMD=Scottish Index of Multiple Deprivation.
Uptake, birth cohort, SIMD, immunisation and rurality
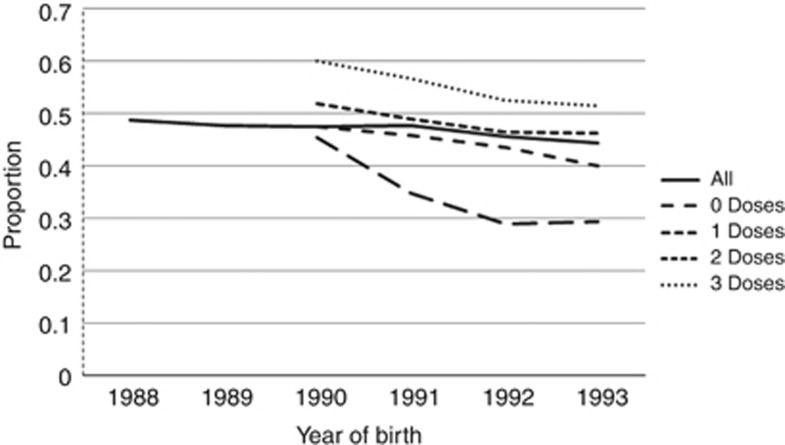
Both unadjusted and adjusted analysis (Table 1) showed significant association between uptake and year of birth, SIMD, immunisation status and rurality (all P<0.05).There was a significant decline in overall attendance from the 1988 cohort to the 1993 cohort with the adjusted attendance ratio for those in the 1988 cohort being 1.49 times (95% CI 1.46–1.52) that of the 1993 cohort. Immunisation compensated for this decrease in uptake with unvaccinated individuals having a reduced ratio of attendance compared with those fully vaccinated (RR=0.65, 95% CI 0.64–0.65) (Table 1); however, the downward trend with the later birth cohorts persisted in those fully vaccinated (Figure 1). Attendance for screening decreased from baseline in the unvaccinated group after the introduction of immunisation compared with the 1988 and 1989 cohorts, who were almost all unimmunised, although there is a suggestion of a levelling off in those born in 1993. Among those vaccinated, there is a clear trend of increased proportions attending with increasing number of doses, though in all groups there is a downward trend over time.
Figure 1.
The proportion of women aged 20 attending for the first screen within 12 months by year of birth and number of doses of vaccine. Note those born before 1990 were not eligible for HPV vaccination.
The relationship between deprivation and screening attendance showed the lowest uptake in the least deprived individuals (Table 1) with statistically significant increased risk of attendance in all SIMD quintiles compared with the least deprived, although the scale of the increase is relatively small (adjusted RR∼1.05 in all other SIMD groups).
Interactions
The most important interactions involved year of birth, SIMD and the number of doses of vaccine, all with P<0.001. There is an interaction between urban/rural status and SIMD (P=0.002), which is characterised by low screening attendance percentage for those in the least deprived groups in very remote areas. The other interactions involving the urban/rural status were not important.
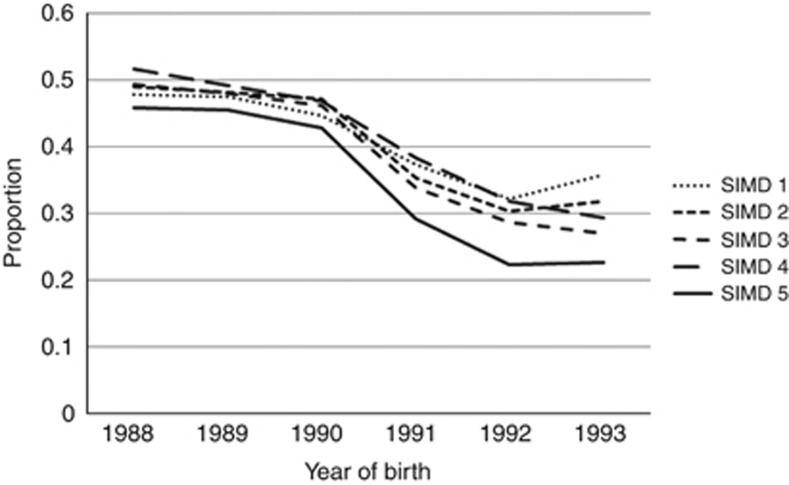
Examination of the interaction between SIMD and vaccination status (Table 2) showed that unimmunised women in SIMD5 (least deprived) were also least likely to attend for screening. This was seen in all year of birth cohorts (Figure 2). Figure 2 also shows that the difference in uptake between the SIMD quintiles is widening in the younger cohorts of unimmunised women. Whereas women in SIMD1–4 born in 1988 and 1989 showed a trend of increasing attendance with decreasing deprivation, there was no consistent effect of SIMD on attendance from 1990 onwards. Uptake was however always lowest in the least deprived group (SIMD5).
Table 2. Screening attendance proportions by SIMD and number of vaccine doses for women born between 1 January 1988 and September 1993 and invited for screening.
| Vaccine dose | SIMD | Total eligible | Attended | % Uptake |
|---|---|---|---|---|
| 0 | 1 | 29 983 | 13 168 | 43.9 |
| 2 | 26 815 | 11 945 | 44.5 | |
| 3 | 24 548 | 10 791 | 44 | |
| 4 | 20 796 | 9598 | 46.2 | |
| 5 | 26 487 | 10 562 | 39.9 | |
| 1 | 1 | 1106 | 483 | 43.7 |
| 2 | 811 | 337 | 41.6 | |
| 3 | 584 | 276 | 47.3 | |
| 4 | 440 | 187 | 42.5 | |
| 5 | 344 | 163 | 47.4 | |
| 2 | 1 | 1908 | 914 | 47.9 |
| 2 | 1478 | 696 | 47.1 | |
| 3 | 1187 | 590 | 49.7 | |
| 4 | 944 | 456 | 48.3 | |
| 5 | 826 | 396 | 47.9 | |
| 3 | 1 | 12 010 | 6338 | 52.8 |
| 2 | 12 551 | 6853 | 54.6 | |
| 3 | 12 650 | 6862 | 54.2 | |
| 4 | 12 063 | 6559 | 54.4 | |
| 5 | 13 492 | 7286 | 54 | |
| 2 01 023 | 94 460 | 47.6 |
Figure 2.
The proportion of unvaccinated women aged 20 attending for first screen within 12 months by year of birth and SIMD. Note those born before 1990 were not eligible for routine HPV vaccination and the whole cohort is represented here. In the post-1990 cohorts, vaccine was offered and unvaccinated women chose not to receive the vaccine.
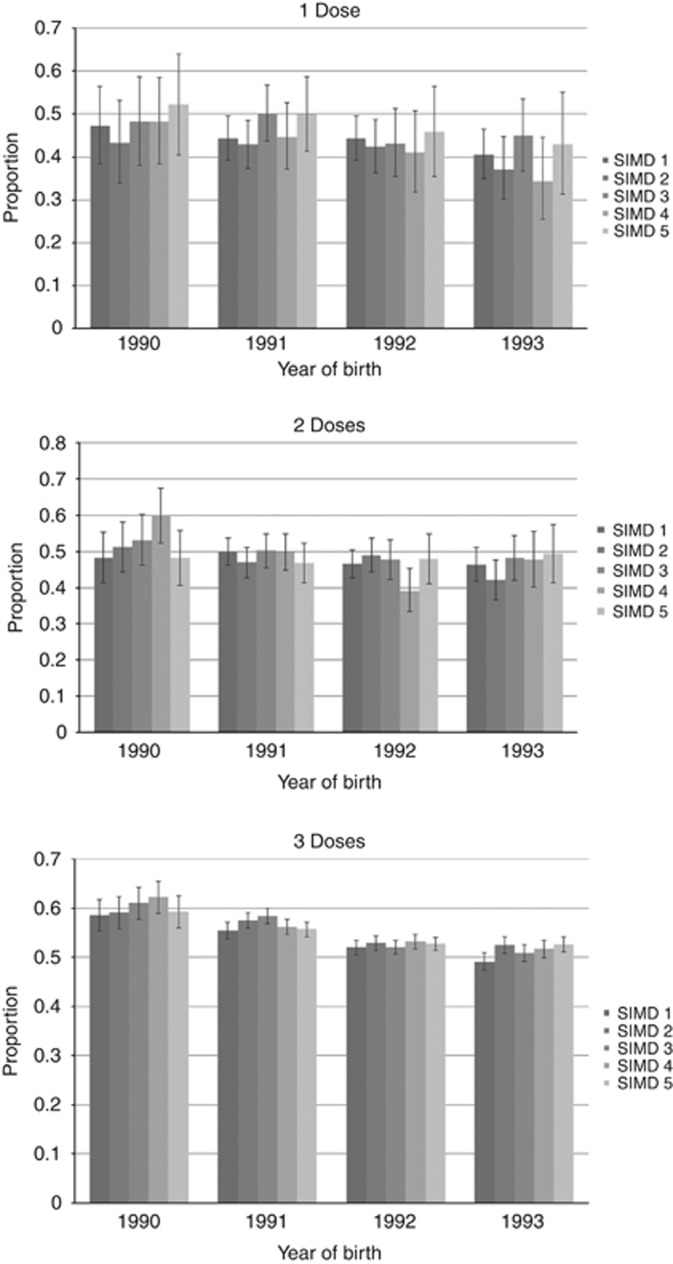
In those immunised during the catch-up vaccination campaign (Figure 3), full immunisation was associated with higher uptake of screening across all SIMD quintiles compared with partial immunisation. The deprivation differential is minimal among women who received one, two or three doses of the vaccine (Figure 3), with no clear trend discernible (P=0.134).
Figure 3.
The proportion of vaccinated women aged 20 attending for first screen within 12 months by year of birth and SIMD. Vaccine was only offered to those born in 1990 or later and women chose to receive one, two or three doses of the vaccine.
Discussion
Immunisation against HPV with the bivalent vaccine is associated with a higher uptake of the first smear at age 20 years. The women subject to analysis had been eligible for the HPV vaccine as part of the catch-up cohort following the introduction of the HPV immunisation programme in Scotland, in September 2008. As the increased uptake was observed with any number of doses received, it may reflect characteristics of the women taking up the opportunity for immunisation, in particular their willingness to take responsibility for their own health. These results are encouraging for cervical screening of immunised populations in view of concerns of a hypothetical reduction in participation in screening and corroborate the effect previously reported from Wales (Beer et al, 2014). It is also consistent with the increased uptake reported in the United States and Sweden (Herweijer et al, 2015; Paynter et al, 2015; Sauer et al, 2015). The intention to participate in screening reported in the United States, Australia and Scotland appears to have been realised (Paul-Ebhohimhen et al, 2010; Price et al, 2011; Brotherton and Mullins, 2012).
Although immunisation is associated with an increased uptake of screening, the downward trend in uptake over the 6 year-cohorts remains. This is worrying for screening as a process. Many factors affect the uptake of cervical screening, including age, individual perception of risk and external influences, such as media coverage and celebrity involvement(Moser et al, 2009; Waller et al, 2012). Deprivation is usually associated with decreased uptake of cervical screening, so the level of uptake in the least deprived quintile, observed in all unimmunised women, is both unexpected and unwelcome. The reasons for this are not clear, but it has been a feature of Scottish cervical screening for some years. It could relate to reduced usage of health services in this group of women when compared with the more deprived quintiles, or to population movements as a result of entering higher education or migration from areas with no linkage of immunisation to screening. Access to opportunistic screening is possible in Scotland, although minimal especially in young women with access to free health care. Ferris and colleagues report an intriguing observation that those who default from screening are more likely to take up immunisation because it will extend screening intervals (Ferris et al, 2012). Whether, having taken up immunisation, the women then will attend for screening is not reported, but our data would indicate that immunised women are more likely to attend.
Immunisation rates in the catch-up cohorts were related to deprivation, with a 5% reduction in vaccine uptake in the most deprived quintile compared with the least deprived (Sinka et al, 2014). A similar trend in uptake of screening was not observed in the immunised cohorts, suggesting that being immunised has a more motivating effect on more deprived women than on more affluent women. Until there is a better understanding of the reasons for the poor uptake in the unimmunised and most affluent women, it is difficult to explain the relationship between uptake of screening and immunisation in this group. The uptake rates in unimmunised women are, however, strikingly low and this group should be considered for further public health intervention.
Close attention was paid to publicity about HPV immunisation and the relationship between HPV, cervical disease and screening during the immunisation campaign in 2008. The information given to young girls and their parents continues to stress the need for continued screening despite being immunised. The campaign was many-pronged, with advertisements on television and in cinemas, as well as written information provided to the girls and their parents directly (Potts et al, 2013). The national screening leaflet for women invited for their first screening test has a section aimed at women who have been vaccinated to highlight the need for vaccinated women to attend for screening. This would appear to have been an effective strategy and suggests that, if appropriate information is given to women at the time of immunisation and when invited for screening, there is an appreciation that immunisation does not confer complete protection from cervical cancer and that screening is still necessary.
However, from April 2016, the age at which young women will be screened in Scotland will increase to 25. Furthermore, in September 2014, the Joint Committee for Vaccination and Immunisation suggested that girls as young as 11 could be offered the HPV vaccine. Consequently, there will be a significant period of time (13 years) between immunisation and invitation for the first screen; therefore, it is critical that regular educational messages are communicated to young women in order to sustain the reduction in cervical disease.
The strengths of this study are that it uses data routinely entered into SCCRS at a national level for the management of women in the Scottish Cervical Screening Programme. Results are entered contemporaneously and are available for any screening episode within Scotland. Data quality is actively managed through the programme. The CHI number allows direct and robust linkage of many aspects of an individual's health record. The use of a national screening database means that the sample size is substantially larger than most previous studies. Although the Swedish study of Herweijer and colleagues was larger overall, there were significantly fewer immunised women (Herweijer et al, 2015).
One of the main limitations of this study is that the women analysed may be a different population, with different motivation, from women immunised routinely at age 12 or 13. Paynter et al have reported that although uptake in recently immunised women is better than unimmunised women of the same age, this effect diminishes as the time between immunisation and eligibility for cervical screening increases (Paynter et al, 2015). Although such a trend is not apparent in this analysis, these results may not be generalisable to all immunised populations. The analysis will therefore need to be repeated when routinely immunised women from the school-based programme enter the Scottish Cervical Screening Programme from September 2015. Other limitations are that the observational nature of this study means we are unable to account for possible confounding due to variation in uptake of vaccination and of screening by factors such as school attendance, educational attainment and employment. The very high uptake of immunisation in Scotland means that the numbers of partially immunised women are small, and thus the confidence limits for those women vaccinated with one and two doses are wide. Further work includes extending these observations to include routinely immunised women. Our results look only at the first invitation to screening and it is important to examine the attendance at second and subsequent routine screens. The comprehensive nature of the SCCRS database makes this eminently possible.
Acknowledgments
We acknowledge funding (reference CZH/4/528) from the Chief Scientist Office (part of the Scottish Government Health and Social Care Directorates), which has supported this programme of work.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
MC has been a member of an Advisory Board (Sanofi Pasteur MSD) and has been an investigator for studies sponsored and funded by GlaxoSmithKline via her institution.
The remaining authors declare no conflict of interest.
References
- Beer H, Hibbitts S, Brophy S, Rahman MA, Waller J, Paranjothy S (2014) Does the HPV vaccination programme have implications for cervical screening programmes in the UK? Vaccine 32: 1828–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhopal RS, Bansal N, Steiner M, Brewster DH Scottish Health and Ethnicity Linkage Study (2012) Does the 'Scottish effect' apply to all ethnic groups? All-cancer, lung, colorectal, breast and prostate cancer in the Scottish Health and Ethnicity Linkage Cohort Study. BMJ Open 2: e001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton JM, Mullins RM (2012) Will vaccinated women attend cervical screening? A population based survey of human papillomavirus vaccination and cervical screening among young women in Victoria, Australia. Cancer Epidemiol 36: 298–302. [DOI] [PubMed] [Google Scholar]
- Cameron RL, Kavanagh K, Pan J, Love J, Cuschieri K, Robertson C, Ahmed S, Palmer TJ, Pollock KGJ (2016) Human papillomavirus prevalence and herd immunity after introduction of vaccination programme, Scotland. Emerg Infect Dis 22: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comber H, Gavin A (2004) Recent trends in cervical cancer mortality in Britain and Ireland: the case for population-based cervical cancer screening. Br J Cancer 91: 1902–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuschieri K, Brewster DH, Williams AR, Millan D, Murray G, Nicoll S, Imrie J, Hardie A, Graham C, Cubie HA (2010) Distribution of HPV types associated with cervical cancers in Scotland and implications for the impact of HPV vaccines. Br J Cancer 102: 930–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet M, Bénard É, Boily MC, Ali H, Baandrup L, Bauer H, Beddows S, Brisson J, Brotherton JM, Cummings T, Donovan B, Fairley CK, Flagg EW, Johnson AM, Kahn JA, Kavanagh K, Kjaer SK, Kliewer EV, Lemieux-Mellouki P, Markowitz L, Mboup A, Mesher D, Niccolai L, Oliphant J, Pollock KG, Soldan K, Sonnenberg P, Tabrizi SN, Tanton C, Brisson M (2015) Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 15: 565–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett T, Bryant A, Martin-Hirsch PP, Forbes CA, Jepson RG (2011) Interventions targeted at women to encourage the uptake of cervical screening. Cochrane Database of Syst Rev(5): CD002834. [DOI] [PubMed]
- Ferris DG, Waller J, Dickinson A, McCracken C, Goebel A (2012) Impact of pap test compliance and cervical cancer screening intervals on human papillomavirus vaccine acceptance. Low Genit Tract Dis 16: 39–44. [DOI] [PubMed] [Google Scholar]
- Herweijer E, Feldman AL, Ploner A, Arnheim-Dahlström L, Uhnoo I, Netterlid E, Dillner J, Sparén P, Sundström K (2015) The participation of HPV-vaccinated women in a national cervical screening program: population-based cohort study. Plos One 10: e0134185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Information Services Division, Scotland Estimate of HPV vaccine uptake in Scotland by year of birth, catch-up cohort. Available at http://www.isdscotland.org/Health-Topics/Child-Health/Publications/2012-09-25/HPV_Catch-up_Programme.xls (accessed on 29 July 2015).
- Information Services Division, Scotland Estimate of HPV vaccine uptake in Scotland, by year of birth. Available at https://isdscotland.scot.nhs.uk/Health-Topics/Child-Health/Publications/2014-09-30/S2_Trend_Data_Aug14.xlsx (accessed on 29 July 2015).
- Kavanagh K, Pollock KG, Potts A, Love J, Cuschieri K, Cubie H, Robertson C, Donaghy M (2014) Introduction and sustained high coverage of the HPV bivalent vaccineleads to a reduction in prevalence of HPV 16/18 and closely related HPV types. Br J Cancer 110: 2804–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser K, Patnick J, Beral V (2009) Inequalities in reported use of breast and cervical screening in Great Britain: analysis of cross sectional survey data. BMJ 338: b2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul-Ebhohimhen V, Huc S, Tissington H, Oates K, Stark C (2010) HPV vaccination: vaccine acceptance, side effects and screening intentions. Community Pract 6: 30–33. [PubMed] [Google Scholar]
- Paynter CA, van Treeck BJ, Verdenius I, Laud AWY, Dhawane T, Lashf KA, Bergaminig EA, Ekekezieh CN, Hilali AM, Jamese KN, Alongia S, Harperj SM, Bonhamk AJ, Baumgartnerl KB, Baumgartnerl RN, Harper DM (2015) Adherence to cervical cancer screening varies by human papillomavirus vaccination status in a high-riskpopulation. Prev Med Rep 2: 711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts A, Sinka K, Love J, Gordon R, McLean S, Malcolm W, Ross D, Donaghy M (2013) High uptake of HPV immunisation in Scotland—perspectives on maximising uptake. Euro Surveill 18(39): pii20593. [DOI] [PubMed] [Google Scholar]
- Price RA, Koshiol J, Kobrin S, Tiro JA (2011) Knowledge and intention to participate in cervical cancer screening after the human papillomavirus vaccine. Vaccine 29: 4238–4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer AG, Jemal A, Simard EP, Fedewa SA (2015) Differential uptake of recent Papanicolaou testing by HPV vaccination status among young women in the United States, 2008–2013. Cancer Epidemiol 24: 637–652. [DOI] [PubMed] [Google Scholar]
- Sinka K, Kavanagh K, Gordon R, Love J, Potts A, Donaghy M, Robertson C (2014) Achieving high and equitable coverage of adolescent HPV vaccine in Scotland. J Epidemiol Community Health 68: 57–63. [DOI] [PubMed] [Google Scholar]
- Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, Clifford GM (2007) Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer 121: 621–632. [DOI] [PubMed] [Google Scholar]
- Tabrizi SN, Brotherton JM, Kaldor JM, Skinner SR, Liu B, Bateson D, McNamee K, Garefalakis M, Phillips S, Cummins E, Malloy M, Garland SM (2014) Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect Dis 14: 958–966. [DOI] [PubMed] [Google Scholar]
- Waller J, Bartoszek M, Marlow L, Wardle J (2009) Barriers to cervical cancer screening attendance in England: a population-based survey. J Med Screen 16: 199–204. [DOI] [PubMed] [Google Scholar]
- Waller J, Jackowska M, Marlow L, Wardle J (2012) Exploring age differences in reasons for nonattendance for cervical screening: a qualitative study. BJOG 119: 26–32. [DOI] [PubMed] [Google Scholar]