Abstract
Background:
To document the effect of bivalent HPV immunisation on cervical cytology as a screening test and assess the implications of any change, using a retrospective analysis of routinely collected data from the Scottish Cervical Screening Programme (SCSP).
Methods:
Data were extracted from the Scottish Cervical Call Recall System (SCCRS), the Scottish Population Register and the Scottish Index of Multiple Deprivation. A total of 95 876 cytology records with 2226 linked histology records from women born between 1 January 1988 and 30 September 1993 were assessed. Women born in or after 1990 were eligible for the national catch-up programme of HPV immunisation. The performance of cervical cytology as a screening test was evaluated using the key performance indicators used routinely in the English and Scottish Cervical Screening Programmes (NHSCSP and SCSP), and related to vaccination status.
Results:
Significant reductions in positive predictive value (16%) and abnormal predictive value (63%) for CIN2+ and the mean colposcopy score (18%) were observed. A significant increase (38%) in the number of women who had to be referred to colposcopy to detect one case of CIN2+ was shown. The negative predictive value of negative- or low-grade cytology for CIN2+ increased significantly (12%). Sensitivity and specificity, as used by the UK cervical screening programmes, were maintained.
Conclusions:
The lower incidence of disease in vaccinated women alters the key performance indicators of cervical cytology used to monitor the quality of the screening programme. These findings have implications for screening, colposcopy referral criteria, colposcopy practice and histology reporting.
Keywords: cervical cancer, screening, cervical cytology, HPV immunisation, colposcopy
The UK cervical screening programme is a success and is estimated to have prevented the deaths from cervical cancer of 1 in 65 women born since 1950 (Peto et al, 2004). This success can be attributed to adherence to national protocols for regular screening and a strong commitment to regular quality assurance and monitoring. The clinical performance of any screening test is crucial and will be influenced not only by fundamental attributes of the test but also by the prevalence of the target disease in the population. This is particularly the case for tests that require subjective interpretation. The primary modality of screening in the UK is liquid-based cytology although primary screening using HPV testing is being piloted at six sites in England (http://www.cancerscreening.nhs.uk/cervical/hpv-primary-screening.html). In Scotland, cytological primary screening is supplemented by image assisted screening (ThinPrep Imaging System, HOLOGIC Inc, Marlborough, MA, USA.
The advent of immunisation against the two most common high-risk types of HPV (HPV16 and 18) is beginning to alter profoundly the prevalence of HPV16 and 18, as well as HPV 31, 33 and 45, in the population (Kavanagh et al, 2014; Pollock et al, 2014; Cameron et al, 2016). Immunisation with the bivalent vaccine began in Scotland in September 2008, with routine immunisation of girls aged 12–13 years in school and a catch-up programme for girls up to the age of 18 years. As a result, the amount of cervical intraepithelial neoplasia (CIN), the precursor of invasive cervical cancer, is also changing in young women attending for cervical screening. Scotland begins cervical screening at age 20 years, so girls immunised as part of catch-up have been screened since 2010. Significant reductions in type specific HPV infection and all grades of CIN, more pronounced with high-grade CIN, have already been demonstrated in Scotland and elsewhere (Kavanagh et al, 2014; Pollock et al, 2014; Drolet et al, 2015).
Much effort is invested in monitoring the effectiveness of cytology to detect high-grade cervical intraepithelial neoplasia (CIN) (ABC, 2013). Various measures have been devised to quantify this for various grades of cytology, including the positive predictive value (PPV) of high-grade dyskaryosis to detect high-grade CIN (HGCIN) and the ability of persistent low-grade cytology to predict HGCIN (abnormal predictive value, APV). Such quality monitoring is particularly relevant given predictions that the performance of cervical cytology may deteriorate as a consequence of immunisation. A reduction in disease in a screened population will directly reduce the PPV of any screening test (Franco et al, 2006). In cervical screening, the relative proportions of high- to low-grade disease influence the PPV for high-grade disease, the target of screening. With cytology as a screening test, this effect may be exaggerated by reader fatigue, with the possibility of missing rare positive events and of overcalling clinically insignificant reactive atypia. These forecasts are theoretical as no data from screening programmes have been published, given the time between vaccination and entry into cervical screening and the challenges of linking data between immunisation and screening databases robustly.
The IT system (Scottish Cervical Call-Recall System, SCCRS) which manages the cervical screening call and recall programme in Scotland holds cytology results, associated histology reports and also immunisation status including the number of doses. Scotland is well placed to assess the impact of immunisation on the performance of cervical cytology. In this paper, we consider the impact of immunisation on the performance of cytology for the detection of CIN.
Materials and Methods
Scottish cervical screening programme (SCSP)
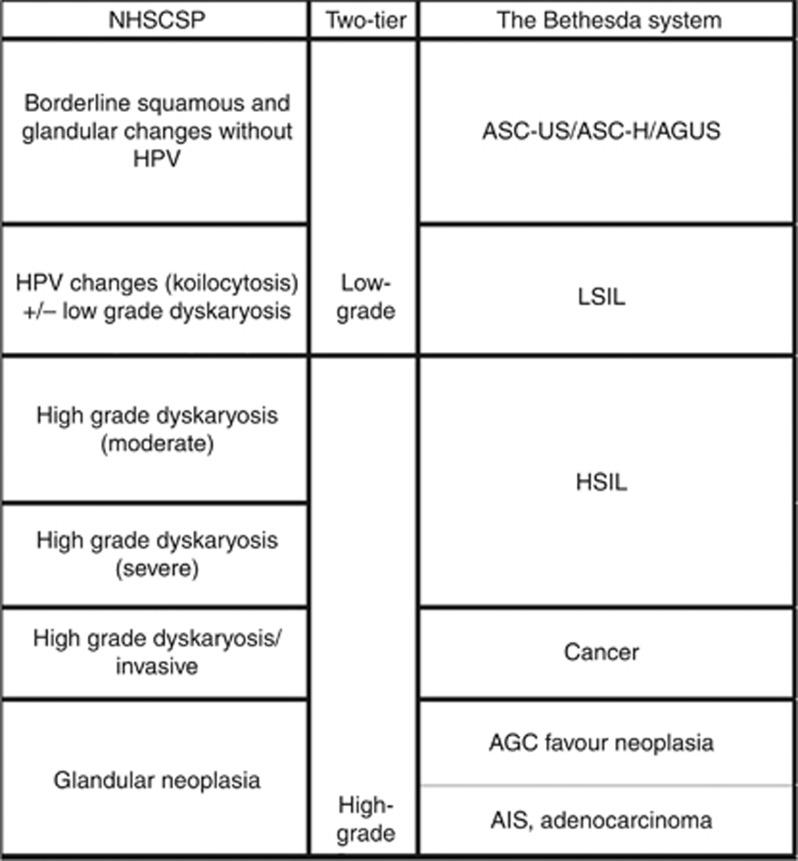
Cervical screening starts at age 20 years in Scotland, with women being called within 4–6 weeks of their 20th birthday. All of Scotland uses Thinprep liquid based cytology with image assisted screening (ThinPrep Imaging System, HOLOGIC Inc). A total of eight NHS cytology laboratories serve the programme and process ∼400 000 samples a year (http://www.sccrs.scot.nhs.uk/lab.html). Cytology and histology classification is performed according to British Association for Cytology and NHS Cervical Screening Programme (NHSCSP) criteria (Denton et al, 2008; NHSCSP, 2010). A comparison between the various reporting systems in use is given in Figure 1.
Figure 1.
Cytology classification according to British Association for Cytology (BAC) and NHS Cervical Screening Programme (NHSCSP) criteria (Denton et al, 2008).
Referral for colposcopy is made after one instance of high-grade disease or borderline, query high-grade (ASC-H) and for persistent low-grade disease. Persistent low-grade disease is defined as two instances of low-grade dyskaryosis or three instances of borderline change during an episode of abnormal follow-up, or three abnormal smears in the last 10 years. In addition, referral for colposcopy is made following three consecutive unsatisfactory smears (SCSP, 2013).
Selection of analysis cohort
The screening records of women resident in Scotland and born between 1 January 1990 and 30 September 1993 who had cytology tests taken after the age of 20 years and before age 21 years, in their first year of eligibility for screening, were examined in this analysis. Data were extracted from SCCRS at the end of September 2014, giving a minimum of 12 months follow-up for each woman. The following data were extracted:
Result of cytology tests taken in the first year of screening
Histology results taken at colposcopy as a consequence of the cytology result
Immunisation status by number of doses received (0, 1, 2 or 3 doses)
Year of birth
Postcode of residence
For most women, the results corresponded to their first smear or first colposcopy examination. For the relatively few women with more than one smear or biopsy the most severe result was used for analysis.
The extract criteria were chosen to obviate bias due to age at time of screening, and due to opportunity for disease detection. The records extracted were compared with the population register to eliminate women who were not resident in Scotland at the time of immunisation. Duplicate records were identified and the cytology and histology results amalgamated into a single patient record. Following these two steps, the postcode of residence was used to derive the SIMD quintile and rurality index. The records were anonymised with preservation of linkage between immunisation, cytology, and histology where appropriate. Caldicott Guardian approval was obtained for the use of patient-identifiable data.
Statistical methods
Impact of immunisation on cytological abnormalities and on histological diagnosis
In order to be able to measure the performance of cytology and place it in context, the impact of immunisation on cytological and histological abnormalities was assessed. The cytology result was recorded as Inadequate, Negative (no evidence of disease), Borderline (including borderline glandular changes), and Low-grade, Moderate or Severe dyskaryosis (including glandular abnormalities). Histology was coded as Normal (no CIN detected), CIN1, CIN2, and CIN3+ (CIN 3 or cancer). The associations between the outcomes of colposcopy and cytology and the demographic variables were estimated using multinomial logistic regression models. Odds ratios (ORs) for the various response levels compared with the baseline or normal levels are reported with 95% confidence intervals (CIs). Univariate and multivariable models were used. In addition to the number of doses of vaccine, we investigated birth cohort, Scottish Index of Multiple Deprivation, (with level 1 corresponding to the most deprived), and an Urban Rural indicator, derived from the Scottish Government 8 level indicator and with three levels: Urban, Accessible Rural (30–60 min drive to a settlement of 10 000 or more), and Remote Rural (>60-min drive to a settlement of 10 000 or more). Two-level interactions were investigated using a Bonferroni correction to the P-value for multiple testing.
Linked histology records and cytology results were tabulated and correlated with the number of doses of vaccine administered. Comparison of the measures of test performance between fully vaccinated and unvaccinated individuals was conducted using a χ2-test of association with a Bonferroni correction used to account for multiplicity of testing based on the different measures and the two end points, CIN2+ and CIN3+. Confidence intervals for binomial proportions were calculated using Wilson's method. In a sensitivity analysis this comparison was adjusted for cohort as the 1988 and 1989 cohorts are unimmunised and only the later ones have some fully immunised individuals. Logistic regression was used here with analysis of deviance tests.
Impact of immunisation on performance of cytology
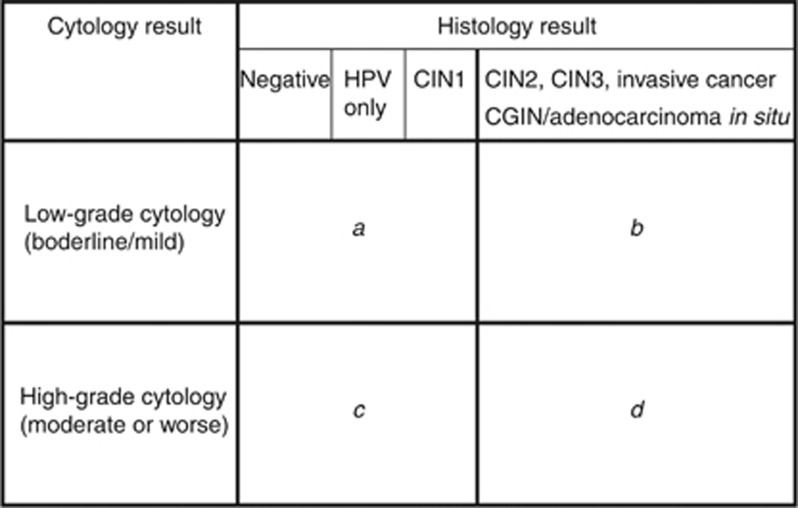
In women who had a satisfactory colposcopic examination the following measures were calculated according to the formulae in the UK Cervical Screening Programme guidance ‘Achievable Benchmarks in Cytology', version 3 (ABC, 2013) (Figure 2). Both CIN2+ and CIN3+ were used as end points:
Sensitivity of high-grade dyskaryosis for CIN2+ and CIN3+
Specificity of negative, borderline or low-grade dyskaryosis for absence of high-grade CIN;
Positive predictive value of high-grade dyskaryosis for HGCIN;
Abnormal predictive value of persistent low-grade dyskaryosis and/or borderline changes for HGCIN;
Referral value, which is the number of women who are referred to colposcopy to detect one case of HGCIN;
Total predictive value of any cytological abnormality for HGCIN;
Negative predictive value of low-grade or negative cytology for HGCIN;
Mean CIN Score—the (weighted) average amount of disease per case seen at colposcopy (MCS).
Figure 2.
Formulae used for derivation of predictive values. Positive predictive value=d/(c+d) Abnormal predictive value=b /(a+b) Total predictive value (TPV)=(b+d) / (a+b+c+d) Negative predictive value=a/(a+b) RV = 1/TPV. Abbreviations: CGIN= cervical glandular intraepithelial neoplasia; CIN= cervical intraepithelial neoplasia.
Results
Data extract
The final data set contained a total of 95 876 cytology records from women aged between 20 years and 20 years and 364 days, of whom 2226 had attended colposcopy. These women with both cytology and histology records were used for the analysis of cytology performance. A total of 34 161 (35.6%) women had received three doses of vaccine (complete schedule of immunisation in the catch-up cohorts), whereas 57 140 (59.6%) were unvaccinated. Only 1475 (1.5%) and 3100 (3.2%) records were associated with women who had received one and two doses of vaccine, respectively (Table 1).
Table 1. Number of women born between 1 January 1988 and 30 September 1993 who attended for cervical screening within 1 year of becoming 20 years, by year of birth: outcome of cytology, outcome of any colposcopy examination and number of doses of HPV vaccine received.
| Year of Birth | 1988 | 1989 | 1990 | 1991 | 1992 | 1993 | Total |
|---|---|---|---|---|---|---|---|
| Total number | 17 139 | 16 451 | 17 040 | 17 125 | 16 382 | 11 739 | 95 876 |
|
Cytology | |||||||
| Normal | 82.1 | 82.6 | 81.6 | 83.5 | 83.6 | 84.5 | 82.9 |
| Borderline | 10.6 | 11.1 | 12.3 | 11.4 | 10.2 | 7.5 | 10.7 |
| Low-grade dyskaryosis | 5.3 | 4.7 | 4.5 | 4.0 | 5.3 | 7.0 | 5.0 |
| High-grade dyskaryosis (Moderate) | 1.2 | 1.0 | 1.1 | 0.8 | 0.6 | 0.7 | 0.9 |
| High-grade dyskaryosis (severe) or worse | 0.7 | 0.6 | 0.5 | 0.4 | 0.3 | 0.4 | 0.5 |
|
Colposcopy attendance (%) with outcome | |||||||
| No colposcopy | 97.09 | 97.22 | 97.32 | 97.83 | 98.38 | 98.50 | 97.68 |
| Colposcopy | 2.91 | 2.78 | 2.68 | 2.17 | 1.62 | 1.50 | 2.32 |
| Normal | 0.78 | 0.72 | 0.70 | 0.57 | 0.41 | 0.32 | 0.60 |
| CIN1 | 0.67 | 0.81 | 0.70 | 0.61 | 0.48 | 0.45 | 0.62 |
| CIN2 | 0.86 | 0.69 | 0.68 | 0.55 | 0.42 | 0.48 | 0.61 |
| CIN3 | 0.61 | 0.57 | 0.59 | 0.44 | 0.32 | 0.25 | 0.47 |
|
Immunisation | |||||||
| Unimmunised | 99.94 | 99.68 | 80.32 | 27.45 | 17.49 | 20.10 | 59.60 |
| Partially immunised 1 dose | 0.02 | 0.05 | 1.29 | 3.09 | 2.53 | 2.56 | 1.54 |
| Partially immunised 2 doses | 0.01 | 0.09 | 2.77 | 6.80 | 5.20 | 5.07 | 3.23 |
| Fully immunised 3 doses | 0.03 | 0.18 | 15.62 | 62.65 | 74.78 | 72.28 | 35.63 |
Abbreviations: CIN=cervical intraepithelial neoplasia; HPV=human papilloma virus.
Impact of immunisation on cytological abnormalities
Complete immunisation with three doses was associated with a highly significant (P<0.001) reduction in all grades of cytological abnormality (Table 2). The reduction in high-grade dyskaryosis was greater than that observed for low-grade abnormalities. When compared with fully vaccinated women, unvaccinated women had an odds ratio of severe dyskaryosis of 2.95 (95% CI 2.17–4.02), for moderate dyskaryosis of 2.43 (95% CI 1.94–3.05), for low-grade dyskaryosis of 1.38 (95% CI 1.26–1.51), and for borderline changes of 1.27 (95% CI 1.19–1.35). Partial immunisation with two doses was also associated with a reduction in low-grade dyskaryosis (OR 1.13, 95% CI 0.95–1.34) compared with unvaccinated (OR 1.38, 95% CI 1.26–1.51) but this was not statistically significant. Immunisation with one dose of vaccine was not associated with a reduction of abnormal cytology when compared with no immunisation.
Table 2. Adjusted multivariate (OR) and 95% confidence limits (LCL lower, UCL upper) for the cytology outcomes of borderline, low-, moderate- and severe-grade dyskaryosis.
|
Low grade |
High-grade |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Borderline |
Low-grade dyskaryosis |
Moderate dyskaryosis |
⩾Severe Dyskaryosis |
||||||||||
| No. of women | n | % | OR (CI) | n | % | OR (CI) | n | % | OR (CI) | n | % | OR (CI) | |
|
Year of birth | |||||||||||||
| 1988 | 17 139 | 1823 | 10.64 | 1.23 (1.12, 1.36) | 910 | 5.31 | 0.61 (0.55, 0.69) | 206 | 1.20 | 0.99 (0.74, 1.33) | 121 | 0.71 | 1.00 (0.68, 1.48) |
| 1989 | 16 451 | 1830 | 11.12 | 1.28 (1.16, 1.41) | 775 | 4.71 | 0.54 (0.48, 0.61) | 164 | 1.00 | 0.82 (0.61, 1.11) | 93 | 0.57 | 0.08 (0.54, 1.20) |
| 1990 | 17 040 | 2098 | 12.31 | 1.49 (1.36, 1.63) | 768 | 4.51 | 0.55 (0.49, 0.62) | 179 | 1.05 | 0.98 (0.73, 1.30) | 88 | 0.52 | 0.84 (0.57, 1.24) |
| 1991 | 17 125 | 1945 | 11.36 | 1.50 (1.38, 1.63) | 681 | 3.98 | 0.56 (0.50, 0.62) | 132 | 0.77 | 1.02 (0.77, 1.34) | 69 | 0.40 | 0.99 (0.68, 1.46) |
| 1992 | 16 382 | 1672 | 10.21 | 1.39 (1.27, 1.51) | 862 | 5.26 | 0.77 (0.69, 0.84) | 91 | 0.56 | 0.82 (0.61, 1.11) | 55 | 0.34 | 0.95 (0.64, 1.42) |
| 1993 | 11 739 | 877 | 7.47 | 1.00 | 822 | 7.00 | 1.00 | 82 | 0.70 | 1.00 | 43 | 0.37 | 1.00 |
|
SIMD | |||||||||||||
| 1 Most deprived | 21 468 | 2459 | 11.45 | 1.13 (1.06, 1.20) | 1168 | 5.44 | 1.16 (1.06, 1.27) | 261 | 1.22 | 1.81 (1.46, 2.25) | 146 | 0.68 | 2.21 (1.62, 3.03) |
| 2 | 20 211 | 2156 | 10.67 | 1.05 (0.98, 1.12) | 1035 | 5.12 | 1.08 (0.99, 1.19) | 205 | 1.01 | 1.52 (1.21, 1.9) | 133 | 0.66 | 2.13 (1.55, 2.92) |
| 3 | 18 743 | 1994 | 10.64 | 1.05 (0.98, 1.12) | 904 | 4.82 | 1.02 (0.92, 1.12) | 154 | 0.82 | 1.21 (0.95, 1.55) | 66 | 0.35 | 1.10 (0.76, 1.58) |
| 4 | 16 957 | 1730 | 10.20 | 1.00 (0.93, 1.07) | 827 | 4.88 | 1.03 (0.93, 1.13) | 114 | 0.67 | 1 (0.78, 1.3) | 70 | 0.41 | 1.31 (0.92, 1.88) |
| 5 Least deprived | 18 497 | 1906 | 10.30 | 1.00 | 884 | 4.78 | 1.00 | 120 | 0.65 | 1.00 | 54 | 0.29 | 1.00 |
|
Dose of vaccine | |||||||||||||
| 0 | 57 140 | 6516 | 11.40 | 1.27 (1.19, 1.35) | 2938 | 5.14 | 1.38 (1.26, 1.51) | 644 | 1.13 | 2.43 (1.94, 3.05) | 363 | 0.64 | 2.95 (2.17, 4.02) |
| 1 | 1475 | 179 | 12.14 | 1.33 (1.13, 1.56) | 89 | 6.03 | 1.38 (1.10, 1.72) | 14 | 0.95 | 1.92 (1.11, 3.34) | 11 | 0.75 | 3.07 (1.63, 5.80) |
| 2 | 3100 | 353 | 11.39 | 1.22 (1.08, 1.37) | 155 | 5.00 | 1.13 (0.95, 1.34) | 35 | 1.13 | 2.28 (1.57, 3.3) | 16 | 0.52 | 2.13 (1.24, 3.65) |
| 3 | 34 161 | 3197 | 9.36 | 1.00 | 1636 | 4.79 | 1.00 | 161 | 0.47 | 1.00 | 79 | 0.23 | 1.00 |
|
Urban rural | |||||||||||||
| Urban | 88 904 | 9589 | 10.79 | 1.21 (1.06, 1.39) | 4460 | 5.02 | 1.07 (0.89, 1.29) | 781 | 0.88 | 0.79 (0.54, 1.16) | 419 | 0.47 | 0.51 (0.33, 0.77) |
| Accessible rural | 4277 | 412 | 9.63 | 1.10 (0.93, 1.3) | 231 | 5.40 | 1.18 (0.94, 1.47) | 44 | 1.03 | 1.03 (0.64, 1.66) | 26 | 0.61 | 0.75 (0.43, 1.32) |
| Very remote rural | 2695 | 244 | 9.05 | 1.00 | 127 | 4.71 | 1.00 | 29 | 1.08 | 1.00 | 24 | 0.89 | 1.00 |
Abbreviations: CI=confidence interval; OR=odds ratio; SIMD=scottish index of multiple deprivation. n is the number of women with the event % is the percentage of women with the event.
The proportions of women with the different grades of abnormal cytology varied by birth cohort (P<0.001). However, once adjusted for number of doses of the vaccine, only the reduction in borderline changes in the 1993 cohort remained significant. There are trends associated with deprivation (P<0.001) with higher odds of disease for all outcomes among the most deprived individuals (Table 2).
Impact of immunisation on performance of cytology
In women with a satisfactory colposcopy (n=2226), the sensitivity of ⩾high-grade dyskaryosis for CIN2+ and the specificity of ⩽low-grade dyskaryosis for <=CIN1 was slightly higher in fully immunised vs non-immunised women although these differences were not significant (Tables 3a and 3b).
Table 3a. Sensitivity, specificity, PPV, NPV, APV, TPV and RV of cytology for colposcopy outcomes (CIN2+) among women attending for a colposcopy within 12 months of their first invitation for screening.
| Measure | Vaccination | N | R | Estimate (95% CI) | P-value |
|---|---|---|---|---|---|
| Sensitivity high-grade dyskaryosis CIN2+ | Unimmunised | 807 | 604 | 74.85 (71.74, 77.72) | 0.793 |
| Fully immunised | 176 | 134 | 76.14 (69.32, 81.83) | ||
| Specificity Neg/Border/LG CIN2+ | Unimmunised | 815 | 630 | 77.30 (74.30, 80.04) | 0.950 |
| Fully immunised | 303 | 233 | 76.90 (71.83, 81.29) | ||
| PPV of high-grade dyskaryosis for CIN2+ | Unimmunised | 789 | 604 | 76.55 (73.47, 79.38) | 0.002 |
| Fully immunised | 204 | 134 | 65.69 (58.94, 71.86) | ||
| NPV Neg/Border/LG for CIN2+ | Unimmunised | 833 | 630 | 75.63 (72.60, 78.42) | 0.002 |
| Fully immunised | 275 | 233 | 84.73 (80.00, 88.50) | ||
| APV of Bl/LG for CIN2+ | Unimmunised | 759 | 179 | 23.58 (20.70, 26.73) | 0.003 |
| Fully immunised | 256 | 37 | 14.45 (10.67, 19.29) | ||
| TPV of all colp for CIN2+ | Unimmunised | 1622 | 807 | 49.75 (47.32, 52.18) | 0.000 |
| Fully immunised | 479 | 176 | 36.74 (32.55, 41.15) | ||
| RV of all colp for CIN2+ | Unimmunised | 1622 | 807 | 2.01 (1.92, 2.11) | 0.000 |
| Fully immunised | 479 | 176 | 2.72 (2.43, 3.07) |
Abbrevaitions: APV=abnormal predictive value; CI=confidence interval; CIN=cervical intraepithelial neoplasia; colp=colposcopy; NPV=negative predictive value; PPV=positive predictive value; RV=referral value; TPV=total predictive value.
Table 3b. Sensitivity, specificity, PPV, NPV, APV, TPV and RV of cytology for colposcopy outcomes (CIN3+) among women attending for a colposcopy within 12 months of their first invitation for screening.
| Measure | Vaccination | N | R | Estimate (95% CI) | P-value |
|---|---|---|---|---|---|
| Sensitivity high-grade dyskaryosis CIN3+ | Unimmunised | 351 | 288 | 82.05 (77.70, 85.71) | 0.427 |
| Fully immunised | 75 | 65 | 86.67 (77.17, 92.59) | ||
| Specificity Neg/Border/LG CIN3+ | Unimmunised | 1271 | 770 | 60.58 (57.87, 63.23) | 0.081 |
| Fully immunised | 404 | 265 | 65.59 (60.83, 70.06) | ||
| PPV of high-grade dyskaryosis for CIN3+ | Unimmunised | 789 | 288 | 36.50 (33.22, 39.92) | 0.249 |
| Fully immunised | 204 | 65 | 31.86 (25.85, 38.54) | ||
| NPV Neg/Border/LG for CIN3+ | Unimmunised | 833 | 770 | 92.44 (90.44, 94.04) | 0.033 |
| Fully immunised | 275 | 265 | 96.36 (93.44, 98.01) | ||
| APV of Bl/LG for CIN3+ | Unimmunised | 759 | 51 | 6.72 (5.15, 8.73) | 0.049 |
| Fully immunised | 256 | 8 | 3.13 (1.59, 6.04) | ||
| TPV of all colp for CIN3+ | Unimmunised | 1622 | 351 | 21.64 (19.70, 23.71) | 0.005 |
| Fully immunised | 479 | 75 | 15.66 (12.68, 19.18) | ||
| RV of all colp for CIN3+ | Unimmunised | 1622 | 351 | 4.62 (4.22, 5.08) | 0.005 |
| Fully immunised | 479 | 75 | 6.39 (5.21, 7.89) |
Abbrevaitions: APV=abnormal predictive value; CI=confidence interval; CIN=cervical intraepithelial neoplasia; colp=colposcopy; NPV=negative predictive value; PPV=positive predictive value; RV=referral value; TPV=total predictive value.
The PPVs of ⩾high-grade dyskaryosis for both CIN2+ and CIN3+ reduced in fully immunised women by 16% (P=0.002) and 14% (P=0.25, NS), respectively. The NPV of ⩽low-grade dyskaryosis was higher in immunised women than in non-immunised women for both CIN2+ (P=0.002) and CIN3+ (P=0.033 (NS)). The APV of low-grade and borderline changes for CIN2+ decreased in fully immunised women for CIN2+ by 63% (P=0.002) and for CIN3+ by 97% (P=0.049 (NS)). The number of women who had to be referred to colposcopy to detect one case of high-grade disease (referral value) was significantly increased for both CIN2+ and CIN3+ in fully immunised women (P<0.001 and P=0.005, respectively). There was a corresponding significant decrease (P<0.0001) in the average amount of disease per case seen at colposcopy (MCS) in fully immunised women (MCS=1.23 (s.d.=1.04)) compared with unimmunised women (MCS=1.46 (s.d.=1.07)). In a sensitivity analysis the comparison of fully immunised women with unimmunised women was adjusted for cohort. There was no evidence of a temporal trend associated with cohort and the conclusions were unaffected (data not shown).
Discussion
Scotland is almost uniquely placed to determine the impact of immunisation on the performance of cytology screening in young women using national data sets, which can be linked effectively. The results show preservation of sensitivity of high-grade cytology for CIN2+ and specificity of negative or low-grade cytology for the absence of CIN2+, yet a deterioration overall in the predictive value of cytology for the detection of CIN2+. These findings confirm the expectation of Franco et al (2006) who predicted a reduction in the overall performance of cytology as a consequence of vaccination. In their 2006 paper, Franco used estimates of sensitivity (51%) and specificity (98%) based on the correlation of HSIL with CIN2+ taken from Nanda et al (2000). In their 2009 modelling, Franco et al, 2009 used a sensitivity of 70% and specificity of 98%, similar to those achieved by the SCSP. Furthermore, these authors examined the effect of variations of sensitivity and specificity on the predictive value at various levels of disease prevalence. When the sensitivity and specificity are maintained, PPV drops sharply and progressively at disease prevalence of <10%. As sensitivity falls, PPV declines even further. The prevalence of high-grade disease in the fully immunised cohorts is significantly lower in younger women, and we expect it to fall still further when the routinely immunised women enter the screening programmes in the UK from September 2015. Positive predictive value is dependent on disease prevalence, but cytology is a subjective technique, relying on pattern recognition. Thus, the effect on PPV may be exaggerated with a knock on effect for colposcopy by failing to identify a population with sufficiently high-risk to warrant further intervention.
The significant reduction in PPV of high-grade dyskaryosis for the detection of CIN2+ contrasts with the maintained PPV for CIN3+. This may reflect the small numbers of CIN3+ cases in the fully immunised women compared with CIN2+ cases, and hence wider confidence limits. Other reasons for the difference between the outcomes for CIN2+ and CIN3+ include difficulty in the interpretation of cytology and difficulty in correctly distinguishing CIN2 from reactive metaplasia on histology in immunised women. The cytological features usually interpreted as dyskaryosis may have different significance in immunised than non-immunised women, being more likely to represent reactive changes and metaplasia than significant disease. The diagnosis of CIN2 is less robust than CIN3, with a greater possibility of over diagnosing reactive viral changes (Robertson et al, 1989; Mesher et al, 2015). The difficulty in interpreting correctly the cytology and histology may be a result of the changing HPV distribution in immunised women. Most of the high-grade disease observed in the UK in non-immunised women has been driven by HPV 16 and 18 (Mesher et al, 2015). HPV 16 particularly is known to have a shorter natural history in the development of CIN3. Lesions related to non-vaccine types may be detected at an earlier stage of development than hitherto, when they are smaller and have less specific features. Further, it may be that the non-vaccine related types have different cytological presentations (Bosch et al, 2008; Thomsen et al, 2015).
The reduction in the APV is also clinically important in cytology based screening, as current management protocols for low-grade cytology are centred on the likelihood of high-grade CIN being present (NHSCSP20, 2010). This reduction was only significant using CIN2+ as an outcome despite a much greater percentage reduction for CIN3+ (54%) than for CIN2+ (39%). The lack of significance at the CIN3+ level could be influenced by the small number of cases in this young age group. The higher negative predictive value (NPV) of low-grade cytology in immunised women is in keeping with the reduced APV. For CIN3+, this offers considerable reassurance as <1 in 20 immunised women with persistent low-grade abnormalities will have CIN3+.
The strengths of this study include the use of routinely collected data from a nationally organised cervical screening programme that uses a single information system, SCCRS. The information in SCCRS is scrutinised regularly as it is used to monitor the performance and quality of the programme. The HPV immunisation programme is also organised at a population level, with high uptake and direct linkage to the cervical screening data. The histological diagnoses of women referred for colposcopy and who had a biopsy are comparable to those already reported from Scotland (Pollock et al, 2014).
A weakness of the study is that the immunised women attending for screening were vaccinated as part of the catch-up programme, and some will have been sexually active before immunisation (Kavanagh et al, 2014; Pollock et al, 2014). This would reduce the effectiveness of immunisation, and also influence the effect of immunisation on cytology performance. The data presented in this paper may therefore underestimate the true effect of immunisation on cytology performance in women immunised as part of the routine programme. In addition, the true immunisation status is only known for women immunised as part of the national programme. Women moving into Scotland and those immunised in the private sector are recorded on SCCRS as unvaccinated. Although relatively few women come into either of these categories, this also leads to a slight underestimate of the true effect of immunisation.
The measures of cytology performance may be confounded by colposcopy performance and disease ascertainment, and by non-attendance at colposcopy. Knowledge of referral cytology is recognised as influencing the colposcopic impression (Shafi et al, 1993). Given that colposcopy performance relies on pattern recognition by the practitioner, unfamiliarity with this new referral population may result in the colposcopist missing disease or, alternatively, increasing the number of interventions, biopsies or treatment with negative histology. This may not be a significant problem at present because there are relatively few fully immunised women in the screening programme, but it will be become increasingly important. This will have to be addressed through colposcopy training and quality assurance.
The key performance indicators have been calculated using known histology outcomes only. An alternative methodology is to presume that all women who attend colposcopy and are not biopsied, and all those who do not attend colposcopy, do not have disease (called ‘predictive value of referral'). Although the use of only histological outcomes will overestimate the predictive values, the use of the referral population, irrespective of attendance, will underestimate predictive values as some of the non-attenders will have significant disease. Referral urgency is graduated according to the cytology and it is possible, for example, that women with persistent low-grade disease, who wait longer for colposcopy, are less likely to attend than women with high-grade disease; similar biases may exist for taking coloposcopic biopsies. It is not possible to account for these factors with the data available in SCCRS but ongoing studies using the national clinical colposcopy database will address this issue.
The findings have implications for colposcopy, for cervical cytology, and for histology reporting. There are clear implications for colposcopy services. The prevalence of significant disease in immunised women seen at colposcopy will reduce and the number of women who need to be referred to detect one case of CIN2+ will increase significantly. At the level of CIN3+, 38% (CIN2+, 35%) more immunised women than non-immunised women have to be referred following abnormal cytology to detect one case. The criteria for referral for colposcopy need revision for immunised women with persistent low-grade disease to avoid over investigation. Colposcopy with or without associated diagnostic and therapeutic interventions brings its own physical and psychological effects (Sharp et al, 2009).
The changing environment in which cytology is practiced is having an adverse effect on its utility as a screening test. Performance is likely to reduce further as the proportion of immunised women in the screened population rises, particularly in routinely immunised women, where a greater reduction in HPV prevalence and associated disease is anticipated. This reduction in performance will be accentuated if the sensitivity of high-grade cytology for CIN2+ declines. There will come a point where the balance of benefit and harm reverses, and cytology will no longer be the screening test of choice. With regards to histology, cervical biopsies from immunised women may be more difficult to interpret, and adjunctive tests may need to be used for accurate classification (Galgano et al, 2010).
Testing for the presence of high-risk HPV is the obvious alternative to cytology as a screening test, although we acknowledge that the performance of HPV-based primary screening has been assessed almost entirely in unimmunised women to date (Dijkstra et al, 2014; Ronco et al, 2014). Consequently, endeavours to assess the impact of immunisation on HPV primary screening are underway in Scotland (Bhatia et al, 2014; Cruickshank et al, 2014). Women positive for high-risk HPV are likely have a much higher prevalence of high-grade disease than the current primary screening population. In these circumstances, cytology could serve as an effective triage test (Franco et al, 2009; Rijkart et al, 2012). Our results should be generalisable to other populations with high vaccine coverage and organised screening. The effect of cytology performance is likely to be less noticeable in populations with low immunisation rates, but may be relevant to forward planning if uptake is expected to increase.
In conclusion, the performance of cervical cytology as a screening test is adversely affected by immunisation, particularly the ability of low-grade cytology to predict clinically significant disease, with consequences for referral criteria and colposcopy practice. Implications for colposcopy services include the challenge of managing a higher proportion of referred women who have no (or clinically insignificant) disease. Continued monitoring of cervical screening performance in immunised women and the assessment of new models of screening (e.g. HPV testing) more adapted to the immunisation era are essential so that the quality of what is arguably the most successful cancer screening programme to date can be maintained.
Acknowledgments
We acknowledge funding (reference CZH/4/528) from the Chief Scientist Office (part of the Scottish Government Health and Social Care Directorates), which has supported this programme of work.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
MC has been a member of an Advisory Board (Sanofi Pasteur MSD) and has been an investigator for studies sponsored and funded by GlaxoSmithKline via her institution. The remaining authors declare no conflict interest.
References
- Achievable standards, Benchmarks for reporting, and Criteria for evaluating cervical cytopathology (3rd edn) (2013) https://www.gov.uk/government/publications/cervical-screening-cytopathology-standards-and-evaluation-criteria (accessed 28 December 2015).
- Bhatia R, Cubie H, Wennington H, Serrano I, Palmer T, Kavanagh K, Hopkins K, Cuschieri K (2014) Performance of clinically validated HPV tests in a fully vaccinated “catch up” cohort in Scotland. 29th International Papillomavirus Conference; Seattle, WA, USA, 21–25 August 2014, CS.OA04.03 p 89. Available at http://ipvsoc.org/sites/default/files/news/HPV-2014-Abstract-eBook.pdf (accessed on 25 August 2015).
- Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, Tortolero-Luna G, Kjaer SK, Muñoz N (2008) Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine Suppl 10: K1–K16. [DOI] [PubMed] [Google Scholar]
- Cameron RL, Kavanagh K, Pan J, Love J, Cuschieri K, Robertson C, Ahmed S, Palmer TJ, Pollock KGJ (2016) Human papilloma virus prevalence and herd immunity after introduction of vaccination program, Scotland, 2009–2013. Emerg Infect Dis 22: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreon JD, Sherman ME, Guillen D, Solomon D, Herrero R, Jerónimo J, Wacholder S, Rodríguez AC, Morales J, Hutchinson M, Burk RD, Schiffman M (2007) CIN2 is a much less reproducible and less valid diagnosis than CIN3: Results from a Histological Review of Population-Based Cervical Samples. Int J Gynecol Pathol 26: 441–446. [DOI] [PubMed] [Google Scholar]
- Colposcopy and programme management: guidelines for the NHS Cervical Screening Programme. NHS CSP Document 20. 2nd Edn (2010) Available at https://www.gov.uk/government/publications/cervical-screening-programme-and-colposcopy-management (accessed 11 January 2016).
- Cruickshank ME, Cotton S, Cubie H, Campbell C, Robertson C, Kavanagh K, Pollock K, Weller D, McNamee P, Sinka K, Choi Y, Cuschieri K (2014) The Scottish Cervical Cancer Prevention Prorgamme (SCCPP): Integrating primary and secondary prevention in national research programme. 29th International Papillomavirus Conference, Seattle, WA, USA, 21-25 August, 2014, PH.PP03.08 p 338. Available at http://ipvsoc.org/sites/defau5)lt/files/news/HPV-2014-Abstract-eBook.pdf (accessed 28 August 2015).
- Denton K, Herbert A, Turnbull L, Waddell C, Desai M, Rana D, Dudding N, Smith JH (2008) The proposed BSCC terminology for abnormal cervical cytology. Cytopathol 19: 137–157. [DOI] [PubMed] [Google Scholar]
- Dijkstra MG, Snijders PJF, Arbyn M, Rijkaart DC, Berkhof J, Meijer CJ (2014) Cervical cancer screening: on the way to a shift from cytology to full molecular screening. Ann Oncol 25: 927–935. [DOI] [PubMed] [Google Scholar]
- Drolet M, Bénard É, Boily MC, Ali H, Baandrup L, Bauer H, Beddows S, Brisson J, Brotherton JM, Cummings T, Donovan B, Fairley CK, Flagg EW, Johnson AM, Kahn JA, Kavanagh K, Kjaer SK, Kliewer EV, Lemieux-Mellouki P, Markowitz L, Mboup A, Mesher D, Niccolai L, Oliphant J, Pollock KG, Soldan K, Sonnenberg P, Tabrizi SN, Tanton C, Brisson M (2015) Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 15: 565–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco EL, Cuzick J, Hildesheim A, de Sanjosé S (2006) Chapter 20: Issues in planning cervical cancer screening in the era of HPV vaccination. Vaccine S3: 171–177. [DOI] [PubMed] [Google Scholar]
- Franco EL, Salaheddin MM, Tota J, Ferenczy A, Coutlée F (2009) The expected impact of HPV vaccination on the accuracy of cervical cancer screening: the need for a paradigm change. Arch Med Res 40: 478–485. [DOI] [PubMed] [Google Scholar]
- Galgano MT, Castle PE, Atkins KA, Brix WK, Nassau SR, Stoler MH (2010) Using biomarkers as objective standards in the diagnosis of cervical biopsies. Am J Surg Pathol 34: 1077–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Histopathology reporting in Cervical Cancer. NHSCSP10. 2nd edn (2010) https://www.gov.uk/government/publications/cervical-screening-histopathology-reporting-handbook (accessed 28 December 2015).
- Kavanagh K, Pollock KG, Potts A, Love J, Cuschieri K, Cubie H, Robertson C, Donaghy M (2014) Introduction and sustained high coverage of the HPV bivalent vaccine leads to a reduction in prevalence of HPV 16/18 and closely related HPV types. Br J Cancer 110: 2804–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesher D, Cuschieri K, Hibbitts S, Jamison J, Sargent A, Pollock KG, Powell N, Wilson R, McCall F, Fiander A, Soldan K (2015) Type specific HPV prevalence in invasive cervical cancer prior to national HPV Immunisation programme: baseline for monitoring the effects of immunisation. J Clin Pathol 68: 135–140. [DOI] [PubMed] [Google Scholar]
- Nanda K, McCroy DC, Myers ER, Bastian LA, Hasselblad V, Hickey JD, Matchar DB (2000) Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med 132: 810–819. [DOI] [PubMed] [Google Scholar]
- Peto J, Gilham C, Fletcher O, Matthews FE (2004) The cervical cancer epidemic that screening has prevented in the UK. Lancet 364: 249–256. [DOI] [PubMed] [Google Scholar]
- Pollock KG, Kavanagh K, Potts A, Love J, Cuschieri K, Cubie H, Robertson C, Cruickshank M, Palmer TJ, Nicoll S, Donaghy M (2014) Reduction of low- and high-grade cervical abnormalities associated with high uptake of the HPV bivalent vaccine in Scotland. Br J Cancer 111: 1824–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijkaart DC, Berkhof J, van Kamenade FJ, Coupe VM, Hesselink AT, Rozendaal L, Heideman DA, Verheijen RH, Bulk S, Verweij WM, Snijders PJ, Meijer CJ (2012) Evaluation of 14 triage strategies for HPV DNA-positive women in population-based cervical screening. Int J Cancer 130: 602–610. [DOI] [PubMed] [Google Scholar]
- Robertson AJ, Anderson JM, Swanson-Beck J, Burnett RA, Howatson SR, Lee FD, Lessells AM, McLaren KM, Moss SM, Simpson JG, Smith GD, Tavadia HB, Walker F (1989) Observer variability in the interpretation of cervical biopsy specimens. J Clin Pathol 42: 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco G, Dillner J, Elfstrom KM, Tunesi S, Snijders PJF, Arbyn M, Kitchener H, Segnan N, Gilham C, Giorgi-Rossi P, Berkhof J, Peto J, Meijer CJLM International HPV screening working group (2014) Efficacy of HPV based screening for the prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet 383: 524–532. [DOI] [PubMed] [Google Scholar]
- SCSP recommendations for management of routine and non-routine smears http://www.sccrs.scot.nhs.uk/Documents/2013-04-01naps1-11April%202013.pdf (accessed on 23 August 2015).
- Shafi MI, Dunn JA, Finn CB, Kehoe S, Buxton EJ, Jordan JA, Luesley DM (1993) Characterization of high- and low-grade cervical intraepithelial neoplasia. Int J Gynecol Cancer 3: 203–207. [DOI] [PubMed] [Google Scholar]
- Sharp L, Cotton S, Cochran C, Gray N, Little J, Neal K, Cruickshank M TOMBOLA (Trial Of Management of Borderline and Other Low-grade Abnormal smears) Group (2009) After-effects reported by women following colposcopy, cervical biopsies and LLETZ: results from the TOMBOLA trial. BJOG 116: 1506–1514. [DOI] [PubMed] [Google Scholar]
- Thomsen LT, Frederiksen K, Munk C, Junge J, Iftner T, Kjaer SK (2015) Long-term risk of cervical intraepithelial neoplasia grade 3 or worse according to high-risk human papillomavirus genotype and semi-quantitative viral load among 33,288 women with normal cervical cytology. Int J Cancer 137: 193–203. [DOI] [PubMed] [Google Scholar]