Abstract
Background:
Mismatch repair-deficient (dMMR) colorectal cancer (CRC) is associated with a conspicuous local immune infiltrate; however, its relationship with systemic inflammatory responses remains to be determined. The present study aims to examine the relationships and prognostic value of assessment of the local and systemic environment in the context of MMR status in patients with CRC.
Methods:
The relationship between MMR status, determined using immunohistochemistry, and the local inflammatory cell infiltrate, differential white cell count, neutrophil : platelet score (NPS), neutrophil : lymphocyte ratio and modified Glasgow Prognostic Score (mGPS), and cancer-specific survival was examined in 228 patients undergoing resection of stage I–III CRC.
Results:
Thirty-five patients (15%) had dMMR CRC. Mismatch repair deficiency was associated with a higher density of CD3+, CD8+ and CD45R0+ T lymphocytes within the cancer cell nests and an elevated mGPS (mGPS2: 23% vs 9%, P=0.007) and NPS (NPS2: 19% vs 3%, P=0.001). CD3+ density (P<0.001), mGPS (P=0.01) and NPS (P=0.042) were associated with survival independent of MMR status (P=0.367) and stratified 5-year survival of patients with MMR-competent CRC from 94% to 67%, 83% to 46% and 78% to 60% respectively.
Conclusions:
Mismatch repair deficiency was associated with local and systemic environments, and in comparison with their assessment, dMMR had relatively poor prognostic value in patients with primary operable CRC. In addition to MMR status, local and systemic inflammatory responses should be assessed in these patients.
Keywords: colorectal cancer, mismatch repair, microsatellite instability, tumour microenvironment, systemic inflammation
Colorectal cancer (CRC) is the second most common cause of cancer-related death in the United Kingdom (Ferlay et al, 2013). Although prognosis and the need for post-operative treatment are presently determined by pathological staging, obvious heterogeneity in outcome exists among patients with similar disease stage (Horgan and McMillan, 2010). Indeed, other tumour-associated characteristics, intrinsic to the tumour cell and pertaining to both the tumour microenvironment and the patient, may similarly influence oncological outcome and be used to determine the need for further treatment (McAllister and Weinberg, 2014).
One such tumour characteristic is loss of mismatch repair (MMR) protein activity. Approximately 15–18% of tumours arise through genomic instability as a result of loss of MMR competency, whereas 2% of MMR-deficient (dMMR) tumours occur through inherited germline mutations, the remaining 13–15% account for sporadic cases of CRC, often as a result of hypermethylation-induced silencing of the hMLH1 promoter region (Boland and Goel, 2010). Tumours arising through dMMR activity accumulate mutations at an exponential rate, in particular within repeating microsatellite regions, and are characterised by the presence of MSI as well as distinct phenotypic characteristics, such as proximal tumour location and poor or mucinous differentiation (Ward et al, 2001; Jass et al, 2002; Greenson et al, 2009). Furthermore, dMMR status is associated with improved survival, in particular in patients with Stage II/III CRC (Popat et al, 2005; Guastadisegni et al, 2010; Saridaki et al, 2014).
In addition to such phenotypic characteristics, dMMR CRC is associated with characteristic features within the tumour microenvironment; in particular, the presence of a high density of tumour-infiltrating lymphocytes, a stage-independent predictor of increased survival in patients with CRC (Mei et al, 2014), has been consistently reported in this patient group (Smyrk et al, 2001; Ward et al, 2001; Greenson et al, 2009; De Smedt et al, 2015). Furthermore, the presence of a low proportion of tumour-associated stroma has similarly been associated with both favourable prognosis and dMMR status (Huijbers et al, 2013). Indeed, it has previously been suggested that the improved prognosis attributed to dMMR status may not be independent of such favourable characteristics within the tumour microenvironment (Ogino et al, 2009; Deschoolmeester et al, 2011; Huijbers et al, 2013).
Despite extensive characterisation of the tumour microenvironment, it is of interest that the relationship between MMR status and the systemic environment remains to be fully defined. Dysregulated systemic inflammatory responses promote cancer progression (McAllister and Weinberg, 2014) and the presence of a systemic inflammatory response, as measured by routinely available biomarkers, such as circulating acute-phase proteins and components of the differential white cell count, is associated with reduced survival independent of pathological staging (Roxburgh and McMillan, 2010). Given the favourable prognosis associated with dMMR status, it would be expected that patients with tumours arising through this pathway would be less likely to exhibit evidence of a cancer-associated systemic inflammatory response at diagnosis. Therefore, the aim of the present study was to characterise the relationships between MMR status, host local and systemic inflammatory responses and survival of patients undergoing elective, potentially curative resection of CRC.
Materials and methods
Clinicopathological characteristics
Patients were identified from a prospectively collected and maintained database of elective and emergency CRC resections in a single surgical unit at Glasgow Royal Infirmary. Patients who, on the basis of preoperative thoracoabdominal computed tomography and laparotomy findings, were considered to have undergone elective, potentially curative resection of Stage I–III CRC between January 1997 and May 2007, and whose tumour resection was included in a previously constructed CRC tissue microarray (TMA) were included. Exclusion criteria were as follows: (1) emergency resection, (2) inflammatory bowel disease-related CRC or known hereditary CRC syndrome, (3) pre-operative chemoradiotherapy, (4) surgery with palliative intent and (5) death within 30 days of surgery.
Tumours were routinely staged by gastrointestinal pathologists using the fifth edition of the tumour, node and metastases classification as is the current practice in the United Kingdom (Loughrey et al, 2014). Tumour differentiation, graded as well/moderate or poor in accordance with Royal College of Pathologists guidelines (Loughrey et al, 2014), and additional data were taken from pathological reports issued following resection. At multidisciplinary meetings following surgery, patients with stage III and high-risk stage II disease were considered for 5-fluorouracil-based adjuvant chemotherapy according to treatment guidelines at the time. Patients were routinely followed up for 5 years following surgery. Date and cause of death were cross-checked with the cancer registration system and the Registrar General (Scotland), and death records were complete until 31 March 2014 that served as the censor date. Cancer-specific survival was measured from date of index surgery until the date of death from recurrent or metastatic disease. Patients were censored at date of non-CRC death or date of last follow-up.
Assessment of the tumour microenvironment
Using routine haematoxylin and eosin-stained sections of the deepest point of invasion, the generalised inflammatory cell infiltrate at the invasive margin was assessed using Klintrup–Mäkinen (KM) grade and the extent of tumour stroma was assessed using tumour stroma percentage (TSP), both as previously described (Klintrup et al, 2005; Mesker et al, 2007). Briefly, using KM grade, the inflammatory cell density at the invasive margin was graded as either low-grade (no increase or mild/ patchy increase in inflammatory cells) or high-grade (prominent inflammatory reaction forming a band at the invasive margin or florid cup-like infiltrate at the invasive edge with destruction of cancer cell islands) (Klintrup et al, 2005; Roxburgh et al, 2009). Tumour stroma percentage was graded as either low (⩽50%) or high (>50%) based on previously derived thresholds (Mesker et al, 2007; Park et al, 2014).
Tumour-infiltrating T-lymphocyte density at the invasive margin and within the cancer cell nests was assessed using immunohistochemistry as previously described (Richards et al, 2014b). Briefly, tumour sections were stained for CD3+ (mature T lymphocyte), CD8+ (cytotoxic T-lymphocyte), CD45R0+ (memory T lymphocyte) and FOXP3+ (regulatory T lymphocyte), and the density of each lymphocyte subset within each compartment graded semi-quantitatively as low (absent or weak) or high (moderate or strong). Investigators blinded to clinicopathological and outcome data performed all assessments with co-scoring by two investigators for immunohistochemistry staining and the KM grade in 100 cases and TSP in 30 cases, to ensure consistency of scoring.
Assessment of the systemic inflammatory responses
Pre-operative C-reactive protein (CRP), serum albumin and differential white cell count measured within 30 days before surgery were recorded prospectively. On the basis of previously derived thresholds, neutrophil count>7.5 × 109 l−1, lymphocyte count >4 × 109 l−1 and platelet count >400 × 109 l−1 were considered elevated (Watt et al, 2015a). The modified Glasgow Prognostic Score (mGPS) was calculated as previously described (Park et al, 2016); patients with a normal CRP (⩽10 mg l−1) were allocated a score of 0, an elevated CRP (>10 mg l−1) alone a score of 1 and an elevated CRP and low albumin (<35 g l−1) a score of 2. The neutrophil : platelet score (NPS) was calculated as previously described (Watt et al, 2015b); patients with a normal platelet count and neutrophil count were allocated a score of 0, either a neutrophil count >7.5 × 109 l−1 or platelet count >400 × 109 l−1 a score of 1 and those with both an elevated neutrophil and platelet count a score of 2.
Assessment of MMR status
Previously constructed TMAs, comprising four 0.6-mm cores of formalin-fixed paraffin-embedded cancer tissue per patient, were used to assess MMR status (Roxburgh et al, 2013). Tissue microarray slides were placed in a ThermoFisher pH 9 PT module solution (Thermo Fisher Scientific Inc., Waltham, MA, USA) at room temperature. Slides were then heated in the PT module to a temperature of 96 °C for 20 min and allowed to cool. Using the ThermoFisher autostainer, slides were incubated in peroxidase block for 5 min and rinsed with TBS before incubating in UV protein blocker for 5 min and rinsing once again with TBS solution. Slides were then incubated in primary antibody for 20 min at a concentration of 1 : 100 for MLH1 and MSH6, and 1 : 50 for MSH2 and PMS2 (product codes: M3640, M3646, M3639 and M3647, respectively; Dako UK Ltd, Cambridgeshire, UK). Following this incubation period, slides were rinsed with TBS and Quanto Amplifier (Thermo Fisher Scientific Inc.) was applied to slides for 10 min followed by a further wash with TBS. Quanto Polymer was then added for 10 min followed by a TBS wash. DAB Quanto substrate was then added for 5 min, slides washed in TBS, counterstained in haematoxylin, blued in Scotts' tap water, dehydrated through a series of graded alcohols and cover slips applied with DPX mounting medium.
Mismatch repair protein expression was established by a single observer (AGP) blinded to clinical outcomes using UK NEQAS scoring guidelines (Arends et al, 2008). Appendix and normal colon were used as positive controls and positive staining within intra-tumoural immune cells serving as an internal positive control. An observer blinded to clinical outcome (JHP) scored 10% of cores. Expression was reported as MMR proficient (tumour cell nuclear expression with positive immune cell expression) or MMR deficient (absent tumour nuclear expression with normal immune cell expression). The use of multiple TMA cores per patient has been shown to be comparable to the use of full sections, even in the presence of known intra-tumoural heterogeneity of protein expression (Zhang et al, 2003). In the present study, four cores were examined per patient for each MMR protein; TMA assessment of MLH1 and MSH2 using three cores per patient has previously been shown to be comparable to full section analysis (Jourdan et al, 2003).
Statistical analysis
The relationship between MMR status, clinicopathological characteristics and the local and systemic inflammatory responses was examined using the χ2 method for linear trend for categorical variables and Mann–Whitney U-test for continuous variables. The relationship between MMR status, local and systemic inflammatory characteristics associated with MMR status and survival was examined by Kaplan–Meier log-rank survival analysis and Cox proportional hazards regression using a multivariate backwards conditional model to calculate hazard ratios and 95% confidence intervals. Variables with a P⩽0.05 on univariate analysis were entered into a multivariate model. A P-value ⩽0.05 was considered statistically significant. All analyses were performed using SPSS version 22.0 (IBM SPSS, Armonk, NY, USA). The West of Scotland Research Ethics Committee approved the study and tissue for analysis of MMR status was obtained from the National Health Service Greater Glasgow and Clyde Tissue Biorepository.
Results
A total of 228 patients who underwent elective, potentially curative resection of stage I–III CRC were included. Almost two thirds of patients were older than 65 years at the time of surgery and 53% were male. Pathological assessment confirmed Stage I disease in 16 patients (7%), stage II disease in 111 patients (49%) and stage III disease in 101 patients (44%). Sixty-six patients (29%) received adjuvant therapy; 1 patient with stage I disease, 15 patients with stage II disease and 50 patients with stage III disease received adjuvant therapy. Mismatch repair deficiency was identified in 35 patients (15%); the frequency of aberrant MMR protein expression in patients with dMMR CRC is displayed in Table 1.
Table 1. Pattern of aberrant MMR protein expression.
| Aberrant protein expression | Number of patients |
|---|---|
| MLH1/PMS2 | 17 |
| MSH6/MSH2 | 8 |
| PMS2 | 7 |
| MSH6 | 1 |
| PMS2/MSH6 | 1 |
| PMS2/MSH6/MSH2 | 1 |
Abbreviations: CRC=colorectal cancer; dMMR=mismatch repair deficient; MMR=mismatch repair.
Pattern of aberrant MMR protein expression in patients undergoing elective, potentially curative resection of dMMR I–III CRC.
Mismatch repair status and clinicopathological characteristics
The relationship between MMR status and clinicopathological characteristics is displayed in Table 2. Patients with dMMR CRC were more likely to have a colonic primary and poor tumour differentiation (both P<0.05). In addition, although not associated with T stage, dMMR status was associated with an increased rate of peritoneal involvement (P<0.05). Detection of dMMR did not differ with year of diagnosis (P=0.290). Furthermore, the age of patients with dMMR CRC did not differ significantly from those with MMR-competent cancer (P=0.707). As such, it is unlikely that a significant proportion of included patients had Lynch syndrome cancer.
Table 2. Relationship between MMR status and clinicopathological characteristics.
| Host characteristics | All n=228 (%) | MMR competent n=193 (%) | dMMR n=35 (%) | P-value |
|---|---|---|---|---|
|
Age (years) | ||||
| <65 | 83 (36) | 71 (37) | 12 (34) | 0.707 |
| 65–74 | 73 (32) | 62 (32) | 11 (32) | |
| >75 | 72 (32) | 60 (31) | 12 (34) | |
|
Sex | ||||
| Male | 108 (47) | 92 (48) | 16 (46) | 0.832 |
| Female | 120 (53) | 101 (52) | 19 (54) | |
|
Diagnosis year | ||||
| 1997–2002 | 142 (62) | 123 (64) | 19 (54) | 0.290 |
| 2003–2007 | 86 (38) | 70 (36) | 16 (46) | |
|
Adjuvant therapy | ||||
| No | 162 (71) | 135 (70) | 27 (77) | 0.389 |
| Yes | 66 (29) | 58 (30) | 8 (23) | |
|
Tumour characteristics | ||||
| Tumour site | ||||
| Colon | 151 (66) | 122 (63) | 29 (83) | 0.024 |
| Rectum | 77 (34) | 71 (37) | 6 (17) | |
| TNM stage | ||||
| I | 25 (11) | 21 (11) | 4 (11) | 0.037 |
| II | 141 (62) | 124 (64) | 17 (49) | |
| III | 62 (27) | 48 (25) | 14 (40) | |
| T stage | ||||
| 1–2 | 127 (55) | 105 (54) | 22 (63) | 0.160 |
| 3 | 77 (34) | 65 (34) | 12 (34) | |
| 4 | 24 (11) | 23 (12) | 1 (3) | |
| N stage | ||||
| 0 | 16 (7) | 14 (7) | 2 (6) | 0.539 |
| 1 | 111 (49) | 91 (47) | 20 (57) | |
| 2 | 101 (44) | 88 (46) | 13 (37) | |
| Differentiation | ||||
| Moderate/well | 200 (88) | 173 (90) | 27 (77) | 0.039 |
| Poor | 28 (12) | 20 (10) | 8 (23) | |
| Venous invasion | ||||
| Absent | 148 (65) | 123 (64) | 25 (71) | 0.381 |
| Present | 80 (35) | 70 (36) | 10 (29) | |
| Margin involvement | ||||
| Absent | 215 (94) | 182 (94) | 33 (94) | 0.997 |
| Present | 13 (6) | 11 (6) | 2 (6) | |
| Peritoneal involvement | ||||
| Absent | 165 (72) | 145 (75) | 20 (57) | 0.029 |
| Present | 63 (28) | 48 (25) | 15 (43) | |
| Tumour perforation | ||||
| Absent | 223 (98) | 188 (97) | 35 (100) | 0.337 |
| Present | 5 (2) | 5 (3) | 0 (0) | |
Abbreviations: CRC=colorectal cancer; dMMR=mismatch repair deficient; MMR=mismatch repair; TNM, tumour, node, metastasis.
The relationship between MMR status and clinicopathological characteristics of patients undergoing elective, potentially curative resection of stage I–III CRC.
Mismatch repair status and the tumour microenvironment
The relationship between MMR status and the tumour microenvironment is displayed in Table 3. Patients with dMMR CRC had an increased density of CD3+ (P<0.01), CD45R0+ (P<0.05) and CD8+ (P=0.071) T lymphocytes within the cancer cell nests. Although not reaching statistical significance, patients with dMMR CRC were less likely to have a high TSP (15% vs 28%, P=0.118). The density of FOXP3+ T lymphocytes within the cancer cell nests, density of T lymphocytes at the invasive margin nor the KM grade showed significant association with MMR status.
Table 3. Relationship between MMR status and tumour microenvironment.
| Tumour microenvironment | All n=228 (%) | MMR competent n=193 (%) | dMMR n=35 (%) | P-value |
|---|---|---|---|---|
|
KM grade | ||||
| Weak | 77 (34) | 63 (33) | 14 (40) | 0.398 |
| Strong | 151 (66) | 130 (67) | 21 (60) | |
|
CD3 margin density (215) | ||||
| Low | 118 (55) | 100 (55) | 18 (56) | 0.867 |
| High | 97 (45) | 83 (45) | 14 (44) | |
|
CD3 cancer cell nest density (224) | ||||
| Low | 146 (65) | 130 (69) | 16 (46) | 0.009 |
| High | 78 (35) | 59 (31) | 19 (54) | |
|
CD8 margin density (216) | ||||
| Low | 127 (59) | 105 (57) | 22 (67) | 0.319 |
| High | 9 (41) | 78 (43) | 11 (33) | |
|
CD8 cancer cell nest density (222) | ||||
| Low | 161 (72) | 140 (75) | 21 (60) | 0.071 |
| High | 61 (28) | 47 (25) | 14 (40) | |
|
CD45R0 margin density (217) | ||||
| Low | 112 (52) | 96 (53) | 6 (47) | 0.564 |
| High | 105 (48) | 87 (47) | 18 (53) | |
|
CD45R0 cancer cell nest density (224) | ||||
| Low | 160 (71) | 141 (75) | 19 (54) | 0.015 |
| High | 64 (29) | 48 (25) | 16 (46) | |
|
FOXP3 margin density (216) | ||||
| Low | 126 (58) | 104 (57) | 22 (65) | 0.413 |
| High | 90 (42) | 78 (43) | 12 (35) | |
|
FOXP3 cancer cell nest density (219) | ||||
| Low | 110 (50) | 92 (50) | 18 (53) | 0.731 |
| High | 109 (50) | 93 (50) | 16 (47) | |
|
TSP (225) | ||||
| Low | 166 (74) | 138 (72) | 28 (85) | 0.118 |
| High | 59 (26) | 54 (28) | 5 (15) | |
Abbreviations: CRC=colorectal cancer; dMMR=mismatch repair deficient; KM=Klintrup–Mäkinen; MMR=mismatch repair; TSP=tumour stroma percentage. The relationship between MMR status and tumour microenvironment of patients undergoing elective, potentially curative resection of stage I–III CRC.
Mismatch repair status and systemic inflammatory responses
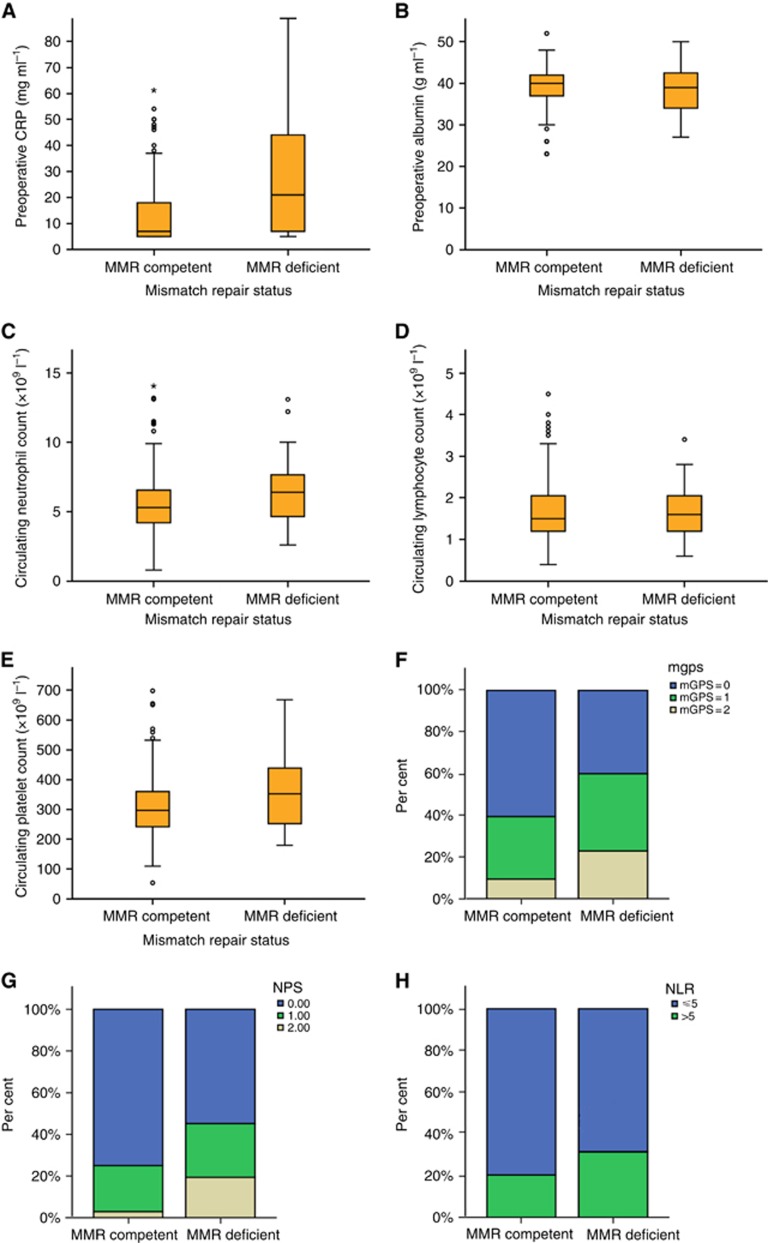
The relationship between MMR status and host systemic inflammatory responses is displayed in Figure 1 and Table 4. Patients with dMMR CRC had a higher median pre-operative CRP (P<0.001) and neutrophil count (P<0.05), and showed a trend towards a higher median platelet count (P=0.091). Serum albumin concentrations and circulating lymphocyte count did not differ with MMR status. Patients with dMMR CRC were more likely to have a neutrophil count >7.5 × 109 l−1 (P<0.01) and platelet count >400 × 109 l−1 (P<0.05). In addition, both the mGPS and NPS were more likely to be elevated in patients with dMMR CRC (both P<0.01).
Figure 1.
Relationship between MMR status and host systemic inflammatory responses. The relationship between MMR status and host systemic inflammatory responses in patients undergoing elective, potentially curative resection of stage I–III CRC (A) serum CRP (P<0.001), (B) serum albumin (P=0.258), (C) circulating neutrophil count (P=0.032), (D) circulating lymphocyte count (P=0.669), (E) circulating platelet count (P=0.091), (F) mGPS (P=0.007), (G) NLS (P=0.001), and (H) neutrophil : lymphocyte ratio (NLR; P=0.145). Boxplots represent median value and interquartile range.
Table 4. Relationship between MMR status and systemic inflammatory responses.
| Systemic inflammatory responses | All n=228 (%) | MMR competent n=193 (%) | dMMR n=35 (%) | P-value |
|---|---|---|---|---|
|
Serum CRP | ||||
| mg l−1 | 8 (6–20) | 7 (5–18) | 21 (7–48) | <0.001 |
|
Serum albumin | ||||
| g l−1 | 40 (36–42) | 40 (37–42) | 39 (34–43) | 0.258 |
|
Modified Glasgow Prognostic Score | ||||
| 0 | 131 (58) | 117 (61) | 14 (40) | 0.007 |
| 1 | 71 (31) | 58 (30) | 13 (37) | |
| 2 | 26 (11) | 18 (9) | 8 (23) | |
|
Neutrophil count (227) | ||||
| × 109 l−1 | 5.4 (4.3–6.7) | 5.3 (4.2–6.6) | 6.4 (4.6–7.7) | 0.032 |
|
Lymphocyte count (227) | ||||
| × 109 l−1 | 1.5 (1.2–2.1) | 1.5 (1.2–2.1) | 1.6 (1.2–2.1) | 0.891 |
|
Platelet count (207) | ||||
| × 109 l−1 | 300 (245–369) | 296 (242–360) | 352 (251–441) | 0.091 |
|
Neutrophil count (227) | ||||
| ⩽7.5 × 109 l−1 | 192 (85) | 168 (87) | 24 (69) | 0.004 |
| >7.5 × 109 l−1 | 35 (15) | 24 (13) | 11 (31) | |
|
Lymphocyte count (227) | ||||
| ⩽4 × 109 l−1 | 171 (83) | 191 (99) | 35 (100) | 0.669 |
| >4 × 109 l−1 | 36 (17) | 1 (1) | 0 (0) | |
|
Platelet count (207) | ||||
| ⩽400 × 109 l−1 | 226 (99) | 150 (85) | 21 (68) | 0.018 |
| >400 × 109 l−1 | 1 (1) | 26 (15) | 10 (32) | |
|
NLR (227) | ||||
| ⩽5 | 177 (78) | 153 (80) | 24 (69) | 0.145 |
| >5 | 50 (22) | 39 (20) | 11 (31) | |
|
NPS (207) | ||||
| 0 | 149 (72) | 132 (75) | 17 (55) | 0.001 |
| 1 | 47 (23) | 39 (22) | 8 (26) | |
| 2 | 11 (5) | 5 (3) | 6 (19) | |
Abbreviations: CRC=colorectal cancer; CRP=C-reactive protein; dMMR=mismatch repair deficient; mGPS=modified Glasgow Prognostic Score; MMR=mismatch repair; NLR=neutrophil : lymphocyte ratio; NPS=neutrophil : platelet score.
The relationship between MMR status and systemic inflammatory responses of patients undergoing elective, potentially curative resection of stage I–III CRC.
Mismatch repair status and survival
The relationship between MMR status, characteristics of the local and systemic inflammatory responses significantly associated with MMR status and cancer-specific survival was subsequently examined (Table 5). The median follow-up of survivors was 143 months (range 87–206 months) with 66 cancer-specific deaths and 5-year cancer-specific survival of 76%. On multivariate survival analysis, dMMR was not significantly associated with cancer-specific survival (P=0.790), whereas the density of CD3+ T lymphocytes within the cancer cell nests (P<0.001), mGPS (P<0.01) and NPS (P<0.05) were independently associated with survival. When analysis was restricted to patients with stage II/III disease only, cancer cell nest CD3+ T-lymphocyte density (P<0.001), mGPS and NPS (both P<0.05) remained associated with survival independent of MMR status (P=0.833).
Table 5. Relationship between tumour microenvironment and systemic inflammatory response characteristics.
| Multivriate analysis HR (95% CI) | P-value | |
|---|---|---|
|
All patients (n=228) | ||
| CD3 cancer cell nest density (low/high) | 0.28 (0.14–0.57) | <0.001 |
| CD45R0 cancer cell nest density (low/high) | 0.69 (0.28–1.72) | 0.430 |
| mGPS (0/1/2) | 1.59 (1.12–2.27) | 0.010 |
| NPS (0/1/2) | 1.47 (1.01–2.14) | 0.042 |
| MMR status (competent/deficient) | 0.69 (0.31–1.54) | 0.367 |
|
Stage II/Stage III only (n=212) | ||
| CD3 cancer cell nest density (low/high) | 0.30 (0.15–0.61) | 0.001 |
| CD45R0 cancer cell nest density (low/high) | 0.77 (0.30–1.95) | 0.578 |
| mGPS (0/1/2) | 1.52 (1.06–2.19) | 0.023 |
| NPS (0/1/2) | 1.46 (1.01–2.13) | 0.047 |
| MMR status (competent/deficient) | 0.71 (0.32–1.58) | 0.399 |
Abbreviations: CRC=colorectal cancer; CI=confidence interval; HR=hazard ratio; mGPS=modified Glasgow Prognostic Score; MMR=mismatch repair; NPS=neutrophil : platelet score.
The relationship between tumour microenvironment and systemic inflammatory response characteristics associated with MMR status and cancer-specific survival of patients undergoing elective, potentially curative resection of stage I–III CRC.
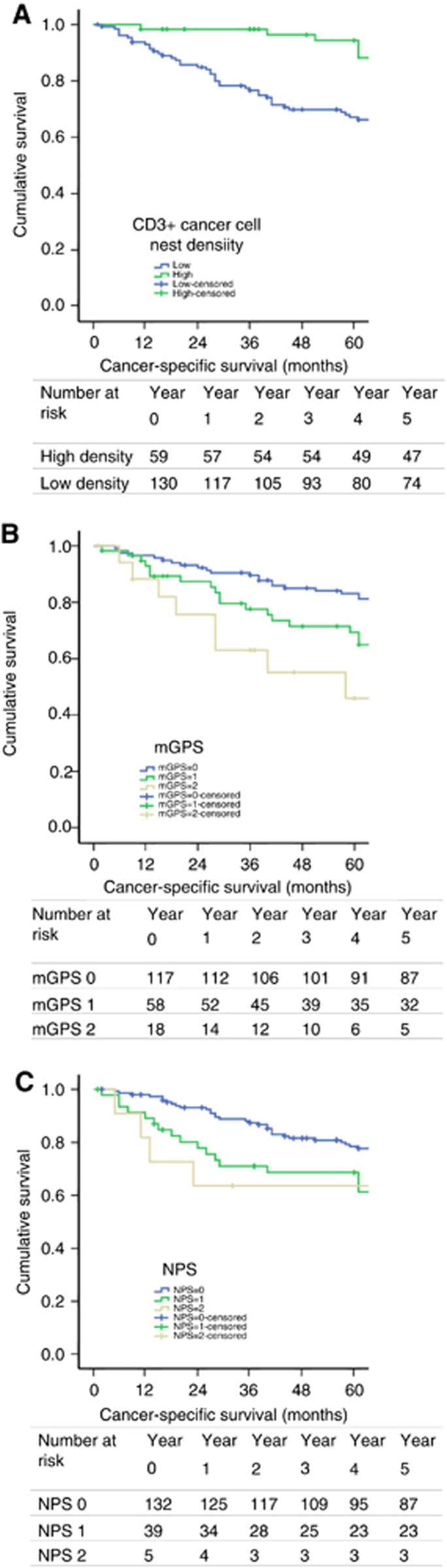
As cancer cell nest density of CD3+ T lymphocytes, mGPS and NPS were all associated with survival independent of MMR status, the relationship between these characteristics and cancer-specific survival of patients with MMR-competent CRC was subsequently examined (Figure 2). Five-year cancer-specific survival was stratified from 94% to 67% by cancer cell nest CD3+ T-lymphocyte density (P<0.001), from 83% to 46% by mGPS (P=0.002) and from 78% to 60% by NPS (P=0.054).
Figure 2.
Relationship between tumour and host characteristics. The relationship between tumour and host characteristics associated with survival independent of MMR status and cancer-specific survival of patients undergoing elective, potentially curative resection of MMR competent, stage I–III CRC (A) cancer cell nest CD3+ T-lymphocyte density (P<0.001), (B) mGPS (P=0.002) and (C) NPS (P=0.054).
Discussion
The present study describes the distinct tumour and host phenotypic characteristics associated with MMR deficiency in patients undergoing elective, potentially curative resection of CRC. Patients with dMMR CRC were more likely to have a high density of T lymphocytes within the tumour microenvironment and evidence of an elevated host systemic inflammatory response as evidenced by components of the differential white cell count and serum acute phase proteins. Furthermore, these characteristics were associated with cancer-specific survival independent of MMR status. Taken together with the previous literature (Ogino et al, 2009; Deschoolmeester et al, 2010; Huijbers et al, 2013; Vayrynen et al, 2014; Park et al, 2015b), this provides further evidence that the prognostic benefit associated with dMMR CRC is not necessarily independent of such characteristics.
Patients with dMMR CRC were more likely to have a high density of intratumoural CD3+, CD8+ and CD45R0+ T lymphocytes; however, dMMR status did not appear to influence FOXP3+ T-regulatory lymphocyte density. Furthermore, it was of interest that the inflammatory cell infiltrate at the invasive margin, as measured by either T-lymphocyte density or KM grade, did not differ with MMR status. Given that the KM grade is reflective of components of both adaptive and innate local immune responses (Vayrynen et al, 2013; Park et al, 2015a), the present study would favour an association between dMMR status and development primarily of a co-ordinated, adaptive intratumoural immune response. Indeed, this is consistent with recent work addressing the nature of the immune microenvironment in patients with dMMR CRC (De Smedt et al, 2015; Maby et al, 2015). De Smedt et al (2015) recently reported that MSI-associated colon cancers primarily elicited an intratumoural, lymphocytic inflammatory response with little change in the peritumoural generalised inflammatory infiltrate. Second, Maby et al (2015) reported that an increased burden of MSI-associated frameshift mutations predominantly favoured tumour infiltration by CD8+ T lymphocytes but not FOXP3+ T lymphocytes. Taken together with these prior studies, the present results further support the role of dMMR/MSI status in promoting tumour infiltration by a co-ordinated, adaptive anti-tumour lymphocytic response (Llosa et al, 2015).
An unexpected finding was an association between dMMR status and the presence of an elevated systemic inflammatory response. In particular, dMMR status was associated with an elevated CRP, neutrophil count and platelet count, as well prognostic scores derived from these markers. Of interest however, and consistent with recent work by Pine et al (2015), neither circulating lymphocyte count nor neutrophil : lymphocyte ratio were associated with MMR status. Although Pine et al (2015) hypothesised that the peritumoural lymphocytosis associated with dMMR CRC may translate into an increase in circulating lymphocyte count, the results of the present study more closely reflect our understanding of the nature of the systemic inflammatory response in cancer. However, whereas the presence of a conspicuous inflammatory cell infiltrate within the tumour microenvironment primarily reflects the presence of an adaptive, anti-tumour immune response, it is increasingly appreciated that cancer-associated perturbances of the systemic inflammatory response primarily reflects upregulation of mediators of innate immunity, which in turn promote tumour progression and dissemination (McAllister and Weinberg, 2014). As such, it would be expected that any association between tumour characteristics and the systemic inflammatory response would be reflected by changes in markers of innate immunity, such as circulating CRP concentrations and neutrophil and platelet counts.
The mechanism underlying an association between systemic inflammation and MMR status is not clear. Although dMMR/MSI-associated tumours may be more likely to express an ‘inflammatory response'-type gene signature (Missiaglia et al, 2014), another possible explanation is that the presence of a chronic systemic inflammatory response may predispose patients to sporadic development of dMMR tumours (Boland and Goel, 2010; Fuseya et al, 2012). For example, the pro-inflammatory cytokine interleukin-6 has previously been implicated in the initiation of MMR defects in colon cancer cell lines (Tseng-Rogenski et al, 2015) and a similar relationship between systemic inflammation and MMR status has been observed in patients with gynaecological malignancies (Fuseya et al, 2012). Furthermore, despite dMMR tumours eliciting a profound anti-tumour lymphocytic immune response, it has recently been shown that this is counterbalanced by upregulation of multiple immune checkpoints (Llosa et al, 2015). Indeed, whether the systemic inflammatory response reflects underlying immune checkpoint activation, or may be indicative of an activated common upstream precursor, such as the JAK/STAT3 pathway, would be of considerable interest (Pardoll, 2012).
On multivariate survival analysis, characterisation of host local and systemic inflammatory responses was a stronger predictor of survival than assessment of MMR status, and showed prognostic value in patients with MMR competent CRC, consistent with previous reports (Ogino et al, 2009; Sinicrope et al, 2009; Dahlin et al, 2011; Vayrynen et al, 2013; Vayrynen et al, 2014; Park et al, 2015b). Furthermore, a considerable proportion of patients with MMR-competent CRC had a high density of intraepithelial T lymphocytes. Given that assessment of MMR status alone would have failed to identify these patients, it is clear that combined assessment of host local and systemic inflammatory response, in conjunction with MMR status and standard pathological staging could potentially lead to better risk stratification of patients following potentially curative resection of CRC.
The present study is perhaps limited by its use of immunohistochemistry to identify loss of MMR activity rather than genetic sequencing for microsatellite instability. Indeed, not all MSI pathway tumours will be identifiable by loss of MMR proteins (Shia, 2008). Immunohistochemical detection of MLH1 and MSH2 however has an acceptable sensitivity and specificity for microsatellite instability screening (Lindor et al, 2002) and this is further improved by the use of the additional markers, PMS2 and MSH6, as used in the present study (Shia, 2008). In addition, previous studies have found that immunohistochemical assessment of MMR status using TMA sections is comparable to full-section analysis (Hendriks et al, 2003; Jourdan et al, 2003). Whereas prior studies have recommended the use of three cores per tumour (Jourdan et al, 2003), the present analysis was performed using four cores for each protein. Furthermore, although the use of older, archival tissue can influence the results of immunohistochemistry, there was no difference in the frequency of detection of MMR deficiency with year of surgery, suggesting that this was not an issue in the present study. Finally, manual semi-quantitative assessment of the local inflammatory cell infiltrate was presently employed; however, this has been shown to have excellent inter-operator agreement (Richards et al, 2014a) and correlates strongly with automated digital assessment (Forrest et al, 2014; De Smedt et al, 2015).
In summary, the present study further highlights the complexities of the relationship between the local and systemic tumour environment and MMR status in patients with CRC. Furthermore, these results confirm the importance of the tumour microenvironment and host inflammatory responses, in addition to the intrinsic properties of tumour cells, in determining outcome of patients with CRC.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
References
- Arends M, Ibrahim M, Happerfield L, Frayling I, Miller K (2008) Interpretation of immunohistochemical analysis of mismatch repair (MMR) protein expression in tissue sections for investigation of suspected Lynch/Hereditary Non-Polyposis Colorectal Cancer (HNPCC) syndrome. UK NEQAS ICC ISH Recommendations.
- Boland CR, Goel A (2010) Microsatellite instability in colorectal cancer. Gastroenterology 138(6): 2073–2087 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin AM, Henriksson ML, Van Guelpen B, Stenling R, Oberg A, Rutegard J, Palmqvist R (2011) Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod Pathol 24(5): 671–682. [DOI] [PubMed] [Google Scholar]
- De Smedt L, Lemahieu J, Palmans S, Govaere O, Tousseyn T, Van Cutsem E, Prenen H, Tejpar S, Spaepen M, Matthijs G, Decaestecker C, Moles Lopez X, Demetter P, Salmon I, Sagaert X (2015) Microsatellite instable vs stable colon carcinomas: analysis of tumour heterogeneity, inflammation and angiogenesis. Br J Cancer 113(3): 500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschoolmeester V, Baay M, Lardon F, Pauwels P, Peeters M (2011) Immune cells in colorectal cancer: prognostic relevance and role of MSI. Cancer Microenviron 4(3): 377–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschoolmeester V, Baay M, Van Marck E, Weyler J, Vermeulen P, Lardon F, Vermorken JB (2010) Tumor infiltrating lymphocytes: an intriguing player in the survival of colorectal cancer patients. BMC Immunol 11(1): 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F (2013) Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49(6): 1374–1403. [DOI] [PubMed] [Google Scholar]
- Forrest R, Guthrie GJ, Orange C, Horgan PG, McMillan DC, Roxburgh CS (2014) Comparison of visual and automated assessment of tumour inflammatory infiltrates in patients with colorectal cancer. Eur J Cancer 50(3): 544–552. [DOI] [PubMed] [Google Scholar]
- Fuseya C, Horiuchi A, Hayashi A, Suzuki A, Miyamoto T, Hayashi T, Shiozawa T (2012) Involvement of pelvic inflammation-related mismatch repair abnormalities and microsatellite instability in the malignant transformation of ovarian endometriosis. Hum Pathol 43(11): 1964–1972. [DOI] [PubMed] [Google Scholar]
- Greenson JK, Huang SC, Herron C, Moreno V, Bonner JD, Tomsho LP, Ben-Izhak O, Cohen HI, Trougouboff P, Bejhar J, Sova Y, Pinchev M, Rennert G, Gruber SB (2009) Pathologic predictors of microsatellite instability in colorectal cancer. Am J Surg Pathol 33(1): 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastadisegni C, Colafranceschi M, Ottini L, Dogliotti E (2010) Microsatellite instability as a marker of prognosis and response to therapy: a meta-analysis of colorectal cancer survival data. Eur J Cancer 46(15): 2788–2798. [DOI] [PubMed] [Google Scholar]
- Hendriks Y, Franken P, Dierssen JW, De Leeuw W, Wijnen J, Dreef E, Tops C, Breuning M, Brocker-Vriends A, Vasen H, Fodde R, Morreau H (2003) Conventional and tissue microarray immunohistochemical expression analysis of mismatch repair in hereditary colorectal tumors. Am J Pathol 162(2): 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgan PG, McMillan DC (2010) Surgeons and selection of adjuvant therapy for node-negative colonic cancer. Br J Surg 97(10): 1459–1460. [DOI] [PubMed] [Google Scholar]
- Huijbers A, Tollenaar RA, v Pelt GW, Zeestraten EC, Dutton S, McConkey CC, Domingo E, Smit VT, Midgley R, Warren BF, Johnstone EC, Kerr DJ, Mesker WE (2013) The proportion of tumor-stroma as a strong prognosticator for stage II and III colon cancer patients: validation in the VICTOR trial. Ann Oncol 24(1): 179–185. [DOI] [PubMed] [Google Scholar]
- Jass JR, Whitehall VL, Young J, Leggett BA (2002) Emerging concepts in colorectal neoplasia. Gastroenterology 123(3): 862–876. [DOI] [PubMed] [Google Scholar]
- Jourdan F, Sebbagh N, Comperat E, Mourra N, Flahault A, Olschwang S, Duval A, Hamelin R, Flejou JF (2003) Tissue microarray technology: validation in colorectal carcinoma and analysis of p53, hMLH1, and hMSH2 immunohistochemical expression. Virchows Arch 443(2): 115–121. [DOI] [PubMed] [Google Scholar]
- Klintrup K, Makinen JM, Kauppila S, Vare PO, Melkko J, Tuominen H, Tuppurainen K, Makela J, Karttunen TJ, Makinen MJ (2005) Inflammation and prognosis in colorectal cancer. Eur J Cancer 41(17): 2645–2654. [DOI] [PubMed] [Google Scholar]
- Lindor NM, Burgart LJ, Leontovich O, Goldberg RM, Cunningham JM, Sargent DJ, Walsh-Vockley C, Petersen GM, Walsh MD, Leggett BA, Young JP, Barker MA, Jass JR, Hopper J, Gallinger S, Bapat B, Redston M, Thibodeau SN (2002) Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol 20(4): 1043–1048. [DOI] [PubMed] [Google Scholar]
- Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, Zhang M, Papadopoulos N, Kinzler KW, Vogelstein B, Sears CL, Anders RA, Pardoll DM, Housseau F (2015) The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 5(1): 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughrey MB, Quirke P, Shepherd NA (2014) Dataset for Colorectal Cancer Histopathology Reports 3rd edn. The Royal College of Pathologists: London, UK. [Google Scholar]
- Maby P, Tougeron D, Hamieh M, Mlecnik B, Kora H, Bindea G, Angell HK, Fredriksen T, Elie N, Fauquembergue E, Drouet A, Leprince J, Benichou J, Mauillon J, Le Pessot F, Sesboue R, Tuech JJ, Sabourin JC, Michel P, Frebourg T, Galon J, Latouche JB (2015) Correlation between density of CD8+ T-cell infiltrate in microsatellite unstable colorectal cancers and frameshift mutations: a rationale for personalized immunotherapy. Cancer Res 75(17): 3446–3455. [DOI] [PubMed] [Google Scholar]
- McAllister SS, Weinberg RA (2014) The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol 16(8): 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Z, Liu Y, Liu C, Cui A, Liang Z, Wang G, Peng H, Cui L, Li C (2014) Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer 110(6): 1595–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesker WE, Junggeburt JM, Szuhai K, de Heer P, Morreau H, Tanke HJ, Tollenaar RA (2007) The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell Oncol 29(5): 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaglia E, Jacobs B, D'Ario G, Di Narzo AF, Soneson C, Budinska E, Popovici V, Vecchione L, Gerster S, Yan P, Roth AD, Klingbiel D, Bosman FT, Delorenzi M, Tejpar S (2014) Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol 25(10): 1995–2001. [DOI] [PubMed] [Google Scholar]
- Ogino S, Nosho K, Irahara N, Meyerhardt JA, Baba Y, Shima K, Glickman JN, Ferrone CR, Mino-Kenudson M, Tanaka N, Dranoff G, Giovannucci EL, Fuchs CS (2009) Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res 15(20): 6412–6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4): 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, McMillan DC, Edwards J, Horgan PG, Roxburgh CSD (2015. a) Comparison of the prognostic value of measures of the tumor inflammatory cell infiltrate and tumor-associated stroma in patients with primary operable colorectal cancer. Oncoimmunology (In Press). [DOI] [PMC free article] [PubMed]
- Park JH, McMillan DC, Powell AG, Richards CH, Horgan PG, Edwards J, Roxburgh CS (2015. b) Evaluation of a tumor microenvironment-based prognostic score in primary operable colorectal cancer. Clin Cancer Res 21(4): 882–888. [DOI] [PubMed] [Google Scholar]
- Park JH, Richards CH, McMillan DC, Horgan PG, Roxburgh CS (2014) The relationship between tumour stroma percentage, the tumour microenvironment and survival in patients with primary operable colorectal cancer. Ann Oncol 25(3): 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Watt DG, Roxburgh CS, Horgan PG, McMillan DC (2016) Colorectal cancer, systemic inflammation, and outcome: staging the tumor and staging the host. Ann Surg 263(2): 326–336. [DOI] [PubMed] [Google Scholar]
- Pine JK, Morris E, Hutchins GG, West NP, Jayne DG, Quirke P, Prasad KR (2015) Systemic neutrophil-to-lymphocyte ratio in colorectal cancer: the relationship to patient survival, tumour biology and local lymphocytic response to tumour. Br J Cancer 113(2): 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popat S, Hubner R, Houlston RS (2005) Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 23(3): 609–618. [DOI] [PubMed] [Google Scholar]
- Richards CH, Roxburgh CS, Powell AG, Foulis AK, Horgan PG, McMillan DC (2014. a) The clinical utility of the local inflammatory response in colorectal cancer. Eur J Cancer 50(2): 309–319. [DOI] [PubMed] [Google Scholar]
- Richards CH, Roxburgh CS, Powell AG, Foulis AK, Horgan PG, McMillan DC (2014. b) The clinical utility of the local inflammatory response in colorectal cancer. Eur J Cancer 50(2): 309–319. [DOI] [PubMed] [Google Scholar]
- Roxburgh CS, McMillan DC (2010) Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 6(1): 149–163. [DOI] [PubMed] [Google Scholar]
- Roxburgh CS, Richards CH, Macdonald AI, Powell AG, McGlynn LM, McMillan DC, Horgan PG, Edwards J, Shiels PG (2013) The in situ local immune response, tumour senescence and proliferation in colorectal cancer. Br J Cancer 109(8): 2207–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roxburgh CS, Salmond JM, Horgan PG, Oien KA, McMillan DC (2009) Comparison of the prognostic value of inflammation-based pathologic and biochemical criteria in patients undergoing potentially curative resection for colorectal cancer. Ann Surg 249(5): 788–793. [DOI] [PubMed] [Google Scholar]
- Saridaki Z, Souglakos J, Georgoulias V (2014) Prognostic and predictive significance of MSI in stages II/III colon cancer. World J Gastroenterol 20(22): 6809–6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shia J (2008) Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn 10(4): 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ (2009) Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology 137(4): 1270–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyrk TC, Watson P, Kaul K, Lynch HT (2001) Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer 91(12): 2417–2422. [PubMed] [Google Scholar]
- Tseng-Rogenski SS, Hamaya Y, Choi DY, Carethers JM (2015) Interleukin 6 alters localization of hMSH3, leading to DNA mismatch repair defects in colorectal cancer cells. Gastroenterology 148(3): 579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vayrynen JP, Sajanti SA, Klintrup K, Makela J, Herzig KH, Karttunen TJ, Tuomisto A, Makinen MJ (2014) Characteristics and significance of colorectal cancer associated lymphoid reaction. Int J Cancer 134(9): 2126–2135. [DOI] [PubMed] [Google Scholar]
- Vayrynen JP, Tuomisto A, Klintrup K, Makela J, Karttunen TJ, Makinen MJ (2013) Detailed analysis of inflammatory cell infiltration in colorectal cancer. Br J Cancer 109(7): 1839–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R, Meagher A, Tomlinson I, O'Connor T, Norrie M, Wu R, Hawkins N (2001) Microsatellite instability and the clinicopathological features of sporadic colorectal cancer. Gut 48(6): 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt DG, Martin JC, Park JH, Horgan PG, McMillan DC (2015. a) Neutrophil count is the most important prognostic component of the differential white cell count in patients undergoing elective surgery for colorectal cancer. Am J Surg 210(1): 24–30. [DOI] [PubMed] [Google Scholar]
- Watt DG, Proctor MJ, Park JH, Horgan PG, McMillan DC (2015. b) The neutrophil-platelet score (NPS) predicts survival in primary operable colorectal cancer and a variety of common cancers. PLoS ONE 10(11): e0142159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Salto-Tellez M, Putti TC, Do E, Koay ES (2003) Reliability of tissue microarrays in detecting protein expression and gene amplification in breast cancer. Mod Pathol 16(1): 79–84. [DOI] [PubMed] [Google Scholar]