Abstract
A detailed knowledge of the capsid assembly pathways of viruses from their coat protein building blocks is required to devise novel therapeutic strategies to inhibit such assembly. In the quest for understanding how assembly of single-stranded RNA viruses is achieved at the molecular level, HDX-MS has been used to locate regions of a coat protein dimer that exhibit conformational/dynamical changes, and hence changes in their HDX kinetics, upon binding to a genomic RNA stem-loop known to trigger assembly initiation. The HDX-MS data highlight specific areas within the coat protein dimer that alter their exchange kinetics in the presence of the RNA. These include the known RNA-binding sites, β-strands E and G, which have a lower susceptibility to HDX when ligand-bound, as may have been expected. In contrast, several exposed regions are unaffected by ligand binding. Significantly in this example, the loop between β-strands F and G exhibits reduced HDX propensity when the RNA is bound, consistent with previous inferences from NMR and normal mode analysis that suggested a local conformational change at this loop induced by dynamic allostery. These results demonstrate the potential utility of HDX to probe conformational and dynamical changes within non-covalently bound protein-ligand complexes which are of widespread importance in many biomolecular systems.
Introduction
A major problem in structural virology is how spherical viruses regulate the conformations of their coat protein subunits (CPs) during assembly of capsid shells based on icosahedral surface lattices.1 Such lattices tesselate the surface of a sphere and their allowed configurations have been predicted using quasi-equivalence theory.2 This permits identical CPs to adopt different symmetry-related conformations, defined by the triangulation number (T), at appropriate positions in the icosahedral shell, allowing larger capsids to be built from multiple copies of smaller CPs, and thus increasing the genetic economy within the viral genome.3 How this conformational switching is regulated at the molecular level has until recently been a mystery for all viruses. We have made major contributions to this understanding using a model single-stranded (ss) RNA virus, bacteriophage MS2.4-6
ssRNA viruses such as MS2 represent one of the largest groups of all known viruses and include major human, animal and plant pathogens.7 The MS2 phage capsid consists of a T = 3 icosahedral protein lattice comprising 180 copies of the viral coat protein (CP) in the form of 90 non-covalent dimers (CP2) (Fig. 1). The quasi-symmetry is accommodated by the CP adopting three distinct conformations, defined by the geometry of the polypeptide loop connecting the F and G β-strands in each monomer: in the B subunit the FG-loop is folded back towards the protein subunit, whilst it is fully extended but in slightly differing orientations in A and C conformers.8,9 The FG-loops define the type of symmetry axis formed in the icosahedral shell: five B-type loops surrounding the particle five-fold axis with three interdigitating A-type and C-type loops creating the three-fold axis. Recently, using a combination of mass spectrometry (MS), NMR and size-exclusion chromatography, we have shown that the RNA-free CP2 in solution is symmetrical (i.e., C/C-like) and that binding of a 19-nucleotide RNA stem-loop (denoted TR) from within the genomic sequence promotes a conformational switch to an asymmetric (A/B-like) dimer.6 Both C/C- and A/B-like dimers are required for eefficient assembly of T = 3 capsids and multiple switching events would be required to promote formation of a completed capsid. We have shown that non-TR stem-loops will also promote the conformational switching, consistent with cryo-EM structures of the wild-type phage.4,10
Fig. 1.
(a) The MS2 capsid consists of 180 coat protein (CP) monomers co-populating three quasi-equivalent conformers denoted A (blue), B (green), and C (pink) in the form of 90 non-covalent dimers arranged within an icosahedral surface lattice; (b) the two types of dimers (CP2): the asymmetric A/B and the symmetric C/C, which form the basic building blocks of the T = 3 capsid. The main site of variation between these conformers occurs between the F and G β-strands (FG-loop) (structures taken from pdb 2MS28); (c) conformational switching between the two types of dimer occurs upon binding of a 19-nucleotide RNA stem loop (TR).4,6,11 The sequence of the stem loop is shown and is also depicted as a yellow ribbon in complex with an A/B CP2 dimer (CP2: TR).
If RNA acts like an allosteric effector of CP2 conformation, the problem of regulating assembly becomes one of understanding how this is achieved at the molecular level. We have used whole atom normal mode analysis of CP2 with and without TR bound and shown that the effect could be due to dynamic allostery; addition of the RNA stem-loop differentially stiffens or loosens regions of the protein, consistent with the required conformational change from C/C to A/B.11 Our goal here was to obtain experimental support of this dynamic allostery model. To do this we have used hydrogen/deuterium exchange-mass spectrometry (HDX-MS) to probe the differences in the protein dynamics for the RNA-free CP2 and RNA-bound CP2, (CP2: TR), and thus determine the specific regions of the protein sequence which differ in the ligand-bound and ligand-free forms of the CP2. Amide HDX has been used to good effect to obtain information on both protein structure and dynamics.12 Typically, the unlabelled protein is diluted into an excess of D2O at neutral pH at a pre-determined temperature and the deuterium uptake monitored over time. HDX at unprotected backbone amides occurs rapidly, whilst the rate of exchange is reduced by up to 108-fold for sites that are hydrogen bonded and/or shielded from the solvent. The slow exchange of these protected hydrogen atoms occurs via unfolding/refolding events that cause transient disruption of hydrogen bonds and allow solvent access. MS is an ideal technique to use to monitor the deuterium uptake and, furthermore, HDX-MS enables the distinction between EX1 and EX2 modes of exchange, representing global and local unfolding events, respectively.12 In EX2 kinetics, peaks in the mass spectra gradually increase in mass over time during protein exposure to D2O, whereas in EX1 kinetics, multiple amide hydrogen atoms in a specific region can become deuterated simultaneously during a single unfolding event.13-15 Here we have used a continuous labelling HDX-MS method16,17 in which, after protein dilution into D2O and subsequent HDX, the pH and temperature of the reaction mixture was then lowered to minimize back-exchange of the amide backbone residues during the subsequent analysis.18 Proteolysis of the protein under conditions of slow exchange was then implemented using an immobilised pepsin column which was followed by a rapid separation of the protein fragments using reversed-phase HPLC en route to the mass spectrometer. Thus, the number of deuterium atoms incorporated into each peptide was determined. Side-chain deuterons undergo rapid back-exchange during the analysis but, importantly, labelling information is retained for the majority of the protein backbone.19
Results and discussion
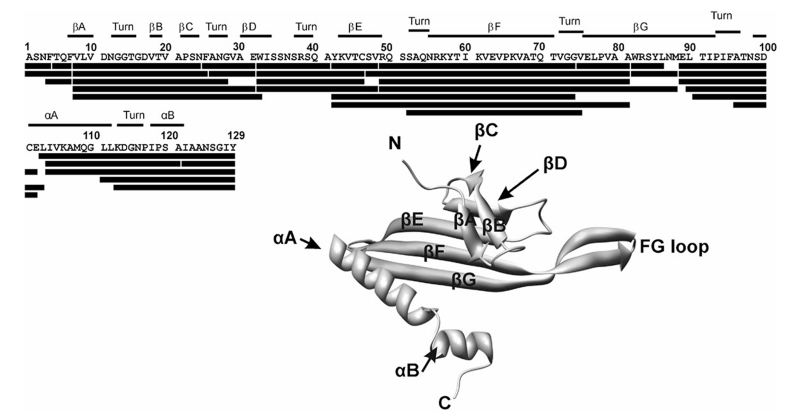
In order to compare the conformational differences between the two non-covalently bound complexes, i.e., RNA-free CP2 and RNA-bound CP2 (CP2: TR), HDX-MS was used to characterise the respective hydrogen exchange rates with peptide level resolution. A specifically developed, automated HDX-MS system described in detail elsewhere16,17 was employed for the experiments. Briefly, the system comprises a robotic autosampler capable of performing HDX solvent manipulations and a dual column HPLC system coupled to an FT-ICR mass spectrometer, and has been shown to produce sensitive, highly reproducible HDX measurements.17 After HDX, rapid proteolysis produced a mixture of peptic peptides which were identified by exact mass measurements (≤1 ppm accuracy). An initial experiment indicated that a pepsin digest of the CP2 carried out with this system (but without any HDX) provided complete sequence coverage, generating peptide fragments which covered the entire protein backbone in a partially over-lapping fashion (Fig. 2).
Fig. 2.
Peptide map of the CP2 peptides identified from the pepsin proteolysis experiments by ESI-MS exact mass measurements. The CP sequence and numbering is shown below a summary of the secondary structure. Black bars below the sequence show the peptides identified post-proteolysis. Inset: A ribbon cartoon of the C conformer of the coat protein (CP) monomer (taken from pdb 2MS28). The MS2 coat protein is folded as a five stranded antiparallel β-sheet (βC to βG) with two antiparallel β-strands (βA and βB) folding over at the N-terminus (N) and a kinked α-helix (αA, αB) at the C-terminus (C).
For both the CP2 and CP2: TR complexes, HDX-MS measurements were determined for duplicate experiments under identical conditions, with aliquots of each protein solution being removed and subjected to HDX for the stated length of time. HDX, followed by pepsin digestion, peptide separation and MS analysis allowed deuterium incorporation as a function of time for each peptic peptide to be determined and the results indicated a high level of reproducibility (see Table 1 and Table 2). The RNA-free CP2 is expected to be predominantly C/C-like with very little of the A/B conformer, making eefficient assembly dependent on RNA interaction.4,10,11,20 In contrast, the CP2: TR would be expected to be in the A/B conformation only following the proposed allosteric conformational switch in one of the FG-loops induced by RNA binding6 (Fig. 1). As the HDX-MS methodology cannot differentiate between the two protein monomers within a CP2, the data therefore represent an average of all conformers present within each species. Thus, even small changes in HDX behaviour may represent significant conformational or dynamical changes.
Table 1.
Peptides selected to cover the sequence of the coat protein (CP) showing the percentage exchange and the difference of exchange between two repeat measurements monitoring HDX-MS of CP2
| Peptide | Exchange measured |
Difference between repeat measurements |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time of exchange (seconds) | ||||||||||||||
| 30 | 100 | 300 | 1000 | 3000 | 10000 | 30000 | 30 | 100 | 300 | 1000 | 3000 | 10000 | 30000 | |
| [1–4] | 22.5% | 23.3% | 25.8% | 23.9% | 25.2% | 25.2% | 27.4% | 1.4% | 0.7% | 0.1% | 1.3% | 0.4% | 0.9% | 1.8% |
| [5–7] | 0.2% | 3.0% | 4.2% | 11.2% | 12.0% | 15.9% | 18.4% | 0.9% | 2.0% | 0.2% | 0.9% | 2.2% | 3.8% | 0.2% |
| [8–24] | 30.0% | 32.8% | 32.6% | 36.0% | 39.3% | 42.4% | 43.5% | 0.8% | 2.9% | 0.4% | 1.1% | 0.6% | 0.9% | 0.3% |
| [25–31] | 37.6% | 43.4% | 41.0% | 46.6% | 48.6% | 50.5% | 51.9% | 1.2% | 3.4% | 0.7% | 3.0% | 0.2% | 1.0% | 0.3% |
| [32–41] | 56.9% | 61.8% | 63.1% | 63.7% | 66.1% | 68.0% | 69.0% | 2.5% | 2.7% | 0.2% | 0.4% | 0.4% | 1.9% | 0.8% |
| [42–48] | 21.6% | 21.7% | 21.1% | 26.9% | 32.3% | 37.7% | 42.8% | 3.1% | 1.1% | 1.0% | 1.4% | 2.1% | 1.7% | 0.9% |
| [49–74] | 38.2% | 39.4% | 40.2% | 42.9% | 44.2% | 46.8% | 49.5% | 0.3% | 0.4% | 0.2% | 0.4% | 1.2% | 1.3% | 0.2% |
| [75–81] | 55.9% | 59.2% | 64.9% | 67.6% | 72.1% | 70.5% | 72.7% | 1.8% | 0.9% | 1.7% | 0.4% | 4.2% | 4.1% | 0.8% |
| [82–88] | 10.4% | 13.1% | 14.1% | 21.1% | 30.6% | 41.1% | 52.4% | 2.6% | 1.7% | 0.2% | 4.4% | 0.1% | 1.1% | 0.4% |
| [89–102] | 21.4% | 26.3% | 30.1% | 31.9% | 35.9% | 36.3% | 38.3% | 0.9% | 1.6% | 0.3% | 1.9% | 0.0% | 4.8% | 1.9% |
| [103–129] | 26.2% | 29.7% | 29.2% | 33.8% | 39.9% | 45.0% | 48.9% | 1.4% | 4.0% | 0.0% | 2.3% | 0.0% | 1.7% | 0.2% |
| [104–121] | 12.8% | 12.9% | 13.9% | 17.1% | 21.7% | 27.7% | 31.8% | 0.1% | 0.6% | 0.3% | 0.1% | 1.1% | 4.1% | 1.2% |
| [122–129] | 44.2% | 50.0% | 51.2% | 58.3% | 61.9% | 66.5% | 70.8% | 1.4% | 4.0% | 0.3% | 1.7% | 1.2% | 0.1% | 0.9% |
Table 2.
Peptides selected to cover the sequence of the coat protein (CP) showing the percentage exchange and the difference of exchange between two repeat measurements monitoring HDX-MS of CP2: TR
| Peptide | Exchange measured |
Difference between repeat measurements |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time of exchange (seconds) | ||||||||||||||
| 30 | 100 | 300 | 1000 | 3000 | 10000 | 30000 | 30 | 100 | 300 | 1000 | 3000 | 10000 | 30000 | |
| [1–4] | 25.9% | 23.6% | 22.8% | 22.4% | 23.0% | 23.8% | 23.4% | 4.5% | 3.2% | 0.4% | 1.3% | 1.0% | 0.5% | 0.1% |
| [5–7] | 1.7% | −1.3% | 1.8% | 15.4% | 22.4% | 20.4% | 21.5% | 8.7% | 0.1% | 0.7% | 0.5% | 1.8% | 0.9% | 0.9% |
| [8–24] | 29.2% | 29.5% | 30.6% | 31.8% | 36.0% | 40.4% | 45.1% | 1.1% | 1.1% | 1.2% | 0.3% | 1.1% | 1.3% | 0.7% |
| [25–31] | 34.0% | 35.2% | 36.1% | 37.9% | 45.1% | 52.3% | 57.6% | 7.4% | 0.2% | 0.1% | 0.4% | 2.8% | 0.4% | 0.2% |
| [32–41] | 58.1% | 62.1% | 65.3% | 66.0% | 67.5% | 66.2% | 67.9% | 2.5% | 1.0% | 1.0% | 0.3% | 2.3% | 3.8% | 0.8% |
| [42–48] | 17.7% | 18.5% | 19.1% | 17.5% | 17.6% | 19.7% | 21.0% | 4.8% | 1.7% | 2.5% | 0.5% | 0.8% | 2.5% | 1.4% |
| [49–74] | 38.4% | 39.0% | 40.5% | 41.5% | 42.8% | 43.8% | 43.9% | 0.4% | 0.0% | 0.1% | 0.6% | 1.0% | 1.6% | 1.1% |
| [75–81] | 61.7% | 67.8% | 67.2% | 66.2% | 66.9% | 66.6% | 65.5% | 11.9% | 1.9% | 0.8% | 1.6% | 2.7% | 1.2% | 2.1% |
| [82–88] | 5.8% | 5.0% | 5.5% | 7.0% | 11.8% | 20.7% | 27.2% | 4.4% | 1.6% | 0.7% | 1.0% | 0.7% | 1.9% | 0.2% |
| [89–102] | 20.9% | 27.1% | 28.1% | 29.6% | 29.2% | 31.7% | 32.3% | 2.9% | 1.1% | 1.0% | 0.7% | 2.9% | 0.3% | 1.1% |
| [103–129] | 23.9% | 24.4% | 25.9% | 27.4% | 31.0% | 35.2% | 39.0% | 3.2% | 0.3% | 0.9% | 0.2% | 0.9% | 1.9% | 0.3% |
| [104–121] | 11.9% | 13.0% | 12.8% | 12.9% | 14.8% | 18.7% | 23.8% | 1.1% | 0.2% | 0.4% | 0.6% | 0.8% | 0.5% | 0.6% |
| [122–129] | 46.1% | 53.1% | 60.4% | 64.2% | 68.6% | 69.4% | 72.4% | 3.8% | 4.4% | 1.0% | 2.4% | 0.0% | 2.1% | 1.7% |
The deuterium uptake curves for example peptides are shown in Fig. 3 and 4. Due to an unavoidable but small degree of back-exchange that occurs during proteolysis (involving the free N-terminal and its adjacent residue of each peptide19) and subsequent chromatographic separation, 100% deuterium incorporation is never detected. Regions that are highly dynamic and solvent exposed undergo a high degree of HDX rapidly; this can be seen for some of the loops connecting elements of secondary structure. As an example peptide 26–31, which comprises the loop between β-strands C and D, shows a high level of deuterium incorporation for both the CP2 and CP2: TR (~64–67%). For such a six-residue peptide, we would expect to detect the incorporation of up to four deuteriums due to back exchange at the proteolysis step19 i.e., a maximum of ~67% HDX, implying that this loop exchanges completely within the time of the experiment, consistent with it being solvent-exposed (Fig. 3a). Interestingly, although this loop does not change its conformation on binding TR and is distal from any RNA atoms, it exchanges significantly more rapidly in the absence of RNA-binding at early time-points. This result is consistent with the conclusion of normal mode analysis which implied that ligand binding results in widespread dynamical changes within the protein subunits of the dimer.11 A clear difference in the extent of deuterium incorporation in the presence and absence of RNA is also evident in β-strand E (peptide residues 42–48) with exchange increasing steadily over time to ≤45% (out of a theoretical maximum for this method of ~70%) in CP2, whilst in CP2: TR it has a much lower exchange level (only ~20%) which does not increase significantly over time (Fig. 3b). Residues 82–86 from β-strand G (Fig. 3d), also show a difference in exchange between CP2 and CP2: TR, with significantly more (~15%) deuterium incorporation observed for the unliganded protein. These two regions of the CP are part of the RNA binding site and make polar contacts with TR specifically at residues Lys43, Thr45 and Ser4721,22 and a stacking contact with the loop pyrimidine at position-5 (Fig. 1) on residue Tyr85.21,23 The higher degree of amide backbone protection observed by HDX-MS in CP2: TR is consistent with these contacts.
Fig. 3.
Time-course of HDX-MS showing the percentage of deuterium incorporation as a function of exchange time for specific peptides in CP2 (open circles) and CP2: TR (filled circles). (a) residues 26–31 of the loop region linking β-strands C and D; (b) residues 42–48 from β-strands E; (c) residues 75–81 of the loop linking β-strands F and G, and (d) residues 82–86 from β-strands G.
Fig. 4.
HDX-MS of the (M+3H)3+ ions (m/z 881.5) of the peptide fragment (residues 104–129) covering the α-helices A and B together with their connecting turn and the C-terminus, showing the differences in the isotope patterns over time. The isotope patterns are indicative of EX1 kinetics for the CP2 (upper spectrum) and EX2 kinetics for the CP2: TR (lower spectrum).
The conformation of the FG-loops defines the quasi-conformers of coat protein subunits in MS2 and is the basis for our current molecular model of the assembly pathway. The dynamic nature of the CP FG-loops has been probed previously by NMR using measurements of 1H–15N T1/T2 relaxation rates and heteronuclear nOe measurements.6,24 Observed changes in the chemical shifts confirm that upon RNA binding, conformational changes in the FG-loops occur creating asymmetry in the protein dimer i.e., this complex becomes A/B-like. No RNA atoms contact the residues in the FG-loop so this effect appears indirect. All atom normal mode analysis suggests that dynamical modes within the coat protein dimer are differentially altered by binding RNA stem-loops, leading to a proposal that the conformational change is the result of dynamic allostery. Consistent with this view, the peptide 75–81 from the FG-loop region exhibits a high degree of deuterium incorporation (~65% out of a theoretical maximum for this method of ~72%) for both ligand-bound and free CP2 at early time points (<30 s) (Fig. 3c). After the initial HDX uptake, consistently more exchange (~7%) has taken place within this region in the CP2 compared with the CP2: TR complex. In the A and C monomeric conformations of the A/B and C/C CP2 the FG-loops are flexible and exposed to exchange, whereas in the CP2: TR complex with solely the asymmetrical A/B arrangement of CP2, the FG-loop of the B conformer is less flexible and folded back towards the main body of the protein. The HDX-MS experiments detect an average exchange over both monomers in each of the CP2 and CP2: TR complexes, hence the recorded 7% difference is noteworthy and consistent with a lower degree of exposure to HDX on average within the FG-loops of the CP2: TR.
The peptide fragment 104–129, encompasses the C-terminal region of the protein, including the A and B α-helices together with their connecting turn. It is located on the face of the protein opposite to the one that binds RNA. It also displays noticeably different kinetic behaviour for CP2 and CP2: TR, with clear differences in the widths of the respective isotopic distributions (Fig. 4). From the width of the isotope pattern of the (M+3H)3+ ions, covering the range m/z 881–889, the CP2 can be seen to display EX1 kinetics. This implies that a portion of this peptide undergoes a co-operative unfolding event simultaneously exposing a specific number of residues to solvent. When the rate of refolding of this region of the protein is slower than the rate of amide HDX, exchange of all the exposed residues occurs before the protein refolds and results in a characteristic bimodal isotope pattern. In this case ~6 hydrogen atoms undergo labelling in such a manner, accounting for the appearance of a second isotopic distribution peaking at m/z 886.5 (~8 h time-point) co-existing with the original isotopic distribution peaking at m/z 884.5. The more common EX2 kinetics, however, are observed for this peptide in CP2: TR, implying that in this complex the peptide simply undergoes a series of minor, local unfolding events. Since the probability of exchange during a single event is small, a binomial isotope pattern is observed. This is shown by the isotope pattern from m/z 881–886 with a single maximum at m/z 884.5 (~8 h time-point; Fig. 4). These findings indicate that RNA binding to the CP2 stabilises the coat protein throughout its tertiary structure, not just in regions in direct contact with the RNA or adjacent to secondary structure elements making up the RNA binding site.
The HDX data for the CP2 and CP2: TR are summarized in Fig. 5, in which the relative percentage of deuterium incorporation in a series of peptides selected to provide full sequence coverage throughout the CP is mapped onto the crystal structure of a monomer (shown arbitrarily as the C conformer). The colour gradient of the CP2 and CP2: TR time-courses represents the extent of exchange, with blue indicating the lowest, and red the highest, amount of exchange. Measuring the deuterium content of the pepsin fragments over the exchange time for both ligand-free and ligand-bound complexes allowed identification of the specific regions within the protein that exhibit different exchange kinetics (Fig. 5 and Tables 1, 2 and 3). The differences between the complexes are also shown (Fig. 5 and Table 3). The central segment of β-strand G (residues 82–86) and also β-strand E (residues 42–48) were both found to be significantly more accessible to HDX in the CP2 compared with the CP2: TR. Similarly, the FG-loop (residues 75–81) and the C-terminal α-helices A and B (residues 104–121), were also found to exchange faster. Conversely, the CD-loop situated between β-strands C and D underwent HDX at similar rates in both CP2 and CP2: TR, whilst the C-terminal region (residues 122-129) is slightly more accessible to HDX in the CP2: TR. For many peptides, exchange is only ~50% complete after the longest time point in these experiments (~8 h), indicating considerable protection in these regions from HDX due to H-bonding within the secondary structure of the subunits.
Fig. 5.
(left and centre columns) A comparison of deuterium incorporation by the non-covalently bound CP2 and CP2: TR complexes over time (30 s to 30 000 s). A series of peptide fragments covering the entire coat protein (namely residues: 1–4, 5–7, 8–25, 26–31, 32–41, 42–48, 49–74, 75–81, 82–88, 89–102, 103–121, and 122–129) were used to map the HDX-MS data onto the crystal structure of the monomer (taken from pdb 2MS28). The relative percentage of deuterium incorporation for all residues is shown at the times indicated. Colour coding is shown at the top of the figure, black representing 0% exchange to red representing 75% exchange; (right column) maps highlighting the differences in deuterium uptake between RNA-free and RNA-bound CP2, where red indicates less exchange in CP2, and blue less exchange in CP2: TR.
Table 3.
Differences in percentage exchange of CP2 vs. CP2: TR for peptides selected to cover the coat protein (CP) sequence. Positive values correspond to more exchange in CP2 compared with CP2: TR; negative values correspond to more exchange in CP2: TR compared with CP2
| Peptide | Time of exchange (seconds) |
||||||
|---|---|---|---|---|---|---|---|
| 30 | 100 | 300 | 1000 | 3000 | 10000 | 30000 | |
| [1–4] | −3.4% | −0.3% | 2.9% | 1.5% | 2.3% | 1.4% | 4.0% |
| [5–7] | −1.5% | 4.4% | 2.4% | −4.3% | −10.4% | −4.5% | −3.0% |
| [8–24] | 0.8% | 3.3% | 2.0% | 4.1% | 3.2% | 1.9% | −1.7% |
| [25–31] | 3.6% | 8.2% | 4.9% | 8.7% | 3.5% | −1.8% | −5.7% |
| [32–41] | −1.3% | −0.4% | −2.2% | −2.2% | −1.4% | 1.8% | 1.1% |
| [42–48] | 3.9% | 3.2% | 2.1% | 9.4% | 14.7% | 18.0% | 21.8% |
| [49–74] | −0.3% | 0.4% | −0.3% | 1.4% | 1.5% | 3.0% | 5.6% |
| [75–81] | −5.8% | −8.6% | −2.3% | 1.4% | 5.2% | 3.9% | 7.2% |
| [82–88] | 4.6% | 8.2% | 8.6% | 14.1% | 18.9% | 20.4% | 25.1% |
| [89–102] | 0.5% | −0.8% | 2.0% | 2.4% | 6.7% | 4.6% | 6.0% |
| [103–129] | 2.4% | 5.3% | 3.3% | 6.3% | 9.0% | 9.8% | 9.9% |
| [104–121] | 0.8% | −0.1% | 1.0% | 4.2% | 6.8% | 8.9% | 8.0% |
| [122–129] | −1.8% | −3.1% | −9.2% | −5.9% | −6.7% | −2.8% | −1.6% |
Conclusion
HDX-MS has been used to compare subtle conformational/dynamical differences between RNA-free and RNA-bound forms of a viral coat protein subunit. In general, the rate of HDX was higher in the RNA-free CP2 state, consistent with the expected decrease in dynamics and increase in stability on protein–ligand binding. There is excellent correlation between the HDX-MS data and the known structural data for CP2: TR. Specifically, the previously determined RNA binding sites in β-strands E and G21,23,25 show distinct differences between the CP2 and CP2: TR, as anticipated, with the CP2 showing a significantly higher degree of deuterium incorporation than CP2: TR. Additionally, the data show that for certain regions of the coat protein, e.g., the loop in between β-strands C and D (peptide residues 26–31), the degree of deuterium incorporation is similarly high for both the CP2 and CP2: TR, indicating little difference in the dynamics and solvent accessibility of this region in both complexes.
Importantly, HDX-MS has allowed us to gain additional insights into the mechanism of the allosteric conformational changes that occur upon RNA binding to the CP2 that are an essential feature of the viral assembly process and cannot be determined by other analytical techniques. At early time-points during HDX, the FG-loop can be seen to undergo a high degree of exchange in both the CP2 and the CP2: TR, although the maximum amount of deuterium incorporation is consistently ~7% lower for the CP2: TR complex. This latter observation provides strong evidence that RNA binding does indeed cause an allosteric switch in the FG-loop to take place. Also noteworthy is the behaviour of the α-helices A and B towards the C-terminal of the protein. In the case of the CP2, EX1 HDX behaviour is observed suggesting a significant unfolding event can occur which is not possible in the case of CP2: TR, indicating that RNA binding leads to stabilisation of the kinked α-helix thereby preventing the global unfolding event to occur, despite the RNA binding site being distant to this region.
Together these data illustrate that subtle differences in the conformation or dynamics of biomolecular complexes, brought about by allosteric effects that occur on protein-ligand binding, can be located by HDX-MS. Such effects are believed to be widespread in nature and to underpin many important biological features. This technology is not limited by the size of the complexes being studied and therefore has the potential to detect and map such behaviour in species beyond the range of techniques such as NMR. HDX-MS uses very small amounts of material allowing structural information to be obtained without large scale protein purification.
Experimental
Sample preparation
Wild-type recombinant MS2 coat protein in the form of T = 3 capsid shells was prepared by over-expression in E. coli as previously described.26 These capsids were purified and then treated with glacial acetic acid followed by exchange into 20 mM acetic acid to generate disassembled coat protein dimers (CP2). To form the complex [CP2: RNA], the CP2 was mixed with an equimolar concentration of the 19-nucleotide RNA (TR) (5′-ACA UGA GGA UUA CCC AUG U-3′).20
Automated hydrogen/deuterium exchange analysis
The system used to perform the HDX-MS analysis has been described previously in detail.16,17 Briefly, a HTS-PAL robotic autosampler (CTC Analytics AG, Zwingen, Switzerland) was used to perform solvent manipulations, while columns and switching valves were maintained at 0 °C in a cooled water bath. Samples were exchanged in D2O for 30 s, 100 s, 300 s, 1000 s, 3000 s, 10 000 s and 30 000 s by mixing the protein complex (10 μL; 37 μM) with D2O (190 μL)). To quench the exchange, 45 μL of the deuterated solution was mixed with 45 μL of 5 M guanidine hydrochloride containing HCl such that the pH of the quenched solution was 2.5. 40 μL of this solution was then analysed as described previously.17 Briefly, a dual column approach was used for increased sample throughput. This employed two Atlantis dC18 analytical columns of dimensions 2.1 × 50 mm with particle sizes of 5 μm (Waters Corpn., Milford, CT, USA) for the separation and two Atlantis dC18 columns of dimensions 2.1 × 15 mm (Waters Corpn., Milford, CT, USA) which were used as trap columns. For the enzymatic digestion, an in-house packed column of dimensions 2.1 × 50 mm containing immobilized pepsin (Pierce Biotechnology Inc., Rockford, IL, USA) was used. Two Smartline 1000 solvent delivery pumps (Knauer, Berlin, Germany) were used to supply the solvent flow rate and gradient compositions required for the HPLC. One pump was used to equilibrate the columns and to wash the digested peptides from the immobilised pepsin column, while the second pump ran a water/acetonitrile gradient to elute the peptides from the reverse phase column to the mass spectrometer. The solvents used for the chromatography contained 0.3% aqueous formic acid to reduce the back exchange of the peptides during analysis. The gradient used to elute the peptides from the analytical column was from 0 to 60% aqueous acetonitrile at 200 μL min−1 over 10 min. Each time point was measured twice for both CP2 and CP2: TR, with the analyses being undertaken in a semi-random order.
Mass spectrometry
Mass spectrometry measurements for the HDX-MS experiments were performed on a 4.7 T Apex III Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometer (Bruker Daltonics, Billerica, MA, USA) using positive mode electrospray ionisation (ESI) with a mass accuracy of ≤1 ppm.16 Data acquisitions were initiated by contact closure, one spectrum was collected approximately once every three seconds over a ten-minute period, during which time the peptides were eluted into the ion source of the mass spectrometer. Peptide identification was achieved from the exact peptide masses obtained from internally calibrated mass spectra.
Calculation of exchange data
The number of deuterium atoms incorporated into each peptide was calculated from the difference in the mass measured for the peptide before and after exchange. The percentage deuterium exchange was calculated from the measured number of deuteriums incorporated into the peptide and the maximum number of amide hydrogens for which the exchange was expected. The maximum number of measureable exchangeable hydrogens was calculated taking N, where N is the number of amino acid residues, and subtracting P, the number of proline residues excluding those present in the first two N-terminal residues. This calculation assumes that the amide on the second residue exchanges rapidly and is not observed in the measurements.19
Acknowledgements
VLM was supported by a grant from the Biotechnology and Biological Sciences Research Council [BB/E008070/1]. We are also grateful for financial support of this work from the Biotechnology and Biological Sciences Research Council, The Wellcome Trust, The Leverhulme Trust, and the University of Leeds. We would like to thank members of the Ashcroft, Stockley and Stonehouse groups for helpful discussions.
References
- 1.Harrison SC, Olson AJ, Schutt CE, Winkler FK, Bricogne G. Nature. 1978;276:368–373. doi: 10.1038/276368a0. [DOI] [PubMed] [Google Scholar]
- 2.Caspar DL, Klug A. Cold Spring Harb. Symp. Quant. Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- 3.Crick FH, Watson JD. Nature. 1956;177:473–475. doi: 10.1038/177473a0. [DOI] [PubMed] [Google Scholar]
- 4.Basnak G, Morton VL, Rolfsson O, Stonehouse NJ, Ashcroft AE, Stockley PG. J. Mol. Biol. 2010;395:924–936. doi: 10.1016/j.jmb.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stockley PG, Ashcroft AE, Francese S, Thompson GS, Ranson NA, Smith AM, Homans SW, Stonehouse NJ. J. Theor. Med. 2005;6:119–125. [Google Scholar]
- 6.Stockley PG, Rolfsson O, Thompson GS, Basnak G, Francese S, Stonehouse NJ, Homans SW, Ashcroft AE. J. Mol. Biol. 2007;369:541–552. doi: 10.1016/j.jmb.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneemann A. Annu. Rev. Microbiol. 2006;60:51–67. doi: 10.1146/annurev.micro.60.080805.142304. [DOI] [PubMed] [Google Scholar]
- 8.Golmohammadi R, Valegard K, Fridborg K, Liljas L. J. Mol. Biol. 1993;234:620–639. doi: 10.1006/jmbi.1993.1616. [DOI] [PubMed] [Google Scholar]
- 9.Valegard K, Liljas L, Fridborg K, Unge T. Nature. 1990;345:36–41. doi: 10.1038/345036a0. [DOI] [PubMed] [Google Scholar]
- 10.Toropova K, Basnak G, Twarock R, Stockley PG, Ranson NA. J. Mol. Biol. 2008;375:824–836. doi: 10.1016/j.jmb.2007.08.067. [DOI] [PubMed] [Google Scholar]
- 11.Dykeman EC, Stockley PG, Twarock R. J. Mol. Biol. 2010;395:916–923. doi: 10.1016/j.jmb.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Marcsisin SR, Engen JR. Anal. Bioanal. Chem. 2010;397:967–972. doi: 10.1007/s00216-010-3556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng Y, Zhang Z, Smith DL. J. Am. Soc. Mass Spectrom. 1999;10:675–684. doi: 10.1016/S1044-0305(99)00038-0. [DOI] [PubMed] [Google Scholar]
- 14.Miranker A, Robinson CV, Radford SE, Aplin RT, Dobson CM. Science. 1993;262:896–900. doi: 10.1126/science.8235611. [DOI] [PubMed] [Google Scholar]
- 15.Weis DD, Wales TE, Engen JR, Hotchko M, Ten Eyck LF. J. Am. Soc. Mass Spectrom. 2006;17:1498–1509. doi: 10.1016/j.jasms.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Burkitt W, Domann P, O’Connor G. Protein Sci. 2010;19:826–835. doi: 10.1002/pro.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burkitt W, O’Connor G. Rapid Commun. Mass Spectrom. 2008;22:3893–3901. doi: 10.1002/rcm.3794. [DOI] [PubMed] [Google Scholar]
- 18.Bai Y, Milne JS, Mayne L, Englander SW. Proteins: Struct., Funct., Genet. 1993;17:75–86. doi: 10.1002/prot.340170110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Smith DL. Anal. Biochem. 2003;314:46–53. doi: 10.1016/s0003-2697(02)00620-6. [DOI] [PubMed] [Google Scholar]
- 20.Morton VL, Dykeman EC, Stonehouse NJ, Ashcroft AE, Twarock R, Stockley PG. J. Mol. Biol. 2010;401:298–308. doi: 10.1016/j.jmb.2010.05.059. [DOI] [PubMed] [Google Scholar]
- 21.Valegard K, Murray JB, Stockley PG, Stonehouse NJ, Liljas L. Nature. 1994;371:623–626. doi: 10.1038/371623a0. [DOI] [PubMed] [Google Scholar]
- 22.Valegard K, Murray JB, Stonehouse NJ, van den Worm S, Stockley PG, Liljas L. J. Mol. Biol. 1997;270:724–738. doi: 10.1006/jmbi.1997.1144. [DOI] [PubMed] [Google Scholar]
- 23.Valegard K, Murray JB, Stonehouse NJ, van den Worm S, Stockley PG, Liljas L. J. Mol. Biol. 1997;270:724–738. doi: 10.1006/jmbi.1997.1144. [DOI] [PubMed] [Google Scholar]
- 24.Stockley PG, Ashcroft AE, Francese S, Thompson GS, Ransom NS, Smith AM, Homans SW, Stonehouse NJ. Comp. & Math. Meths. in Medicine. 2005;6:119–125. [Google Scholar]
- 25.Stockley PG, Stonehouse NJ, Murray JB, Goodman ST, Talbot SJ, Adams CJ, Liljas L, Valegard K. Nucleic Acids Res. 1995;23:2512–2518. doi: 10.1093/nar/23.13.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mastico RA, Talbot SJ, Stockley PG. J. Gen. Virol. 1993;74:541–548. doi: 10.1099/0022-1317-74-4-541. [DOI] [PubMed] [Google Scholar]