Abstract
Spleen tyrosine kinase (SYK) has been known to relay adaptive immune receptor signalling. However, recent reports indicate that SYK also mediates other, unexpectedly diverse biological functions including cellular adhesion, innate immune recognition, osteoclast maturation, platelet activation and vascular development. SYK is activated by C-type lectins and integrins, and activates novel targets including the CARD9/CARMA1–BCL10–MALT1 pathway and the NLRP3 inflammasome. Drosophila studies indicate evolutionary ancient origin of SYK-mediated signalling. Moreover, SYK has a crucial role in autoimmune diseases and haematological malignancies. This Review summarizes our current understanding of SYK functions and the translation of this knowledge for therapeutic purposes.
Introduction
Our understanding of signal transduction in the immune system was dramatically increased during the 1990s when it was shown that B cell receptors (BCRs), T cell receptors (TCRs) and Fc receptors (FcRs) (collectively termed classical immunoreceptors) signal by a conceptually similar mechanism. All these receptors associate with transmembrane proteins with cytoplasmic domains containing immunoreceptor tyrosine-based activation motifs (ITAMs)1, short peptide sequences with two tyrosine residues 6-12 amino acids apart. ITAMs are rapidly phosphorylated following receptor engagement, leading to the recruitment and activation of SYK or the related ζ-chain-associated protein kinase of 70 kDa (ZAP70). This signalling model soon entered immunology textbooks and became the central paradigm of immune cell signalling.
SYK is a 72 kDa non-receptor tyrosine kinase, which contains two SRC homology 2 (SH2)-domains and a kinase domain (BOX 1), and is most highly expressed by haematopoietic cells. Mammals also express a SYK homologue, ZAP70, which is mostly restricted to T- and NK-lineage cells. SYK-related kinases are also found in invertebrates. Further facts about SYK and SYK-related molecules can be found in BOX 1.
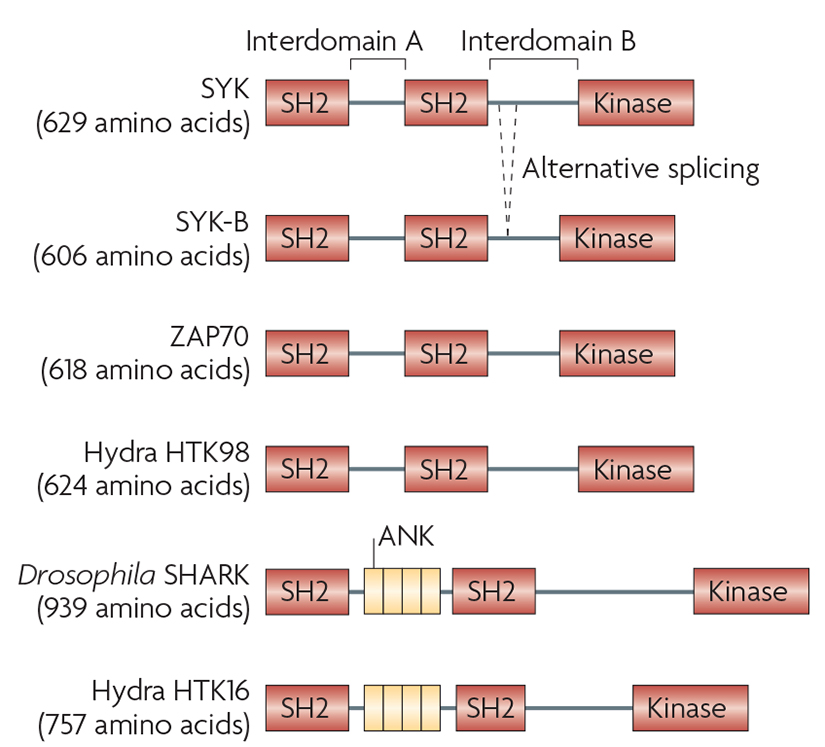
Box 1. Facts and figures on SYK and SYK-related molecules.
SYK contains two tandem SH2 domains and a C-terminal tyrosine kinase domain. These domains are linked by two linker regions: interdomain A between the two SH2 domains and interdomain B between the C-terminal SH2 domain and the kinase domain. An alternatively spliced form of SYK (known as SYK-B) lacks 23 amino acids of interdomain B, including a nuclear localization signal9,185.
SYK is highly expressed by all haematopoietic lineage cells. While it was initially thought to be a haematopoietic cell-specific kinase, later studies showed its expression in other tissues, as well145. Though the expression of SYK is tightly regulated (see REF. 30) the mechanism of this regulation, or that of the generation of the SYK-B isoform, is poorly understood.
Mammals also express the SYK-related molecule ZAP70, the expression of which is mostly confined to the T and NK cell lineages. Readers interested in further details of ZAP70 biology are referred to an excellent recent review36.
SYK-family kinases have an evolutionary ancient origin but they have appeared and disappeared during the evolution of animals186. Drosophila express a single SYK-related molecule, SHARK4, which contains ankyrin-like (ANK) repeats between its two SH2 domains. Interestingly, Hydrae and sponges express two SYK-related kinases, one (HTK98) similar to the vertebrate SYK, and the other (known as HTK16) more closely related to Drosophila SHARK186,187. By contrast, the Caenorhabditis elegans genome does not contain any SYK-related tyrosine kinases188.
The Syk gene has been disrupted in mice by two independent groups23,24. The major phenotypes displayed by SYK-deficient mice are perinatal lethality, a petechiated in utero appearance and the lack of mature B cells. Two groups have also reported mutations allowing the Cre-mediated conditional deletion of Syk189,190. To the best of our knowledge, no human patients or animal strains with a spontaneous germline mutation in SYK have been described.
The SYK signalling pathway was initially thought to be restricted to classical immunoreceptors of the adaptive immune response. However, later studies showing that glycoprotein VI (GpVI), a collagen-receptor expressed by platelets, also signals by a similar mechanism2, and that thepetechiated appearance of SYK-deficient embryos was due to a defect in lymphatic vascular development3 provided evidence for the role of SYK outside the adaptive immune response. The discovery of ITAM-based signalling in Drosophila4 indicated the role of such pathways beyond adaptive immunity (insects do not have an adaptive immune system). Additional studies have revealed that SYK is required for several functions of innate immune cells and for certain non-immune functions such as bone resorption by osteoclasts5. SYK has also been shown to mediate signaling by novel classes of receptors, including integrins6 and C-type lectins7, that do not contain conventional ITAM sequences. Collectively, these studies have dramatically changed our view of the SYK tyrosine kinase. This Review summarizes our current understanding of the diverse roles of SYK, in both immune and non-immune biological responses, and describes how this knowledge is being translated for therapy of human diseases.
ITAM-based signaling by classical immunoreceptors and C-type lectins
Classical immunoreceptors include BCRs, TCRs and various FcRs which mediate, either directly or indirectly, the adaptive recognition of self and foreign antigens. Immunoreceptor-associated transmembrane adaptors or, in the case of FcγRIIA, the ligand binding receptor chain itself contain one or more ITAMs (FIG. 1A), and receptor ligation leads to the phosphorylation of these ITAMs, primarily by members of the SRC tyrosine kinase family. Through interaction with their tandem SH2 domains, dual-phosphorylated ITAMs recruit SYK, in the case of the BCR or FcR, or ZAP70, in the case of the TCR, triggering kinase activation and downstream signalling. It should be mentioned that ZAP70 is significantly more dependent on SRC-family kinases than SYK, likely because SYK can phosphorylate the ITAM tyrosines itself8,9.
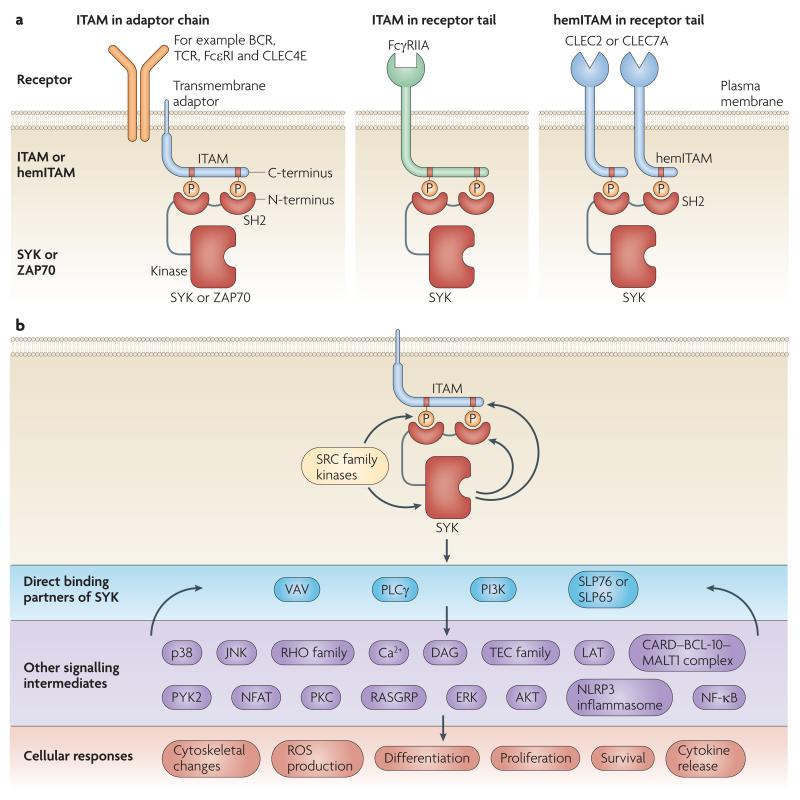
Figure 1. General mechanism of SYK-mediated signalling.
a ∣ Recruitment of SYK or ZAP-70 to plasma membrane receptors through binding of the tandem SH2 domains of SYK/ZAP-70 to two phosphotyrosine residues in the receptor complex. The two phosphorylated tyrosines are either within a single ITAM motif or in two hemITAMs on two separate receptor peptide chains. The ITAM motifs are either present in receptor-associated transmembrane adaptors or in the cytoplasmic tail of the receptor chain itself. b ∣ General scheme of signal transduction through SYK. The signal transduction is mostly initiated by phosphorylation of ITAM tyrosines by SRC-family kinases. Recruitment of SYK to dually phosphorylated ITAMs triggers activation of SYK and its direct binding to members of the VAV and PLCγ families, the p85α subunit of PI3-kinases (PI3K), as well as the SLP76/SLP65 adaptors. These direct binding partners activate downstream signaling components which eventually trigger various cellular responses. The SYK-mediated signaling pathways are also regulated by several feedback-mechanisms such as the phosphorylation of ITAM tyrosines by SYK or the regulation of direct SYK binding partners by further downstream molecules. SYK activation by hemITAM-containing receptors likely proceeds through similar mechanisms.
Signalling downstream of SYK and ZAP70
Of the several intermediates implicated in relaying SYK- or ZAP70-mediated downstream signalling, VAV family members, phospholipase Cγ (PLCγ) isoforms, the regulatory subunits of phosphoinositide 3-kinases (PI3Ks) and the SH2 domain-containing leukocyte protein (SLP) family members SLP76 and SLP65 are directly associated with SYK (FIG. 1B) and/or ZAP70. These molecules probably participate in forming receptor-proximal signalling complexes and trigger downstream processes including Ca2+ and protein kinase C (PKC) signalling, RAS homologue (RHO)-family and Pyk2-mediated cytoskeletal rearrangement, reactive oxygen species (ROS) production and phagocytosis, PI3K-mediated TEC-family and AKT signaling pathways, as well as transcriptional regulation through the RAS–extracellular signal-regulated kinase (ERK) and Ca2+-NFAT pathways (FIG. 1B). While the transmembrane adaptor linker for activation of T cells (LAT) is an important component of TCR and FcεRI signalling, it is at present unclear whether any LAT-related molecules play similar roles in other lineages10.
Ligation of classical immunoreceptors also triggers pro-inflammatory transcriptional programmes, through activation of the nuclear factor-κB (NFκB) transcription factor. Recent studies have revealed that ITAM-based signalling events lead to caspase-recruitment domain (CARD)-mediated heterotypic aggregation of CARD9 or CARMA1 (also known as CARD11) with the adaptor protein B cell lymphoma 10 (BCL10) and the associated paracaspase MALT111-16, triggering activation of the NF-κB pathway. Whereas CARMA1 relays antigen receptor-mediated activation of the BCL10–MALT1 complex in B cells, T cells, NK cells and mast cells, CARD9 is responsible for BCL10–MALT1 activation in macrophages and dendritic cells (DCs)11-17. The role of this pathway in fungal recognition will be discussed below whereas further details of CARMA1/CARD9 signaling can be found in an excellent recent review18.
Negative regulation of SYK function
The action of SYK is often counteracted by phosphatases such as PTPN6 (SHP-1), and it is the balance of SYK and PTPN6 activities that determines the ultimate signalling output19. The expression of SYK is also negatively regulated by the E3 ubiquitin ligase Casitas B-lineage lymphoma (CBL), leading to ubiquitination and degradation of SYK20-22.
Role of SYK and ZAP70 in leukocyte function
As B cell and T cell development requires signal transduction by properly rearranged antigen receptors, SYK deficiency leads to complete absence of mature B cells23,24 and ZAP70-deficient humans and mice have severe defects in T cell development25-27. More detailed studies revealed that SYK plays a major though not indispensable role in the transition from the pro-B to pre-B cell stage, whereas it is completely required to pass a novel Rac-dependent checkpoint controlling the entry of immature B-cells into the splenic white pulp (termed transitional type 0 or T0 stage)23,28. Initially, signalling by the BCR and TCR was thought to be specifically mediated by SYK and ZAP70, respectively. Unexpectedly, ZAP70 was recently found to be expressed throughout B cell development and to participate in pre-BCR signalling, and therefore also in allelic exclusion and transition from the pro-B to the pre-B cell stage29. On the other hand, SYK was shown to have a considerable role inpre-TCR signalling, which occurs during the transition from the double-negative 3 (DN3) to the DN4 stage of early thymocyte development30. Hence, SYK and ZAP70 have overlapping functions in early lymphocyte development.
SYK is also required for FcεRI signalling in mast cells31 and FcγR signalling in neutrophils and macrophages32,33, whereas FcγR-mediated antibody-dependent cell-mediated cytotoxicity (ADCC) reactions are defective in natural killer (NK) cells that lack both SYK and ZAP7034. The reader is referred to excellent recent and past reviews on further aspects of those signal transduction processes9,35-38.
ITAM-mediated tonic signalling
ITAM-mediated signalling was initially thought to only occur following ligand-induced receptor crosslinking. However, recent studies revealed that the disruption of BCR signalling by inactivation of the ITAM-bearing Igα chain leads to a rapid loss of resting mature B cells39. These studies indicated that the maintenance of mature B cells requires ITAM-dependent constitutive (“tonic”) signalling, apparently in the absence of a BCR ligand. It has yet to be tested whether this tonic signaling requires SYK, though the effect of SYK inhibitors on BCR-expressing B-cell lymphomas suggest that this is the case (see below).
Recent studies have also revealed antigen-independent signal transduction by FcεRI on mast cells40,41. Since these responses require receptor aggregation and SYK, they appear to conform to conventional ITAM–SYK-mediated signaling.
Signaling by hemITAM-containing C-type lectins
It has long been thought that the two phosphorylated tyrosine residues have to reside within a single peptide chain (as in a classical ITAM) to be able to recruit and activate SYK. However, a novel family of C-type lectins, including the fungal recognition receptor CLEC7 (Dectin-1) or the platelet receptor CLEC2 are able to activate SYK even though these receptors only have a single Tyr-X-X-Leu motif (termed “hemITAM”) in their cytoplasmic tail42,43. Further studies have indicated the role of the tyrosine residue in the Tyr-X-X-Leu motif, as well as both SYK SH2 domains in signal transduction43,44, suggesting that hemITAM-containing C-type lectins activate SYK by dimerisation followed by engagement of the two SH2-domains of SYK by phoshorylated hemITAMs on two separate receptor chains (FIG. 1A). Evidence for this stoichiometry has recently been provided for the CLEC2-SYK interaction45. The functional role of SYK in signaling by C-type lectins will be discussed below; other aspects of these receptors can be found in excellent recent reviews7,46.
Structural basis of SYK function
Three different SYK activity states
Immunoreceptor signalling through SYK requires the kinase activity of SYK, as well as both SH2 domains. The kinase domain of SYK is inactive in the resting state of the protein but can be activated by binding of both SH2 domains to dually phosphorylated ITAMs (FIG 2A). Phosphorylation of tyrosine residues within the linker regions (interdomain A or B) also results in kinase activation even in the absence of phospholytaled ITAM binding (see below). This observation led to the proposal that SYK functions as an “OR” logic gate switch47 (FIG. 2A). The physiological relevance of this activation will be discussed below.
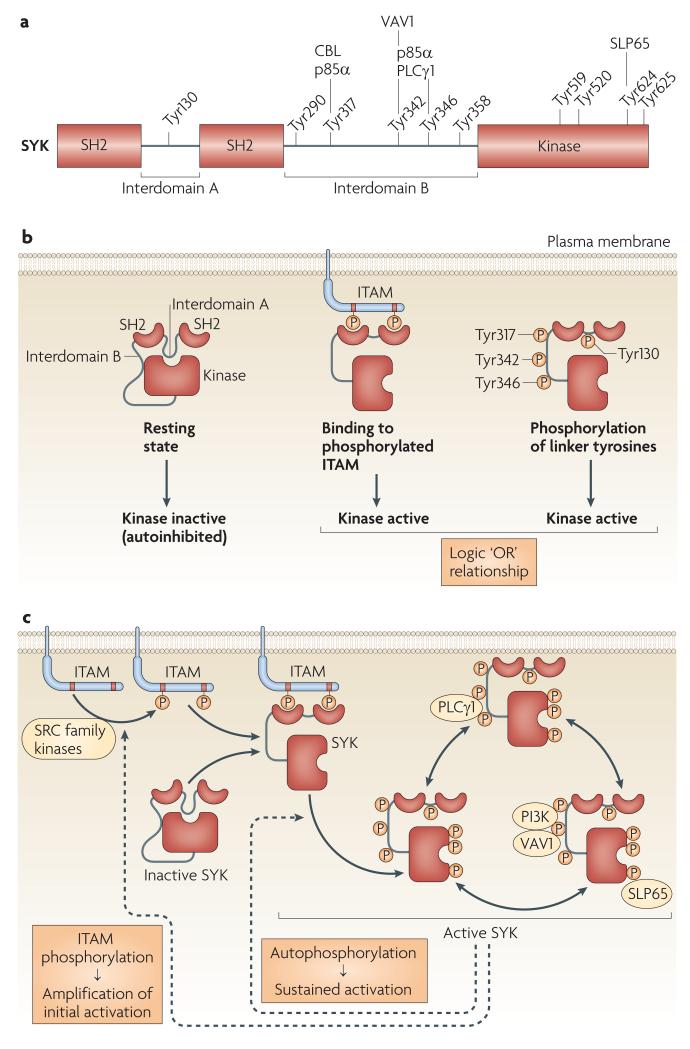
Figure 2. Structural basis of SYK activation.
a ∣ Three different states of SYK activation. SYK is autoinhibited in its resting state, due to the binding of interdomain A (“A”) and interdomain B (“B”) to the kinase domain, in particular, the C-terminal end. This autoinhibited conformation can be suspended by binding of the two SH2 domains to dually phosphorylated ITAMs or by phosphorylation of linker tyrosines in interdomain A or B. There is a logic “OR” relationship between those two activation mechanisms. b ∣ The domain structure of SYK with tyrosine residues shown to be sites of autophosphorylation. Proteins shown to bind to phosphorylated tyrosines are indicated above the tyrosine residues. p85α, the 85 kDa PI3K regulatory subunit. c ∣ Combined mechanism of SYK activation. Prior to activation, SYK is held in an autoinhibited conformation by interactions between interdomains A and B and the kinase domain. Initial phosphorylation of ITAM tyrosines by SRC-family kinases provides docking sites for the SYK SH2 domains inducing conformational changes and kinase activation. SYK activation results in autophoshorylation on multiple residues, leading to release from the ITAM and sustained SYK activation. SYK then recruits direct binding partners to its phosphorylated tyrosine residues, triggering downstream signaling. Phosphorylation of ITAM tyrosine by SYK provides a positive feedback loop. Amino acid numbers correspond to the sequence of mouse SYK. Phosphorylation of the linker tyrosines are highlighted in orange colour.
Autoinhibition of the kinase domain
Although no high-resolution 3D structure of the entire SYK protein is available, the structure for the related ZAP70 has shown that the kinase adopts an autoinhibited conformation in the absence of phosphorylated ITAM binding48,49. In this structure, binding between interdomain A, interdomain B and parts of the kinase domain hold the catalytic centre in an inactive conformation. Engagement of the SH2 domains by a phosphorylated ITAM disassembles this tripartite binding, allowing the kinase domain to adopt an active conformation.
Several studies indicate that, analogous to ZAP70, SYK is also regulated by similar autoinhibition (FIG. 2A). A low-resolution 3D structure of SYK shows a compact structure, similar to the autoinhibited ZAP7050. Deletion of both SH2 domains, or of interdomain A alone51,52, as well as binding of a phosphorylated ITAM to the SH2 domains52 results in SYK activation, consistent with being relieved from an autoinhibited conformation.
Interaction of SYK with phosphorylated ITAMs
Binding of the SH2 domains of SYK to phosphorylated ITAMs is a critical step in SYK activation and downstream signalling. A high-resolution three-dimensional structure of the SH2 domains of SYK in complex with the doubly phosphorylated ITAM of CD3ε showed that the N-terminal SH2 domain binds the C-terminal phosphorylated tyrosine of the ITAM and vice versa53 (FIG. 1A). The structure shows significant flexibility53,54, which may account for the ability of SYK, in contrast to ZAP70, to bind to phosphorylated ITAMs with a broad range of spacing between the phosphorylated tyrosine residues (including the long-spaced FcγRIIA ITAM55), as well as to bridge two hemITAMs in C-type lectins43-45 (FIG. 1A).
Role of phosphorylated tyrosines in SYK
There are ten tyrosines in SYK that are sites of autophosphorylation (FIG. 2B)56, most of which have been shown to participate in SYK-mediated signal transduction. Unless otherwise stated, amino acids are numbered as in mouse Syk.
BCR activation triggers phosphorylation of Tyr130 in interdomain A56, leading to dissociation of SYK from the phosphorylated ITAMs of the BCR complex57,58 (FIG. 2C). Mutation of Tyr130 to the phosphomimetic glutamate also decreases ITAM binding and results in increased kinase activity, once again pointing to an inhibitory role for interdomain A57.
Phosphorylated Tyr317 is a binding site for the E3 ubiquitin ligase CBL (FIG. 2B), which is involved in the ubiquitination and degradation of SYK20,21. Accordingly, mutation of this residue results in augmented FcεRI- and BCR-mediated signalling59,60. Phosphorylated Tyr317 also binds the C-terminal SH2 domain of the PI3K regulatory subunit p85α, whereas the N-terminal SH2 domain of p85α binds phosphorylated Tyr342 and phosphorylated Tyr34661 (FIGS 2B-C). Mutation of all three tyrosines abrogates FcεRI-induced AKT activation, consistent with an inability to activate PI3K62.
In the ZAP70 structure, Tyr315 and Tyr319 in interdomain B and Tyr597 and Tyr598 in the kinase domain stabilize the autoinhibited conformation48. The mutation of these residues in ZAP70 or the mutations of the analogous residues in SYK (Tyr342, Tyr346, Tyr624 and Tyr625) results in kinase activation, further indicating that SYK adopts an autoinhibited conformation63-65. All four residues are phosphorylated following BCR stimulation56, likely contributing to kinase activation by destabilizing the autoinhibited conformation of SYK (FIG. 2A), as well as providing docking sites for other signalling molecules which stabilise the active conformation and initiate downstream signaling (FIG. 2C).
Phosphorylated Tyr342 binds to the SH2 domain of VAV1 and both phosphorylated Tyr342 and phosphorylated Tyr346 bind to the C-terminal SH2 domain of PLCγ166,67 (FIGS 2B-C). Interestingly, this latter binding is mediated by two phosphorylated tyrosines binding to one SH2 domain68. Mutation of these residues inhibits FcεRI signalling in mast cells62,69 and phenocopies the cytoskeletal changes observed in SYK-deficient neutrophils70.
When phosphorylated, Tyr624 binds the SH2 domain of SLP6571 (FIGS 2B-C). This binding activates the kinase, which is consistent with the putative role of Tyr624 in stabilizing the autoinhibitory conformation. This SLP65–SYK association is also important for BCR signalling and B cell development71. Although no direct interaction between Tyr624 and the SLP65-related SLP76 adaptor has been reported, such a direct functional association would not be surprising given the strikingly similar phenotypes of SYK-deficient3,23,24,33,72,73 and SLP76-deficient mice3,74,75.
Integration of SYK activation
The most likely physiological relevance of the dual activation of SYK either by ITAM binding or by phosphorylation of linker tyrosines is that initial ITAM binding triggers rapid activation of Syk whereas subsequent phosphorylation leads to prolonged activation and downstream signaling even in the absence of binding to phosphorylated ITAMs (FIG. 2C). Importantly, SYK itself can catalyze the autophoshorylation of its linker tyrosines, leading to sustained SYK activation after a transient ITAM phosphorylation. Inaddition, SYK itself can phosphorylate ITAMs, suggesting the existence of a positive-feedback loop during initial ITAM-mediated SYK activation52 (FIG. 2C).
The role of SYK in cellular adhesion
Integrin signal transduction
Integrins are a family of heterodimeric transmembrane receptors that participate in leukocyte adhesion and migration (through the β2 integrins lymphocyte function-associated antigen 1 (LFA1) and macrophage receptor 1 (MAC1)), platelet activation (αIIbβ3 integrin) and osteoclast development and function (αVβ3 integrin). Given their structural and functional differences, integrins and classical immunoreceptors were long thought to signal by conceptually different mechanisms.
Evidence for the use of similar signalling mechanisms by integrins and immunoreceptors came from studies showing defective integrin-mediated signalling in SYK-deficient neutrophils72, monocytes/macrophages76,77, platelets73 and osteoclasts78. Further in vivo studies showed that SYK is necessary for firm leukocyte adhesion to the inflamed endothelium79 and the development of a MAC1-dependent vasculopathy reaction80.
The mechanism of SYK activation by integrins has long been debated, primarily since initial studies in heterologous expression systems indicated that the αIIbβ3 integrin activates SYK by an ITAM-independent mechanism through direct association between the cytoplasmic tail of the integrin β-chain and the non-ITAM-binding surfaces of the N-terminal SH2 domain of SYK81-83 (FIG. 3A).
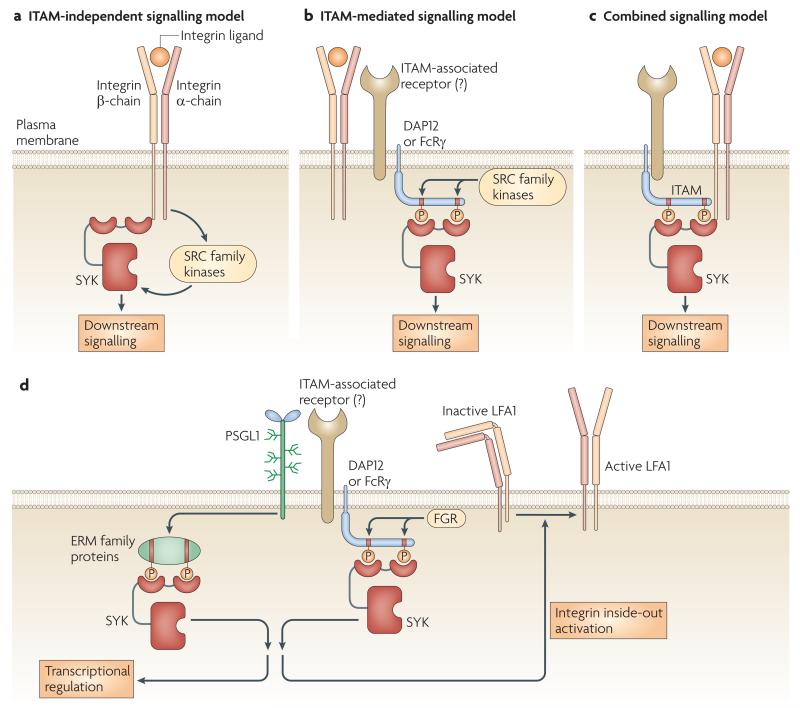
Figure 3. The role of SYK in integrin and PSGL1 signalling.
a-c ∣ Mechanism of integrin-mediated SYK activation. a ∣ In the original, ITAM-independent model, integrins activate SYK without phosphorylated tyrosine binding to the SYK SH2 domains. The interaction is mediated by the intracellular domain of the integrin β-chain and the non-phosphorylated tyrosine-binding surface of the N-terminal SH2-domain of SYK. b ∣ In the ITAM-mediated model, integrin ligation triggers SRC-family-mediated phosphorylation of ITAM-bearing transmembrane adaptor molecules (DAP12 and FcRγ), leading to recruitment of SYK through its tandem SH2 domains. This signalling likely involves DAP12- and/or FcRγ-associated transmembrane receptors. c ∣ In the combined model, the non-phosphorylated tyrosine-binding surface of the SYK SH2 domains bind to the C-terminal end of the integrin β-chain while the two SH2 domains bind to the phosphorylated ITAM tyrosines. d ∣ SYK-mediated signalling by PSGL1. Ligation of leukocyte PSGL1 triggers phosphorylation of the ITAM tyrosines of ERM family proteins which recruit SYK via its tandem SH2 domains. A supposedly parallel mechanism under flow conditions leads to phosphorylation of the ITAM tyrosines of the DAP12 and FcRγ transmembrane adaptors by the FGR tyrosine kinase, leading to recruitment of SYK through its tandem SH2 domains. SYK activation triggers transcriptional changes and inside-out activation of LFA1.
More recent analyses of primary cells (rather than heterologous expression systems) came to a very different conclusion. Structure-function studies indicated a crucial role of phosphorylated tyrosine binding by the SH2 domains of SYK for integrin-mediated functions in neutrophils, macrophages and platelets77,84. The ITAM-containing proteins linking β2 integrin signalling to SYK activation in neutrophils and macrophages have been identified as the DAP12 and the FcR γ-chain (FcRγ) transmembrane adaptor molecules77 (FIG. 3B). Later studies indicated that integrin signalling in osteoclasts85, DCs86, microglia87 and platelets88 also require ITAM-containing transmembrane adaptors. However, the coupling between integrins and the ITAM-containing adaptor molecules is poorly understood, even though preliminary studies77 suggested the involvement of as yet unidentified DAP12--associated molecules (FIG. 3B).
Importantly, the above ITAM-independent81,84 and ITAM-mediated77,84-88 mechanisms of integrin–SYK coupling are not mutually exclusive. Indeed, the most likely scenario is that the two mechanisms jointly regulate the activity of SYK6,85 (FIG. 3C), allowing the fine control of adhesive interactions of haematopoietic lineage cells.
It should also be noted that whereas SYK is crucial for several integrin-mediated functions, it makes limited contribution to β2 integrin-mediated migration of neutrophils70,72.
Leukocyte selectin functions
Selectins are transmembrane glycoproteins that are involved in leukocyte rolling on the endothelium. Endothelial P-selectins mediate steady-state leukocyte rolling, whereas slow rolling in an inflammatory environment is triggered by endothelial E-selectins. Recent evidence indicates a role for SYK in signal transduction by P-selectin glycoprotein ligand 1 (PSGL1), the major selectin receptor on the leukocyte surface. SYK is activated by, and associated with, PSGL1 in leukocytes89,90. Abrogation of SYK activity inhibits PSGL1-mediated transcriptional regulation89, whereas SYK-deficient neutrophils show a defect in slow rolling, suggesting that SYK is involved in PSGL1-induced inside-out signalling that activates LFA190 (FIG. 3D). Both groups concluded that PSGL1-induced SYK activation proceeds through an ITAM-mediated pathway89,91. However, one report concluded that SYK is activated through the ITAM-like motif-containing ezrin, radixin and moesin (ERM) family proteins89, whereas the other study suggested the role of the ITAM-bearing adaptors DAP12 and FcRγ phosphorylated by the SRC-family kinase FGR91 (FIG. 3D).
Role of SYK in innate pathogen recognition
The innate immune system uses germline-encoded pattern recognition receptors (PRRs) to detect pathogen-associated molecular patterns (PAMPs) and activate immune responses. Recently, SYK has emerged as a key component of these pathways (FIG. 4A).
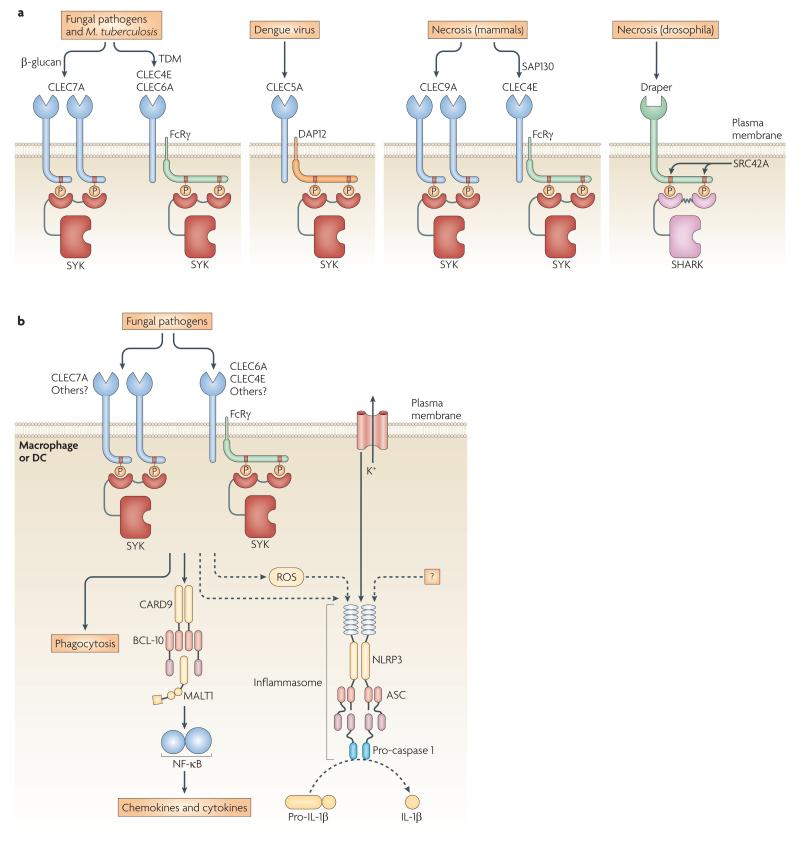
Figure 4. SYK-dependent innate pathogen- and damage-recognition pathways.
a ∣ Innate immune receptors coupled to SYK or SYK-related molecules sense foreign pathogens and tissue damage. Fungal pathogens and M. tuberculosis engage CLEC7A (Dectin-1), CLEC6A (Dectin-2) and CLEC4E (Mincle); Dengue virus activates CLEC5A; necrotic cells activate the mammalian CLEC9A and CLEC4E, and the Drosophila Draper receptors. The ligand-binding chains of those receptors either have a hemITAM or a complete ITAM, or associate with the ITAM-bearing transmembrane adaptors FcRγ or DAP12. All of these receptors activate SYK-family kinases (SYK or SHARK) by SH2-mediated recruitment to phosphorylated tyrosines. The kinase responsible for phosphorylation of the Draper ITAM is the Drosophila SRC-family kinase SRC42A. b ∣ Mechanism of pro-inflammatory responses triggered by recognition of fungal pathogens by C-type lectins. CLEC7A binds SYK directly through an intracellular hemITAM, whereas CLEC6A and CLEC4E engage SYK through the ITAM-containing FcRγ adaptor. Pathogens trigger SYK-mediated phagocytosis, cytokine and chemokine production, and ROS generation. SYK-mediated NF-κB signalling is controlled by the CARD9–BCL10–MALT1 complex. Additionally, SYK is required for IL-1β processing by the NLRP3 inflammasome, which is activated by SYK-dependent ROS production, potassium ion (K+) efflux and presumably additional unknown danger signals (“?”).
Fungal recognition
The first insights into SYK-dependent PRR signalling came from studies of the C-type lectin CLEC7A (Dectin-1), a major mammalian PRR for fungal β-glucans43 with a hemITAM in its intracellular tail (FIG. 4A). Agonist binding triggers hemITAM phosphorylation resulting in direct recruitment of SYK to CLEC7A. As with other hemITAM-containing C-type lectins, CLEC7A-mediated SYK activation requires a single tyrosine residue in a Tyr-X-X-Leu motif. It is reasonable to assume that two hemITAM-phosphorylated CLEC7A molecules engage the tandem SH2 domains of a single SYK molecule in a 2:1 stoichiometry7, as has recently been shown for the CLEC7A-related CLEC2 receptor in platelets45.
SYK-mediated signalling following CLEC7A ligation triggers ROS production92,93 and phagocytosis of yeast particles by DCs43. Moreover, SYK couples PAMP recognition to de novo gene transcription and induces chemokine and cytokine production through several downstream pathways including the ERK signalling cascade and activation of transcription factors such as NFAT94 and NF-κB11,43,93,95-97. Although the precise mechanisms of SYK-mediated phagocytosis and ROS production are poorly understood, the CLEC7A–SYK-induced NF-κB response is transduced through CARD9 which engages the BCL10 adaptor and the MALT1 paracaspase11,98,99 (FIG. 4B). Consistent with an essential role of the CLEC7A–SYK–CARD9 axis for antifungal host defence, CARD9-deficient mice and humans are highly susceptible to fungal infections11,100. It is at present unclear how SYK activates CARD9 but this mechanism likely differs from the PKC-mediated activation of CARMA1 in lymphocytes since the critical PKC phosphorylation sites of CARMA1 are not conserved in CARD918. Moreover, the specific role of SYK-triggered ERK or NFAT activation in anti-fungal immunity has not yet been defined either.
Although the CLEC7A–SYK–CARD9 pathway is a bona fide PRR pathway that can couple innate to adaptive immunity, it induces a distinct pattern of DC cytokines with particularly robust IL-2, IL-10 and IL-23 production96, leading to the initiation of potent T helper 17 (TH17) cell responses96,101.
SYK-deficient DCs have a more profound block in IL-2 or IL-10 synthesis upon yeast stimulation than those lacking CLEC7A, suggesting redundancy at the receptor level96. Indeed, two additional SYK-coupled PRRs for fungi, CLEC6A (Dectin-2) and CLEC4E (Mincle), were recently identified102-105. Instead of containing a cytoplasmic hemITAM, CLEC6A and CLEC4E associate with and signal through the ITAM-containing FcRγ adaptor103,105 (FIGS 4A-B). Both CLEC6A–SYK and CLEC4E–SYK signalling induce CARD9- and NF-κB-mediated pro-inflammatory responses which promote adaptive immunity and robust TH17-type responses102,106,107.
Inflammasome activation
IL-1β is a SYK-dependent cytokine with essential role in antifungal immunity93. Production of mature IL-1β requires NF-κB-mediated transcription of pro-IL-1β and proteolytic processing of pro-IL-1β by caspase-1-containing multiprotein complexes termedinflammasomes108. Live yeast cells specifically activate the NLRP3 inflammasome93. SYK controls both pro-IL-1β synthesis and NLRP3 activation in response to fungal infection93. Whereas pro-IL-1β synthesis is regulated through the SYK–CARD9 pathway, NLRP3 activation occurs through a SYK-dependent but largely CARD9-independent mechanism93 (FIG. 4B). Since NLRP3 activation by malarial haemozoin also requires SYK109, the role of SYK in NLRP3 activation is not restricted to antifungal immunity.
The NLPR3 inflammasome is activated in response to various distinct danger signals108. Upstream mechanisms that trigger NLRP3 activation in multiple scenarios are ROS production, potassium efflux and sometimes release of lysosomal proteases into the cytoplasm108. NLRP3 activation by fungi requires SYK-triggered ROS generation and potassium efflux93 (FIG. 4B). However, since SYK activation by zymosan or non-viable yeast is not sufficient for inflammasome activation93,110, yet unidentified other components are likely also required for the activation of the NLRP3 inflammasome following yeast infection (FIG. 4B).
Bacterial and viral recognition
There is increasing evidence that SYK-coupled PRRs also have important roles in the innate recognition of bacteria, with CLEC7A, CLEC6A and CLEC4E all implicated in mycobacterial PAMP sensing46 (FIG. 4A). Although the mycobacterial structures recognized by CLEC7A and CLEC6A remain to be identified, CLEC4E has been reported to be the long-sought receptor for the mycobacterial cord factor trehalose-6,6′-dimycolate (TDM), the most thoroughly studied immunostimulant of Mycobacterium tuberculosis107,111. Myeloid cell activation by TDM, or its less toxic analogue trehalose-6,6-dibehenate, uses ITAM–SYK signalling through FcRγ, SYK and the CARD9–BCL10–MALT1 complex106,107.
In addition to CLEC7A, CLEC6A and CLEC4E, there are further myeloid C-type lectin receptors with still unidentified functions46, and other innate immune receptors have also been proposed to signal through SYK112. Furthermore, genetic deletion of SYK has been shown to attenuate antibacterial host defence in mice113. The dengue virus is detected by the C-type lectin CLEC5A (myeloid DAP12-associating lectin 1(MDL1)), which induces pro-inflammatory cytokine production through the ITAM-containing DAP12 adaptor114 (FIG. 4A). Therefore, SYK is presumably also involved in signaling PRR-mediated recognition of certain viruses.
Signalling tissue damage in mammals and invertebrates
The innate immune system is also activated by non-infectious stimuli, such as cell death caused by sterile injury or developmental processes, or sterile inflammation during autoimmune diseases.
There is accumulating evidence that SYK-coupled C-type lectins can detect host-derived molecules from damaged cells. CLEC4E, an FcRγ-associated fungal and mycobacterial PRR (see above), can sense necrotic cells after prolonged culture in vitro115 and it is reasonable to assume that this response also requires the ITAM-mediated activation of SYK (FIG. 4A). The CLEC4E ligand expressed by necrotic cells has been identified as the spliceosome-associated protein 130 (SAP130)115. A second SYK-coupled receptor for dead cell recognition is CLEC9A (dendritic cell NK lectin-group receptor 1 (DNGR1)). Similar to CLEC7A, CLEC9A has a cytoplasmic hemITAM with a single Tyr-X-X-Leu motif (FIG. 4A). CLEC9A is highly expressed by subsets of DCs and monocytes and recognizes as yet unidentified ligands that are exposed during cell necrosis116. CLEC9A promotes DC-mediated cross-presentation of dead cell-derived antigens through a hemITAM-SYK interaction117.
The (hem)ITAM–SYK-mediated damage-recognition pathway likely represents an ancient mechanism as it is also found in invertebrates. The clearance of neuronal cell corpses by phagocytic glial cells in the Drosophila brain requires Draper, an ITAM-containing phagocytic receptor118 (FIG. 4A). Draper ligation by phagocytic targets triggers ITAM phosphorylation by the SRC-related kinase SRC42A, leading to subsequent association with the SYK-related kinase SH2 domain ankyrin repeat kinase (SHARK) (FIG. 4A) which is essential for Draper function in glial cells in vivo4.
Taken together, the above pathways indicate that the (hem)ITAM–SYK-mediated damage recognition pathway is an ancient mechanism that it shared between insects and mammals, and that this pathway overlaps with innate recognition of pathogens.
Other immune functions of SYK
SYK has been implicated in several other signalling pathways within immune cells. The DAP12-associated triggering receptor expressed on myeloid cells (TREM) molecules participate in a number of inflammation-related biological activities, including modulation of Toll-like receptor function, through activation of SYK119. CLEC4C (blood dendritic cell antigen 2 (BDCA2)), an FcRγ-associated receptor expressed on DCs, also signals through SYK but, in contrast to C-type lectins involved in pathogen recognition, does not activate the NFκB46. A number of other well-known immune cell receptors do not contain ITAMs, yet appear to also utilize ITAM–SYK-mediated accessory signals. Interferon-γ (IFNγ)-mediated enhancement of IFNα-induced STAT1 activation requires DAP12, FcRγ and SYK, possibly through interaction of DAP12- and FcRγ-containing receptors with the IFNα receptor120. Basophils lacking either FcRγ or SYK show reduced IL-3-induced IL-4 secretion (but not proliferation), supposedly through direct association between FcRγ and the IL-3 receptor βc subunit121 and DAP12 have been implicated in signaling from the macrophage colony-stimulating factor (M-CSF) receptor cFMS in an ITAM-dependent manner also requiring PYK278,122. MHC class II molecules on B cells associate with, and trigger the phosphorylation of, the ITAM-bearing Igα and Igβ adaptors123.
NK cells express SYK and ZAP70, both of which are involved in FcγR-mediated ADCC responses34. NK cells also express the ITAM-bearing transmembrane adaptor DAP12, which is coupled to several activating NK cell receptors. However, although SYK and/or ZAP70 are required for calcium signalling and cytokine release following activation of DAP12-associated receptors124,125, NK cells lacking both SYK and ZAP70 are able to kill target cells through a DAP10- and PI3K-mediated parallel pathway125,126.
Crystals of monosodium urate activate DCs by a receptor-independent pathway127, which involves aggregation of cholesterol and activation of a SYK-dependent signalling pathway. The mechanism of this activation is incompletely understood, but might involve the recruitment of ITAM-associated receptors. Importantly, this mechanism may contribute to the development of crystal-induced diseases such as gout.
SYK had also been proposed to participate in G-protein-coupled receptor signalling128. However, genetic studies in neutrophils and mast cells128 argue against that possibility.
Non-immune functions of SYK
Although ITAM–SYK signalling has long been regarded to be specific for the immune system, recent studies indicate several non-immune functions of SYK.
Bone metabolism
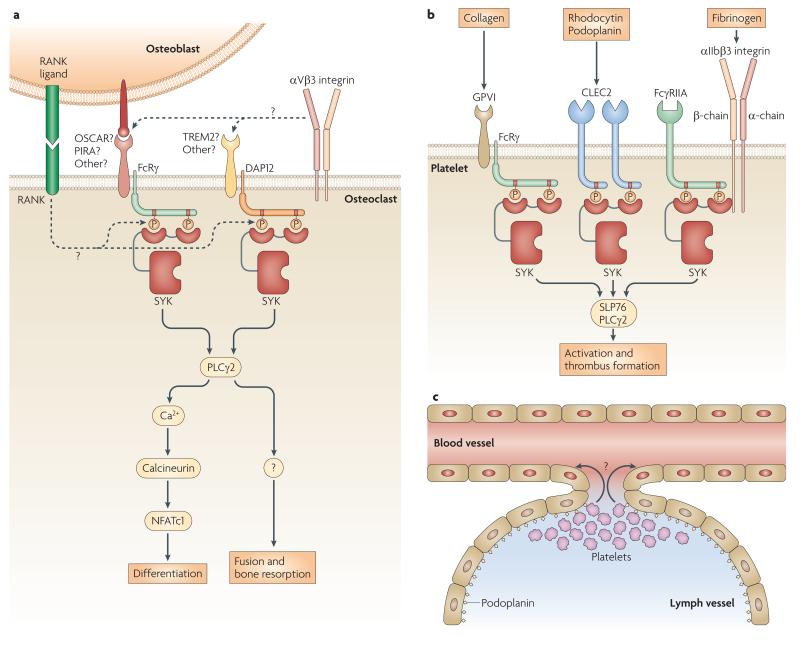
Osteoclasts are macrophage-related bone-resorbing cells that develop from early myeloid precursors under the control of the TNF-related protein receptor activator of NF-κB (RANK) ligand expressed by osteoblasts. Differentiated preosteoclasts fuse to form large multi-nucleated cells, which then bind to and resorb the bone surface (FIG. 5A, inset). Importantly , the αVβ3 integrin participates in diverse functions of osteoclasts, including the sealing the resorption area from the surrounding environment (FIG. 5A, inset). Loss-of-function mutations of DAP12 (Nasu-Hakola disease) cause bone abnormalities in humans129, indicating a possible role of ITAM-based signalling in bone metabolism. Indeed, DAP12-deficient mice show moderate osteopetrosis130,131 while mice lacking both DAP12 and FcRγ are severely osteopetrotic5,132. While FcRγ is involved in osteoblast–osteoclast interactions, DAP12 relays an osteoblast-independent signal5,132 (FIG. 5A). Importantly, SYK, which is activated downstream of DAP12 and FcRγ in an ITAM-dependent manner, is also required for osteoclast development and function5,85,133 (FIG. 5A). Although the precise mechanism of DAP12 and FcRγ activation is poorly understood, it likely involves the FcRγ-associated receptors osteoclast-associated receptor (OSCAR) and paired immunoglobulin-like receptor A (PIR-A), and the DAP12-associated TREM2 molecule132 (FIG. 5A). This ITAM-mediated pathway has been proposed to be a costimulatory pathway to RANK ligand signaling132, as well as a mediator of αVβ3 integrin signal transduction5,85 (FIG. 5A).
Figure 5. Non-immune functions of SYK.
a ∣ The role of DAP12 and FcRγ in osteoclasts. In addition to a RANK–RANK ligand interaction, osteoblasts also express yet unidentified ligands that engage a putative FcR-associated receptor (most likely OSCAR or PIR-A) on the osteoclast surface. Osteoclasts also express putative DAP12-associated receptors (most likely TREM2) which bind to yet unidentified ligands that are not expressed by osteoblasts. Both FcRγ and DAP12 promote osteoclast development and function through ITAM-mediated SYK activation and the PLCγ2-Calcineurin-NFATc1 pathway. Inset: Mechanism of osteoclast development. Osteoclasts develop from myeloid progenitors re-programmed by osteoblast-derived RANK ligand, leading to biochemical differentiation. Pre-osteoclasts then fuse to generate mature multi-nucleated osteoclasts and resorb the bone surface. αVβ3 integrins are required for sealing the resorption area. b ∣ SYK-mediated signaling in platelets. Collagen activates the FcRγ-associated GpVI receptor on platelets and triggers Syk activation by an ITAM-mediated manner. The snake venom rhodocytin, the endogenous podoplanin and possible other platelet agonists activate the CLEC2 receptor which recruits SYK to phosphorylated tyrosine in the CLEC2 hemITAMs at a 2:1 stoichiometry. Fibrinogen engages the αIIbβ3 integrin which associates with the ITAM-containing FcγRIIA receptor. This complex likely activates SYK through both conventional ITAM-mediated SYK activation pathways and by binding of the integrin β-chain to the N-terminal SH2-domain of SYK in a phosphotyrosine-independent manner. All three cases of SYK activation lead to platelet activation through PLCγ2. c ∣ The role of SYK in separation of blood and lymphatic vessels. Lymphatic vessels express podoplanin which trigger platelet activation and aggregation. This and possible other mechanisms (“?”) lead to closing off any blood-lymphatic shunts. The mechanism of podoplanin-mediated platelet activation involves the CLEC2 receptor which activates SYK through hemITAM phosphorylation, as well as SLP76 and PLCγ2 acting downstream of SYK.
Platelet functions
Several platelet functions rely on SYK signalling. GpVI, the major collagen receptor of platelets, is an FcRγ-associated receptor closely related to FcαRs, which may explain that GpVI, like other FcR family members, signals through SYK2 (FIG. 5B). The C-type lectin CLEC2 has also been shown to signal through SYK in platelets42. Similar to other hemITAM-cotaining receptors, CLEC2 has a single cytoplasmic Tyr-X-X-Leu motif which engages both SH2-domains of Syk by dimerisation of the receptor45 (FIG. 5B). SYK also mediates outside-in signalling by the αIIbβ3 integrin of platelets73. The mechanism of SYK activation by αIIbβ3 was shown to be ITAM-dependent84 (see above) and to require, at least in human cells, the ITAM-containing FcγRIIA88 molecule (FIG. 5B). Since CLEC2134 and the SYK substrate SLP-76135 are required for arterial thrombus formation, it is likely that SYK also participates in in vivo haemostatic functions of platelets.
Vascular development
One of the most striking phenotypes of Syk-deficient fetuses is the appearance of blood-filled subcutaneous structures originally described as petechial haemorrhages23,24 but later identified as blood-filled lymphatic vessels3, indicating that SYK is required for the separation of lymphatic vessels from the general circulation. Surprisingly, this vascular defect could be transferred to lethally-irradiated wild-type recipients by bone marrow transplantation33, indicating the role of SYK expression within the haematopoietic compartment. Two subsequent studies proposed very different explanations, suggesting either that the defect lies in circulating endothelial progenitor cells (which share their origin with true blood cells)136 or that the vascular phenotype is due to a gain-of function effect whereby tissue accumulation of Syk–/– myeloid cells leads to excessive release of pro-angiogenic factors triggering hyperproliferation of the lymphatic vasculature137. However, the former mechanism contradicts the lack of SYK expession in the endothelial lineage137 whereas the latter mechanism does not appear to explain the correction of the vascular phenotype by the presence of as little as 5-10% wild type cells in the hematopoietic compartment in mixed bone marrow chimeras3.
A series of very recent studies propose an exciting third explanation (FIG. 5C). Blood and lymph vessels fail to separate in platelet-deficient fetuses138, as well as in mice lacking either the platelet receptor CLEC2139, or the CLEC2 ligand podoplanin which is expressed on lymphatic (but not blood) endothelial cells139-141. A similar vascular phenotype is also induced by platelet-specific deletion of SLP76139 (the deficiency of which also leads to blood-lymphatic vascular separation defect3) or of SYK itself (E. Schweighoffer and V. T., unpublished observation; and C. Bertozzi and M. Kahn, personal communication). Lymphatic endothelial cells also induce aggregation of platelets in a SLP76-dependent manner139. Together with the ability of podoplanin to activate platelets via CLEC2142 and the fact that CLEC2 signals through SYK and PLCγ242, these results suggest that podoplanin on lymphatic endothelial cells triggers platelet activation and aggregation through CLEC2, SYK, SLP76 and PLCγ2 (FIG 5C), thereby closing off any blood-lymphatic shunts by a yet unidentified mechanism that possibly involves platelet aggregation. As a consequence, newly formed lymphatic sacks are separated from the blood vasculature, and a long-term incompatibility between the two vascular systems is establishes.
Other non-haematopoietic functions
SYK is also expressed by cells of non-haematopoietic origin including normal mammary glands and mammary gland epithelial cell lines143, as well as various other tissues (reviewed in REFS 144,145). While sporadic studies have proposed that SYK plays a role in processes including signaling by integrins and TNF-receptors, transcriptional regulation and mitotic progression in these cells (reviewed in REF.144), this area is still poorly understood with very little mechanistic information available.
A SYK-related kinase has also been proposed to participate in gonadal development in the parasitic helminth Schistosoma mansoni146, suggesting that the role of SYK-related kinases in non-hematopoietic tissues may also have an ancient evolutionary origin.
SYK in disease pathogenesis and therapy
Allergy and autoimmunity
The diverse roles of SYK in the immune system suggest that it may participate in allergic and/or autoimmune diseases. Indeed, SYK-deficient bone marrow chimaeras are protected from reverse passive Arthus reaction70 and autoantibody-mediated experimental arthritis147. A supposedly SYK-selective inhibitor, R112, alleviates the symptoms of allergic rhinitis in human patients148. An R112-related inhibitor, R406, as well as its orally bioavailable prodrug, R788 (also known as fostamatinib), delayed the onset and reduced the severity of autoimmune arthritis in animal models149,150 and provided clinical benefit in rheumatoid arthritis patients151. More recently, R788/fostamatinib has shown beneficial effects in autoantibody-induced thrombocytopenia in mice, and autoimmune thrombocytopenic purpura in humans152. Various aspects of the mechanism of action of SYK-inhibitors in clinical use are discussed in BOX 2.
Box 2. What is the mechanism of action of SYK inhibitors in clinical use?
While SYK inhibitors have shown positive effects in allergy148, autoimmune diseases151,152 and B-lineage malignancies166, the mechanism of their action is incompletely understood. This is in part due to the diverse roles of SYK in immunological functions. As an example, B-cell-mediated antigen presentation and autoantibody formation, Fc-receptor-mediated myeloid cell functions and β2 integrin-mediated leukocyte activation have all been implicated in the pathogenesis of rheumatoid arthritis and they all have been shown to require SYK (see ref. 147 for further details). The rapid effects of R112 treatment in allergic rhinitis148 and the effectiveness of SYK inhibitors in autoantibody-induced passive models of arthritis149 and autoimmune thrombocytopenia152 also suggest that SYK inhibitors target disease components other than BCR-mediated B-cell functions. Likewise, though initial studies suggested that the only role of SYK in B-cell lymphomas is to relay ITAM-mediated tonic BCR signaling, a recent report suggested that SYK also contributes to the survival of B-cell lymphomas through processing BCR-independent adhesive and homing signals163.
A further complicating issue is that R406, the active metabolite of the R788/fostamatinib prodrug, is an ATP-competitive kinase inhibitor and, similar to most such inhibitors, has rather limited specificity towards SYK. Indeed, R406 has been shown to inhibit a number of other kinases and non-kinase targets at concentrations comparable to those inhibiting SYK149. Of those targets, the Flt3, c-Kit, Lck and Jak1/3 tyrosine kinases and the adenosine A3 receptor have been implicated in autoimmune diseases, providing additional or alternative explanations for the clinical effect of R788/fostamatinib in human patients (see ref. 147 for further details).
Taken together, it is reasonable to assume that the clinical effects of R788/fostamatinib are likely mediated by inhibition of a number of SYK-dependent and SYK-independent immune signaling pathways.
Haematological malignancies
SYK has been linked to the development or maintenance of hematological malignancies including B-lineage leukemias and lymphomas (FIG. 6).
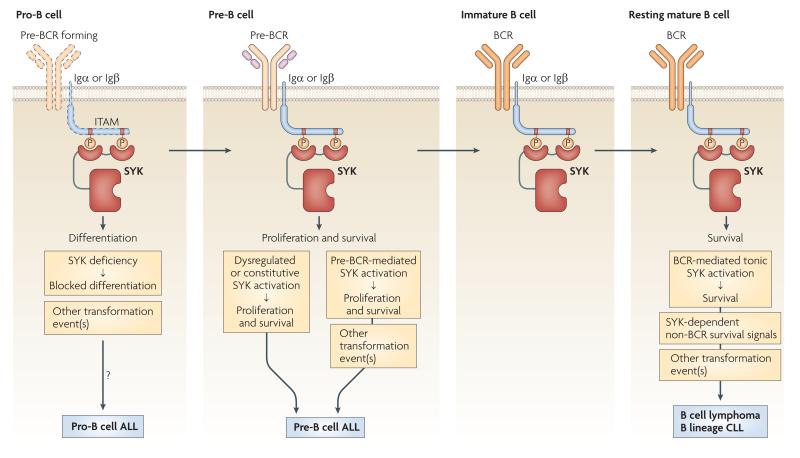
Figure 6. The Role of SYK in malignancies of the B-cell lineage.
SYK is required for various processes of B-cell development, including transition from pro-B to pre-B cells, proliferation and survival of pre-B cells, as well as the survival of mature B-cells. When other transforming factors are present, loss of SYK in pro-B cells may contribute to development of pro-B cell ALL, possibly by blocking further differentiation. Deregulated or constitutive activation of SYK is able to transform pre-B cells to pre-B cell ALL. In addition, pre-BCR-induced SYK activation is required for transformation of pre-B cells to pre-B cell ALL by other transforming factors (e. g. c-MYC). BCR-mediated tonic activation of SYK, as well as other SYK-dependent but BCR-independent signals are required for survival of mature B-cell lymphomas and B-lineage CLLs.
The first study linking SYK to a haematological malignancy described marked reduction of SYK expression in highly aggressive childhood pro-B cell acute lymphoblastic leukaemia (ALL) but not in more differentiated pre-B cell ALL153. Since SYK is required for the transition of pro-B to pre-B cells23, it is tempting to speculate that the loss of SYK contributes to transformation of B cell precursors by preventing further differentiation (FIG. 6), as has been proposed for SLP65, the lack of which contributes to pre-B cell leukemia in both humans and mice154,155. However, the lack of an independent confirmation of SYK deficiency in pro-B ALL, and the uncertainties related to extrapolating prior findings on SLP65 to SYK point to the need for further studies on the role of SYK in pro-B cell leukemia.
SYK has very different and much better characterised roles in lymphomas derived from B-lineage cells beyond the pro-B cell stage. Likely related to the role of SYK in promoting proliferation and blocking apoptosis in pre-B cells, exogenous expression of SYK was able to transform normal pre-B cells, and endogenous SYK was required for transformation of pre-B cells by exogenous c-MYC156 (FIG. 6). Furthermore, SYK inhibition promoted apoptosis of primary human B-cell precursor ALL (most likely pre-B cell ALL) cells both in vitro and in vivo, suggesting a potential therapeutic application of SYK inhibitors in certain B-cell ALL patients157.
Transformation of mature B-cells mostly leads to various forms of B-cell lymphoma or B-lineage chronic lymphocytic leukemia (CLL). ITAM-mediated tonic BCR signaling is required for the survival of resting mature B cells and certain B cell lymphomas39,158,159. This signal likely acts through SYK which could explain the expression of constitutively active SYK in B-cell lymphomas and B-lineage CLL cells160-162. SYK is also required for processing stroma cell-derived pro-survival signals, such as adhesion and homing, by CLL cells independently of BCR signaling163. These results suggest that SYK contributes to the survival and maintenance of B cell malignancies (FIG. 6). Accordingly, abrogation of SYK activity inhibited the growth of various B cell lymphomas160,164,165, and R788/fostamatinib therapy prolonged the survival of patients with diverse types of B cell lymphoma or B-lineage CLL166.
SYK may also participate in haematological malignancies of non-B-cell origin. T-cell lymphomas often overexpress enzymatically active SYK167, may contain chromosomal translocations leading to the expression of an IL-2-inducible T cell kinase (ITK)–SYK fusion protein168, and are sensitive to silencing of SYK expression169. A chromosomal translocation generating a TEL–SYK fusion protein causes myelodysplastic syndrome, a preleukemic state predisposing for the development of acute myeloid leukaemia170, while SYK appears to be an important component of disease pathogenesis in acute myeloid leukaemia itself171. These results may further extend the possible use of SYK inhibitors in human therapy.
SYK signalling by viral oncogenes
SYK is also required for the oncogenic activity of several ITAM-containing viruses172. The ITAM of the viral LMP2A protein and host cell SYK are both required for Epstein-Barr virus-induced oncogenic transformation173,174. The ITAM-containing envelope surface protein contributes to mouse mammary tumour virus-induced transformation in an ITAM- and SYK-dependent manner175,176. Furthermore, the ITAM-containing K1 protein of Kaposi's sarcoma-associated herpesvirus initiates ITAM- and, presumably, SYK-dependent signalling pathways to promote lytic viral replication in infected B cells177,178. The possible role of these and other viruses in breast cancer179 raise the possibility of a tumor-promoting role for SYK in certain tumours.
Other non-haematopoietic tumours
In contrast, SYK has been proposed to suppress the growth of other (supposedly non-viral-induced) tumours of non-haematopoietic origin. SYK expression inversely correlated with invasiveness in breast cancer cell lines143 and exogenous expression of SYK inhibited tumour growth, while expression of a dominant-negative SYK was found to promote tumorigenesis143. SYK expression also inversely correlated with the severity of breast cancer and other epithelial cell-derived tumours in human patients143,144,180-182, with particularly poor prognosis when low nuclear levels of SYK was present183,184.
The decreased level of SYK expression in cancer cells likely results from hyper-methylation of the SYK promoter, rather than mutations or genetic deletion of SYK itself144,180,182, while the loss of nuclear localization of SYK is likely due to alternative splicing leading to the expression of the SYK-B isoform, which lacks a nuclear localization signal185. However, it is presently unclear how loss of SYK expression results in poor prognosis in epithelial cell-derived tumours, though it may be related to any of the previously mentioned possible physiological functions of SYK in these lineages (reviewed in ref.144).
Conclusions and perspective
The SYK tyrosine kinase was originally thought to only contribute to signalling responses of immunoreceptors of the adaptive immune response. However, more recent studies have identified a large number of novel functions of SYK both in the immune system and beyond. Moreover, the identification of a similar pathway in Drosophila indicates that this signalling emerged significantly earlier than the adaptive immune response. Collectively, these studies indicate that SYK plays a much more diverse role in vertebrate and invertebrate biology than previously anticipated.
In addition to the diverse roles of SYK in basic biological processes, it is also involved in the pathogenesis of several human diseases, including allergy, autoimmunity and various haematological malignancies. The promising results of SYK-inhibitors in recently completed human clinical trials suggest that SYK may become a major therapeutic target in those diseases in the future
The new observations of diverse biological functions for SYK also raise a number of questions. How do the initial steps of ITAM signalling occur? How do integrins and selectins “hook up” to an ITAM-based signalling pathway? How are DAP12 and FcRγ coupled to osteoclast development? How does SYK in platelets contribute to vascular development? What downstream pathways mediate the diverse functions of SYK? What are the roles of SYK in epithelial cells and are ITAMs involved in these functions? Is SYK a suitable target in thrombocytic diseases or pathological bone loss? Would targeting SYK be effective in treating metabolic disorders of inflammatory origin? Will long-term treatment with SYK inhibitors result in unexpected side effects, e. g. related to the role of SYK in innate immunity and its tumor suppressor function in epithelial cells? Answering these and other questions will keep scientists interested in the biological functions of SYK busy for many years to come.
Online summary.
SYK is a nonreceptor tyrosine kinase that has long been thought to exclusively mediate signalling by receptors of the adaptive immune response (BCR and FcR). However, recent studies indicate that it also participates in innate immunity and non-immune functions.
SYK activation proceeds through binding of its two SH2 domains to ITAM tyrosines phosphorylated by SRC family kinases. Binding to phosphorylated ITAMs relieves SYK from an intramolecular autoinhibition and triggers its association with receptor-proximal signalling molecules.
SYK mediates integrin signalling in neutrophils, macrophages and platelets, signalling by the PSGL1 selectin ligand, as well as the development of osteoclasts. These responses are mediated by ITAM-containing adaptor molecules (mostly DAP12 and FcRγ).
SYK participates in innate recognition of fungal and other microbial pathogens, as well as of tissue damage, by C-type lectins. SYK activation by C-type lectins activates the CARD9-BCL10-MALT1 pathway and it is also required for NLRP3 inflammasome activation following fungal infection.
The phosphorylated ITAM-mediated activation of the SYK homologue Shark is required to remove cellular debris in Drosophila. This finding indicates an ancient origin of the ITAMSYK signalling pathway.
SYK is required for the separation of newly formed lymphatic vessels from the general vasculature. This function is likely mediated by the SYK-coupled CLEC2 receptor in platelets.
SYK has a central role in the development of allergic and autoimmune diseases, as well as in haematological malignancies such as B cell lymphomas. A novel SYK inhibitor has shown beneficial effects in human rheumatoid arthritis and B cell lymphomas.
Acknowledgement
We apologize to the many colleagues whose work could not be discussed due to space restriction; as well as for the overt focus of this Review on mouse knockout studies. We thank the members of our laboratories for stimulating discussions and Mark Kahn for sharing unpublished results. The authors' laboratories are supported by the European Research Council, the Wellcome Trust and the Hungarian Office for Research and Technology (A. M.), the German Research Foundation and the German Cancer Aid (J. R.), as well as by the UK Medical Research Council (V. T.).
Glossary terms
- immunoreceptor tyrosine-based activation motifs (ITAMs)
An ITAM is a short peptide motif containing two tyrosine residues that is found in the cytoplasmic tail of several signalling adaptor proteins and is necessary to recruit proteins that are involved in triggering activating signalling proteins. The consensus sequence is Tyr-X-X-(Leu/Ile)-X6–12-Tyr-X-X-(Leu/Ile), where X denotes any amino acid.
- petechia
A small red spot on the skin that results from a small bleeding due to a broken blood vessels. A large number of petechiae usually indicates a platelet defect. Note that, in contrast to the original assumptions, the red spots of Syk-deficient embryos are not bona fide petechiae since they are caused by a vascular developmental defect.
- Glycoprotein VI (GpVI)
One of several collagen receptors expressed on platelets. GpVI is a transmembrane glycoprotein closely related to Fcα-receptors. It associates with, and signals through, the ITAM-containing FcRγ adaptor.
- haemostasis
The combination of events that result in cessation of bleeding. Heamostatic processes include constriction of blood vessels, platelet aggregation and coagulation (clotting) of the blood.
- osteoclasts
Large multinucleated cells that arise from macrophage precursors and are specialized for bone resorption. Once firmly adhered to the bone surface, osteoclasts release hypochlorous acid (HCl) and hydrolytic enzymes to digest the bone tissue.
- antibody-dependent cell-mediated cytotoxicity (ADCC)
A cytotoxic mechanism by which an antibody-coated target cell is directly killed by a leukocyte that expresses Fc receptors, such as a natural killer cell, macrophage or neutrophil.
- pre-B cell receptor (pre-BCR)
A receptor that is formed at the surface of pre-B cells by the pairing of rearranged immunoglobulin heavy chains with surrogate heterodimer of Igα and Igβ. Signalling by the pre-BCR occurs in the absence of known ligands and is a crucial event in B cell development.
- pro-B cells
Cells in the earliest stage of B cell development in the bone marrow. They are characterized by incomplete immunoglobulin heavy-chain rearrangements and are defined as CD19+cytoplasmic IgM– or, sometimes, as B220+CD43+ (by the Hardy classification scheme).
- pre-B cell
Cells in a stage of B cell development in the bone marrow that are characterized by complete immunoglobulin heavy-chain rearrangement in the absence of immunoglobulin light-chain rearrangement. They express the pre-B cell receptor, which comprises a pseudo light chain and a heavy chain.
- transitional type 0 (T0) stage
Transitional B cells are recent emigrants from the bone marrow that are in the process of maturing into follicular and marginal zone B cells. They can be found in the spleen and typically have a half-life of a few days. Transitional B cells have been subdivided into subsets type 0 – 3 (T0 – T3) based on expression of IgM, IgD, CD93 and CD23. T0 B cells are the earliest immigrants from the marrow and are located in the red pulp of the spleen.
- pre-T cell receptor (pre-TCR)
A cell surface receptor complex consisting of TCRβ, pre-TCRα and CD3 that is expressed by immature CD4−CD8− thymocytes. Signalling through this complex is essential for maturation to the CD4+CD8+ stage
- paracaspase
A group of proteolytic enzymes distinct from, but closely related to, the caspases. The most important mammalian paracaspase is the mucosa-associated lymphoid tissue 1 (MALT1) paracaspase.
- E3 ubiquitin ligase
Ubiquitination of proteins target them for proteasomal degradation, hence it determines the half-life of proteins. E3 ubiquitin ligases are proteins responsible for specifically targeting ubiquitin to the proteins to be degraded. The other two ubiquitin ligase families (E1 and E2) are required to supply ubiquitin for E3 ubiquitin ligases.
- hemITAM
A peptide motif in the cytoplasmic tail of various C-type lectins, characterized by the presence of a single Tyr-X-X-Leu sequence (in contrast to the two such sequences in full ITAMs). Phosphorylation of the Tyr residue in the hemITAMs leads to binding to the SYK SH2-domains in a 2:1 stoichiometry.
- phosphomimetic
An amino acid whose conformation resembles that of a phosphate residue, or that of the phosphorylated form of another amino acid. Due to their negative charge, the most important phosphomimetic amino acids are glutamate and aspartate.
- vasculopathy
A disorder of blood vessels, such as the inflammation of the vessel wall.
- heterologous expression systems
An analytical tool relying on the forced expression of a protein in a cell type (usually a long-prpagated cell line) where it is not expressed normally. Heterologous expression systems are highly powerful research tools but may lead to artefactual results due to the expression of proteins in a foreign environment.
- inside-out signalling
The process by which intracellular signalling mechanisms result in the activation of a cell surface receptor, such as integrins. By contrast, outside-in signalling is the process by which ligation of a cell surface receptor activates signalling pathways inside the cell.
- T helper 17 (TH17) cell
A subset of CD4+ T helper cells that produce interleukin-17 (IL-17) and that are thought to be important in inflammatory and autoimmune diseases. Their generation involves IL-23 and IL-21, as well as the transcription factors RORγt (retinoic-acid-receptor-related orphan receptor-γt) and STAT3 (signal transducer and activator of transcription 3).
- inflammasome
An inflammasome is a molecular complex of several proteins that upon assembly cleaves pro-IL-1, thereby producing active IL-1.
- zymosan
A protein-carbohydrate complex prepared from yeast wall extract. Its polysacharide components (β-glucans) contains D-glucose monomers linked by β-type glycosidic bonds. β-glucans are particularly strong activators on anti-fungal host defence.
- monosodium urate
Monosodium salt of uric acid. It has a tendency to precipitate in needle-shaped monosodium urate crystals in the joints, leading to gout, a painful joint inflammatory disease.
- osteoblasts
Bone-lining fibroblast-related cells of non-haematopoietic origin that specialize themselves for the generation of bone matrix and the regulation of bone metabolism. Through expression of receptor-activator of NF-κB (RANK) ligand, they also induce development and activation of osteoclasts.
- receptor activator of NF-κB (RANK)
A cytokine receptor closely related to tumor necrosis factor (TNF) receptors that is primarily expressed in the osteoclast lineage. Ligation of RANK on early marophage precursors by RANK ligand expressed on osteoblasts triggers re-programming of the cell leading to osteoclast development.
- Nasu-Hakola disease
An inherited human disease characterised by presenile dementia and bone cysts. It is also known as polycystic lipomembranous osteodysplasia with sclerosing leucoencephalopathy (PLOSL). Nasu-Hakola disease is caused by loss-of-function mutations in the ITAM-cotaining DAP12 adapter or the DAP12-associated TREM2 receptor. The clinical manifestations of Nasu-Hakola disease are likely due to the loss of DAP12 or TREM2 in osteoclasts and microglia.
- osteopetrosis
A rare inherited disease characterized by pathological accumulation of mineralised bone matrix in the bones. Despite the increased bone mass, osteopetrotic bones are very fragile due to abnormal bone composition and structure. Osteopetrosis is caused by the lack or disfunction of osteoclasts. Therefore, it can be cured by bone marrow transplantation.
- podoplanin
A 43 kDa syaloglycoprotein originally identified from the podocytes of glomerular membrane but later shown to be expressed by several other cell types incuding lymphatic endothelial cells and tumor cells. Its name comes for extensive flattening of podoplanin-deficient podocytes. Podoplanin is a natural ligand of CLEC2 and induces CLEC2-mediated platelet aggregation.
- rhodocytin
A snake venom toxin that triggers platelet activation by binding to the CLEC2 receptor on platelets. Rhodocytin is a disulphide-linked heterodimer reminiscent of certain C-type lectins.
- reverse passive Arthus reaction
An antibody-mediated local hypersensitivity reaction triggered by systemic injection of a antigen, followed by local (e. g. subcutaneous) injection of antibody against the given antigen. The local formation of immune complexes results in local edema and inflammation, which is mediated by cell surface Fc-receptors and activation of the complement cascade.
- C-type lectins
Carbohydrate-recognizing proteins (lectins) that require Ca2+ ions for their proper function. A characteristic feature of their structure is a carbohydrate recognition domain (CRD). C-type lectins include soluble molecules such as the complement-activating mannose binding lectin, as well as cell surface receptors such as various CLEC proteins (e.g. CLEC7A or CLEC4E).
- slow rolling
The reduction of leukocyte rolling velocity in an inflammatory environment. Under normal conditions, leukocytes roll on the surface of endothelial cells at a speed of approx. 40 μm/s, primarily determined by the low-level expression of endothelial P-selectins. At the site of inflammation, E-selectins appear at high density on the endothelial surface, leading to slowing down of the rolling cells to approx. 5 μm/s.
Biographies
Author biographies
Attila Mócsai received his M.D. and Ph.D. degrees from the Semmelweis University, Budapest (Hungary) and his postdoctoral training from the University of California, San Francisco (USA). He then joined the the Semmelweis University School of Medicine in Budapest, where he is currently associate professor at the Department of Physiology and heads the Laboratory of Inflammatory Physiology. He is also an International Senior Research Fellow of the Wellcome Trust. Attila Mócsai's research focuses on signal transduction mechanisms in phagocytic cells (primarily neutrophils, macrophages and osteoclasts), as well as the molecular mechanisms of autoimmune inflammatory diseases.
Victor Tybulewicz is head of the Division of Immune Cell Biology at the Medical Research Council (MRC) National Institute for Medical Research, London, UK. He obtained his Ph.D. at the MRC Laboratory of Molecular Biology, Cambridge, UK, studying ATP synthase, and then trained as a postdoctoral fellow at the Whitehead Institute, Cambridge, Massachusetts, USA, developing mouse gene targeting techniques. His current interests are in lymphocyte signal transduction and the genetics of Down Syndrome.
Jürgen Ruland heads the Molecular Immunology Research Group, and is a clinical immunologist of the Third Department of Medicine, at the Technical University of Munich (Germany). He had previously trained with Tak W. Mak at the Amgen Institute in Toronto (Canada). Jürgen Ruland's main interests are the signalling pathways linking innate immune recognition to transcriptional regulation, as well as the molecular basis of haematological malignancies.
Footnotes
Further information
Attila Mocsai's page on the Phagocytes server: www.phagocytes.net/groups/mocsai.html
Victor Tybulewicz's page at the NIMR: www.nimr.mrc.ac.uk/immcellbiol/tybulewicz
Rigel, Inc., developing SYK inhibitors: www.rigel.com
SYK in the UCSD-Nature Molecule Pages: www.signaling-gateway.org/molecule/query;jsessionid=84f9144b229c8a6520e5196a4aa29a7884b67d528a32?afcsid=A000040
SYK in the Mouse Genome Informatics Database: http://www.informatics.jax.org//searches/accession_report.cgi?id=MGI:99515
References
- 1.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–4. [PubMed] [Google Scholar]
- 2.Poole A, et al. The Fc receptor γ-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. EMBO J. 1997;16:2333–41. doi: 10.1093/emboj/16.9.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abtahian F, et al. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science. 2003;299:247–51. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that genetic deficiency of Syk or SLP-76 leads to defective separation of blood and lymphatic vessels.
- 4.Ziegenfuss JS, et al. Draper-dependent glial phagocytic activity is mediated by Src and Syk family kinase signalling. Nature. 2008;453:935–9. doi: 10.1038/nature06901. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified a key role for the SYK family member Shark in damaged cell recognition signaling in Drosophila.
- 5.Mócsai A, et al. The immunomodulatory adapter proteins DAP12 and Fc receptor γ-chain (FcRγ) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci USA. 2004;101:6158–63. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with refs 132,133, this paper demonstrated that SYK activation through DAP12 and FcRγ is required for osteoclast development and bone resorption
- 6.Jakus Z, Fodor S, Abram CL, Lowell CA, Mócsai A. Immunoreceptor-like signaling by β2 and β3 integrins. Trends Cell Biol. 2007;17:493–501. doi: 10.1016/j.tcb.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Kerrigan AM, Brown GD. Syk-coupled C-type lectin receptors that mediate cellular activation via single tyrosine based activation motifs. Immunol Rev. 2010;234:335–52. doi: 10.1111/j.0105-2896.2009.00882.x. [DOI] [PubMed] [Google Scholar]
- 8.Pao LI, Bedzyk WD, Persin C, Cambier JC. Molecular targets of CD45 in B cell antigen receptor signal transduction. J Immunol. 1997;158:1116–24. [PubMed] [Google Scholar]
- 9.Turner M, Schweighoffer E, Colucci F, Di Santo JP, Tybulewicz VL. Tyrosine kinase SYK: essential functions for immunoreceptor signalling. Immunol Today. 2000;21:148–54. doi: 10.1016/s0167-5699(99)01574-1. [DOI] [PubMed] [Google Scholar]
- 10.Iwaki S, Jensen BM, Gilfillan AM. Ntal/Lab/Lat2. Int J Biochem Cell Biol. 2007;39:868–73. doi: 10.1016/j.biocel.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gross O, et al. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature. 2006;442:651–6. doi: 10.1038/nature04926. [DOI] [PubMed] [Google Scholar]; Together with ref. 99, this study revealed that the CARD9 adaptor protein acting downstream of ITAM-coupled receptors induces myeloid cell activation and regulation of the innate immune response.
- 12.Gaide O, et al. CARMA1 is a critical lipid raft-associated regulator of TCR-induced NF-κB activation. Nat Immunol. 2002;3:836–43. doi: 10.1038/ni830. [DOI] [PubMed] [Google Scholar]
- 13.Hara H, et al. The MAGUK family protein CARD11 is essential for lymphocyte activation. Immunity. 2003;18:763–75. doi: 10.1016/s1074-7613(03)00148-1. [DOI] [PubMed] [Google Scholar]
- 14.Wang D, et al. A requirement for CARMA1 in TCR-induced NF-κB activation. Nat Immunol. 2002;3:830–5. doi: 10.1038/ni824. [DOI] [PubMed] [Google Scholar]
- 15.Ruefli-Brasse AA, French DM, Dixit VM. Regulation of NF-κB-dependent lymphocyte activation and development by paracaspase. Science. 2003;302:1581–4. doi: 10.1126/science.1090769. [DOI] [PubMed] [Google Scholar]
- 16.Gross O, et al. Multiple ITAM-coupled NK-cell receptors engage the Bcl10/Malt1 complex via Carma1 for NF-κB and MAPK activation to selectively control cytokine production. Blood. 2008;112:2421–8. doi: 10.1182/blood-2007-11-123513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klemm S, et al. The Bcl10-Malt1 complex segregates FcεRI-mediated nuclear factor κB activation and cytokine production from mast cell degranulation. J Exp Med. 2006;203:337–47. doi: 10.1084/jem.20051982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hara H, Saito T. CARD9 versus CARMA1 in innate and adaptive immunity. Trends Immunol. 2009;30:234–42. doi: 10.1016/j.it.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Veillette A, Latour S, Davidson D. Negative regulation of immunoreceptor signaling. Annu Rev Immunol. 2002;20:669–707. doi: 10.1146/annurev.immunol.20.081501.130710. [DOI] [PubMed] [Google Scholar]
- 20.Lupher ML, Jr., et al. Cbl-mediated negative regulation of the Syk tyrosine kinase. A critical role for Cbl phosphotyrosine-binding domain binding to Syk phosphotyrosine 323. J Biol Chem. 1998;273:35273–81. doi: 10.1074/jbc.273.52.35273. [DOI] [PubMed] [Google Scholar]
- 21.Yankee TM, Keshvara LM, Sawasdikosol S, Harrison ML, Geahlen RL. Inhibition of signaling through the B cell antigen receptor by the protooncogene product, c-Cbl, requires Syk tyrosine 317 and the c-Cbl phosphotyrosine-binding domain. J Immunol. 1999;163:5827–35. [PubMed] [Google Scholar]
- 22.Rao N, Dodge I, Band H. The Cbl family of ubiquitin ligases: critical negative regulators of tyrosine kinase signaling in the immune system. J Leukoc Biol. 2002;71:753–63. [PubMed] [Google Scholar]
- 23.Turner M, et al. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]; Together with ref. 24, this paper showed that genetic deficiency of SYK leads to perinatal lethality and blocked B-cell development.
- 24.Cheng AM, et al. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;378:303–6. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 25.Chan AC, et al. ZAP-70 deficiency in an autosomal recessive form of severe combined immunodeficiency. Science. 1994;264:1599–601. doi: 10.1126/science.8202713. [DOI] [PubMed] [Google Scholar]
- 26.Elder ME, et al. Human severe combined immunodeficiency due to a defect in ZAP-70, a T cell tyrosine kinase. Science. 1994;264:1596–9. doi: 10.1126/science.8202712. [DOI] [PubMed] [Google Scholar]
- 27.Negishi I, et al. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995;376:435–8. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- 28.Henderson RB, et al. A novel Rac-dependent checkpoint in B cell development controls entry into the splenic white pulp and cell survival. J Exp Med. 2010 doi: 10.1084/jem.20091489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schweighoffer E, Vanes L, Mathiot A, Nakamura T, Tybulewicz VL. Unexpected requirement for ZAP-70 in pre-B cell development and allelic exclusion. Immunity. 2003;18:523–33. doi: 10.1016/s1074-7613(03)00082-7. [DOI] [PubMed] [Google Scholar]
- 30.Palacios EH, Weiss A. Distinct roles for Syk and ZAP-70 during early thymocyte development. J Exp Med. 2007;204:1703–15. doi: 10.1084/jem.20070405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costello PS, et al. Critical role for the tyrosine kinase Syk in signalling through the high affinity IgE receptor of mast cells. Oncogene. 1996;13:2595–605. [PubMed] [Google Scholar]
- 32.Crowley MT, et al. A critical role for Syk in signal transduction and phagocytosis mediated by Fcγ receptors on macrophages. J Exp Med. 1997;186:1027–39. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiefer F, et al. The Syk protein tyrosine kinase is essential for Fcγ receptor signaling in macrophages and neutrophils. Mol Cell Biol. 1998;18:4209–20. doi: 10.1128/mcb.18.7.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colucci F, et al. Natural cytotoxicity uncoupled from the Syk and ZAP-70 intracellular kinases. Nat Immunol. 2002;3:288–94. doi: 10.1038/ni764. [DOI] [PubMed] [Google Scholar]
- 35.Kane LP, Lin J, Weiss A. Signal transduction by the TCR for antigen. Curr Opin Immunol. 2000;12:242–9. doi: 10.1016/s0952-7915(00)00083-2. [DOI] [PubMed] [Google Scholar]
- 36.Au-Yeung BB, et al. The structure, regulation, and function of ZAP-70. Immunol Rev. 2009;228:41–57. doi: 10.1111/j.1600-065X.2008.00753.x. [DOI] [PubMed] [Google Scholar]
- 37.Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–34. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 38.Siraganian RP, Zhang J, Suzuki K, Sada K. Protein tyrosine kinase Syk in mast cell signaling. Mol Immunol. 2002;38:1229–33. doi: 10.1016/s0161-5890(02)00068-8. [DOI] [PubMed] [Google Scholar]
- 39.Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igα/β heterodimer. Cell. 2004;117:787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 40.Kitaura J, et al. Evidence that IgE molecules mediate a spectrum of effects on mast cell survival and activation via aggregation of the FcεRI. Proc Natl Acad Sci U S A. 2003;100:12911–6. doi: 10.1073/pnas.1735525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohno M, Yamasaki S, Tybulewicz VL, Saito T. Rapid and large amount of autocrine IL-3 production is responsible for mast cell survival by IgE in the absence of antigen. Blood. 2005;105:2059–65. doi: 10.1182/blood-2004-07-2639. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki-Inoue K, et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107:542–9. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- 43.Rogers NC, et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–17. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]; This study demonstrated a role for SYK in innate immunity triggered by C-type lectins.
- 44.Fuller GL, et al. The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signaling cascade. J Biol Chem. 2007;282:12397–409. doi: 10.1074/jbc.M609558200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hughes CE, et al. CLEC-2 activates Syk through dimerisation. Blood. 2010 doi: 10.1182/blood-2009-08-237834. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–79. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsang E, et al. Molecular mechanism of the Syk activation switch. J Biol Chem. 2008;283:32650–9. doi: 10.1074/jbc.M806340200. [DOI] [PubMed] [Google Scholar]
- 48.Deindl S, et al. Structural basis for the inhibition of tyrosine kinase activity of ZAP-70. Cell. 2007;129:735–46. doi: 10.1016/j.cell.2007.03.039. [DOI] [PubMed] [Google Scholar]; This paper provided the first high-resolution 3D structure of a full-length SYK-related kinase (ZAP70) and provided an explanation for the activation mechanism of SYK-family kinases.
- 49.Deindl S, Kadlecek TA, Cao X, Kuriyan J, Weiss A. Stability of an autoinhibitory interface in the structure of the tyrosine kinase ZAP-70 impacts T cell receptor response. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0911512106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arias-Palomo E, Recuero-Checa MA, Bustelo XR, Llorca O. 3D structure of Syk kinase determined by single-particle electron microscopy. Biochim Biophys Acta. 2007;1774:1493–9. doi: 10.1016/j.bbapap.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adachi T, Wienands J, Tsubata T, Kurosaki T. Interdomain A is crucial for ITAM-dependent and -independent regulation of Syk. Biochem Biophys Res Commun. 2007;364:111–7. doi: 10.1016/j.bbrc.2007.09.100. [DOI] [PubMed] [Google Scholar]
- 52.Rolli V, et al. Amplification of B cell antigen receptor signaling by a Syk/ITAM positive feedback loop. Mol Cell. 2002;10:1057–69. doi: 10.1016/s1097-2765(02)00739-6. [DOI] [PubMed] [Google Scholar]
- 53.Futterer K, Wong J, Grucza RA, Chan AC, Waksman G. Structural basis for Syk tyrosine kinase ubiquity in signal transduction pathways revealed by the crystal structure of its regulatory SH2 domains bound to a dually phosphorylated ITAM peptide. J Mol Biol. 1998;281:523–37. doi: 10.1006/jmbi.1998.1964. [DOI] [PubMed] [Google Scholar]
- 54.Grucza RA, Futterer K, Chan AC, Waksman G. Thermodynamic study of the binding of the tandem-SH2 domain of the Syk kinase to a dually phosphorylated ITAM peptide: evidence for two conformers. Biochemistry. 1999;38:5024–33. doi: 10.1021/bi9829938. [DOI] [PubMed] [Google Scholar]
- 55.Fodor S, Jakus Z, Mócsai A. ITAM-based signaling beyond the adaptive immune response. Immunol Lett. 2006;104:29–37. doi: 10.1016/j.imlet.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 56.Furlong MT, et al. Identification of the major sites of autophosphorylation of the murine protein-tyrosine kinase Syk. Biochim Biophys Acta. 1997;1355:177–90. doi: 10.1016/s0167-4889(96)00131-0. [DOI] [PubMed] [Google Scholar]
- 57.Keshvara LM, Isaacson C, Harrison ML, Geahlen RL. Syk activation and dissociation from the B-cell antigen receptor is mediated by phosphorylation of tyrosine 130. J Biol Chem. 1997;272:10377–81. doi: 10.1074/jbc.272.16.10377. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, et al. Tyr130 phosphorylation triggers Syk release from antigen receptor by long-distance conformational uncoupling. Proc Natl Acad Sci U S A. 2008;105:11760–5. doi: 10.1073/pnas.0708583105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hong JJ, Yankee TM, Harrison ML, Geahlen RL. Regulation of Signaling in B Cells through the Phosphorylation of Syk on Linker Region Tyrosines. A MECHANISM FOR NEGATIVE SIGNALING BY THE Lyn TYROSINE KINASE. J. Biol. Chem. 2002;277:31703–31714. doi: 10.1074/jbc.M201362200. [DOI] [PubMed] [Google Scholar]
- 60.Sada K, Zhang J, Siraganian RP. Point mutation of a tyrosine in the linker region of Syk results in a gain of function. J. Immunol. 2000;164:338–44. doi: 10.4049/jimmunol.164.1.338. [DOI] [PubMed] [Google Scholar]
- 61.Moon KD, et al. Molecular basis for a direct interaction between the Syk protein-tyrosine kinase and phosphoinositide 3-kinase. J. Biol. Chem. 2004;280:1543–1551. doi: 10.1074/jbc.M407805200. [DOI] [PubMed] [Google Scholar]
- 62.Simon M, Vanes L, Geahlen RL, Tybulewicz VL. Distinct roles for the linker region tyrosines of Syk in FcεRI signaling in primary mast cells. J Biol Chem. 2005;280:4510–7. doi: 10.1074/jbc.M410326200. [DOI] [PubMed] [Google Scholar]
- 63.Brdicka T, Kadlecek TA, Roose JP, Pastuszak AW, Weiss A. Intramolecular regulatory switch in ZAP-70: analogy with receptor tyrosine kinases. Mol Cell Biol. 2005;25:4924–33. doi: 10.1128/MCB.25.12.4924-4933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kulathu Y, Grothe G, Reth M. Autoinhibition and adapter function of Syk. Immunol Rev. 2009;232:286–99. doi: 10.1111/j.1600-065X.2009.00837.x. [DOI] [PubMed] [Google Scholar]
- 65.Zeitlmann L, et al. T cell activation induced by novel gain-of-function mutants of Syk and ZAP-70. J Biol Chem. 1998;273:15445–52. doi: 10.1074/jbc.273.25.15445. [DOI] [PubMed] [Google Scholar]
- 66.Deckert M, Tartare Deckert S, Couture C, Mustelin T, Altman A. Functional and physical interactions of Syk family kinases with the Vav proto-oncogene product. Immunity. 1996;5:591–604. doi: 10.1016/s1074-7613(00)80273-3. [DOI] [PubMed] [Google Scholar]
- 67.Law CL, Chandran KA, Sidorenko SP, Clark EA. Phospholipase C-γ1 interacts with conserved phosphotyrosyl residues in the linker region of Syk and is a substrate for Syk. Mol. Cell. Biol. 1996;16:1305–15. doi: 10.1128/mcb.16.4.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Groesch TD, Zhou F, Mattila S, Geahlen RL, Post CB. Structural basis for the requirement of two phosphotyrosine residues in signaling mediated by Syk tyrosine kinase. J Mol Biol. 2006;356:1222–36. doi: 10.1016/j.jmb.2005.11.095. [DOI] [PubMed] [Google Scholar]
- 69.Zhang J, Berenstein E, Siraganian RP. Phosphorylation of Tyr342 in the Linker Region of Syk Is Critical for FcεRI Signaling in Mast Cells. Mol. Cell. Biol. 2002;22:8144–8154. doi: 10.1128/MCB.22.23.8144-8154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schymeinsky J, et al. The Vav binding site of the non-receptor tyrosine kinase Syk at Tyr 348 is critical for β2 integrin (CD11/CD18)-mediated neutrophil migration. Blood. 2006;108:3919–27. doi: 10.1182/blood-2005-12-030387. [DOI] [PubMed] [Google Scholar]
- 71.Kulathu Y, Hobeika E, Turchinovich G, Reth M. The kinase Syk as an adaptor controlling sustained calcium signalling and B-cell development. EMBO J. 2008;27:1333–44. doi: 10.1038/emboj.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mócsai A, Zhou M, Meng F, Tybulewicz VL, Lowell CA. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16:547–58. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]; Together with refs. 73,76, this study demonstrated the role of SYK in integrin signal transduction in hematopoietic cells
- 73.Obergfell A, et al. Coordinate interactions of Csk, Src, and Syk kinases with αIIbβ3 initiate integrin signaling to the cytoskeleton. J Cell Biol. 2002;157:265–75. doi: 10.1083/jcb.200112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clements JL, et al. Fetal hemorrhage and platelet dysfunction in SLP-76-deficient mice. J Clin Invest. 1999;103:19–25. doi: 10.1172/JCI5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Newbrough SA, et al. SLP-76 regulates Fcγ receptor and integrin signaling in neutrophils. Immunity. 2003;19:761–9. doi: 10.1016/s1074-7613(03)00305-4. [DOI] [PubMed] [Google Scholar]
- 76.Vines CM, et al. Inhibition of β2 integrin receptor and Syk kinase signaling in monocytes by the Src family kinase Fgr. Immunity. 2001;15:507–19. doi: 10.1016/s1074-7613(01)00221-7. [DOI] [PubMed] [Google Scholar]
- 77.Mócsai A, et al. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat Immunol. 2006;7:1326–1333. doi: 10.1038/ni1407. [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with ref. 84, this study provided the first evidence that SYK activation by integrins proceeds through an ITAM-dependent manner.
- 78.Zou W, Reeve JL, Liu Y, Teitelbaum SL, Ross FP. DAP12 Couples c-Fms Activation to the Osteoclast Cytoskeleton by Recruitment of Syk. Mol Cell. 2008;31:422–431. doi: 10.1016/j.molcel.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frommhold D, et al. Spleen tyrosine kinase Syk is critical for sustained leukocyte adhesion during inflammation in vivo. BMC Immunol. 2007;8:31. doi: 10.1186/1471-2172-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirahashi J, et al. Mac-1 signaling via Src-family and Syk kinases results in elastase-dependent thrombohemorrhagic vasculopathy. Immunity. 2006;25:271–83. doi: 10.1016/j.immuni.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 81.Gao J, Zoller KE, Ginsberg MH, Brugge JS, Shattil SJ. Regulation of the pp72syk protein tyrosine kinase by platelet integrin αIIbβ3. Embo J. 1997;16:6414–25. doi: 10.1093/emboj/16.21.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Woodside DG, et al. Activation of Syk protein tyrosine kinase through interaction with integrin β cytoplasmic domains. Curr Biol. 2001;11:1799–804. doi: 10.1016/s0960-9822(01)00565-6. [DOI] [PubMed] [Google Scholar]
- 83.Woodside DG, et al. The N-terminal SH2 domains of Syk and ZAP-70 mediate phosphotyrosine-independent binding to integrin β cytoplasmic domains. J Biol Chem. 2002;277:39401–8. doi: 10.1074/jbc.M207657200. [DOI] [PubMed] [Google Scholar]
- 84.Abtahian F, et al. Evidence for the requirement of ITAM domains but not SLP-76/Gads interaction for integrin signaling in hematopoietic cells. Mol Cell Biol. 2006;26:6936–49. doi: 10.1128/MCB.01040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zou W, et al. Syk, c-Src, the αVβ3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J Cell Biol. 2007;176:877–88. doi: 10.1083/jcb.200611083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Graham DB, et al. An ITAM-signaling pathway controls cross-presentation of particulate but not soluble antigens in dendritic cells. J Exp Med. 2007;204:2889–97. doi: 10.1084/jem.20071283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wakselman S, et al. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J Neurosci. 2008;28:8138–43. doi: 10.1523/JNEUROSCI.1006-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boylan B, et al. Identification of FcγRIIa as the ITAM-bearing receptor mediating αIIbβ3 outside-in integrin signaling in human platelets. Blood. 2008;112:2780–6. doi: 10.1182/blood-2008-02-142125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Urzainqui A, et al. ITAM-based interaction of ERM proteins with Syk mediates signaling by the leukocyte adhesion receptor PSGL-1. Immunity. 2002;17:401–12. doi: 10.1016/s1074-7613(02)00420-x. [DOI] [PubMed] [Google Scholar]
- 90.Zarbock A, Lowell CA, Ley K. Spleen tyrosine kinase Syk is necessary for E-selectin-induced αLβ2 integrin-mediated rolling on intercellular adhesion molecule-1. Immunity. 2007;26:773–83. doi: 10.1016/j.immuni.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zarbock A, et al. PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcRγ to induce slow leukocyte rolling. J Exp Med. 2008;205:2339–47. doi: 10.1084/jem.20072660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood. 2005;106:2543–50. doi: 10.1182/blood-2005-03-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gross O, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–6. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]; This study shows that fungal pathogens activate the NLRP3 inflammasome through SYK in a CARD9-independent manner
- 94.Goodridge HS, Simmons RM, Underhill DM. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol. 2007;178:3107–15. doi: 10.4049/jimmunol.178.5.3107. [DOI] [PubMed] [Google Scholar]
- 95.Slack EC, et al. Syk-dependent ERK activation regulates IL-2 and IL-10 production by DC stimulated with zymosan. Eur J Immunol. 2007;37:1600–12. doi: 10.1002/eji.200636830. [DOI] [PubMed] [Google Scholar]
- 96.LeibundGut-Landmann S, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–8. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 97.Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 98.Hara H, et al. Cell type-specific regulation of ITAM-mediated NF-kappaB activation by the adaptors, CARMA1 and CARD9. J Immunol. 2008;181:918–30. doi: 10.4049/jimmunol.181.2.918. [DOI] [PubMed] [Google Scholar]
- 99.Hara H, et al. The adaptor protein CARD9 is essential for the activation of myeloid cells through ITAM-associated and Toll-like receptors. Nat Immunol. 2007;8:619–29. doi: 10.1038/ni1466. [DOI] [PubMed] [Google Scholar]
- 100.Glocker EO, et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361:1727–35. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Leibundgut-Landmann S, Osorio F, Brown GD, Reis e Sousa C. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood. 2008;112:4971–80. doi: 10.1182/blood-2008-05-158469. [DOI] [PubMed] [Google Scholar]
- 102.Robinson MJ, et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med. 2009;206:2037–51. doi: 10.1084/jem.20082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sato K, et al. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor γ chain to induce innate immune responses. J Biol Chem. 2006;281:38854–66. doi: 10.1074/jbc.M606542200. [DOI] [PubMed] [Google Scholar]
- 104.Wells CA, et al. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol. 2008;180:7404–13. doi: 10.4049/jimmunol.180.11.7404. [DOI] [PubMed] [Google Scholar]
- 105.Yamasaki S, et al. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc Natl Acad Sci USA. 2009;106:1897–902. doi: 10.1073/pnas.0805177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Werninghaus K, et al. Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRγ-Syk-Card9-dependent innate immune activation. J Exp Med. 2009;206:89–97. doi: 10.1084/jem.20081445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ishikawa E, et al. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med. 2009;206:2879–88. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–65. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 109.Shio MT, et al. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS Pathog. 2009;5:e1000559. doi: 10.1371/journal.ppat.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hise AG, et al. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5:487–97. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schoenen H, et al. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J Immunol. 2010;184:2756–60. doi: 10.4049/jimmunol.0904013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sarantis H, Gray-Owen SD. The specific innate immune receptor CEACAM3 triggers neutrophil bactericidal activities via a Syk kinase-dependent pathway. Cell Microbiol. 2007;9:2167–80. doi: 10.1111/j.1462-5822.2007.00947.x. [DOI] [PubMed] [Google Scholar]
- 113.Van Ziffle JA, Lowell CA. Neutrophil-specific deletion of Syk kinase results in reduced host defense to bacterial infection. Blood. 2009;114:4871–82. doi: 10.1182/blood-2009-05-220806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen ST, et al. CLEC5A is critical for dengue-virus-induced lethal disease. Nature. 2008;453:672–6. doi: 10.1038/nature07013. [DOI] [PubMed] [Google Scholar]
- 115.Yamasaki S, et al. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9:1179–88. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]; Together with ref. 116, this study demonstrated important roles for SYK coupled C type lectins in recognition of necrotic cells and the resulting immune activation in mammals.
- 116.Sancho D, et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huysamen C, Willment JA, Dennehy KM, Brown GD. CLEC9A is a novel activation C-type lectin-like receptor expressed on BDCA3+ dendritic cells and a subset of monocytes. J Biol Chem. 2008;283:16693–701. doi: 10.1074/jbc.M709923200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Awasaki T, et al. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron. 2006;50:855–67. doi: 10.1016/j.neuron.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 119.Ford JW, McVicar DW. TREM and TREM-like receptors in inflammation and disease. Curr Opin Immunol. 2009;21:38–46. doi: 10.1016/j.coi.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tassiulas I, et al. Amplification of IFN-α-induced STAT1 activation and inflammatory function by Syk and ITAM-containing adaptors. Nat Immunol. 2004;5:1181–9. doi: 10.1038/ni1126. [DOI] [PubMed] [Google Scholar]
- 121.Hida S, et al. Fc receptor γ-chain, a constitutive component of the IL-3 receptor, is required for IL-3-induced IL-4 production in basophils. Nat Immunol. 2009;10:214–22. doi: 10.1038/ni.1686. [DOI] [PubMed] [Google Scholar]
- 122.Otero K, et al. Macrophage colony-stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and β-catenin. Nat Immunol. 2009;10:734–43. doi: 10.1038/ni.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lang P, et al. TCR-induced transmembrane signaling by peptide/MHC class II via associated Ig-α/β dimers. Science. 2001;291:1537–40. doi: 10.1126/science.291.5508.1537. [DOI] [PubMed] [Google Scholar]
- 124.McVicar DW, et al. DAP12-mediated signal transduction in natural killer cells. A dominant role for the Syk protein-tyrosine kinase. J Biol Chem. 1998;273:32934–42. doi: 10.1074/jbc.273.49.32934. [DOI] [PubMed] [Google Scholar]
- 125.Zompi S, et al. NKG2D triggers cytotoxicity in mouse NK cells lacking DAP12 or Syk family kinases. Nat Immunol. 2003;4:565–72. doi: 10.1038/ni930. [DOI] [PubMed] [Google Scholar]
- 126.Billadeau DD, Upshaw JL, Schoon RA, Dick CJ, Leibson PJ. NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway. Nat Immunol. 2003;4:557–64. doi: 10.1038/ni929. [DOI] [PubMed] [Google Scholar]
- 127.Ng G, et al. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity. 2008;29:807–18. doi: 10.1016/j.immuni.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mócsai A, et al. G-protein-coupled receptor signaling in Syk-deficient neutrophils and mast cells. Blood. 2003;101:4155–63. doi: 10.1182/blood-2002-07-2346. [DOI] [PubMed] [Google Scholar]
- 129.Paloneva J, et al. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat Genet. 2000;25:357–61. doi: 10.1038/77153. [DOI] [PubMed] [Google Scholar]
- 130.Kaifu T, et al. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J Clin Invest. 2003;111:323–32. doi: 10.1172/JCI16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Humphrey MB, et al. The signaling adapter protein DAP12 regulates multinucleation during osteoclast development. J Bone Miner Res. 2004;19:224–34. doi: 10.1359/JBMR.0301234. [DOI] [PubMed] [Google Scholar]
- 132.Koga T, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–63. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 133.Faccio R, Zou W, Colaianni G, Teitelbaum SL, Ross FP. High dose M-CSF partially rescues the Dap12−/− osteoclast phenotype. J Cell Biochem. 2003;90:871–83. doi: 10.1002/jcb.10694. [DOI] [PubMed] [Google Scholar]
- 134.May F, et al. CLEC-2 is an essential platelet-activating receptor in hemostasis and thrombosis. Blood. 2009;114:3464–72. doi: 10.1182/blood-2009-05-222273. [DOI] [PubMed] [Google Scholar]
- 135.Bezman NA, et al. Requirements of SLP76 tyrosines in ITAM and integrin receptor signaling and in platelet function in vivo. J Exp Med. 2008;205:1775–88. doi: 10.1084/jem.20080240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sebzda E, et al. Syk and Slp-76 mutant mice reveal a cell-autonomous hematopoietic cell contribution to vascular development. Dev Cell. 2006;11:349–61. doi: 10.1016/j.devcel.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 137.Bohmer R, et al. Regulation of developmental lymphangiogenesis by Syk+ leukocytes. Dev Cell. 2010;18:437–49. doi: 10.1016/j.devcel.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 138.Carramolino L, et al. Platelets Play an Essential Role in Separating the Blood and Lymphatic Vasculatures During Embryonic Angiogenesis. Circ Res. 2010 doi: 10.1161/CIRCRESAHA.110.218073. in press. [DOI] [PubMed] [Google Scholar]; Together with refs. 139-141, this study identified a podoplanin-CLEC2-SLP76-mediated platelet signaling pathway required for separation of blood and lymphatic vessels.
- 139.Bertozzi CC, et al. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood. 2010 doi: 10.1182/blood-2010-02-270876. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fu J, et al. Endothelial cell O-glycan deficiency causes blood/lymphatic misconnections and consequent fatty liver disease in mice. J Clin Invest. 2008;118:3725–37. doi: 10.1172/JCI36077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Uhrin P, et al. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood. 2010 doi: 10.1182/blood-2009-04-216069. in press. [DOI] [PubMed] [Google Scholar]
- 142.Suzuki-Inoue K, et al. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J Biol Chem. 2007;282:25993–6001. doi: 10.1074/jbc.M702327200. [DOI] [PubMed] [Google Scholar]
- 143.Coopman PJ, et al. The Syk tyrosine kinase suppresses malignant growth of human breast cancer cells. Nature. 2000;406:742–7. doi: 10.1038/35021086. [DOI] [PubMed] [Google Scholar]; This paper identified a tumor suppressor role for SYK in breast cancer
- 144.Coopman PJ, Mueller SC. The Syk tyrosine kinase: a new negative regulator in tumor growth and progression. Cancer Lett. 2006;241:159–73. doi: 10.1016/j.canlet.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 145.Yanagi S, Inatome R, Takano T, Yamamura H. Syk expression and novel function in a wide variety of tissues. Biochem Biophys Res Commun. 2001;288:495–8. doi: 10.1006/bbrc.2001.5788. [DOI] [PubMed] [Google Scholar]
- 146.Beckmann S, Buro C, Dissous C, Hirzmann J, Grevelding CG. The Syk kinase SmTK4 of Schistosoma mansoni is involved in the regulation of spermatogenesis and oogenesis. PLoS Pathog. 2010;6:e1000769. doi: 10.1371/journal.ppat.1000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jakus Z, Simon E, Balázs B, Mócsai A. Genetic deficiency of Syk protects mice from autoantibody-induced arthritis. Arthritis Rheum. 2010 doi: 10.1002/art.27438. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provided the first genetic evidence for the role of SYK in an animal model of a major human disease (rheumatoid arthritis)
- 148.Meltzer EO, Berkowitz RB, Grossbard EB. An intranasal Syk-kinase inhibitor (R112) improves the symptoms of seasonal allergic rhinitis in a park environment. J Allergy Clin Immunol. 2005;115:791–6. doi: 10.1016/j.jaci.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 149.Braselmann S, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks Fc receptor signaling and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther. 2006;319:998–1008. doi: 10.1124/jpet.106.109058. [DOI] [PubMed] [Google Scholar]; This study described and characterized an orally available SYK inhibitor for therapeutic use
- 150.Pine PR, et al. Inflammation and bone erosion are suppressed in models of rheumatoid arthritis following treatment with a novel Syk inhibitor. Clin Immunol. 2007;124:244–57. doi: 10.1016/j.clim.2007.03.543. [DOI] [PubMed] [Google Scholar]
- 151.Weinblatt ME, et al. Treatment of rheumatoid arthritis with a Syk kinase inhibitor: a twelve-week, randomized, placebo-controlled trial. Arthritis Rheum. 2008;58:3309–18. doi: 10.1002/art.23992. [DOI] [PubMed] [Google Scholar]; This paper and ref. 166 reported the results of the first human clinical trials with the SYK inhibitor fostamatinib, showing clinical benefit in rheumatoid arthritis (ref. 151) and B-cell malignancies (ref. 166)
- 152.Podolanczuk A, Lazarus AH, Crow AR, Grossbard E, Bussel JB. Of mice and men: an open-label pilot study for treatment of immune thrombocytopenic purpura by an inhibitor of Syk. Blood. 2009;113:3154–60. doi: 10.1182/blood-2008-07-166439. [DOI] [PubMed] [Google Scholar]
- 153.Goodman PA, Wood CM, Vassilev A, Mao C, Uckun FM. Spleen tyrosine kinase (Syk) deficiency in childhood pro-B cell acute lymphoblastic leukemia. Oncogene. 2001;20:3969–78. doi: 10.1038/sj.onc.1204515. [DOI] [PubMed] [Google Scholar]
- 154.Jumaa H, et al. Deficiency of the adaptor SLP-65 in pre-B-cell acute lymphoblastic leukaemia. Nature. 2003;423:452–6. doi: 10.1038/nature01608. [DOI] [PubMed] [Google Scholar]
- 155.Flemming A, Brummer T, Reth M, Jumaa H. The adaptor protein SLP-65 acts as a tumor suppressor that limits pre-B cell expansion. Nat Immunol. 2003;4:38–43. doi: 10.1038/ni862. [DOI] [PubMed] [Google Scholar]
- 156.Wossning T, et al. Deregulated Syk inhibits differentiation and induces growth factor-independent proliferation of pre-B cells. J Exp Med. 2006;203:2829–40. doi: 10.1084/jem.20060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Uckun FM, Ek RO, Jan ST, Chen CL, Qazi S. Targeting SYK kinase-dependent anti-apoptotic resistance pathway in B-lineage acute lymphoblastic leukaemia (ALL) cells with a potent SYK inhibitory pentapeptide mimic. Br J Haematol. 2010 doi: 10.1111/j.1365-2141.2010.08106.x. in press. [DOI] [PubMed] [Google Scholar]
- 158.Monroe JG. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nat Rev Immunol. 2006;6:283–94. doi: 10.1038/nri1808. [DOI] [PubMed] [Google Scholar]
- 159.Davis RE, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gururajan M, Jennings CD, Bondada S. Cutting edge: constitutive B cell receptor signaling is critical for basal growth of B lymphoma. J Immunol. 2006;176:5715–9. doi: 10.4049/jimmunol.176.10.5715. [DOI] [PubMed] [Google Scholar]
- 161.Buchner M, et al. Spleen tyrosine kinase is overexpressed and represents a potential therapeutic target in chronic lymphocytic leukemia. Cancer Res. 2009;69:5424–32. doi: 10.1158/0008-5472.CAN-08-4252. [DOI] [PubMed] [Google Scholar]
- 162.Carsetti L, et al. Phosphorylation of the activation loop tyrosines is required for sustained Syk signaling and growth factor-independent B-cell proliferation. Cell Signal. 2009;21:1187–94. doi: 10.1016/j.cellsig.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 163.Buchner M, et al. Spleen tyrosine kinase inhibition prevents chemokine- and integrin-mediated stromal protective effects in chronic lymphocytic leukemia. Blood. 2010 doi: 10.1182/blood-2009-07-233692. in press. [DOI] [PubMed] [Google Scholar]
- 164.Chen L, et al. SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood. 2008;111:2230–7. doi: 10.1182/blood-2007-07-100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Young RM, et al. Mouse models of non-Hodgkin lymphoma reveal Syk as an important therapeutic target. Blood. 2009;113:2508–16. doi: 10.1182/blood-2008-05-158618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Friedberg JW, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115:2578–85. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Feldman AL, et al. Overexpression of Syk tyrosine kinase in peripheral T-cell lymphomas. Leukemia. 2008;22:1139–43. doi: 10.1038/leu.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Streubel B, Vinatzer U, Willheim M, Raderer M, Chott A. Novel t(5;9)(q33;q22) fuses ITK to SYK in unspecified peripheral T-cell lymphoma. Leukemia. 2006;20:313–8. doi: 10.1038/sj.leu.2404045. [DOI] [PubMed] [Google Scholar]
- 169.Wilcox RA, et al. Inhibition of Syk protein tyrosine kinase induces apoptosis and blocks proliferation in T-cell non-Hodgkin's lymphoma cell lines. Leukemia. 2009 doi: 10.1038/leu.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kuno Y, et al. Constitutive kinase activation of the TEL-Syk fusion gene in myelodysplastic syndrome with t(9;12)(q22;p12) Blood. 2001;97:1050–5. doi: 10.1182/blood.v97.4.1050. [DOI] [PubMed] [Google Scholar]
- 171.Hahn CK, et al. Proteomic and Genetic Approaches Identify Syk as an AML Target. Cancer Cell. 2009;16:281–94. doi: 10.1016/j.ccr.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lanier LL. Viral immunoreceptor tyrosine-based activation motif (ITAM)-mediated signaling in cell transformation and cancer. Trends Cell Biol. 2006;16:388–90. doi: 10.1016/j.tcb.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 173.Morrison JA, Raab-Traub N. Roles of the ITAM and PY motifs of Epstein-Barr virus latent membrane protein 2A in the inhibition of epithelial cell differentiation and activation of β-catenin signaling. J Virol. 2005;79:2375–82. doi: 10.1128/JVI.79.4.2375-2382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lu J, et al. Syk tyrosine kinase mediates Epstein-Barr virus latent membrane protein 2A-induced cell migration in epithelial cells. J Biol Chem. 2006;281:8806–14. doi: 10.1074/jbc.M507305200. [DOI] [PubMed] [Google Scholar]
- 175.Katz E, et al. MMTV Env encodes an ITAM responsible for transformation of mammary epithelial cells in three-dimensional culture. J Exp Med. 2005;201:431–9. doi: 10.1084/jem.20041471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ross SR, et al. An immunoreceptor tyrosine activation motif in the mouse mammary tumor virus envelope protein plays a role in virus-induced mammary tumors. J Virol. 2006;80:9000–8. doi: 10.1128/JVI.00788-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lagunoff M, Lukac DM, Ganem D. Immunoreceptor tyrosine-based activation motif-dependent signaling by Kaposi's sarcoma-associated herpesvirus K1 protein: effects on lytic viral replication. J Virol. 2001;75:5891–8. doi: 10.1128/JVI.75.13.5891-5898.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lee BS, et al. Characterization of the Kaposi's sarcoma-associated herpesvirus K1 signalosome. J Virol. 2005;79:12173–84. doi: 10.1128/JVI.79.19.12173-12184.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Amarante MK, Watanabe MA. The possible involvement of virus in breast cancer. J Cancer Res Clin Oncol. 2009;135:329–37. doi: 10.1007/s00432-008-0511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yuan Y, et al. Frequent epigenetic inactivation of spleen tyrosine kinase gene in human hepatocellular carcinoma. Clin Cancer Res. 2006;12:6687–95. doi: 10.1158/1078-0432.CCR-06-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Layton T, Stalens C, Gunderson F, Goodison S, Silletti S. Syk tyrosine kinase acts as a pancreatic adenocarcinoma tumor suppressor by regulating cellular growth and invasion. Am J Pathol. 2009;175:2625–36. doi: 10.2353/ajpath.2009.090543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Muthusamy V, et al. Epigenetic silencing of novel tumor suppressors in malignant melanoma. Cancer Res. 2006;66:11187–93. doi: 10.1158/0008-5472.CAN-06-1274. [DOI] [PubMed] [Google Scholar]
- 183.Wang L, Devarajan E, He J, Reddy SP, Dai JL. Transcription repressor activity of spleen tyrosine kinase mediates breast tumor suppression. Cancer Res. 2005;65:10289–97. doi: 10.1158/0008-5472.CAN-05-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nakashima H, et al. Clinical significance of nuclear expression of spleen tyrosine kinase (Syk) in gastric cancer. Cancer Lett. 2006;236:89–94. doi: 10.1016/j.canlet.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 185.Wang L, et al. Alternative splicing disrupts a nuclear localization signal in spleen tyrosine kinase that is required for invasion suppression in breast cancer. Cancer Res. 2003;63:4724–30. [PubMed] [Google Scholar]
- 186.Steele RE, Stover NA, Sakaguchi M. Appearance and disappearance of Syk family protein-tyrosine kinase genes during metazoan evolution. Gene. 1999;239:91–7. doi: 10.1016/s0378-1119(99)00373-x. [DOI] [PubMed] [Google Scholar]
- 187.Suga H, et al. Extensive gene duplication in the early evolution of animals before the parazoaneumetazoan split demonstrated by G proteins and protein tyrosine kinases from sponge and hydra. J Mol Evol. 1999;48:646–53. doi: 10.1007/pl00006508. [DOI] [PubMed] [Google Scholar]
- 188.Ruvkun G, Hobert O. The taxonomy of developmental control in Caenorhabditis elegans. Science. 1998;282:2033–41. doi: 10.1126/science.282.5396.2033. [DOI] [PubMed] [Google Scholar]
- 189.Saijo K, et al. Essential role of Src-family protein tyrosine kinases in NF-κB activation during B cell development. Nat Immunol. 2003;4:274–9. doi: 10.1038/ni893. [DOI] [PubMed] [Google Scholar]
- 190.Yasuda T, et al. Erk kinases link pre-B cell receptor signaling to transcriptional events required for early B cell expansion. Immunity. 2008;28:499–508. doi: 10.1016/j.immuni.2008.02.015. [DOI] [PubMed] [Google Scholar]