Abstract
Background
High-dose methotrexate (HDMTX) is used in the treatment of certain malignancies, including leptomeningeal metastases, systemic non-Hodgkin lymphoma, acute lymphoblastic leukemia, and osteosarcoma. High circulating levels of methotrexate can cause severe myelosuppression. The present study aimed to examine the differences in plasma MTX concentrations measured by two immunoassay systems currently available in the Japanese market, a TDX/FLX analyzer and a TBA-25FR analyzer.
Methods
A total of 69 plasma samples from 16 patients were assayed by a fluorescence polarization immunoassay technique using a TDx/FLx analyzer (Abbott Diagnostics, Chicago, Illinois, U.S.A.) and a homogeneous enzyme immunoassay technique using a TBA-25FR analyzer (Toshiba Medical Systems, Tokyo, Japan).
Results
Assay results were very consistent between the two systems, with good correlation 24 h after the start of treatment (TBA-25FR = 1.06・TDX/FLX, −1.31, r = 0.99), 48 h after the start of treatment (TBA-25FR = 1.00・TDX/FLX, +0.027, r > 0.99), and 72 h after the start of treatment (TBA-25FR = 1.09・TDX/FLX, +0.011, r > 0.99).
Conclusions
The calibration curve spanned one order of magnitude with a linear working range from the lowest to the highest standard. The standard deviations show the excellent reproducibility of repeated measurements at each standard level for both immunoassay systems. However, when using the TBA-25FR, it is necessary to perform measurements in the low-concentration range with care.
Keywords: Methotrexate, High-dose Methotrexate, Therapeutic drug monitoring, Automatic immunoassay systems
Background
Methotrexate (MTX; (2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methylmethylamino] benzoyl] amino] pentanedioic acid)) is an antimetabolite drug that acts by inhibiting dihydrofolate reductase, disrupting purine synthesis, and preventing cell division. This results in the depletion of intracellular pools of reduced folates required for DNA synthesis. Rapidly dividing malignant cells that require greater amounts of reduced folates are preferentially affected by MTX, resulting in the cessation of DNA synthesis and eventual cell death. High-dose MTX (HDMTX) is used in the treatment of certain malignancies, including leptomeningeal metastases, systemic non-Hodgkin lymphoma, acute lymphoblastic leukemia, and osteosarcoma [1]. Methotrexate is predominantly eliminated by the kidneys [2–4]. Indeed, 70–90 % of the administered MTX dose is excreted unchanged in the urine [3]. Despite the fact that HDMTX is a very effective treatment, plasma MTX concentrations may become excessively high in a small proportion of patients, resulting in toxicity and elimination delay [5]. High circulating levels of methotrexate can cause severe myelosuppression. Wide inter-individual variability has been reported for the pharmacokinetic profiles of MTX in cases treated with HDMTX therapy [6]. The plasma concentration of MTX at 24 h can predict efficacy, whereas the plasma concentrations at 48 and 72 h can reflect the excretion of MTX. Elimination delay is indicated by MTX concentrations > 1.0 μmol/L at 48 h and > 0.1 μmol/L at 72 h [7, 8]. Therefore, frequent determination of MTX concentrations is needed to safely manage individual patients receiving HDMTX therapy [9, 10]. This determination is usually carried out by automated immunoassay because urgent analysis may be required. A TDX/FLX analyzer had been used but we were changed to TBA-25FR analyzer for discontinued measuring reagent for use in a TDX/FLX analyzer. There is a possibility that results of blood concentrations in same sample measured by the different assay systems may differ by those measurement equipment. However, information about the correlation of the measured values of MTX between TDX/FLX analyzer and TBA-25FR analyzer is small.
The present study aimed to examine differences in plasma MTX concentrations measured by two immunoassay systems currently available in the Japanese market. We compared the plasma MTX concentrations measured by a TDX/FLX analyzer and a TBA-25FR analyzer.
Methods
Patients
Medical records were reviewed to identify hospitalized Japanese patients treated with methotrexate at Oita University Hospital between May 2013 and December 2013. Patients who received HDMTX therapy for certain malignancies including leptomeningeal metastases, systemic non-Hodgkin lymphoma, acute lymphoblastic leukemia, and osteosarcoma were included. This study was approved by the Ethics Committee of Oita University. Since blood samples were collected for therapeutic drug monitoring and laboratory testing as part of routine patient care, written informed consent was not necessary.
Automatic immunoassay systems
The plasma MTX concentrations were determined by a fluorescence polarization immunoassay technique using a TDX/FLX analyzer (Abbott Diagnostics, Chicago, Illinois, U.S.A.) and a homogeneous enzyme immunoassay technique using a TBA-25FR analyzer (Toshiba Medical Systems, Tokyo, Japan). For the measurement of the a TDX/FLX analyzer and a TBA-25FR analyzer we were using the TDx-Methotrexate Dynapac. II (Abbott Diagnostics, Chicago, Illinois, U.S.A. Lot No.: 35351 M500) and Nanopia eTDM Methotrexate (SEKISUI MEDICAL CO., LTD. Tokyo, Japan. Lot No.: 804REL). Assays were performed at Oita University Hospital according to manufacturer instructions.
Measurement of plasma methotrexate concentrations
Plasma MTX concentrations were measured by TDX/FLX and TBA-25FR at 24, 48, and 72 h after the start of treatment. According to manufacturer’s information for the TDX/FLX analyzer, the lower limit of quantification of the assay is 0.02 μmol/L. Blood samples exceeding the upper limit of the calibration range (0.00–1.00 μmol/L) were diluted according to the manufacturer’s protocol. According to manufacturer’s information for the TDx-Methotrexate Dynapac. II, the intra- and inter-day accuracy were lower than 115 % at the 0.07, 0.40, 0.80, 5, 50 and 500 μmol/L concentration. And coefficient of variation values were less than 15 %. According to manufacturer’s information for the TBA-25FR analyzer, the lower limit of quantification of the assay was 0.04 μmol/L. Blood samples exceeding the upper limit of the calibration range (0.00–1.20 μmol/L) were diluted according to the manufacturer’s protocol. According to manufacturer’s information for the Nanopia eTDM Methotrexate, the intra- and inter-day accuracy were lower than 115 % at the 0.00, 0.05, 0.15, 0.25, 0.50 and 1.20 μmol/L concentration. And coefficient of variation values were less than 15 %.
Comparisons between the same samples
They were evaluated for accuracy intra-day, inter-day and interobserver using the same sample. We used 0.07 (CONTROL Low), 0.4 (CONTROL Middle) and 0.8 (CONTROL High) μmol/L of The Methotrexate II Controls (Abbott, Lot 37001 M100) as spiked MTX concentration. This study performed according to the FDA or the PMDA guidance for bioanalytical method validation.
Findings
Results
A total of 16 patients (12 men and 4 women) were included in the study. Their median (range) age was 32 (8–73) years and mean body weight was 50.8 (26.6–75.6) kg (Table 1). The number of total samples were 69. The number of samples that were measured at each time point 24, 48, and 72 h after the start of treatment was 23, 18, 18. The number of samples measured at other than 24, 48, and 72 hours after the start of treatment was 10.
Table 1.
Patient characteristics and clinical laboratory data
| Age (years) | 32 (8–73) |
| Gender [male/female] | 12/4 |
| Height (cm) | 165 (124.4–178) |
| Body weight (kg) | 50.8 (26.6–75.6) |
| WBC | 5.8 (1.3–22.5) |
| RBC | 3.4 (2.8–4.6) |
| HGB | 10.6 (8.3–15.1) |
| HCT | 31.6 (24.1–41.7) |
| CRP (mg/dL) | 0.20 (0.02–1.85) |
| Alb (g/dL) | 3.8 (2.7–4.5) |
| TP (g/dL) | 6.4 (4.7–7.4) |
| T.Bil (mg/dL) | 0.66 (0.19–1.74) |
| AST (IU/L) | 28.2 (14.2–177.7) |
| ALT (IU/L) | 33.8 (12.5–198.4) |
| ALP | 322.0 (189.0–514.0) |
| γ-GTP | 49.4 (11.1–222.8) |
| BUN (mg/dL) | 12.2 (2.9–30.6) |
| SCr (mg/dL) | 0.60 (0.23–2.05) |
| CCr | 99.6 (43.5–219.1) |
Data are expressed as median and interquartile range unless otherwise stated
Assay results were very consistent between the two immunoassay systems, with good correlation total samples after the start of treatment (TBA-25FR = 1.05*TDX/FLX, −0.29, r = 0.99; Fig. 1a), 24 h after the start of treatment (TBA-25FR = 1.06*TDX/FLX, −1.31, r = 0.99; Fig. 1b), 48 h after the start of treatment (TBA-25FR = 1.00*TDX/FLX, +0.027, r > 0.99; Fig. 1c), and 72 h after the start of treatment (TBA-25FR = 1.09*TDX/FLX, +0.011, r > 0.99; Fig. 1d). The data for intra- and interday precision and accuracy are presented in Table 2.
Fig. 1.
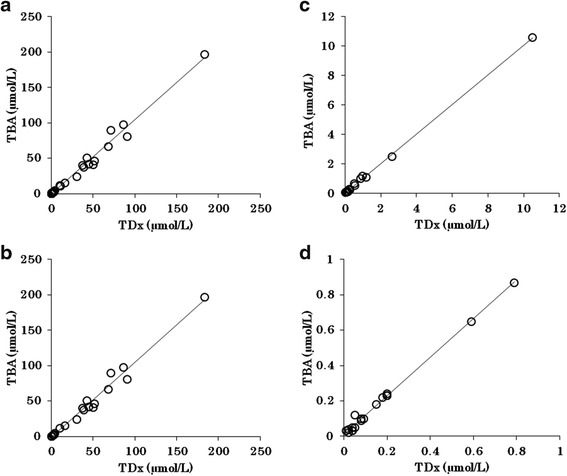
Scatter plots with fits correlating methotrexate results (μmol/L) obtained by the reference method TBA-25FR with results obtained by TDX/FLX immunoassay at total samples (a), 24 h (b), 48 h (c), and 72 h (d) after the start of treatment. Assay results were very consistent between the two immunoassay systems, with good correlation total samples after the start of treatment (TBA-25FR = 1.05*TDX/FLX, −0.29, r = 0.99; Fig. 1a), 24 h after the start of treatment (TBA-25FR = 1.06*TDX/FLX, −1.31, r = 0.99; Fig. 1b), 48 h after the start of treatment (TBA-25FR = 1.00*TDX/FLX, +0.027, r > 0.99; Fig. 1c), and 72 h after the start of treatment (TBA-25FR = 1.09*TDX/FLX, +0.011, r > 0.99; Fig. 1d)
Table 2.
Intra- and interday precision and accuracy
| Intraday variation | ||||||
| Spiked MTX concentration (μmol/L) | TDX/FLX | TBA-25FR | ||||
| Measured concentration (μmol/L) (mean ± S.D.) | Accuracy (%) | CV (%) | Measured concentration (μmol/L) (mean ± S.D.) | Accuracy (%) | CV (%) | |
| 0.07 | 0.077 ± 0.013 | 110 | 16.3 | 0.096 ± 0.011 | 137.1 | 11.2 |
| 0.4 | 0.41 ± 0.025 | 102.5 | 6.2 | 0.41 ± 0.016 | 102.5 | 4.0 |
| 0.8 | 0.85 ± 0.041 | 106.3 | 4.9 | 0.92 ± 0.040 | 115 | 4.4 |
| Interday variation | ||||||
| Spiked MTX concentration (μmol/L) | TDX/FLX | TBA-25FR | ||||
| Measured concentration (μmol/L) (mean ± S.D.) | Accuracy (%) | CV (%) | Measured concentration (μmol/L) (mean ± S.D.) | Accuracy (%) | CV (%) | |
| 0.07 | 0.073 ± 0.010 | 104.3 | 14.5 | 0.96 ± 0.013 | 137.1 | 13.1 |
| 0.4 | 0.41 ± 0.018 | 102.5 | 4.3 | 0.43 ± 0.020 | 107.5 | 4.6 |
| 0.8 | 0.83 ± 0.045 | 103.8 | 5.4 | 0.93 ± 0.051 | 116.3 | 5.4 |
Discussion
The time calibration curve showed excellent linearity, with correlation coefficients generally ≥ 0.99. The calibration curve spanned one order of magnitude with a linear working range from the lowest to the highest standard. The standard deviations show the excellent reproducibility of repeated measurements at each standard level. Linear regression of correlation data revealed that the homogeneous enzyme immunoassay technique using a TBA-25FR analyzer produced positive bias compared with the fluorescence polarization immunoassay technique using a TDX/FLX analyzer.
In the low-concentration and high-concentration regions, the TBA-25FR showed less variation when compared to TDX/FLX, but the positive bias needs to be taken into consideration.
The coefficient of variation values were less than 15 % for the TBA-25FR. Results were reproducible, although intra- and inter day accuracy was higher than 130 % at the 0.07 μmol/L concentration. This slight change means that although the variation was low and results were reproducible, the 130 % accuracy at the 0.07 concentration is an indication of less accuracy at this concentration (or a positive bias, as mentioned above) (Table 2). Interobserver precision and accuracy are result similar to the intra- and inter day accuracy (Table 3). Cause of the results it was thought to be due to the use of TDx’s QC sample. Because I perform the examination of the accuracy intra-day, inter-day and interobserver in this study in TDx-Methotrexate Dynapac. II which is QC for TDx, it is expected that TDx turned out good in comparison with TBA. The need that I evaluated using a standard reagent from this result became clear. TBA-25FR methotrexate assays have inherent limitations at the 0.07 μmol/L and 0.8 μmol/L spiked MTX concentrations, giving higher measured concentrations compared to the TDX/FLX. At the 0.07 μmol/L concentration, the accuracy of TBA-25FR immunoassay was lower than immunoassay by TDX/FLX. The cause is thought to be that the TBA-25FR analyzer has a higher lower limit of quantification concentration than the TDX/FLX. Because an actual value becomes higher than a theoretical value in the vicinity of detection limit of 0.04 g/mL in A, in A, it is thought that it is with a high value than B in the low-concentrated neighborhood.
Table 3.
Interobsever precision and accuracy
| Interobsever (n = 10) | ||||||
|---|---|---|---|---|---|---|
| Spiked MTX concentration (μmol/L) | TDX/FLX | TBA-25FR | ||||
| Measured concentration (μmol/L) (mean ± S.D.) | Accuracy (%) | CV (%) | Measured concentration (μmol/L) (mean ± S.D.) | Accuracy (%) | CV (%) | |
| 0.07 | 0.067 ± 0.005 | 95.7 | 7.9 | 0.103 ± 0.012 | 147.1 | 11.6 |
| 0.4 | 0.40 ± 0.021 | 100 | 5.3 | 0.43 ± 0.021 | 107.5 | 4.8 |
| 0.8 | 0.80 ± 0.052 | 100 | 6.4 | 0.96 ± 0.053 | 120 | 5.5 |
Conclusions
The TBA-25FR immunoassay system can play a valuable diagnostic role in patient care as a valuable adjunct to therapeutic and clinical treatment. It is able to distinguish underdosing from overdosing in HDMTX therapy. However, when using the TBA-25FR, it is necessary to perform measurements in the low-concentration range with care.
Footnotes
Competing interests
We have no competing interests with regard to our report.
Authors’ contributions
We examined differences in plasma MTX concentrations measured by two immunoassay systems currently available in the Japanese market. The present study in the blood concentration measurement of MTX in clinical practice TBA suggested potentially useful as successor instrument of TDx. All authors read and approved the final manuscript.
References
- 1.Pui CH. Recent research advances in childhood acute lymphoblastic leukemia. J Formos Med Assoc. 2010;109:777–787. doi: 10.1016/S0929-6646(10)60123-4. [DOI] [PubMed] [Google Scholar]
- 2.Skärby T, Jönsson P, Hjorth L, Behrentz M, Björk O, Forestier E, et al. High-dose methotrexate: on the relationship of methotrexate elimination time vs renal function and serum methotrexate levels in 1164 courses in 264 Swedish children with acute lymphoblastic leukaemia (ALL) Cancer Chemother Pharmacol. 2003;51:311–320. doi: 10.1007/s00280-002-0552-1. [DOI] [PubMed] [Google Scholar]
- 3.Pizzo PA, Poplack DG. Principles and practice of pediatric oncology. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 4.Fukuhara K, Ikawa K, Morikawa N, Kumagai K. Population pharmacokinetics of high-dose methotrexate in Japanese adult patients with malignancies: a concurrent analysis of the serum and urine concentration data. J Clin Pharm Ther. 2008;33:677–684. doi: 10.1111/j.1365-2710.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- 5.Rask C, Albertioni F, Bentzen SM, Schroeder H, Peterson C. Clinical and pharmacokinetic risk factors for high-dose methotrexate-induced toxicity in children with acute lymphoblastic leukemia—a logistic regression analysis. Acta Oncol. 1998;37:277–284. doi: 10.1080/028418698429586. [DOI] [PubMed] [Google Scholar]
- 6.Relling MV, Fairclough D, Ayers D, Crom WR, Rodman JH, Pui CH, et al. Patient characteristics associated with high-risk methotrexate concentrations and toxicity. J Clin Oncol. 1994;12:1667–1672. doi: 10.1200/JCO.1994.12.8.1667. [DOI] [PubMed] [Google Scholar]
- 7.Perez C, Wang YM, Sutow WW, Herson J, et al. Significance of the 48-hour plasma level in high-dose methotrexate regimens. Cancer Clin Trials. 1978;1:107–111. [PubMed] [Google Scholar]
- 8.Stoller RG, Hande KR, Jacobs SA, Rosenberg SA, Chabner BA, et al. Use of plasma pharmacokinetics to predict and prevent methotrexate toxicity. N Engl J Med. 1977;297(12):630. doi: 10.1056/NEJM197709222971203. [DOI] [PubMed] [Google Scholar]
- 9.Evans WE, Crom WR, Abromowitch M, Dodge R, Look AT, Bowman WP, et al. Clinical pharmacodynamics of high-dose methotrexate in acute lymphocytic leukemia. Identification of a relation between concentration and effect. N Engl J Med. 1986;314:471–477. doi: 10.1056/NEJM198602203140803. [DOI] [PubMed] [Google Scholar]
- 10.Galpin AJ, Evans WE. Therapeutic drug monitoring in cancer management. Clin Chem. 1993;39:2419–2430. [PubMed] [Google Scholar]


