Abstract
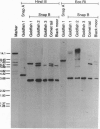
The common goldfish Carassius auratus is tetraploid and has 100 chromosomes. We describe here goldfish cDNA clones for SNAP-25, a 200-amino-acid synaptosome-associated protein that has remained highly conserved during evolution. SNAP-25 occurs as a single-copy gene in mouse, chicken, and Drosophila melanogaster. Sequences of six distinct goldfish cDNA clones and Southern hybridizations show that the goldfish has three, or possibly four, SNAP-25 loci rather than two as expected. A gene duplication early in actinopterygian fish evolution gave rise to the loci SnapA and SnapB. The proteins SNAP-A and SNAP-B are 94% and 91% identical to the mouse protein but are only 91% identical to each other. SNAP-B has a larger number of unique amino acid replacements than SNAP-A and also has more dramatic replacements. The tetraploidization resulted in two SnapB loci whose divergence from each other is consistent with a tetraploidization event 15-20 million years ago. The presence of duplicate SnapA loci has not yet been possible to confirm, possibly because they are still very similar to each other. Two of the SnapA cDNA clones and one SnapB cDNA clone have frameshift mutations. As these aberrant alleles otherwise display high sequence identity to the functional alleles, they probably became nonfunctional recently. The findings of allelic variability and aberrant alleles emphasize the importance of characterizing multiple DNA clones in tetraploid species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bark I. C. Structure of the chicken gene for SNAP-25 reveals duplicated exon encoding distinct isoforms of the protein. J Mol Biol. 1993 Sep 5;233(1):67–76. doi: 10.1006/jmbi.1993.1485. [DOI] [PubMed] [Google Scholar]
- Blomqvist A. G., Söderberg C., Lundell I., Milner R. J., Larhammar D. Strong evolutionary conservation of neuropeptide Y: sequences of chicken, goldfish, and Torpedo marmorata DNA clones. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2350–2354. doi: 10.1073/pnas.89.6.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catsicas S., Larhammar D., Blomqvist A., Sanna P. P., Milner R. J., Wilson M. C. Expression of a conserved cell-type-specific protein in nerve terminals coincides with synaptogenesis. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):785–789. doi: 10.1073/pnas.88.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel W., Schmidtke J., Vogel W., Wolf U. Genetic polymorphism of lactate dehydrogenase isoenzymes in the carp (Cyprinus carpio) apparently due to a "null allele". Biochem Genet. 1973 Mar;8(3):281–289. doi: 10.1007/BF00486181. [DOI] [PubMed] [Google Scholar]
- Hess D. T., Slater T. M., Wilson M. C., Skene J. H. The 25 kDa synaptosomal-associated protein SNAP-25 is the major methionine-rich polypeptide in rapid axonal transport and a major substrate for palmitoylation in adult CNS. J Neurosci. 1992 Dec;12(12):4634–4641. doi: 10.1523/JNEUROSCI.12-12-04634.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986 Sep;3(5):418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- Ohno S., Wolf U., Atkin N. B. Evolution from fish to mammals by gene duplication. Hereditas. 1968;59(1):169–187. doi: 10.1111/j.1601-5223.1968.tb02169.x. [DOI] [PubMed] [Google Scholar]
- Oyler G. A., Higgins G. A., Hart R. A., Battenberg E., Billingsley M., Bloom F. E., Wilson M. C. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol. 1989 Dec;109(6 Pt 1):3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz-Gutierrez A., Ono S. The evidence of gene duplication for S-form NADP-linked isocitrate dehydrogenase in carp and goldfish. Biochem Genet. 1970 Feb;4(1):93–99. doi: 10.1007/BF00484021. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993 Mar 25;362(6418):318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]