Abstract
Cardiovascular complications are a major cause of morbidity and mortality in patients with end-stage liver disease (ESLD) undergoing liver transplantation. Identifying candidates at the highest risk of postoperative cardiovascular complications is the cornerstone for optimizing the outcome. Ischaemic heart disease contributes to major portion of cardiovascular complications and therefore warrants evaluation in the preoperative period. Patients of ESLD usually demonstrate increased cardiac output, compromised ventricular response to stress, low systemic vascular resistance and occasionally bradycardia. Despite various recommendations for preoperative evaluation of cardiovascular disease in liver transplant candidates, a considerable controversy on screening methodology persists. This review critically focuses on the rapidly expanding body of evidence for diagnosis and risk stratification of cardiovascular disorder in liver transplant candidates.
Keywords: Cardiovascular system evaluation, liver transplant, preoperative evaluation
INTRODUCTION
Over last three decades, liver transplantation (LT) has emerged as the definitive treatment for patients with decompensated end-stage liver disease (ESLD). Improved surgical techniques along with better perioperative management and advances in postoperative immunosuppression have transformed LT from being a high-risk and high-mortality procedure to a routinely performed surgery.[1,2] The candidates for LT usually have multiple comorbidities including cardiovascular disease.[3] Presence of cardiovascular disease is a predictor of poor prognosis within this patient population.[1] Hence, the identification of those at risk remains a key clinical priority and requires a systematic and exhaustive pretransplantation cardiovascular assessment.
The authors searched articles published on liver transplant between 1990 and December 2013 on PubMed with keywords: Liver transplant, preoperative evaluation, preoperative cardiovascular evaluation and preoperative cardiac evaluation. The full text articles published in English language were considered. The relevant references cited in the bibliography of selected articles were also retrieved. The authors reviewed the available literature to analyse the association between preoperative cardiovascular evaluation techniques and postoperative outcomes.
Cardiovascular disease consequent to porto-pulmonary hypertension and hepato-pulmonary syndrome were not included.
SPECTRUM OF CARDIOVASCULAR DISEASES IN PATIENTS FOR LIVER TRANSPLANT
The cardiovascular involvement in ESLD patients may vary from subtle electrocardiogram (ECG) changes to heart failure [Table 1]. The presence of high resting cardiac output (CO) and low systemic vascular resistance (SVR) in ESLD and perioperative haemodynamic alterations during LT adversely affect the outcome of patients with cardiovascular involvement.[4]
Table 1.
Spectrum of cardiovascular involvement in ESLD

CORONARY ARTERY DISEASE
The overall prevalence of coronary artery disease (CAD) in chronic liver disease (CLD) has been reported to be 2–28% with highest prevalence in patients aged over 50 years.[5,6] In liver disease, chronic inflammation and decreased SVR along with increased blood flow can predispose to plaque rupture and thus, precipitate the acute coronary syndrome. Furthermore, increased metabolic demand may worsen these adverse conditions. The degree of coronary artery stenosis does not always determine the symptomatology of CAD.[7]
Risk factors for CAD are also prevalent in liver transplant candidates.[8] Age >50 years, male gender, hypertension, altered lipid metabolism, diabetes mellitus and obesity are the most prevalent clinical attributes. The presence of two or more factors (other than age) places these patients at a moderate to severe risk of CAD.[8] Further, the diagnosis of nonalcoholic steatohepatitis (NASH) independently increases the risk of CAD with critical CAD occurring in approximately 23% of patients.[9]
Despite the advances in diagnosis and management of CAD and improved perioperative techniques of LT, the mortality and morbidity rates still remain significantly high.[1,2] Hence, identification of asymptomatic patients with critical CAD continues to be a big challenge.
CARDIOMYOPATHY
Alcohol abuse can potentially damage both liver and the heart. Heart failure may occur even before the occurrence of significant liver damage.[10] The spectrum of cardiac involvement in heavy drinkers varies from diastolic abnormality of left ventricle with or without ventricular hypertrophy, alcoholic nonischaemic dilated cardiomyopathy involving both ventricles to cirrhosis associated cardiomyopathy.[11] The latter is characterised by impaired contractile responsiveness to stress or altered diastolic function with electrophysiological abnormalities without any other known cardiac disease.[12] The cardiac manifestations of cirrhosis can occur regardless of the aetiology of cirrhosis.[13] However, no significant difference has been reported in cardiac structural and functional parameters between alcoholic and nonalcoholic cirrhosis.[14] The features of cirrhotic cardiomyopathy are summarized in Table 2.[15]
Table 2.
Diagnostic criteria for cirrhotic cardiomyopathy

Systolic dysfunction
Systolic dysfunction in cirrhotics is characterized by inability of heart to increase ejection fraction under stress.[16] Systolic dysfunction also explains the high incidence of pulmonary oedema (18%) in cirrhotics after LT.[13]
Diastolic dysfunction
The trans-mitral blood flow is altered in about half of patients with cirrhosis.[17] Diastolic dysfunction is more severe in patients with ascites and usually precedes systolic dysfunction.[18] It may result in pulmonary oedema and heart failure following orthotopic liver transplantation (OLT) and transjugular intrahepatic portosystemic shunt (TIPSS) and thus contributes to high morbidity and mortality.[19]
Electrophysiological abnormality
Prolonged corrected QT (QTc) interval is present in 45% of cirrhotics.[16] Prolongation of the QTc may lead to electromechanical uncoupling. This may lead to sudden cardiac death in cirrhotics following stressful condition like OLT and TIPSS.[16] Mohamed et al. found higher incidence of prolonged QTc in patients who died after LT than the survivors.[20] On long term, OLT often improves or normalizes QTc prolongation in 50% of the patient population.[21]
Autonomic dysfunction
The majority (87%) of patients who undergo LT suffer from autonomic dysfunction.[22] Four years mortality is 30% in patients of ESLD with dysautonomia compared to 6% without dysautonomia.[23] The degree of dysautonomia usually improves after LT in up to 63% of patients within 6–7 months.[22]
HYPERTENSION
Chronic alcohol intake may contribute to blood pressure elevation.[11] In addition, Kadayifci et al. observed prevalence of hypertension significantly higher in patients with NASH related cirrhosis.[24] However, due to decreased SVR in ESLD, most patients do not require pretransplant treatment for hypertension.[25]
CARDIAC EVALUATION IN LIVER TRANSPLANTATION CANDIDATE
It is recommended to evaluate a LT candidate for any active cardiac condition by completing a detailed history and physical examination (Class I). Generally, routine preoperative cardiovascular tests are performed in the form of ECG and two-dimensional echocardiography [Table 3]. Further investigations are done as per the individual characteristics and transplant center protocols. Studies of clinical importance that have evaluated various modalities for preoperative cardiac assessment in LT recipients have been summarized in Table 4. Currently, there is scarcity of guidelines that outline the optimal cardiovascular risk stratification strategy for LT candidates. This is perhaps because there is still a significant knowledge gap in the study of cardiovascular outcomes.
Table 3.
Cardiovascular evaluation for liver transplant
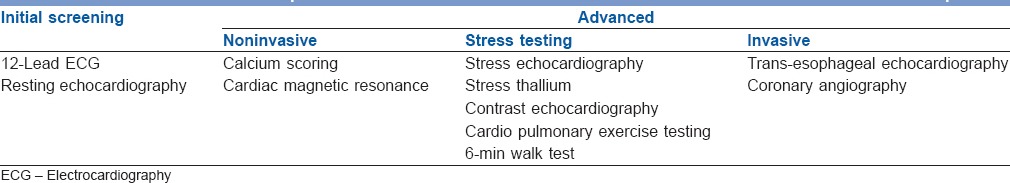
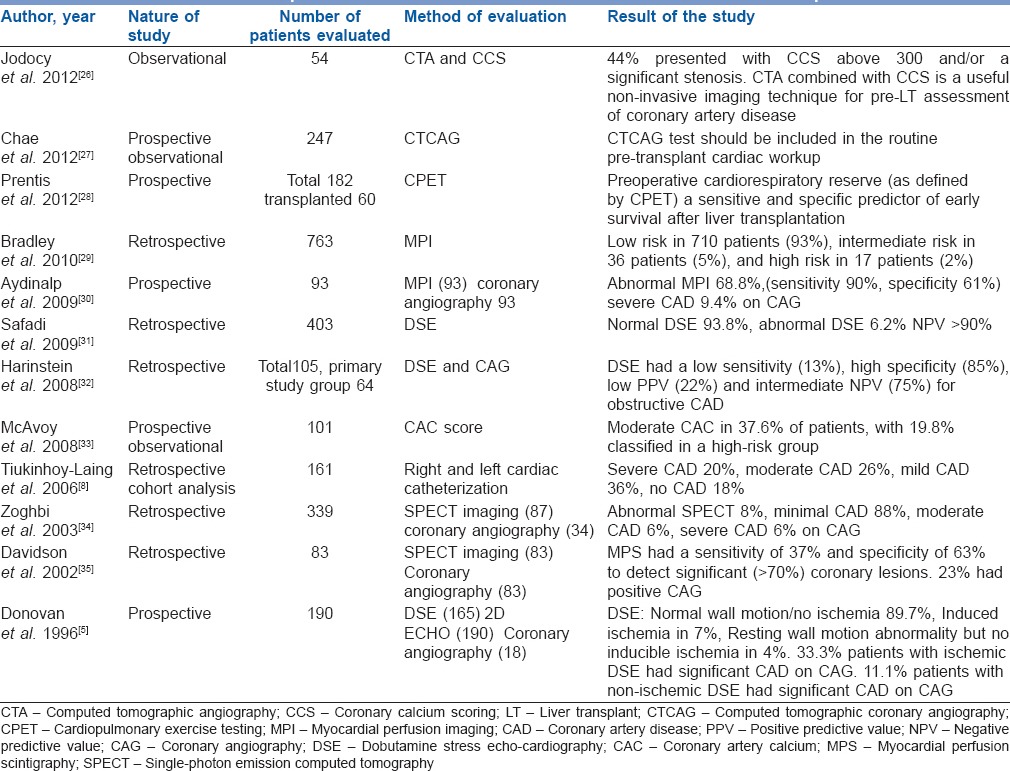
Table 4.
Studies that report evaluation for cardiovascular disease in liver transplant patients
Electrocardiography
A 12-lead ECG is part of routine cardiac evaluation in liver transplant candidate. Prolonged QTc interval is the common ECG abnormality in patients with CLD.[16]
Two-dimensional echocardiography
Echocardiography is helpful in detecting structural and functional heart abnormalities.[36] Right ventricular systolic pressure or pulmonary artery pressure can also be measured with Doppler echocardiography. The presence of hepatopulmonary syndrome may also be evaluated by echocardiography with bubble contrast.[37]
Stress testing
High prevalence of CAD, inability of CLD patients to perform exercise and significant cardiovascular instability during LT necessitates pharmacological stress testing before LT. However, literature provides controversial evidence in terms of both the optimum stress imaging modality in this patient group and its utility in predicting outcomes.[38]
DOBUTAMINE STRESS ECHOCARDIOGRAPHY
Dobutamine stress echocardiography (DSE) is the most widely used screening tool for risk stratification. The clinical effect of dobutamine mimics haemodynamic conditions encountered during LT. The sensitivity and specificity of DSE in patients of CLD vary from 12-100% to 57–100% respectively.[39] This appears secondary to the inability to reach target heart rate possibly due to chronotropic incompetency and beta blocker therapy. In a retrospective analysis, Harinstein et al. observed sensitivity, specificity and negative predictive value (NPV) of DSE to be 13%, 85% and 75% respectively in CAD with obstruction >70%. The authors concluded that coronary angiography (CAG) should be performed in liver transplant recipients who are at high-risk for CAD.[32] Williams et al. concluded that DSE positivity did not correlate with intraoperative cardiac events.[40] Safadi et al. examined the correlation between preoperative DSE and adverse cardiac events at 30 days and concluded that an abnormal stress echocardiography was not associated with adverse cardiac outcome but normal stress test had a very high NPV (>90%) in all patients undergoing LT.[31]
NUCLEAR MYOCARDIAL PERFUSION SCANNING
Dipyridamole or adenosine also demonstrate conflicting results as chronic vasodilatory state in CLD limits the drug induced vasodilation and necessary increase in coronary blood flow. In an observational analysis, Bradley et al. reported approximately 93% patients have a low-risk myocardial perfusion imaging (MPI), 5% have an intermediate-risk MPI, and 2% have a high-risk MPI study. They concluded that stress MPI results should not be considered for determining cardiac risk and eligibility for LT.[29] In a single-photon emission computed tomography (SPECT) imaging study where all perfusion abnormalities were accepted as positive, sensitivity and specificity was 37%, and 63% respectively in severe CAD (stenosis >70%).[35] In a prospective study, Aydinalp et al. found the specificity and sensitivity of myocardial perfusion scintigraphy (MPS) to be 61% and 90% in severe CAD (stenosis >70%). Results of myocardial perfusion scanning were abnormal in 68.8% and normal in 31.2%. Of patients with abnormal scans, only 9.4% had severe CAD when CAG was done in patients with abnormal MPS results.[30]
Thus, noninvasive stress testing may help in identifying patients who are at very low-risk for poor cardiac outcomes.
REAL-TIME STRESS MYOCARDIAL CONTRAST PERFUSION ECHOCARDIOGRAPHY
Several studies have shown that real-time stress myocardial contrast perfusion echocardiography (RTMCE) increases the sensitivity and accuracy of DSE in detection of angiographically significant CAD.[41] Tsutsui et al. studied wall motion and myocardial perfusion in 230 patients with RTMCE. Eighty five of these patients underwent LT. They found that 2 years mortality was 24% among patients with normal myocardial perfusion scanning and 45% among those with abnormality.[42] This preliminary observation points toward the increased value of this new imaging technique but further studies are warranted to assess the value in stratifying cardiovascular-risk.
CARDIOPULMONARY EXERCISE TESTING
Cardiopulmonary exercise testing (CPET) simultaneously evaluates the cardiovascular and respiratory system during exercise. Epstein et al. found that markers of cardiovascular reserve, peak oxygen consumption (VO2) and oxygen consumption at anaerobic threshold (VO2-AT), are associated with 100 days outcome following hepatic surgery, whereas resting cardiac and pulmonary function are not.[43] Prentis et al. concluded that submaximal CPET predicts 90 days survival after LT.[28] Though, CPET may provide interesting information, its role in evaluation of LT candidates requires further investigation.
6-MIN WALK TEST
6-min walk test (6MWT) has been found to be significantly lower in ESLD patients than in healthy adults.[44] In a prospective study, Carey et al. observed that 6MWT was inversely related to model for end-stage liver disease score and pretransplant 6MWT<250 m was associated with risk of death on wait list.[45] In another study, Beyer et al. observed improvement in 6MWT post-LT.[46] 6MWT has not been compared to more established modes of investigations and prediction of posttransplant outcomes. Hence, it is of limited value.
CORONARY CALCIUM SCORING
Coronary artery calcium using cardiac computed tomography is an accurate tool to identify early atherosclerotic disease in asymptomatic individuals.[47,48] Calcium score (Agatston Score) directly correlates with the risk of CAD.[48] In an observational study including 54 patients, Jodocy et al. found that 44% presented with coronary calcium scoring (CCS) above 300 and/or a significant stenosis. Remaining 56% patients with normal computed tomographic angiography (CTA) findings were listed for LT without further tests. None of the 54 patients developed cardiovascular events perioperatively. The authors indicated that CTA combined with CCS is a useful noninvasive imaging technique for pre-LT assessment of CAD.[26] Nonetheless, the usefulness of CAC in predicting perioperative and postoperative cardiovascular events in patients undergoing OLT requires further prospective evaluation.
CARDIAC MAGNETIC RESONANCE IMAGING
The clinical applications of Cardiac Magnetic Resonance Imaging (CMR) include assessment of ventricular function, myocardial viability, myocardial perfusion, cardiomyopathy, valvular and other structural heart disease. A prospective trial by Greenwood et al. demonstrated that stress CMR has high diagnostic accuracy in detecting significant CAD and is superior to nuclear stress testing.[49] In the setting of disease affecting both heart and liver like haemochromatosis, CMR may be useful to certain the severity of iron load. Studies are required to establish the role of CMR in the pretransplant evaluation.
CORONARY ANGIOGRAPHY
Coronary angiography is performed to evaluate the presence of CAD in patients with known CAD, more than one cardiovascular-risk factor (other than age) and a positive stress test. According to the American College of Cardiology and the American Heart Association, performing routine CAG in this patient population carries more risk than benefit (class III recommendation).[50] High incidence of clotting disorders and renal insufficiency in the LT candidates may theoretically result in more procedural complications. However, the complications of angiography can be minimized by correcting the coagulopathy before the procedure and by adopting a transradial approach.[51]
Computed tomographic CAG (CTCAG) is emerging as a new modality in noninvasive assessment of CAD and now being used as a useful alternative to CAG. But there is only limited data available for its utility in preoperative evaluation of liver transplant patients.[52] Chae et al. assessed the clinical value of CTCAG and concluded that CTCAG test should be included in the routine pretransplant cardiac workup, along with thallium SPECT.[27]
SUMMARY
A systematic and algorithmic approach to evaluate preoperative cardiac risk in LT candidates may be helpful to optimize post-LT outcome. The same used at authors’ respective institutions is depicted in Figure 1. Furthermore a large multi-centric study to compare different modalities for preoperative cardiac evaluation may help in elucidating best approach to patients with occult or overt cardiac dysfunction.
Figure 1.
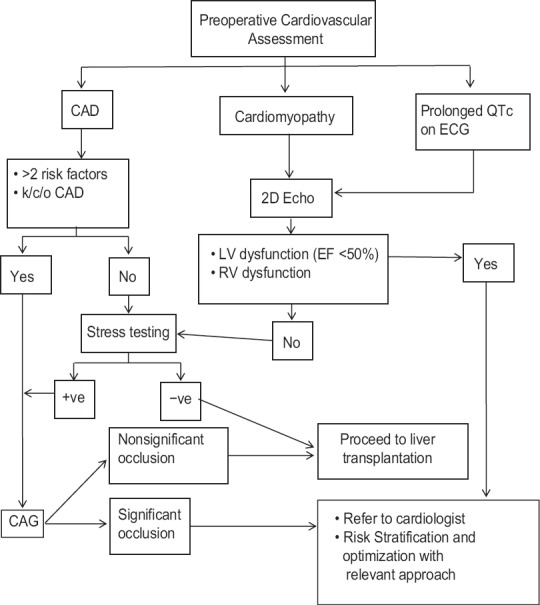
Algorithmic approach towards preoperative cardiovascular evaluation of liver transplant candidates. ECG – electrocardiogram; CAD – Coronary artery disease; LV – Left ventricle; RV – Right ventricle; EF – Ejection fraction
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Plotkin JS, Scott VL, Pinna A, Dobsch BP, De Wolf AM, Kang Y. Morbidity and mortality in patients with coronary artery disease undergoing orthotopic liver transplantation. Liver Transpl Surg. 1996;2:426–30. doi: 10.1002/lt.500020604. [DOI] [PubMed] [Google Scholar]
- 2.Diedrich DA, Findlay JY, Harrison BA, Rosen CB. Influence of coronary artery disease on outcomes after liver transplantation. Transplant Proc. 2008;40:3554–7. doi: 10.1016/j.transproceed.2008.08.129. [DOI] [PubMed] [Google Scholar]
- 3.Xia VW, Taniguchi M, Steadman RH. The changing face of patients presenting for liver transplantation. Curr Opin Organ Transplant. 2008;13:280–4. doi: 10.1097/MOT.0b013e328300a070. [DOI] [PubMed] [Google Scholar]
- 4.Bernardi M, Fornalè L, Di Marco C, Trevisani F, Baraldini M, Gasbarrini A, et al. Hyperdynamic circulation of advanced cirrhosis: A re-appraisal based on posture-induced changes in hemodynamics. J Hepatol. 1995;22:309–18. doi: 10.1016/0168-8278(95)80284-3. [DOI] [PubMed] [Google Scholar]
- 5.Donovan CL, Marcovitz PA, Punch JD, Bach DS, Brown KA, Lucey MR, et al. Two-dimensional and dobutamine stress echocardiography in the preoperative assessment of patients with end-stage liver disease prior to orthotopic liver transplantation. Transplantation. 1996;61:1180–8. doi: 10.1097/00007890-199604270-00011. [DOI] [PubMed] [Google Scholar]
- 6.Plotkin JS, Benitez RM, Kuo PC, Njoku MJ, Ridge LA, Lim JW, et al. Dobutamine stress echocardiography for preoperative cardiac risk stratification in patients undergoing orthotopic liver transplantation. Liver Transpl Surg. 1998;4:253–7. doi: 10.1002/lt.500040415. [DOI] [PubMed] [Google Scholar]
- 7.Nagoshi T, Koiwaya Y, Doi H, Eto T. Angiographic coronary morphology in patients with ischemic heart disease. J Cardiol. 2000;36:91–102. [PubMed] [Google Scholar]
- 8.Tiukinhoy-Laing SD, Rossi JS, Bayram M, De Luca L, Gafoor S, Blei A, et al. Cardiac hemodynamic and coronary angiographic characteristics of patients being evaluated for liver transplantation. Am J Cardiol. 2006;98:178–81. doi: 10.1016/j.amjcard.2006.01.089. [DOI] [PubMed] [Google Scholar]
- 9.Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis. 2007;191:235–40. doi: 10.1016/j.atherosclerosis.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Estruch R, Fernández-Solá J, Sacanella E, Paré C, Rubin E, Urbano-Márquez A. Relationship between cardiomyopathy and liver disease in chronic alcoholism. Hepatology. 1995;22:532–8. [PubMed] [Google Scholar]
- 11.George A, Figueredo VM. Alcoholic cardiomyopathy: A review. J Card Fail. 2011;17:844–9. doi: 10.1016/j.cardfail.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Zaky A, Bendjelid K. Appraising cardiac dysfunction in liver transplantation: An ongoing challenge. Liver Int. 2015;35:12–29. doi: 10.1111/liv.12582. [DOI] [PubMed] [Google Scholar]
- 13.Fouad TR, Abdel-Razek WM, Burak KW, Bain VG, Lee SS. Prediction of cardiac complications after liver transplantation. Transplantation. 2009;87:763–70. doi: 10.1097/TP.0b013e318198d734. [DOI] [PubMed] [Google Scholar]
- 14.Alexander J, Mishra P, Desai N, Ambadekar S, Gala B, Sawant P. Cirrhotic cardiomyopathy: Indian scenario. J Gastroenterol Hepatol. 2007;22:395–9. doi: 10.1111/j.1440-1746.2006.04507.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, Song D, Lee SS. Cirrhotic cardiomyopathy. Gastroenterol Clin Biol. 2002;26:842–7. [PubMed] [Google Scholar]
- 16.Wong F. Cirrhotic cardiomyopathy. Hepatol Int. 2009;3:294–304. doi: 10.1007/s12072-008-9109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong F, Villamil A, Merli M. Prevalence of diastolic dysfunction in cirrhosis and its clinical significance. Hepatology. 2011;54:475A. [Google Scholar]
- 18.Lee RF, Glenn TK, Lee SS. Cardiac dysfunction in cirrhosis. Best Pract Res Clin Gastroenterol. 2007;21:125–40. doi: 10.1016/j.bpg.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Rabie RN, Cazzaniga M, Salerno F, Wong F. The use of E/A ratio as a predictor of outcome in cirrhotic patients treated with transjugular intrahepatic portosystemic shunt. Am J Gastroenterol. 2009;104:2458–66. doi: 10.1038/ajg.2009.321. [DOI] [PubMed] [Google Scholar]
- 20.Mohamed R, Forsey PR, Davies MK, Neuberger JM. Effect of liver transplantation on QT interval prolongation and autonomic dysfunction in end-stage liver disease. Hepatology. 1996;23:1128–34. doi: 10.1002/hep.510230529. [DOI] [PubMed] [Google Scholar]
- 21.Møller S, Henriksen JH. Cirrhotic cardiomyopathy: A pathophysiological review of circulatory dysfunction in liver disease. Heart. 2002;87:9–15. doi: 10.1136/heart.87.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lhuillier F, Dalmas ED, Gratadour PM, Cividjian AA, Boillot OC, Quintin L, et al. Spontaneous baroreflex cardiac sensitivity in end-stage liver disease: Effect of liver transplantation. Eur J Anaesthesiol. 2006;23:426–32. doi: 10.1017/S0265021506000184. [DOI] [PubMed] [Google Scholar]
- 23.Hendrickse MT, Thuluvath PJ, Triger DR. Natural history of autonomic neuropathy in chronic liver disease. Lancet. 1992;339:1462–4. doi: 10.1016/0140-6736(92)92042-e. [DOI] [PubMed] [Google Scholar]
- 24.Kadayifci A, Tan V, Ursell PC, Merriman RB, Bass NM. Clinical and pathologic risk factors for atherosclerosis in cirrhosis: A comparison between NASH-related cirrhosis and cirrhosis due to other aetiologies. J Hepatol. 2008;49:595–9. doi: 10.1016/j.jhep.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Lentine KL, Costa SP, Weir MR, Robb JF, Fleisher LA, Kasiske BL, et al. Cardiac disease evaluation and management among kidney and liver transplantation candidates: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2012;60:434–80. doi: 10.1016/j.jacc.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Jodocy D, Abbrederis S, Graziadei IW, Vogel W, Pachinger O, Feuchtner GM, et al. Coronary computer tomographic angiography for preoperative risk stratification in patients undergoing liver transplantation. Eur J Radiol. 2012;81:2260–4. doi: 10.1016/j.ejrad.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Chae WY, Hwang S, Yoon YI, Kang MC, Moon DB, Song GW, et al. Clinical value of preoperative coronary risk assessment by computed tomographic arteriography prior to adult living donor liver transplantation. Transplant Proc. 2012;44:415–7. doi: 10.1016/j.transproceed.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 28.Prentis JM, Manas DM, Trenell MI, Hudson M, Jones DJ, Snowden CP. Submaximal cardiopulmonary exercise testing predicts 90-day survival after liver transplantation. Liver Transpl. 2012;18:152–9. doi: 10.1002/lt.22426. [DOI] [PubMed] [Google Scholar]
- 29.Bradley SM, Soine LA, Caldwell JH, Goldberg SL. Screening stress myocardial perfusion imaging and eligibility for liver transplantation. Am J Cardiol. 2010;105:1010–3. doi: 10.1016/j.amjcard.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 30.Aydinalp A, Bal U, Atar I, Ertan C, Aktas A, Yildirir A, et al. Value of stress myocardial perfusion scanning in diagnosis of severe coronary artery disease in liver transplantation candidates. Transplant Proc. 2009;41:3757–60. doi: 10.1016/j.transproceed.2009.06.219. [DOI] [PubMed] [Google Scholar]
- 31.Safadi A, Homsi M, Maskoun W, Lane KA, Singh I, Sawada SG, et al. Perioperative risk predictors of cardiac outcomes in patients undergoing liver transplantation surgery. Circulation. 2009;120:1189–94. doi: 10.1161/CIRCULATIONAHA.108.847178. [DOI] [PubMed] [Google Scholar]
- 32.Harinstein ME, Flaherty JD, Ansari AH, Robin J, Davidson CJ, Rossi JS, et al. Predictive value of dobutamine stress echocardiography for coronary artery disease detection in liver transplant candidates. Am J Transplant. 2008;8:1523–8. doi: 10.1111/j.1600-6143.2008.02276.x. [DOI] [PubMed] [Google Scholar]
- 33.McAvoy NC, Kochar N, McKillop G, Newby DE, Hayes PC. Prevalence of coronary artery calcification in patients undergoing assessment for orthotopic liver transplantation. Liver Transpl. 2008;14:1725–31. doi: 10.1002/lt.21540. [DOI] [PubMed] [Google Scholar]
- 34.Zoghbi GJ, Patel AD, Ershadi RE, Heo J, Bynon JS, Iskandrian AE. Usefulness of preoperative stress perfusion imaging in predicting prognosis after liver transplantation. Am J Cardiol. 2003;92:1066–71. doi: 10.1016/j.amjcard.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Davidson CJ, Gheorghiade M, Flaherty JD, Elliot MD, Reddy SP, Wang NC, et al. Predictive value of stress myocardial perfusion imaging in liver transplant candidates. Am J Cardiol. 2002;89:359–60. doi: 10.1016/s0002-9149(01)02244-5. [DOI] [PubMed] [Google Scholar]
- 36.Keller H, Bezjak V, Stegaru B, Buss J, Holm E, Heene DL. Ventricular function in cirrhosis and portasystemic shunt: A two-dimensional echocardiographic study. Hepatology. 1988;8:658–62. doi: 10.1002/hep.1840080337. [DOI] [PubMed] [Google Scholar]
- 37.Rodríguez-Roisin R, Krowka MJ, Hervé P, Fallon MB. ERS Task Force Pulmonary-Hepatic Vascular Disorders (PHD) Scientific Committee. Pulmonary-Hepatic vascular Disorders (PHD) Eur Respir J. 2004;24:861–80. doi: 10.1183/09031936.04.00010904. [DOI] [PubMed] [Google Scholar]
- 38.Dec GW, Kondo N, Farrell ML, Dienstag J, Cosimi AB, Semigran MJ. Cardiovascular complications following liver transplantation. Clin Transplant. 1995;9:463–71. [PubMed] [Google Scholar]
- 39.Ehtisham J, Altieri M, Salamé E, Saloux E, Ollivier I, Hamon M. Coronary artery disease in orthotopic liver transplantation: Pretransplant assessment and management. Liver Transpl. 2010;16:550–7. doi: 10.1002/lt.22035. [DOI] [PubMed] [Google Scholar]
- 40.Williams K, Lewis JF, Davis G, Geiser EA. Dobutamine stress echocardiography in patients undergoing liver transplantation evaluation. Transplantation. 2000;69:2354–6. doi: 10.1097/00007890-200006150-00023. [DOI] [PubMed] [Google Scholar]
- 41.Peltier M, Vancraeynest D, Pasquet A, Ay T, Roelants V, D’hondt AM, et al. Assessment of the physiologic significance of coronary disease with dipyridamole real-time myocardial contrast echocardiography. Comparison with technetium-99m sestamibi single-photon emission computed tomography and quantitative coronary angiography. J Am Coll Cardiol. 2004;43:257–64. doi: 10.1016/j.jacc.2003.07.040. [DOI] [PubMed] [Google Scholar]
- 42.Tsutsui JM, Mukherjee S, Elhendy A, Xie F, Lyden ER, O’Leary E, et al. Value of dobutamine stress myocardial contrast perfusion echocardiography in patients with advanced liver disease. Liver Transpl. 2006;12:592–9. doi: 10.1002/lt.20651. [DOI] [PubMed] [Google Scholar]
- 43.Epstein SK, Ciubotaru RL, Zilberberg MD, Kaplan LM, Jacoby C, Freeman R, et al. Analysis of impaired exercise capacity in patients with cirrhosis. Dig Dis Sci. 1998;43:1701–7. doi: 10.1023/a:1018867232562. [DOI] [PubMed] [Google Scholar]
- 44.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–7. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 45.Carey EJ, Steidley DE, Aqel BA, Byrne TJ, Mekeel KL, Rakela J, et al. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transpl. 2010;16:1373–8. doi: 10.1002/lt.22167. [DOI] [PubMed] [Google Scholar]
- 46.Beyer N, Aadahl M, Strange B, Kirkegaard P, Hansen BA, Mohr T, et al. Improved physical performance after orthotopic liver transplantation. Liver Transpl Surg. 1999;5:301–9. doi: 10.1002/lt.500050406. [DOI] [PubMed] [Google Scholar]
- 47.Budoff MJ. Screening for ischemic heart disease with cardiac CT: Current recommendations. Scientifica (Cairo) 2012. 2012:812046. doi: 10.6064/2012/812046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blaha M, Budoff MJ, Shaw LJ, Khosa F, Rumberger JA, Berman D, et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2:692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 49.Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): A prospective trial. Lancet. 2012;379:453–60. doi: 10.1016/S0140-6736(11)61335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof EL, Fleischmann KE, et al. ACC/AHA 2007 Guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2007;50:161. [Google Scholar]
- 51.Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: A systematic review and meta-analysis of randomized trials. Am Heart J. 2009;157:132–40. doi: 10.1016/j.ahj.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 52.Appleton CP, Hurst RT. Reducing coronary artery disease events in liver transplant patients: Moving toward identifying the vulnerable patient. Liver Transpl. 2008;14:1691–3. doi: 10.1002/lt.21660. [DOI] [PubMed] [Google Scholar]


